The original cohort
Prostate Cancer data Base Sweden (PCBaSe) 2.0 1,2 is based on linkages of different national health care registers with the Swedish National Prostate Cancer Register, and contains more than 110 000 registered cases since 1998. It also includes a selection of two control series of men free of prostate cancer (PCa) at the time of samples, as well as information on brothers of men diagnosed with PCa.
What is the reason for the new data collection?
PCBaSe Traject includes new linkages, so that we cannot only study primary treatment but also the entire PCa treatment trajectory. Prostate cancer has become a chronic disease in a large proportion of afflicted men, since its fatality rate is low, but statistical cure is never attained. 3 Thus, many men with PCa receive multiple subsequent treatments and their treatment trajectory often spans decades. In PCBaSe Traject , the PCa treatment trajectory is delineated by use of data on primary treatment in the National Prostate Cancer Register (NPCR) of Sweden, verified by data obtained by linkages with the Prescribed Drug Registry 4 (i.e. use of androgen deprivation therapy (ADT)) and the National Patient Registry (i.e. surgical procedures and hospital admissions). In addition, data were collected on radiotherapy (RetroRad) from oncology information systems and local databases at radiotherapy departments for treatments performed before 2008 ( Figure 1 ).
Figure 1.
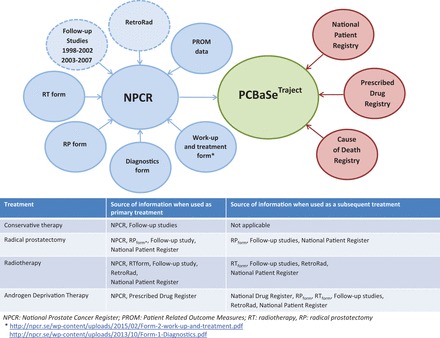
Overview of registers providing data for PCBaSe Traject . Data files in blue are part of the National Prostate Cancer Register. Registers with dashed lines are retrospectively collected. Data files in red are held at the Swedish Board of Health and Welfare.
What will be new areas of research?
Previously all our studies were limited to treatment at time of diagnosis. The new focus of PCBaSe Traject is to outline the full treatment trajectory for a nationwide, population-based cohort of men with PCa. PCBaSe Traject will also continue to serve as a platform for studies on patterns of care, outcomes and adverse effects of treatment in post-authorization surveillance studies.
Who is in the cohort?
PCBaSe Traject focuses specifically on men diagnosed with PCa between 1992 and 2012 with available information on their complete treatment trajectory. It is an update of PCBaSe 3.0, which is already an update of PCBaSe 2.0 through inclusion of two additional years of follow-up. 1,2 However, since the Prescribed Drug Registry started on 1 July 2005, 4 ADT prescriptions could only be captured as of 1 January 2006, allowing for a 6-month run-in period. Therefore, only men who were alive and living in Sweden on 1 January 2006 were eligible ( n = 116 962) for PCBaSe Traject . We excluded: 6146 men who, according to the National Prostate Cancer Register (NPCR), received ADT as primary treatment but had no corresponding filled prescription; 938 men who had undergone curative treatment according to NPCR, but with no corresponding information in other registers; 1948 men who had received ADT according to the Prescribed Drug Registry in the run-in period (July 2005–January 2006), without ADT as primary treatment in NPCR and thus unknown date of ADT initiation; and 1008 men with missing information on treatment in the NPCR as well as in other registers. A total of 106 922 men with PCa with complete and coherent information on treatment were included in the final study population ( Figure 2 , Table 1 ). In addition, a comparison cohort of PCa-free men matched by birth year and county of residence was selected from the background population, as previously described in detail. 1
Figure 2.
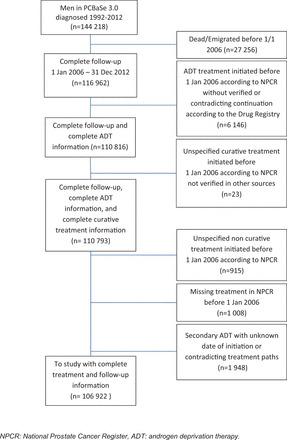
Flow chart of patient selection for PCBaSe Traject .
