Abstract
Background: The Ebola virus disease outbreak that started in Western Africa in 2013 was unprecedented because it spread within densely populated urban environments and affected many thousands of people. As a result, previous advice and guidelines need to be critically reviewed, especially with regard to transmission risks in different contexts.
Methods: Scientific and grey literature were searched for articles about any African filovirus. Articles were screened for information about transmission (prevalence or odds ratios especially). Data were extracted from eligible articles and summarized narratively with partial meta-analysis. Study quality was also evaluated.
Results: A total of 31 reports were selected from 6552 found in the initial search. Eight papers gave numerical odds for contracting filovirus illness; 23 further articles provided supporting anecdotal observations about how transmission probably occurred for individuals. Many forms of contact (conversation, sharing a meal, sharing a bed, direct or indirect touching) were unlikely to result in disease transmission during incubation or early illness. Among household contacts who reported directly touching a case, the attack rate was 32% [95% confidence interval (CI) 26–38%]. Risk of disease transmission between household members without direct contact was low (1%; 95% CI 0–5%). Caring for a case in the community, especially until death, and participation in traditional funeral rites were strongly associated with acquiring disease, probably due to a high degree of direct physical contact with case or cadaver.
Conclusions: Transmission of filovirus is unlikely except through close contact, especially during the most severe stages of acute illness. More data are needed about the context, intimacy and timing of contact required to raise the odds of disease transmission. Risk factors specific to urban settings may need to be determined.
Keywords: Ebola virus disease, Marburg virus, risk factors, bodily fluids, systematic review
Key Messages
Human-to-human transmission of filoviruses usually requires direct contact with a symptomatic individual.
Transmission through indirect contact has been reported, but appears to be uncommon.
There is a need for more primary epidemiological research in urban communities.
During outbreaks, provision of appropriate care through designated specialist health facilities reduces transmission rates.
Introduction
The 2013–15 epidemic of Ebola virus disease (EVD) in Western Africa is by far the largest and most widespread outbreak of this disease to date and case numbers far exceed the total from all previous EVD emergences. It is the first outbreak of the Zaire species of Ebola in this region and the first in urban high population density settings where sustained transmission has occurred. In previous outbreaks the main focus of attention was on nosocomial transmission of the disease and on risks associated with funeral practices. However, the occurrence of cases in high population density urban environments raised concern about alternative transmission pathways. The size of the 2013–15 epidemic was often unmatched by sufficient clinical capacity, which resulted in community-based care rather than hospitalization of cases. 1
Ebola virus is part of the Filoviridae family which also includes Marburg viruses. Both Ebola and Marburg diseases are generally understood to be zoonotic infections whose primary hosts are thought to be bats. 2–4 Once the virus crosses from wildlife into humans, subsequent person-to-person spread propagates the outbreak until it is brought under control. Given the experiences of previous human filovirus infections, the primary focus of interest has been in nosocomial spread and spread associated with funeral practices. 5,6 The 2013–15 epidemic differs from previous outbreaks not only in number of people afflicted and geographical spread, but also in its setting. Many of the reported cases have been among people living in high density and impoverished urban environments. Indeed, because of the lack of adequate health care facilities, many people remained in their home community during the entire course of their illness, receiving care from family members and neighbours. Hence, the scale of the Western Africa outbreak resulted in many Ebola treatment centres being built within or close to these newly-affected urban communities.
Concerns have been raised that the shift in the 2013–15 epidemic towards infected patients being managed in urban communities exacerbated disease transmission. 7 Consequently there is a greater need to better understand the mechanisms and risk factors behind intra-community disease transmission. Only then can appropriate community control measures be implemented. Although there are some previous reviews on Ebola viruses and their epidemiology, 8,9 systematic evaluation of evidence on community human-to-human transmission risks has been limited. With the development of a highly effective vaccine against Ebola, 10 such rigorous evaluation will be essential to designing optimal strategies for immunization campaigns.
In this systematic review we searched for all published evidence which identified and/or quantified the risk factors for community acquisition of filovirus infection. We also included papers where the authors expressed an opinion as to how patients acquired infection, even if the evidence to support this suggestion would not usually meet standards of acceptable epidemiological evidence.
Methods
Medline and Scopus were searched from inception through 13 August 2015 using the search string filovir*.af. OR ebola.af. OR ebolavir*.af OR Marburg-virus.af ( af means ‘all fields’ including all text words and relevant indexing) without restrictions for date or language. Twelve sources of grey literature were searched (see Supplementary Data , available as Supplementary data at IJE online) and screened by a single investigator. Included papers were also checked for further studies.
Inclusion criteria
The preferred study for inclusion in this review provided data that enabled us to assess the odds of filoviral infection transmission between humans according to particular characteristics, behaviours or contacts (data could be presented as odds ratios, risk ratios or raw numbers). In addition, we also included papers where the authors expressed an opinion about how infection was acquired, although not based on analytical epidemiology (i.e. anecdotal observations). Eligible filoviral infections were Marburg virus, Ravn virus, Zaire, Sudan, Taï Forest and Bundibugyo species of Ebola. Species of filovirus not present in Africa or not known to be dangerous to humans were excluded. The filovirus disease outbreak had to be laboratory confirmed (using RT-PCR, NAAT or Vero culture tests), as antibodies or inflammatory factors in body fluids were deemed inadequate by themselves to verify the outbreak, because they are widespread in the regional population including in many people with no relevant clinical history. 9,11–15 Data were included from mixed patient groups where all or some had laboratory confirmation of disease cause, all had a compatible clinical history and survivors were confirmed as having filovirus antibodies.
Titles and abstracts were screened for inclusion by a single reviewer and verified by a second researcher. Conference presentations, protocols, news reports, commentaries and editorials were excluded. Where titles were not accompanied by abstracts they were only assessed in full text if they included the word(s) risk or transmission . Full-text articles were assessed for inclusion independently in duplicate. Decision differences at all stages were resolved by discussion. Where several articles reported on the same primary data, the articles were grouped to ensure data were not duplicated within the review.
Data were extracted into tables and verified by a second researcher. Extracted data included bibliographic details, viral species, date and place of outbreak, risk or exposure factor(s) identified, assay methods and calculated odds, hazard or risk ratios or relevant raw data. Unadjusted and adjusted data (where available) were extracted and unadjusted odds ratios calculated from raw data. Where anecdotal opinions of acquisition were presented, these were also extracted into a separate list. Our study validity assessment was based on attributes most likely to undermine the utility of the studies, including delay in investigating the cause of disease transmission (for studies identified at low risk of bias this was ≤ 3 months from contact), study aim (to quantify human-to-human transmission or not), whether there was a standard methodology for data collection and whether the risk factors assessed were pre-specified. A positive answer suggested low risk of bias for all of these. Validity was assessed by a single researcher (see Supplementary Data , available as Supplementary data at IJE online). The review is reported in accordance with PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses; a PRISMA checklist was included with submission). 16
In order to assess the risk of household transmission, we identified papers that presented incidence rates in household contacts (where possible) with and without direct contact with another case. A random-effects meta-analysis of proportions was conducted using Stats Direct ™ and heterogeneity checked visually. Separate meta-analyses were conducted of incidence rates in household contacts with and without a history of direct contact.
