Abstract
Purpose
The aim of this study was to investigate the prognostic value of preoperative neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and lymphocyte–monocyte ratio (LMR) in patients with upper urinary tract urothelial carcinoma (UUTUC).
Methods
We retrospectively analyzed the clinical data of 140 patients with UUTUC who underwent radical nephroureterectomy from January 2005 to December 2011. We plotted receiver operating characteristic curves of NLR, PLR, and LMR for the diagnosis of tumor recurrence. Survival analysis was performed using the Kaplan–Meier method and log-rank test. Independent risk factor analysis was performed using a Cox proportional hazards regression model.
Results
Receiver operating characteristic curves showed that NLR was superior to PLR and LMR as a predictive factor in patients with UUTUC undergoing radical nephroureterectomy. Univariate analysis revealed that NLR (P<0.001 and P<0.001), PLR (P=0.01 and P<0.001), and LMR (P<0.001 and P<0.001) were significantly associated with disease-free survival and progression-free survival (PFS), respectively. Multivariate analysis identified NLR and LMR as independent prognostic factors for disease-free survival (P=0.035 and P=0.002) and PFS (P=0.005 and P=0.002), respectively.
Conclusion
NLR and LMR could be independent predictors of disease-free survival and PFS, and NLR is a superior predictive factor to LMR.
Keywords: prognostic factors, neutrophil–lymphocyte ratio, platelet–lymphocyte ratio, lymphocyte–monocyte ratio
Introduction
Upper urinary tract urothelial carcinoma (UUTUC) is a rare genitourinary malignancy that accounts for ~2% of all urinary tract tumors and 5% of urothelial carcinoma (UC).1,2 Radical nephroureterectomy (RNU) with bladder cuff excision represents the standard treatment for localized UUTUC.3 However, the prognosis of patients who received RNU was poorer than that of those with bladder urothelial carcinoma. For patients with UUTUC with local muscular invasion, the 5-year disease-free survival (DFS) rate was 50%, and for those who had advanced disease, the rate decreased to 10%.4
Currently, recognized independent prognostic factors include tumor stage, tumor grade, extensive tumor necrosis, sessile tumor architecture, and lymphovascular invasion (LVI), which are mostly derived from postoperative data.5–8 Some preoperative biomarkers, including serum creatinine and hemoglobin levels and Eastern Cooperative Oncology Group performance status, are recognized as independent prognostic factors in patients with UUTUC.9 However, they are not sufficient to guide clinical decision making. Therefore, other reliable pretreatment prognostic factors are urgently needed. There is an increasing amount of evidence to support the role of systemic inflammation and inflammatory microenvironment in carcinogenesis and the development and progression of tumor.10–13
Some systemic inflammatory indicators have been introduced as prognostic markers in several types of cancer. Some cell types, such as neutrophils and lymphocytes, have been shown to predict the prognosis of various cancers.14–16 Based on the numbers of circulating inflammatory cells, some indexes have been calculated and used as valuable prognostic predictors. For example, the neutrophil–lymphocyte ratio (NLR) has been suggested as a prognostic indicator for lung, colorectal, breast, and urinary cancers and hepatocellular carcinoma.17–21 Platelet–lymphocyte ratio (PLR) is another valuable predictor, which has been validated in gastric cancer, intrahepatic cholangiocarcinoma, pancreatic ductal adenocarcinoma, and ovarian cancer.22–26 In addition, the lymphocyte–monocyte ratio (LMR) has diagnostic value in cervical cancer, renal cell carcinoma, UUTUC, lung cancer, and esophageal squamous cell carcinoma.27–30 Although NLR, PLR, and LMR can be used as outcome predictors, few studies have compared their prognostic value in UUTUC simultaneously.
In this study, we compared the prognostic value of preoperative NLR, PLR, and LMR in patients with UUTUC.
Materials and methods
Study subjects
We retrospectively reviewed the clinicopathological data of patients with UUTUC who underwent RNU with bladder cuff excision at the Affiliated Hospital of Qingdao University between January 2005 and December 2011. Patients who received adjuvant chemotherapy or radiotherapy, had clinical evidence of infection, or had advanced disease were excluded from the study. We assessed the data from a final total of 140 patients. Data regarding age, sex, smoking status, history of hypertension and diabetes, history of adjuvant chemotherapy and radiotherapy, tumor location, previous or concomitant bladder cancer, LVI, tumor necrosis, hematuria, hydronephrosis, tumor–node–metastasis staging, and differential grade were obtained from medical records. Staging was assessed according to the tumor–node–metastasis classification, and grading was assessed according to the World Health Organization guidelines.31 We obtained cell counts from routine blood tests that were carried out within 3 days before surgery. Disease recurrence was defined as local treatment failure and lymph node and distant metastases. Bladder recurrence was excluded from the analysis of progression-free survival (PFS). The last follow-up date was March 30, 2015.
All patients gave written informed consent. The study was approved by the Institutional Review Board of the Affiliated Hospital of Qingdao University.