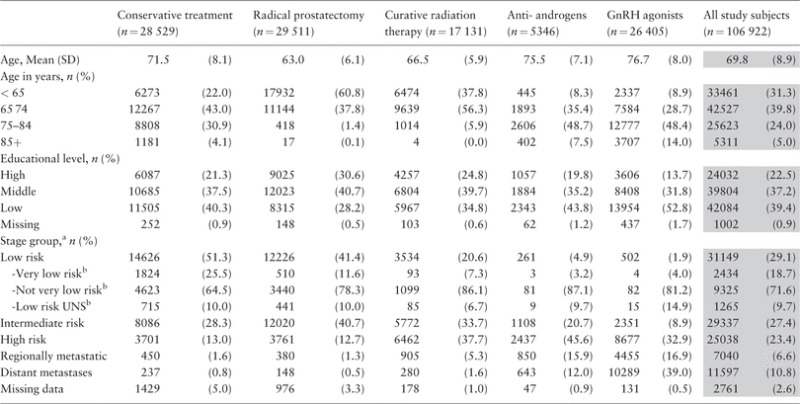
Table 1.
Characteristics of men with prostate cancer in PCBaSe Traject
|
GnRH: Gonadotropin Releasing Hormone
a Based on a modification of the guidelines of the National Comprehensive Cancer Network (1): Low risk (T1–2, Gleason score 2–6 and PSA < 10 ng/ml); intermediate risk (T1–2, Gleason score 7 and/or PSA 10 to < 20 ng/ml); High risk (T3 and/or Gleason score 8–10 and/or PSA 20 to < 50 ng/ml); regionally metastatic disease (T4 and/or N1 and/or PSA 50 to < 100 ng/ml in the absence of distant metastases (M0 or Mx)); distant metastases (M1 and/or PSA = 100 ng/ml and above).
b For men diagnosed as of 2008.
What has been measured?
PCBaSe Traject is designed to capture information on the treatment trajectory following the order of treatments: conservative treatment (CT) → radical prostatectomy → radiotherapy → anti-androgens (AA) → gonadotropin-releasing hormone (GnRH) agonists, in which any treatment may be omitted.
Information about conservative treatment was collected from NPCR and the NPCR Follow-up study 5 in three categories ( Figure 3 ): active surveillance [i.e. regular follow-up with prostate-specific antigen (PSA) testing and repeated biopsies with the intent to convert to curative treatment if signs of progression occur]; watchful waiting (i.e. a treatment strategy where ADT is used if symptomatic progression occurs); and unspecified conservative treatment (CT UNS ) which is a mixture of active surveillance and watchful waiting because the NPCR did not make the distinction between these two types of conservative treatment before 2007. 5 Men registered in the NPCR with active surveillance were only considered to be on active surveillance if they met the inclusion criteria from the Study on Active Monitoring in Sweden (SAMS), i.e. low-risk cancer in men below age 70. 6 Men registered in the NPCR with watchful waiting, who underwent radical prostatectomy or radiotherapy within 2 years of diagnosis were reclassified as curative treatment. Curative treatment initiated more than 2 years after diagnosis, for men originally registered with watchful waiting, were registered as non-standard procedures. Watchful waiting was reclassified to primary ADT when GnRH agonists were used less than 2 months after diagnosis ( Figure 3 ).
Figure 3.
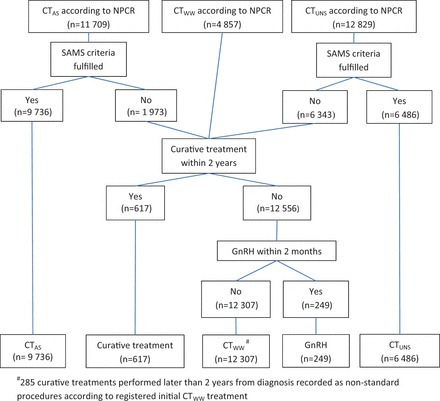
Flow chart of data collection for conservative treatment in PCBaSe Traject .
NPCR: National Prostate Cancer Register, CT WW : watchful waiting, CT AS : active surveillance, CT UNS : Unspecified conservative treatment, SAMS: Study on Active Monitoring in Sweden.
Information on curative treatment was collected from the NPCR, the NPCR Follow-up studies, 5 the National Patient Registry, and RetroRad to define radical prostatectomy and radiotherapy. The majority of primary radical prostatectomies and radiotherapies registered in NPCR were verified by data in other registers ( Figure 4 ). A total of 679 men registered with curative treatment in NPCR, but with no corresponding treatment in other registers, and who had received ADT according to the Prescribed Drug Registry within 9 months of date of diagnosis, were considered to be treated with primary ADT.
Figure 4.
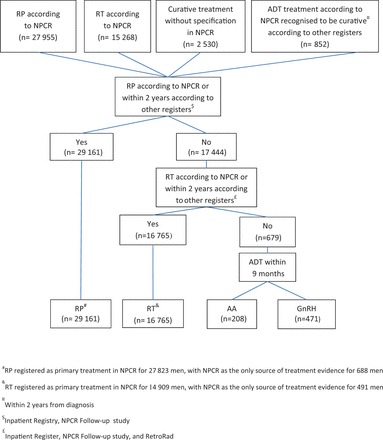
Flow chart of data collection for curative treatment in PCBaSe Traject .