Results
Of 6552 mostly unique articles found in Medline or Scopus, 114 were immediately excluded for being obvious conference abstracts, protocols, news reports, commentaries or editorials ( Figure 1 ). A further 2001 items lacked an abstract and,on brief review, most of these appeared to be short commentaries, news summaries and possible conference presentations. Of the 2001 items, only nine were screened directly because their titles contained keywords most relevant to our research questions. Thus, 4560 scientific articles were duplicate screened on title and abstract, of which 52 were not excluded. The grey literature search (see Supplementary Appendix A1 ) yielded two inclusions. 17,18 One additional article with potential primary data 19 was identified in the discussion text of selected articles; 55 articles were thus chosen for full-text review. Full text was unavailable for one article 20 and, after full-text review, 23 articles did not meet eligibility criteria. Four articles 11,17,19,21 reported at least partly duplicated information on two patient groups (a list of outbreaks in the selected articles to check for duplicated data is in Supplementary Appendix A3 , available at IJE online). The final number of articles included in the final review was 31 covering 29 distinct patient groups, for which data extraction was undertaken. Study quality and validity assessment for all selected studies are shown in Supplementary Appendix A2 .
Figure 1.
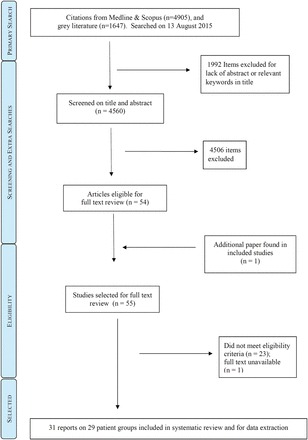
Study selection procedure.
Characteristics of included studies
Most data ( Table 1 ) came from retrospectively administered interviews with survivors or their close contacts or from clinical notes, using standardized questionnaires, and usually collected less than 3 months after illness. Community disease transmission occurred from 1967 to 2015 in 10, primarily African, countries (Angola, Democratic Republic of Congo (DRC)/Zaire, Guinea, Liberia, Republic of Congo, Sierra Leone, South Africa, Sudan, Uganda, West Germany). Quantitative data were available for BUDV, EBOV, MARV and SUDV species, but accounts for how people contracted disease were overwhelmingly anecdotal for EBOV. Laboratory methods [culture or polymerase chain reaction (PCR) tests] confirmed filovirus infection as the cause of disease in each outbreak; but most studies included some cases identified from clinical history and antibody presence only.
Table 1.
Included study characteristics, table ordered by filovirus speciesand chronological date of relevant outbreaks
| Species | Outbreak date, location, authors | Type of information relevant to this review |
|---|---|---|
| BUDV | Aug–Dec 2007, Bundibugyo Uganda 38 | Delayed recognition; unconfirmed, risks of attending childbirth |
| Aug–Dec 2007, Bundibugyo Uganda 27 | Numerical risk ratio data, various attributes, OR | |
| MARV | 1967, Germany and Yugoslavia 17,21 | Documents transmission of disease from sexual contact |
| Feb–Mar 1975, Johannesburg South Africa 85 | Likely transmission moment = handling wet paper tissues from bereaved incubator | |
| Mar–Jul 2005, Uige, Angola 26 | OR data | |
| SUDV | 31 Jul–6. Nov 1979, Nzara, Yambio, Sudan 25 | 34 patients, concentration in blood, one OR + anecdotal, during & after illness |
| Aug 2000–Jan 2001, Gulu, Uganda 22 | PPRs, fomites suggested, many factors | |
| Aug 2000–Jan 2001, Gulu, Uganda 40 | Children under 18 survive better, close contact risk | |
| EBOV | 1 Sep–24 Oct 1976, Bumba, Yambuku, Zaire 11,19 | ORs (also in Breman et al. ) Non-intimate contact risk, touching dry skin, sexual partners, attending childbirth or a funeral, intimate funeral tasks, needle sharing, bedbugs, rats? |
| 1976–77, Sud-Ubangi subregion, Zaire (Tandala) 12 | 1981–85 surveillance report: direct contact implicated, asymptomatic, antibody prevalence | |
| Jan–Jul 1995, Kikwit DRC 24 | PPRs, not recognized until May 1995; households of 27 cases interviewed 17 May–3 June about risk factors (no risk after 1 May); stage of illness relevance | |
| Apr–May 1995, Mosango DRC 28 | Related to Kikwit outbreak, 23 only in Mosango; forms of dangerous contact | |
| Jan-Jul 1995, Kikwit DRC 23 | Matched ORs | |
| 1994–96, Gabon 29 | Occupation and economic activity | |
| 2002–03, Rep. of Congo 35 | Cases linked to direct contact between people following primary contacts with wildlife | |
| 2005, Etoumbi DRC 41 | Gender factors, funerals, cremation controversy | |
| May–Nov 2007, Occidental Kasaı, DRC 37 | Suggests via sweat, dead animals | |
| 2014, Sierra Leone 39 | Transmission after caring for ill patient, organizing funeral, caring for infant or attending during childbirth | |
| 2014, Sierra Leone patient taken to Germany 36 | Believed transmission in office or lavatory; high levels of virus detected in sweat | |
| 2014, Sierra Leone 34 | Funerals, health care workerss affected; people who left clinic but had EVD after all | |
| 2014–15, Sierra Leone 32,45 | Touching bodies at funerals, contact with or caring for patients, touching cadavers | |
| 2014, Conakry, Guinea 33 | Reproduction numbers, chains of transmission | |
| 2014–15 Guinea 18 and Liberia 30,42–44 | Care in community, funerals and cremation. Also, assistance into taxi | |
| 2015 Liberia 31 | Following sexual contact |
Seven papers gave different forms of numerical odds or risk ratios for developing disease. 22–28 Two further articles 11,19 gave data that enabled us to calculate unadjusted odds ratios for one outbreak. The available odds, risk and prevalence ratio data are summarized in Table 2 . The analysis of Dowell et al24 is unique because it broke down risk of transmission by stage of illness at exposure (incubation period, early or late illness). Other sources do not have detailed linkage to disease onset, but still can be used to support observations about overall trends of evidence. Most of the numbers in Table 2 are crude odds ratios (not adjusted for confounding variables). Data from two papers 22,24 are mostly adjusted for co-variates, and we consequently have more confidence in these results than others in our discussion.
Table 2.
Numerical odds, risk or prevalence ratios for filovirus disease transmission
| Risk factor | Details | Unadjusted effect size (95% CI) | Adjusted effect size (95% CI) |
|---|---|---|---|
| Demographics and personal attributes | |||
| Age | Being > 18 years 24 | PRR* 6.8 | PRR* 3.6 (1.3-10.1) a |
| Being > 30 years old 22 | PPR 1.38 (0.64-2.97) | ||
| Being ≥30 years old 26 | OR 1.32 (0.60-2.92) | ||
| Being ≥34 years old 29 | OR 0.83 (0.35-1.95) | ||
| Being 41–60 years old 27 | OR 2.0 (0.8–4.9) | Not reported b | |
| Being ≥40 years old 26 | OR 0.99 (0.37-2.68) | ||
| Sex | Being female 27 | OR 0.63 (0.28–1.43) | Not reported b |
| Being female 22 | PPR 1.54 (0.7-3.6) | ||
| Being female 24 | PRR* 2.1 | PRR* 1.0 (0.5-2.1) a | |
| Being female 26 | OR 2.46 (1.03 – 5.90) | ||
| Occupation | Working in forest 23 | MOR 1.3 (0.4-6.0) | |
| Fishing 23 | MOR 3.0 (0.04-235) | ||
| Fisherman 29 | OR 3.12 (0.59-16.41) | ||
| Health care worker 23 | MOR 9 . 0 (1 . 6-91 . 2) | ||
| Health care worker 26 | OR 1.52 (0.41-5.64) | ||
| Student 26 | OR 0.81 (0.34-1.94) | ||
| Housewife 26 | OR 1.23 (0.50-3.04) | ||
| Housewife 29 | OR 0.87 (0.24-3.09) | ||
| Farmer 29 | OR 1.27 (0.15 -10.81) | ||
| Trader 29 | OR 0.77 (0.22 -2.75) | ||
| Gold-panner 29 | OR 1.33 (0.56-3.17) | ||
| Setting | Urban or suburban (versus rural) 26 | OR 0.82 (0.23-2.89) | |
| Recent travel | To areas with known cases 27 | OR 1.4 (0.5–3.8) | Not reported b |
| Outside own local area 23 | MOR 3.0 (0.2-41.4) | ||
| Recurring non-intimate contact | |||
| Commerce-related | Frequenting markets 23 | MOR 1.1 (0.3-4.5) | |
| Conversation with case | During incubation period 24 | PRR* 1.5 | PRR* 0.7 (0.2-3.0) a |
| During early illness 24 | PRR* 3.3 | PRR* 0.7 (0.3-2.0) a | |
| During late illness 24 | PRR* 10.6 | PRR* 3.9 (1.2-12.2) a | |
| Washing clothes of a case | (Point of disease onset unclear) 22 | PPR 1.68 (0.78-3.60) | PPR 1.02 (0.47-2.2) d |
| Indirect contact with case | Household or similar contact without direct physical touching 26 | OR 6 . 88 (1 . 35-35 . 1) | |
| Sharing same hut | Without sharing bed/sleeping mat 22 | PPR 2.16 (0.90-5.19) | PPR 2 . 34 (1 . 13-4 . 8)d |
| Entered same room but no physical contact 25 | OR 0.06 (0.00-1.06) | ||
| Slept in same room 11 | OR 1.65 (0.95-2.85) | ||
| Visiting cases | In hospital or their own home, before or after diagnosis 27 | OR 8 . 7 (3 . 0–26 . 3) | Not reported b |
| Visit to ill (with fever and bleeding) friend (in own home) 23 | MOR 10 . 6 (3 . 8-36 . 3) | ||
| Recurring intimate contact | |||
| Shared a meal | During early illness 24 | PRR* 2.5 | PRR* 1.2 (0.5-2.7) a |
| During late illness 24 | PRR* 7.0 | PRR* 2.2 (1.2-4.0) a | |
| With index patient 22 | PPR 1.94 (0.89-4.22) | PPR 1.69 (1.0-2.8) d | |
| Sharing a bed or sleeping mat | During incubation 24 | PRR* 2.9 | PRR* 1.4 (0.8-2.4) a |
| During early illness 24 | PRR* 3.8 | PRR* 1.3 (0.7-2.5) a | |
| During late illness 24 | PRR* 7.4 | PRR* 2.2 (1.2-4.2) a | |
| Point of disease onset unclear 22 | PPR 2.78 (1.15-6.70) | PPR 2 . 93 (1 . 2-7 . 4)d | |
| Direct physical contact – touching | During incubation period 24 | PRR* 2.9 | PRR* 0.8 (0.4-1.8) a |
| During early illness 24 | PRR* 12.5 | ||
| During late illness 24 | PRR* 12.5 | ||
| With person who had fever or bleeding, at work or in the market 23 | MOR 24.0 (3 . 2-1065) | ||
| Contact with body or body fluids of a suspected case 26 | OR 11.0 (2 . 6-46 . 1) | ||
| Touched case 11 | OR 1.45 (0.73-2.87) | ||
| Touching during illness 22 | PPR 3.53 (0.52-24.11) | PPR 1.56 (0.2-13.0) c | |
| Touching but no nursing care 25 | OR 0.40 (0.11-1.45) | ||
| Contact with body fluids | Contact with body fluids 22 | PPR 5.30 (2.14-13.14 ) | PPR 4 . 61 (1 . 7-12 . 3)c |
| Direct contact with individuals potentially infected with MHF or their bodily fluids or direct contact during funeral 26 | OR 12.0 (3 . 6-39 . 6) | ||
| Body fluid contact in early illness 24 | PRR* 6.1 | ||
| Body fluid contact in late illness 24 | PRR* 5.9 | ||
| Likely sexual contact | Being spouse of index case 24 | PRR* 3.8 | PRR* 1.3 (0.7-2.5) a |
| Caring for patient | Nursing a patient 25 | OR 8.9 (3.1-25.4) | |
| Cared for case 11 | OR 0.99 (0.56-1.76) | ||
| Early care at home, not until death 22 | PPR 6 (1 . 3-27 . 1) | P for trend for these 3 < 0.001 | |
| At hospital until death 22 | PPR 8 . 57 (1 . 9-37 . 7) | ||
| In home until death 22 | PPR 13 . 33 (3 . 2-55 . 6) | ||
| Aided patient in childbirth 11 | OR 2.46 (1 . 02-5 . 92) | ||
| Funeral-related activities | |||
| Viewed body | Without touching 24 | PRR* 4.8 | PRR* 1.6 (0.5-4.9) a |
| Attended | Special (pre-funeral) rituals 23 | MOR 0.8 (0.2-3.2) | |
| Funeral itself 23 | MOR 3.0 (1 . 2-7 . 6) | ||
| Funeral itself 11 | OR 0.86 (0.41-1.79) | ||
| Communal meal | As part of funeral event 22 | PPR 2.84 (1.35-5.98) | PPR 1.5 (0.98-2.28) d |
| Touched body | Before or during funeral 22 | PPR 1.95 (0.91-4.17) | PPR 1.84 (0.95-3.55) c |
| Before or during ceremony 24 | PRR* 4.9 | PRR* 2.1 (1.1-4.2) a | |
| Ritual handwashing 22 | PPR 2.25 (1.08-4.72) | PPR 1.16 (0.54-2.49) d | |
| Washing and dressing body 27 | OR 7 . 4 (2 . 9–19 . 3) | OR 3.83 (1.78-8.23) b | |
| Direct contact with corpse, its body fluids or soiled items 26 | OR 38 . 5 (4 . 2-352 . 1) | ||
| Prepared for burial 23 | MOR 13 . 1 (1 . 4-631) | ||
| Prepared cadaver | OR 1.07 (0.63-1.82) | ||
| Previous use of health services (nocosomial indicators) | |||
| Taking regular medication | Kikwit outbreak 1995 23 | MOR 2.0 (0.5-9.8) | |
| Admitted previously to hospital for something else | Before outbreak was recognized, Kikwit outbreak, 1995 23 | MOR 9 . 9 (3 . 1-41.0) | |
| Received injection | Before outbreak was recognized, Kikwit, 1995 23 | MOR 30.0 (4 . 3-1302) | |
| Admission or visit to hospital | For any reason 27 | OR 8 . 7 (3 . 0-26 . 3) | Not reported b |
| Number of types of direct contact (touching ill patient, touching dead body, touching body fluid) | No direct contact 22 | PPR 1.0 | P for trend for these 4 < 0.001 |
| One type of contact 22 | PPR 0.18 (0.01-2.45) | ||
| Two types of contact 22 | PPR 1.94 (0.30-12.44) | ||
| Three types of contact 22 | PPR 4.00 (0.64-25.02) | ||
PRR*, prevalence rate ratio; PPR, prevalence proportion ratio; MOR, matched odds ratio; OR, odds ratio. Bold text indicates a 95% confidence interval that is entirely above 1.0. Otherwise, where figures are missing, figures were not provided or not possible to calculate.
a Adjusted for direct physical contact during illness and contacts with body fluids.
b Dropped from multivariate logistic regression by authors due to lack of significance at P < 0.05.
c Using multivariate log-binomial regression models, factors included touching patient during illness, touching dead body and contact with patient fluids.
d Using multivariate log-binomial regression models, factors included shared meals, washed clothes, slept in same hut or mat, ritual handwashing during funeral and communal meal during funeral, and also controlled for intensity of contacts (two or more indirect contacts versus less).