To obtain information on deferred radical prostatectomy following a period of conservative treatment, we used the NPCR Follow-up study and information from the Patient Registry ( Figure 2 ). Since 1987, the National Patient Registry has collected information regarding inpatient care. Each record contains medical information on surgical procedures, hospital department, and discharge diagnoses coded according to ICD-9 or ICD-10. 2 A common classification of surgical procedures has been used in Sweden since 1997. 7
To collect information on deferred curative radiotherapy following a period of conservative treatment or as adjuvant/salvage therapy following radical prostatectomy, we used information from the NPCR, the NPCR Follow-up study, RetroRad and the National Patient Registry ( Figure 2 ). In 2008, the NPCR created a specific form to collect information on radiotherapy (RT Form ) (Appendix A1, available as Supplementary data at IJE online).There is little information regarding radiotherapy in the National Patient Registry, although this information has gradually become more detailed in recent years. To overcome this lack of information, mainly for the time period before 2008, a retrospective data collection was performed (RetroRad). Radiotherapy data were retrieved from the verification/oncology information systems and local databases of radiotherapy departments in Sweden, including information on type of radiotherapy, treatment dates, total dose and fractionation. RetroRad captures information from the early 1990s until 2008–12, depending on the availability of databases at each centre.
Information about primary ADT was collected from the NPCR, the NPCR Follow-up study, 5 the National Patient Registry and the Prescribed Drug Registry ( Figure 2 ). First, we assessed whether a prescription had been filled for an anti-androgen or a GnRH agonist within 3 months after date of diagnosis. If this was not the case, the treatment strategy was defined as watchful waiting ( Figure 5 ). Hence, ADT used as adjuvant or neoadjuvant in combination with curative treatment was ignored. To distinguish neoadjuvant and adjuvant ADT from use of ADT due to disease progression, we retrieved information on ADT from the Prescribed Drug Registry and compared with information for ADT on the RT Form and RP Form in the NPCR. ADT given before curative treatment was considered as neoadjuvant if delivered less than 18 months prior to that treatment. If ADT was initiated more than 18 months prior to curative treatment, it was considered to be primary ADT. With respect to adjuvant ADT for men who did not have information on the RT Form or RP Form in the NPCR, use of ADT without a discontinuation of at least 9 months during the first 3 years after curative treatment was considered to be adjuvant. For men receiving curative treatment with no information from the RT Form or RP Form , dates were retrieved from RetroRAD or the Patient Registry. Furthermore, a distinction was required between anti-androgen monotherapy and flare protection. Anti-androgens initiated less than 3 months before subsequent GnRH agonists were considered to be flare protection, whereas other time frames for anti-androgen prescriptions were considered as indicators of anti-androgen monotherapy. Additional treatment information was obtained from the National Patient Registry, the Prescribed Drug Registry and the RetroRad ( Figure 2 ).
Figure 5.
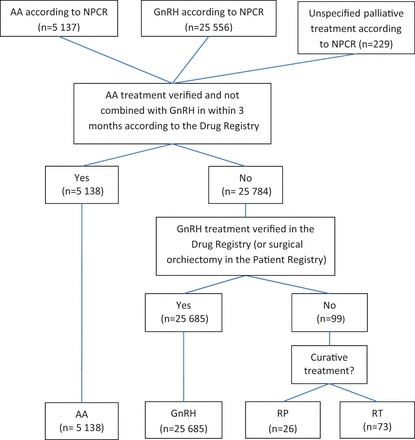
Flow chart of data collection for androgen deprivation therapy in PCBaSe Traject .
NPCR: National Prostate Cancer Register, RP: radical prostatectomy RT: curative radiotherapy, ADT: androgen deprivation therapy, AA: anti-androgen monotherapy, GnRH: GnRH agonists.
Follow-up in PCBaSe for data from the Prescribed Drug Registry, the National Patient Registry and the Cause of Death Registry are annually updated. All data management was conducted with SAS 9.3 (SAS Institute, Cary, NC), and statistical modelling was performed with R 2.13.2 (R Foundation for Statistical Computing, Vienna). The Research Ethics Board at Umeå University Hospital approved the study.
What has it found?