A further 23 articles are included in the review although they provide only anecdotal or contact-tracing observations on how disease transmission probably occurred in confirmed cases. 12,17,18,21,29–45 Only one paper covering the 2013–15 epidemic had a primary aim to quantify aspects of human-to-human transmission, 33 which publication calculated R 0 numbers rather than identifying risk ratios for individual risk factors. Details of the information provided by this anecdotal group of articles are in Supplementary Data (available as Supplementary data at IJE online).
Demographic variables
Outbreak reports often state 40,46,47 that, compared with other age groups, children were less frequently infected and more frequently survived; but this is not consistently confirmed by statistical analysis on EBOV. 24,48 There is mixed evidence (minimum OR 0.83, 95% CI 0.35–1.95, to maximum prevalence rate ratio 6.8, no 95% CI stated), 22,24,27 as to whether increased age among adults is a risk factor for contracting filovirus illness. Gender-related risk ratios ranged from OR 0.63 (95% CI 0.28–1.43) to OR 2.46 (95% CI 1.03–5.90). 22,26,27,47 Recent travel yielded OR 1.4 (95% CI 0.5–3.8) or matched odds ratio 3.0 (95% CI 0.2–41.4). 23,27 Although high prevalence of Marburg virus antibodies has been reported among forest workers 49 and miners, 29,50 these occupations (or being a housewife) were not associated with raised risk of contracting disease (ORs range from 0.87, 95% CI 0.24–3.09, to 3.12, 95% CI 0.59–16.41). 29
Intensity of contact
Within Table 2 , contact was put in order of (approximately) increasing intimacy: non-intimate or intimate with live cases, or with cadavers. It is notable that even within the same categories, there was often substantial heterogeneity in reported risk ratios, making it difficult to provide single estimates of risk. This difficulty is compounded by the fact that many studies did not adequately control for confounding. In the discussion below, stated risk ratios are unadjusted for any confounding factors except where noted.
Only one article 24 provided data for prevalence of risk ratios according to stage of infection (incubation, early or late illness; late illness was defined as post-hospitalization). The adjusted relative risks associated with sharing a bed with a case during the incubation period was PRR 1.4 (95% CI 0.8–2.4) and during early illness PRR 1.3 (95% CI 0.7–2.5), but bed-sharing in later illness yielded a much higher risk ratio (PRR 2.2, 95% CI 1.2–4.2). 24 Such chronological data were missing from other reports, which only gave combined risks from contact at all stages of incubation or illness.
Faye et al . 33 observed that 72% of transmission was between family members. These and other data strongly suggest that non-intimate contact such as frequenting markets (MOR 1.1, 95% CI 0.3–4.5), 23 conversation with a case in early illness [adjusted (adj.) PRR 0.7, 0.3–2.0] 24 or sharing a home but not sleeping space 11 had low risk of transmission before and during early illness. Even touching dry skin (adj. PRR, 0.8 95% CI 0.4–1.8), sharing meals (adj. PRR 1.2, 0.5–2.7) or a bed (adj. PRR 1.3, 0.7–2.5) during early disease stages lacked a significant risk of contracting acute infection. 24 Washing clothes of a case yielded adj. PPR 1.02 (95% CI 0.47–2.2). 22 Disease transmission to people who had regular but non-intimate contact with cases (household or workplace) had quite variable association with disease transmission (OR range from 0.06, 95% CI 0-1.06, 25 to OR 6.88, 95% CI 1.35–35.1 26 ).
Disease contraction from casual contact with sweat has been suggested, 37 although most studies fail to detect virus in sweat. 36,51–55 Shared office or lavatory facilities was implicated by Kreuels et al36 but was not the only possible opportunity for the relevant case. Sexual partners of convalescents are potentially at chronic but low risk of getting disease, 11,56 due to persistence of detectable virus for many weeks in semen and vaginal excretions 57 of convalescents. Filovirus transmission from sexual contact with a convalescent has been documented only twice since 1967. 17,31 Being a spouse of a victim 24 was not shown to be risky as it had a 95% CI for prevalence rate ratio between 0.7 and 2.5 when adjusted for other factors.
During later stages of illness (when bodily fluids with high viral loads are mostly likely to be shed), even relatively non-intimate contact posed some remaining risks even after adjustment for direct contact (e.g. conversation, PRR 3.9, 95% CI 1.2–12.2). 24 During all active disease stages, sharing a meal had PPR 1.69 (95% CI 1.0–2.8) and sharing a bed had PPR 2.93 (95% CI 1.2–7.4). 22 During late disease, sharing a meal had adjusted PPR 2.2 (95% CI 1.2–4.0) and sharing a bed had adjusted PRR 2.2 (95% CI 1.2–4.2). 24 Touching feverish people had MOR 24 (95% CI 3.2–1065) 23 and touching bodily fluids had OR 11 (95% CI 2.6–46.1). 26 That disease often follows directly touching body fluids is corroborated by much anecdotal evidence. 18,21–23,26,28,44 ) Visiting known patients in their homes raised risk of acquiring disease (MOR 10.6, 95% CI 3.8–36.3). 23 Attending the birth of a child to an ill patient conferred OR 2.46 (95% CI 1.02–5.92) 11,19 for contracting disease, and transmission from attendant to mother-in-labour has also been implicated. 39 Other forms of direct physical contact with ill patients were identified as being associated with a high risk of transmission. 12,35,38,39 However, the greatest risks are associated with caring for an actively ill patient (minimum OR 0.99, 95% CI 0.56–1.76, and maximum OR 8.9, 95% CI 3.1–25.4) 22,25 or visiting patients receiving care (OR 8.7, 95% CI 3.0–26.3 and MOR 10.6, 95% CI 3.8–36.3). 23,27 Caring for patients at home until death carries very high risk of disease transmission (OR 13.33, 95% CI 3.2–55.6). 22,30
The quantitative and adjusted data presented in Table 2 suggest that in themselves neither viewing a body(adj. PRR 1.6, 95% CI 0.5–4.9) 24 nor attending a funeral (MOR ranges from 0.8, 95% CI 0.2–3.2, to 3.0, 95% CI 1.2–7.6) 11,19,23,33 pose elevated risks, but rather that it is the nature of traditional funeral-associated activities that increases risk. One anecdotal report 18 found that among cases that arose after a specific (traditional) funeral ceremony, 21% of cases had directly touched the cadaver and the other 79% of linked cases had physical contact with those who touched the body either during the service or afterwards. However, briefly touching the cadaver is not consistently associated with increased risk (range of adj. PPR 1.16, 95% CI 0.54-2.49, to adj. PRR 2.1, 95% CI 1.1–4.2). 22,24 Sharing a communal meal during the funeral was found to pose an elevated risk (adj. PPR 1.5, 95% CI = 0.98–2.28), 22 perhaps due to crowded conditions. The intimate tasks (washing and dressing) associated with preparing a body for funeral and burial tend to confer a very high risk of disease transmission, although again data are inconsistent (range from OR 1.07, 95% CI 0.63–1.82, to MOR 13.1, 95% CI 1.4–631). 11,19,23,26,27,30,41
Contact with health services and other possible risks
Elevated transmission risk following contact with health services appears in Table 2 and is also recounted in Supplementary Appendix A4 , particularly for the 1995 Kikwit outbreak in the DRC. 23 Transmission rates associated with health service contact were high in these outbreaks, partly because the disease was recognized belatedly with subsequent delayed implementation of control measures. 33,58 Unsafe needle-sharing practices as part of a vaccination programme were also specifically blamed for rapid disease spread during the 1976 emergence of EBOV. 11,19 Visiting a hospital or caring for filovirus cases in hospital raised transmission risks dramatically in most outbreaks (OR 8.7, 95% CI 3–26.3, and MOR 9.9, 95% CI 3.1–41.0). 22–25,27
Meta-analysis
Three papers gave sufficient data to calculate incidence rates in household contacts with and without a history of direct contact. 22,24,25 The proportion meta-analyses ( Figures 2 and 3 ) drew on a total of 254 direct contacts and 135 indirect contacts. Among those household contacts reporting direct contact with a case, the attack rate was 32% (95% CI 26–38%) without important heterogeneity. In household contacts with no history of direct contact, the attack rate was 1% (95% CI 0–5%). Only one confirmed case with no direct contact in any of the three studies was reported. 22 This person ‘slept wrapped up in a blanket left by his brother’, recently deceased from EVD. It was possible that this was a case of transmission from direct contact with body fluids. Breman et al . 19 also reported data sufficient to calculate attack rates in household contacts, but without distinction between direct and indirect contact. Instead, they reported that the attack rate was 27% between spouses, brothers and sisters or between parents and children. In all other relatives the attack rate was only 8.0%.
Figure 2.
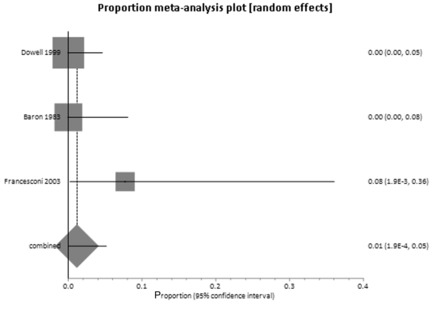
Attack rates without direct contact between household members.
Figure 3.
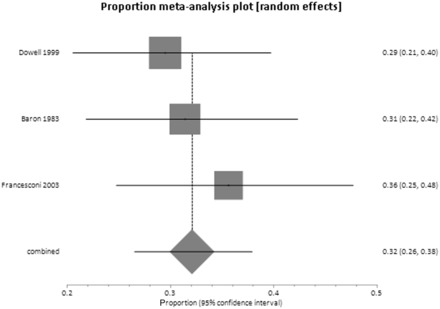
Attack rates after direct contact between household members.
Discussion
We present the first systematic review that addresses the behavioural and other risk factors associated with filovirus (Ebola and Marburg disease) infection within the community. The key findings of this review are that infection risk is primarily associated with three behaviours: (i) close contact in the later stages of infection; (ii) caring for a sick person; or (iii) when preparing the recently deceased for burial. These findings are not surprising, and our review has strengthened the evidence base for them. Importantly, we provide a more nuanced understanding of the risks, especially around risks associated with indirect contact. For example, we have found no evidence of risk associated with casual contact with asymptomatic individuals outside the home. Even between household contacts who did not have direct physical contact, the risk of disease transmission is relatively minor (1%, CI = 0-5%), although not zero. We have also confirmed that there is negligible if any risk of contracting disease during the incubation period and only low risk in the first week of symptomatic illness. Our review also confirms the high risk of transmission associated with funerals, and that disease transmission appears to most often follow after touching the body of a case.
Visiting or caring for filovirus cases in hospital raised transmission risks dramatically across most outbreaks. 22–25,27 This is probably due to high viral loads associated with severe disease and insufficient protection measures. It has been suggested 33 that earlier hospitalization and longer hospital stays (provided sufficient isolation and protection procedures are followed) could significantly shorten the duration of filovirus outbreaks.
Although this review concerned person-to-person transmission in the community, it was not always possible to distinguish primary from secondary cases. We excluded reports where disease clearly resulted from wildlife contacts, but some risk ratios were undoubtedly calculated for mixed groups of primary cases (disease acquired from wildlife or wildlife environments) and secondary cases (from humans). The ecological niche that each filovirus occupies would certainly impact on some risk factors for primary infection 59 (such as occupation). However, the ratio of secondary to primary cases in most outbreaks is so large 9 that ecological factors responsible for primary disease acquisition are unlikely to have a major influence on calculated risk ratios.
Why adulthood would increase the risk of illness is uncertain, although the risk of illness does not appear to depend on lower total viral load, and contradicts intuition that small children would tend to have greater risks due to more physical contact with their carers. 60 The association with adulthood may be primarily explained by the fact that adults tend to be the carers. 25 Funeral-related events are another well-recognized opportunity for infection transmission. 18,32–34,39,41,61 Viable virus has been isolated from animal tissues or fluids in the laboratory as late as 7 days post-mortem. 62 However, not all of the studies found such an association between attending funerals and disease risk. Furthermore, even for those attending funerals where transmission occurs, only people who engage in certain behaviours are particularly at risk. It is possible for dignified funerals to be held without high risk to those attending. 63 Unfortunately, efforts to persuade local populations to change funeral traditions during outbreaks, and in particular to allow cremation, often meet cultural resistance. 35,41,44,64
Of particular relevance to policy is the strong evidence that risk to family members is high in those caring for their relatives up to death, and that this risk is much higher in those caring for people within the home (unadj. PPR 13.33, 95% CI 3.2–55.6). 22 Such an observation strengthens the need to ensure that sufficient spaces are available in health care facilities so that no one suspected of filovirus infection has to be cared for in their own home until death.
Our review focused on community human-to-human infection, and excluded transmissions to health care workers within clinical environments actively treating filovirus, 25,28,34,65,66 accidents in research laboratories 67 or wildlife contact. We omitted cases in health care or laboratory workers because they tend to lead to few subsequent cases 33 (with notable exceptions particularly when EVD has not yet been identified 68–70 ). The risk of transmission in laboratory or clinical environments can clearly be greatly minimized with stringent control measures 33,66,71 that are unlikely to be replicable in the community. Wildlife contacts are extremely important in filovirus epidemiology because outbreak starts are nearly always linked to contact with animals, but calculating the risks of contracting disease is very difficult due to lack of data on wildlife contacts in Africa that do not result in filovirus disease. Wildlife contacts may be at least partly responsible for widespread filovirus antibody seroprevalence after asymptomatic illness. 11–14,49,50,72–74 Genetic analysis strongly suggests that the 2013–15 outbreak was driven by human-to-human transmission rather than new imports after the initial emergence. 75
Limitations
We were able to produce only limited pooled estimates of risk of transmission because the included papers were inconsistent in defining risk factors or which measures of association were used; plus, many of the earlier studies presented only unadjusted estimates. The partial exceptions to this are the data on disease transmission risk to household contacts with, and without, direct contact. Consequently we are unable to specify with sufficient degree of certainty how risky specific behaviours may be. Nevertheless, the general findings of low risk for contracting disease from relatively casual contact is reassuring. It is also important to emphasise that low risk of contracting disease (e.g. from casual contact), which we discuss at length in this paper, is not the same as saying that such contact poses low risk overall to human health after disease has developed. The total risk to human health results from risk of transmission combined with likely consequences of disease, and filovirus diseases are usually very dangerous.
We excluded articles which identified cases solely from symptomology or antibody counts 13,15,49,50,60,73,74,76–78 The symptoms of Ebola and Marburg virus can mimic other diseases endemic to sub-Saharan Africa, 9 and filovirus antibodies from asymptomatic infection are in 2–15% of the regional population not otherwise known to have ever had relevant illness. 9,11–14,49,72,73 Concerns have been raised about problems of high rates of false-positives from Ebola virus antibody tests, 79,80 which is why, ideally, we wanted to only report results from patients who had at least one positive laboratory test for filovirus infection. In reality, too few articles met that strict criterion. Some reports also did not clearly state whether interviews, contact tracing and risk ratios were calculated only with laboratory-confirmed cases. Therefore, our rule was to include risk ratio and anecdotal data in articles where at least some of the patients among the identified cases in the disease cohort were laboratory confirmed, with others identified by clinical and contact history. We are also not in a position to comment on the impacts of possible virus mutations on transmission risks; there is mixed evidence 13,81,82 about whether the large 2013–15 epidemic may have caused the virus to become more or less infectious or deadly to humans. Although it is common practice in epidemiological reviews, grouping Marburg and Ebola viruses together may not be ideal. Better risk assessments may result as more detailed information emerges about the transmission risk factors for each individual filovirus. Nevertheless, there was no evidence within the studies we found that suggested that the risk factors for human-to-human transmission differ between the two genera.