We found that the time to treatment changes was dependent on type of primary treatment and subsequent treatments ( Figure 6 ). For example, men treated with primary radical prostatectomywere more likely to receive adjuvant or salvage radiotherapy than men who underwent deferred prostatectomy after an initial period of active surveillance. There was also a difference in timing and proportion of men who received androgen deprivation therapy due to disease progression after primary or secondary radiotherapy, as ADT was introduced earlier and to a larger proportion of men who had received primary radiotherapy. One-third of the men who were registered as active surveillance had converted to curative treatment 6 years after diagnosis, and most of these men had undergone radical prostatectomy (24%) or radiotherapy (12%) (results not shown). The proportion of men who died of Pca was higher among those who receivedGnRH agonists as primary treatment, compared with men who received GnRH as second-line treatment. Overall,17% of men received two subsequent treatments and 2% received three treatments ( Table 2 ).
Figure 6.
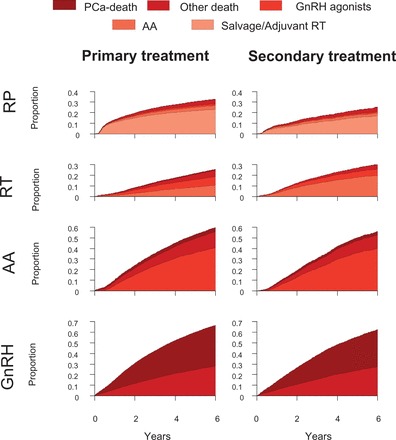
Time to treatment changes based on different primary and subsequent treatments for men with prostate cancer as registered in PCBaSe Traject .
PCa: prostate cancer, AA: anti-androgen monotherapy, RT: curative radiotherapy, RP: radical prostatectomy.
Table 2.
Treatment trajectories in PCBaSe Traject
| Men with one treatment | n | (%) |
|---|---|---|
| CT AS | 6609 | (6.2) |
| CT WW | 8280 | (7.7) |
| CT UNS | 3667 | (3.4) |
| RP | 23 252 | (21.7) |
| CurRT | 14 525 | (13.6) |
| AA | 3534 | (3.3) |
| GnRH | 26 405 | (24.7) |
| Men with two treatments | ||
| CT AS → RP | 1584 | (1.5) |
| CT AS → RT | 824 | (0.8) |
| CT AS → AA | 271 | (0.3) |
| CT AS → GnRH | 186 | (0.2) |
| CT WW → AA | 1274 | (1.2) |
| CT WW → GnRH | 2402 | (2.2) |
| CT UNS → RP | 674 | (0.6) |
| CT UNS → RT | 523 | (0.5) |
| CT UNS → AA | 495 | (0.5) |
| CT UNS → GnRH | 746 | (0.7) |
| RP → RT | 3829 | (3.6) |
| RP → AA | 720 | (0.7) |
| RP → GnRH | 429 | (0.4) |
| CurRT → AA | 1065 | (1.0) |
| CurRT → GnRH | 1098 | (1.0) |
| AA → GnRH | 1812 | (1.7) |
| Men with three treatments | ||
| Starting in CT | 916 | (0.9) |
| Starting in RP | 1077 | (1.0) |
| Starting in RT | 443 | (0.4) |
| Men with four treatments | ||
| Starting in CT | 72 | (0.1) |
| Starting in RP | 204 | (0.2) |
| Men with five treatments | ||
| Starting in CT | 6 | (0.0) |
CT, conservative treatment; CT WW , watchful waiting; CT AS , active surveillance; CT UNS , unspecified conservative treatment; RP, radical prostatectomy; RT, curative radiotherapy; AA, anti-androgen monotherapy; GnRH, gonadotropin-releasing hormone agonist.
Figure 7 shows how misclassification of treatment due to incomplete information may lead to erroneous conclusions. Under the assumption that ADT implies disseminated PCa, using data from the Prescribed Drug Registry only would over-estimate the prevalence of disseminated disease. However, using multiple data sources, PCBaSe Traject identified men on adjuvant ADT, i.e. combined with radiotherapy, thus avoiding this misclassification. Furthermore, combined treatment data in PCBaSe Traject correctly demonstrated the number of men with low-risk cancer on GnRH agonists as secondary treatment, who died of PCa, whereas data from the Prescribed Drug Registry only would have underestimated this number.
Figure 7.
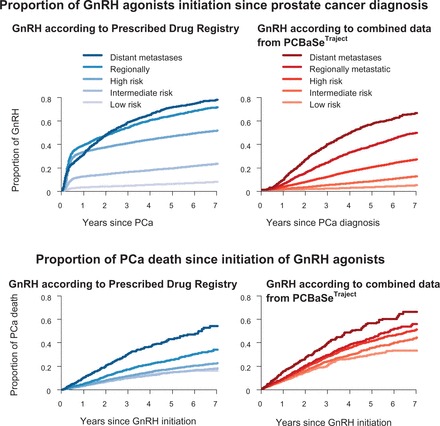
Time to initiation of GnRH agonists and time to PCa death since time of GnRH agonists initiation for men with prostate cancer, as registered in PCBaSe Traject .