It is also unfortunate that we do not have specific odds or risk ratio data for the 2013–15 outbreak. Most of the information emerging from this epidemic is still anecdotal. Undoubtedly, useful risk prevalence or odds ratio figures will emerge from the large Western Africa outbreak. However, this may not be until after vaccination programmes have commenced these inoculation programmes need information as soon as possible about the best strategy for containing the current and likely future outbreaks.
Implications
The 2013–15 epidemic was unprecedented due to the large number of cases in densely populated urban settings. Only one of our selected papers 33 discussed factors which may be especially relevant in this but not previous outbreaks, including (but not limited to): population density in home area, level of education, income or affluence levels, urban occupation group, type of home or household construction materials, religious practices, funeral practices and proximity to medical care. Better future disease containment in urban areas may depend on identification of risk factors specific to urban Africa. Reducing possible risks may also mean long-term cultural changes (e.g. traditional funeral practices) that are difficult for local populations to accept.
The implications of our review are that when vaccination is not widely available, the primary control measure for filovirus infections should remain the early identification of cases, contact tracing and subsequent quarantine and care of cases in suitably equipped treatment centres. When vaccination is possible, then priority should be given to individuals engaged in activities that involve high-risk direct contact with confirmed or suspected cases (or bodily waste): e.g. those providing physical care within the community and treatment centres. A second-level priority ring for vaccination would for those who have other forms of direct contact with known or suspected cases. Such a ring approach to disease containment is already in use, in the 2013–15 EBOV outbreak. 83
Conclusions
We have shown that risk of acquisition of filovirus infections primarily follows from only close personal contact and generally only in later stages of illness. By making this statement, in no way do we deny that filovirus infections are dangerous. The EVD transmission paradox (colloquially summarized as ‘Hard to catch, Easy to die from’) has been discussed previously 84 but never summarized with as much quantitative and documentary evidence as we provide. Caring for patients until death is particularly risky, especially within domestic settings. Among people experiencing only indirect contact, even when living in the same house, the risk of contracting disease is actually quite low. There is little evidence that more distant contact or that contact with people incubating the disease poses any risks. More studies are needed that correlate context, timing and intimacy of contact with days after disease onset and external symptoms or severity of illness. There is evidence that transmission from non-intimate contact is low during early illness, but there is no simple indicator for the transition to late illness when disease transmission is highly likely from any contact without adequate protective measures. Meta-analysis showed that transmission is very unlikely without direct physical contact. Once an outbreak has been identified, care for patients in well-equipped health care facilities cuts transmission rates. There is wide variation in the confidence intervals and magnitude, in many suggested risk factors even when adjusted for confounders, suggesting that understanding of community filovirus transmission could be greatly improved.
Funding
This research was funded by the National Institute for Health Research (NIHR) Health Protection Research Unit in Emergency Preparedness and Response in partnership with Public Health England (PHE). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or PHE.
Supplementary Material
Acknowledgements
Many thanks to MSF staff and filovirus experts who fielded our emails. We are also grateful for the diligent reading skills of two anonymous reviewers.
Conflict of interest : None declared.
References
- 1. Kucharski A, Camacho A, Checchi F, Waldman R, Grais R, Cabrol J . Evaluation of the Benefits and Risks of Introducing Ebola Community Care Centers . Washington, DC: : Himmelfarb Health Sciences Library, The George Washington University; , 2014. . [Google Scholar]
- 2. World Health Organization . Ebola Virus Disease: Fact Sheet No. 103 . Geneva: : WHO; , 201515 . [Google Scholar]
- 3. Olival KJ, Hayman DT . Filoviruses in bats: current knowledge and future directions . Viruses 2014. ; 6 : 1759 – 88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Marburg fever outbreak leads scientists to suspected disease reservoir . Bull World Health Organ 2007. ; 85:659 – 732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacNeil A, Rollin PE . Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl Trop Dis 2012. ; 6 : e1546 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feldmann H, Geisbert TW . Ebola haemorrhagic fever . Lancet 2011. ; 377 : 849 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . The Ebola Outbreak in Liberia is Over. WHO Statement . Geneva: : WHO; , 2015. . [Google Scholar]
- 8. Van Kerkhove MD, Bento AI, Mills HL, Ferguson NM, Donnelly CA . A review of epidemiological parameters from Ebola outbreaks to inform early public health decision-making . Sci Data 2015. ; 2 ; doi: 10.1038/sdata.2015.19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuhn J, Calisher CH . Filoviruses: a Compendium of 40 Years of Epidemiological, Clinical, and Laboratory Studies . New York, NY: : Springer Science & Business Media; ; 2008. . [PubMed] [Google Scholar]
- 10. Henao-Restrepo AM, Longini IM, Egger M, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial . Lancet 2015. ; 386 : 857 – 66 . [DOI] [PubMed] [Google Scholar]
- 11. Burke J, Declerq R, Ghysebrechts G, et al. . Ebola hemorragic-fever in Zaire, 1976. Report of an International Commission . Bull World Health Organ 1978. ; 56 : 271 – 93 . [PMC free article] [PubMed] [Google Scholar]
- 12. Jezek Z, Szczeniowski M, Muyembe-Tamfum J, McCormick J, Heymann D . Ebola between outbreaks: intensified Ebola hemorrhagic fever surveillance in the Democratic Republic of the Congo, 1981–1985 . J Infect Dis 1999. ; 179 : S60 – S64 . [DOI] [PubMed] [Google Scholar]
- 13. Leroy E, Baize S, Volchkov V, et al. . Human asymptomatic Ebola infection and strong inflammatory response . Lancet 2000. ; 355 : 2210 – 15 . [DOI] [PubMed] [Google Scholar]
- 14. Nkoghe D, Padilla C, Becquart P, et al. . Risk factors for Zaire ebolavirus–specific IgG in rural Gabonese populations . J Infect Dis 2011. ; 204 : S768 – S75 . [DOI] [PubMed] [Google Scholar]
- 15. Moyen N, Thirion L, Emmerich P, et al. . Risk Factors Associated with Ebola and Marburg Viruses Seroprevalence in Blood Donors in the Republic of Congo . PLoS Negl Trop Dis 2015. ; 9 : e0003833 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration . Ann Intern Med 2009. ; 151 : W – 65–W-94 . [DOI] [PubMed] [Google Scholar]
- 17. Martini G, Schmidt H . Spermatogene Übertragung des ‘Virus Marburg’. [Spermatogenesis Transmission of Marburg Virus] . Klin Wochenschr 1968. ; 46 : 398 – 400 . [DOI] [PubMed] [Google Scholar]
- 18. Victory KR, Coronado F, Ifono SO, Soropogui T, Dahl BA . Ebola Transmission Linked to a Single Traditional Funeral Ceremony—Kissidougou, Guinea, December, 2014–January 2015 . MMWR Morb Mortal Wkly Rep 2015. ; 64 : 386 – 88 . [PMC free article] [PubMed] [Google Scholar]
- 19. Breman J, Piot P, Johnson K, et al. . T he Epidemiology of Ebola Hemorrhagic Fever in Zaire, 1976 . Amsterdam: : Elsevier/North Holland; , 1978. . [Google Scholar]
- 20. Kunii O, Kita E, Shibuya K . Epidemics and related cultural factors for Ebola hemorrhagic fever in Gabon. [Nihon koshu eisei zasshi] . Jpn J Public Health 2001. ; 48 : 853 – 59 . [PubMed] [Google Scholar]
- 21. Martini G . Marburg virus disease . Postgrad Med J 1973. ; 49 : 542 – 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Francesconi P, Yoti Z, Declich S, et al. . Ebola hemorrhagic fever transmission and risk factors of contacts, Uganda . Emerg Infect Dis 2003. ; 9 : 1430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roels T, Bloom A, Buffington J, et al. . Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure . J Infect Dis 1999. ; 179 : S92 – S97 . [DOI] [PubMed] [Google Scholar]
- 24. Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters C . Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995 . J Infect Dis 1999. ; 179 : S87 – S91 . [DOI] [PubMed] [Google Scholar]
- 25. Baron RC, McCormick JB, Zubeir OA . Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread . Bull World Health Organ 1983. ; 61 : 997 . [PMC free article] [PubMed] [Google Scholar]
- 26. Roddy P, Thomas SL, Jeffs B, et al. . Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola . J Infect Dis 2010. ; 201 : 1909 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wamala JF, Lukwago L, Malimbo M, et al. . Ebola hemorrhagic fever associated with novel virus strain, Uganda, 2007–2008 . Emerg Infect Dis 2010. ; 16 : 1087 – 92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ndambi R, Akamituna P, Bonnet M-J, Tukadila AM, Muyembe-Tamfum J-J, Colebunders R . Epidemiologic and clinical aspects of the Ebola virus epidemic in Mosango, Democratic Republic of the Congo, 1995 . J Infect Dis 1999. ; 179 : S8 – S10 . [DOI] [PubMed] [Google Scholar]
- 29. Bertherat E, Renaut A, Nabias R, Dubreuil G, Georges-Courbot M-C . Leptospirosis and Ebola virus infection in five gold-panning villages in northeastern Gabon . Am J Trop Med Hyg 1999. ; 60 : 610 – 15 . [DOI] [PubMed] [Google Scholar]
- 30. Blackley DJ, Lindblade KA, Kateh F, et al. . Rapid intervention to reduce Ebola transmission in a remote village – Gbarpolu county, Liberia, 2014 . MMWR Morb Mortal Wkly Rep 2015. ; 64 : 175 – 78 . [PMC free article] [PubMed] [Google Scholar]
- 31. Christie A, Davies-Wayne GJ, Cordier-Lasalle T, et al. . Possible sexual transmission of Ebola virus—Liberia, 2015 . MMWR Morb Mortal Wkly Rep 2015. ; 64 : 1 – 3 . [PMC free article] [PubMed] [Google Scholar]
- 32. Dietz P, Jambai A, Paweska JT, Yoti Z, Ksaizek TG . Epidemiology and risk factors for Ebola virus infection in Sierra Leone–May 23, 2014-January 31, 2015 . Clin Infect Dis 2015. , Jul 15. pii: civ568. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 33. Faye O, Boëlle P-Y, Heleze E, et al. . Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: an observational study . Lancet Infect Dis 2015. ; 3 : 320 – 26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fitzpatrick G, Vogt F, Moi Gbabai O, et al. . Describing readmissions to an Ebola case management centre (CMC), Sierra Leone, 2014 . Euro Surveill 2014. ; 19 . [PubMed] [Google Scholar]
- 35. Formenty P, Libama F, Epelboin A, et al. . [Outbreak of Ebola hemorrhagic fever in the Republic of the Congo, 2003: a new strategy?] Med Trop (Mars) 2002. ; 63 : 291 – 95 . [PubMed] [Google Scholar]
- 36. Kreuels B, Wichmann D, Emmerich P, et al. . A case of severe Ebola virus infection complicated by gram-negative septicemia . N Engl J Med 2014. ; 371 : 2394 – 401 . [DOI] [PubMed] [Google Scholar]
- 37. Leroy EM, Epelboin A, Mondonge V, et al. . Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007 . Vector Borne Zoonotic Dis 2009. ; 9 : 723 – 28 . [DOI] [PubMed] [Google Scholar]
- 38. MacNeil A, Farnon EC, Morgan OW, et al. . Filovirus outbreak detection and surveillance: lessons from Bundibugyo . J Infect Dis 2011. ; 204 : S761 – S67 . [DOI] [PubMed] [Google Scholar]
- 39. Moreau M, Spencer C, Gozalbes J, et al. . Lactating mothers infected with Ebola virus: EBOV RT-PCR of blood only may be insufficient . Euro Surveill 2015. ; 20 : 3 . [DOI] [PubMed] [Google Scholar]
- 40. Mupere E, Kaducu O, Yoti Z . Ebola haemorrhagic fever among hospitalised children and adolescents in nothern Uganda: Epidemiologic and clinical observations . Afr Health Sci 2001. ; 1 : 60 – 65 . [PMC free article] [PubMed] [Google Scholar]
- 41. Nkoghe D, Kone ML, Yada A, Leroy E . A limited outbreak of Ebola haemorrhagic fever in Etoumbi, Republic of Congo, 2005 . Trans R Soc Trop Med Hyg 2011. ; 105 : 466 – 72 . [DOI] [PubMed] [Google Scholar]
- 42. Nyenswah T, Blackley DJ, Freeman T, et al. . Community Quarantine to Interrupt Ebola Virus Transmission—Mawah Village, Bong County, Liberia, August–October, 2014 . MMWR Morb Mortal Wkly Rep 2015. ; 64 : 179 – 82 . [PMC free article] [PubMed] [Google Scholar]
- 43. Nyenswah T, Fallah M, Sieh S, et al. . Controlling the last known cluster of Ebola virus disease—Liberia, January–February 2015 . MMWR Morb Mortal Wkly Rep 2015. ; 64 : 500 – 4 . [PMC free article] [PubMed] [Google Scholar]
- 44. Nyenswah TG, Fallah M, Calvert GM, et al. . Cluster of Ebola Virus Disease, Bong and Montserrado Counties, Liberia . Emerg Infect Dis 2015. ; 21 : 1253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richards P, Amara J, Ferme MC, et al. . Social pathways for Ebola virus disease in rural Sierra Leone, and some implications for containment . PLoS Negl Trop Dis 2015. ; 9 : e0003567 . doi: 10.1371/journal.pntd.0003567 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Olupot-Olupot P . Ebola in children: Epidemiology, clinical features, diagnosis and outcomes . Pediatr Infect Dis J 2015. ; 34:314 – 16 . [DOI] [PubMed] [Google Scholar]
- 47. Dowell SF . Ebola hemorrhagic fever: why were children spared? Pediatr Infect Dis J 1996. ; 15 : 189 – 91 . [DOI] [PubMed] [Google Scholar]
- 48. WHO Ebola Response Team . Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections . N Engl J Med 2014. ; 371 : 1481 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Busico KM, Marshall KL, Ksiazek TG, et al. . Prevalence of IgG antibodies to Ebola virus in individuals during an Ebola outbreak, Democratic Republic of the Congo, 1995 . J Infect Dis 1999. ; 179 : S102 – S07 . [DOI] [PubMed] [Google Scholar]
- 50. Bausch DG, Borchert M, Grein T, et al. . Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo . Emerg Infect Dis 2003. ; 9 : 1531 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rowe AK, Bertolli J, Khan AS, et al. . Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo . J Infect Dis 1999. ; 179 : S28 – S35 . [DOI] [PubMed] [Google Scholar]