The additional data from PCBaSe Traject have already allowed us to investigate the association between types and duration of ADT and risk of cardiovascular disease (CVD) in greater detail than was previously possible. 8 We found an increased risk of CVD, in particular within the first year of ADT, which was strongest for men with a previous history of CVD.
In another study, we investigated long-term complications for men who were curatively treated with radiotherapy or radical prostatectomy. We specifically evaluated urinary incontinence, lower urinary tract symptoms and gastrointestinal symptoms up to 12 years after treatment. It was found that these complications mostly occurred within the first 3 years after radical prostatectomy, whereas complications after radiotherapy were more frequent at a later date after treatment. 9
An overview of all publications based on PCBaSe Sweden can be found at [ www.npcr.se/forskning ].
What are the main strengths and weaknesses?
PCBaSe Traject contains comprehensive data on the treatment trajectory for men with PCa. Our results demonstrate that both clinical knowledge and ‘register know how’ are necessary to correctly perform analysis and interpret data from such register-based studies. PCBaSe Traject showed high concordance of treatment classification between different registers. One limitation of PCBaSe Traject is the lack of information on indications for additional treatment. Continuous registration of PSA levels would be a useful way to assess disease progression and efforts to obtain such data are under way. Finally, it is worth noting that due to the later start date of the Prescribed Drug Registry (1 July 2005), PCBaSe Traject requires use of left-truncation in all time-to-event analysis.
Can I get hold of the data? Where can I find out more?
The steering groups of the NPCR and PCBaSe welcome external collaborations. For more information please see [ www.npcr.se/in-english ], where registration forms, manuals and annual reports from the NPCR are found as well as a full list of publications from PCBaSe.
Funding
Funding came from the Swedish Research Council 825-2012-5047, Stockholm Cancer Society, the Swedish Council for Working Life and Social Research, and Västerbotten County Council.
Supplementary Material
Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg Karlsson, Ove Andrén, Anna Bill Axelson, Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm Eriksson,Bill Pettersson, David Robinson, Mats Andén, Jan-Erik Damber, Jonas Hugosson, Ingela Franck Lissbrant, Maria Nyberg, Göran Ahlgren, Ola Bratt, René Blom, Lars Egevad, Calle Waller, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin, Hans Garmo, Mats Lambe, Karin Hellström.
Conflict of interest: None declared.
References
- 1. Van Hemelrijck M, Wigertz A, Sandin F, et al. . Cohort Profile: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0 . Int J Epidemiol 2013. ; 42:956 – 67 . [DOI] [PubMed] [Google Scholar]
- 2. Statistics in the Areas of Health and Medical Care. 2007. http://www.socialstyrelsen.se/en/Statistics/Statistical_databases.htm .
- 3. Center MM, Jemal A, Lortet-Tieulent J, et al. . International variation in prostate cancer incidence and mortality rates . Eur Urol 2012. ; 61:1079 – 92 . [DOI] [PubMed] [Google Scholar]
- 4. National Board of Health and Wellfare . Swedish Prescribed Drug Register 2010 . http://www.socialstyrelsen.se/register/halsodataregister/lakemedelsregistret . [Google Scholar]
- 5. Stattin P, Holmberg E, Johansson JE, et al. . Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study . J Natl Cancer Inst 2010. ; 102:950 – 58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bratt O, Carlsson S, Holmberg E, et al. . The Study of Active Monitoring in Sweden (SAMS): A randomized study comparing two different follow-up schedules for active surveillance of low-risk prostate cancer . Scand J Urol 2013. ; 47:347 – 55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Socialstyrelsen . Klassifikation av kirurgiska åtgärder 1997 . 2004. . [Google Scholar]
- 8. O'Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M . Risk and Timing of Cardiovascular Disease After Androgen-Deprivation Therapy in Men With Prostate Cancer . J Clin Oncol 2015. ; 33:1243 – 51 . [DOI] [PubMed] [Google Scholar]
- 9. Thellenberg-Karlsson C, Fridriksson J, Folkvaljon Y, et al. . Lag time to adverse events after radical prostatectomy and curative radiotherapy. 2015 Genitourinary Cancers Symposium, 26–28 February, Orlando, FL. : Journal of Clinical Oncology; 2015. p. 49 . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