- 52. Bausch DG, Towner JS, Dowell SF, et al. . Assessment of the risk of Ebola virus transmission from bodily fluids and fomites . J Infect Dis 2007. ; 196 : S142 – S47 . [DOI] [PubMed] [Google Scholar]
- 53. Liddell AM, Davey RT, Jr, Mehta AK, et al. . Characteristics and clinical management of a cluster of 3 patients with Ebola virus disease, including the first domestically acquired cases in the United States . Ann Intern Med 2015. ; 163 : 81 – 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mora-Rillo M, Arsuaga M, Ramírez-Olivencia G, et al. . Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain . Lancet Respir Med 2015. ; 3 : 554 – 62 . [DOI] [PubMed] [Google Scholar]
- 55. Schibler M, Vetter P, Cherpillod P, et al. . Clinical features and viral kinetics in a rapidly cured patient with Ebola virus disease: a case report . Lancet Infect Dis 2015. ; 15 : 103 – 40 . [DOI] [PubMed] [Google Scholar]
- 56. Mackay IM, Arden KE . Ebola virus in the semen of convalescent men . Lancet Infect Dis 2014. ; 15 : 149 – 50 . [DOI] [PubMed] [Google Scholar]
- 57. Rodriguez L, De Roo A, Guimard Y, et al. . Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995 . J Infect Dis 1999. ; 179 : S170 – S76 . [DOI] [PubMed] [Google Scholar]
- 58. Bonnet M-J, Akamituna P, Mazaya A . Unrecognized Ebola hemorrhagic fever at Mosango Hospital during the 1995 epidemic in Kikwit, Democratic Republic of the Congo . Emerg Infect Dis 1998. ; 4 : 508 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Changula K, Kajihara M, Mweene AS, Takada A . Ebola and Marburg virus diseases in Africa: Increased risk of outbreaks in previously unaffected areas? Microbiol Immunol 2014. ; 58 : 483 – 91 . [DOI] [PubMed] [Google Scholar]
- 60. McElroy AK, Erickson BR, Flietstra TD, et al. . Biomarker correlates of survival in pediatric patients with Ebola virus disease . Emerg Infect Dis 2014. ; 20 : 1683 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Legrand J, Grais R, Boelle P, Valleron A, Flahault A . Understanding the dynamics of Ebola epidemics . Epidemiol Infect 2007. ; 135 : 610 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Prescott J BT, Fischer R, Miazgowicz K, Judson S, Munster VJ . Postmortem stability of Ebola virus . Emerg Infect Dis 2015. ; 21 : 856 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. World Health Organization . Field Situation: How to Conduct Safe and Dignified Burial of a Patient who Has Died from Suspected or Confirmed Ebola Virus Disease . geneva: : WHO; , 2014. . [Google Scholar]
- 64. De Roo A, Ado B, Rose B, Guimard Y, Fonck K, Colebunders R . Survey among survivors of the 1995 Ebola epidemic in Kikwit, Democratic Republic of Congo: their feelings and experiences . Trop Med Int Health 1998. ; 3 : 883_85 . [DOI] [PubMed] [Google Scholar]
- 65. Khan AS, Tshioko FK, Heymann DL, et al. . The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995 . J Infect Dis 1999. ; 179 : S76 – S86 . [DOI] [PubMed] [Google Scholar]
- 66. Kerstiëns B, Matthys F . Interventions to control virus transmission during an outbreak of Ebola hemorrhagic fever: experience from Kikwit, Democratic Republic of the Congo, 1995 . J Infect Dis 1999. ; 179 : S263 – S67 . [DOI] [PubMed] [Google Scholar]
- 67. Emond R, Evans B, Bowen E, Lloyd G . A case of Ebola virus infection . Br Med J 1977. ; 2 : 541 – 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fasina F, Shittu A, Lazarus D, et al. . Transmission dynamics and control of Ebola virus disease outbreak in Nigeria, July to September 2014 . Euro Surveill 2014. ; 19 : 20920 . [DOI] [PubMed] [Google Scholar]
- 69. Borchert M, Mutyaba I, Van Kerkhove MD, et al. . Ebola haemorrhagic fever outbreak in Masindi District, Uganda: outbreak description and lessons learned . BMC Infect Dis 2011. ; 11 : 357 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baize S, Pannetier D, Oestereich L, et al. . Emergence of Zaire Ebola virus disease in Guinea . N Engl J Med 2014. ; 371 : 1418 – 25 . [DOI] [PubMed] [Google Scholar]
- 71. Hall R . The 1995 Kikwit Ebola outbreak – model of virus properties on system capacity and function: a lesson for future viral epidemics . Am J Disaster Med 2006. ; 2 : 270 – 76 . [PubMed] [Google Scholar]
- 72. Ikegami T, Niikura M, Saijo M, et al. . Antigen capture enzyme-linked immunosorbent assay for specific detection of Reston Ebola virus nucleoprotein . Clin Diagn Lab Immunol 2003. ; 10 : 552 – 57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heffernan RT, Pambo B, Hatchett RJ, Leman PA, Swanepoel R, Ryder RW . Low seroprevalence of IgG antibodies to Ebola virus in an epidemic zone: Ogooue-Ivindo region, northeastern Gabon, 1997 . J Infect Dis 2005. ; 191 : 964 – 68 . [DOI] [PubMed] [Google Scholar]
- 74. Heymann D, Weisfeld J, Webb P, Johnson K, Cairns T, Berquist H . Ebola hemorrhagic fever: Tandala, Zaire, 1977–1978 . J Infect Dis 1980. ; 142 : 372 – 76 . [DOI] [PubMed] [Google Scholar]
- 75. Park DJ, Dudas G, Wohl S, et al. . Ebola virus epidemiology, transmission, and evolution during seven months in Sierra Leone . Cell 2015. ; 161 : 1516 – 26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Knobloch J, Albiez E, Schmitz H . A Serological Survey on Viral Haemorrhagic Fevers in Liberia . paris: : Institut Pasteur/Virologie; , 1982. . [Google Scholar]
- 77. Towner JS, Sealy TK, Khristova ML, et al. . Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda . PLoS Pathog 2008. ; 4 : e1000212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. MacNeil A, Reed Z, Rollin PE . Serologic cross-reactivity of human IgM and IgG antibodies to five species of Ebola virus . PLoS Negl Trop Dis 2011. ; 5 : e1175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Perkins MD, Kessel M . What Ebola tells us about outbreak diagnostic readiness . Nat Biotechnol 2015. ; 33 : 464 – 69 . [DOI] [PubMed] [Google Scholar]
- 80. Vladyko A, Zaĭtseva V, Trofimov N, et al. . [False-positive reactions in laboratory diagnosis of Lassa, Marburg, and Ebola viral hemorrhagic fevers and AIDS] . Vopr Virusol 1996. ; 42 : 66 – 70 . [PubMed] [Google Scholar]
- 81. Olabode AS, Jiang X, Robertson DL, Lovell SC . Ebola virus is evolving but not changing: no evidence for functional change in EBOV from 1976 to the 2014 outbreak . Virology 2015. ; 482 : 202_07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McSpadden K . The Ebola Virus is Mutating, Say Scientists . Time , 29 January 2015. . [Google Scholar]
- 83. Nyenswah T, Massaquoi M, Gbanya M, et al. . Initiation of a ring approach to infection prevention and control at non-ebola health care facilities – Liberia, January-February 2015 . MMWR Morb Mortal Wkly Rep 2015. ; 64 : 505 – 08 . [PMC free article] [PubMed] [Google Scholar]
- 84. Klompas M, Yokoe DS . The Ebola transmission paradox . Am J Infect Control 2015. ; 43 : 786 . [DOI] [PubMed] [Google Scholar]
- 85. Gear S, Cassel GA, Gear AJ, et al. . Outbreak of Marburg virus disease in Johannesburg . Br Med J 1975. ; 4 : 489 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