Statistical analysis
NLR was obtained by dividing absolute neutrophil count by absolute lymphocyte count, LMR by dividing absolute lymphocyte count by absolute monocyte count, and PLR by dividing absolute platelet count by absolute lymphocyte count. The study endpoint was DFS. We plotted receiver operating characteristic (ROC) curves of NLR, PLR, and LMR for the diagnosis of tumor recurrence. The relationships between NLR, LMR, and PLR and other clinicopathological parameters were compared by Pearson’s χ2 test. DFS and PFS curves were drawn by the Kaplan–Meier method and evaluated by the log-rank test. Univariate and multivariate analyses were performed using the log-rank test and Cox proportional hazards regression models. A value of P<0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS Version 19.0 (IBM Corporation, Armonk, NY, USA).
Results
Among the 140 patients, there were 86 males and 54 females, with a median age of 67 years (range: 39–81 years), and the median survival time was 45 months (range: 11–108 months). Thirty patients were lost to follow-up >10 years. Twenty-seven patients died from cancer-related causes, and six from a traffic accident, myocardial infarction, or natural causes. Thirty-five patients had tumor recurrence or metastasis.
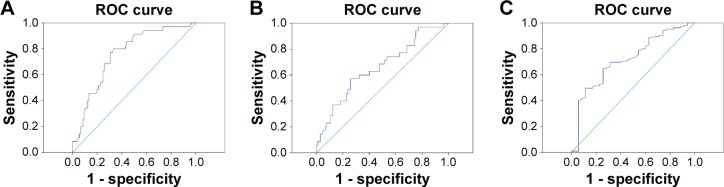
For the 140 patients with UUTUC, an NLR of 2.2 calculated by ROC curve showed the best sensitivity and specificity (Figure 1A). Based on the cutoff value of 2.2, 63 patients (45%) had an elevated preoperative NLR. The area under the curve (AUC) for NLR was 0.759 (95% confidence interval [CI]: 0.671–0.846, P<0.001). A PLR of 128 showed the best sensitivity and specificity on the ROC plot (Figure 1B). Forty-seven patients (33.6%) had an elevated preoperative PLR. The AUC for PLR was 0.659 (95% CI: 0.553–0.765, P=0.005). The cutoff value of LMR was 3.6, with an AUC of 0.717 (95% CI: 0.620–0.814, P<0.001; Figure 1C). Our data showed that NLR was superior to PLR and LMR as a predictive factor in patients with UUTUC undergoing RNU.
Figure 1.
ROC curves for survival prediction.
Notes: ROC curves were plotted to verify the accuracy of NLR, PLR, and LMR for survival. (A) ROC curves of NLR for survival prediction. (B) ROC curves of PLR for survival prediction. (C) ROC curves of LMR for survival prediction.
Abbreviations: LMR, lymphocyte–monocyte ratio; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; ROC, receiver operating characteristic.
The cutoff values of LMR, NLR, and PLR derived from the ROC curves were used to divide the patients into high- and low-LMR, low-NLR and low-PLR groups. The relationships between clinicopathological parameters and NLR, LMR, or PLR are shown in Table 1. Preoperative NLR was significantly higher in pathological tumor (pT) 3/4 stage UUTUC (P<0.001). For patients with pT 3/4 stage UUTUC or LVI, preoperative PLR was elevated significantly (P=0.002, P=0.028). Decreased preoperative LMR was significantly associated with old age (P=0.004) and advanced pT stage (P<0.001).
Table 1.
Relationships between clinicopathological parameters and NLR, LMR, or PLR
| Variable | NLR ≥2.2 | NLR <2.2 | P-value | PLR ≥128 | PLR <128 | P-value | LMR ≥3.6 | LMR <3.6 | P-value |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| n | n | n | n | n | n | ||||
| Age (years) | 0.618 | 0.427 | 0.004 | ||||||
| <70 | 35 | 46 | 25 | 56 | 28 | 53 | |||
| ≥70 | 28 | 31 | 22 | 37 | 35 | 24 | |||
| Sex | 0.197 | 0.491 | 0.807 | ||||||
| Male | 35 | 51 | 27 | 59 | 38 | 48 | |||
| Female | 28 | 26 | 20 | 34 | 25 | 29 | |||
| Smoking status | 0.707 | 0.571 | 0.133 | ||||||
| Yes | 17 | 23 | 12 | 28 | 22 | 18 | |||
| No | 46 | 54 | 35 | 65 | 41 | 59 | |||
| Hypertension | 0.071 | 0.415 | 0.27 | ||||||
| Yes | 29 | 24 | 20 | 33 | 27 | 26 | |||
| No | 34 | 53 | 27 | 60 | 36 | 51 | |||
| Diabetes | 0.225 | 0.599 | 0.794 | ||||||
| Yes | 12 | 9 | 6 | 15 | 10 | 11 | |||
| No | 51 | 68 | 41 | 78 | 53 | 66 | |||
| Tumor location | 0.551 | 0.134 | 0.268 | ||||||
| Left | 31 | 34 | 26 | 39 | 26 | 39 | |||
| Right | 32 | 43 | 21 | 54 | 37 | 38 | |||
| Tumor necrosis | 0.205a | 1a | 0.819 | ||||||
| Present | 3 | 2 | 2 | 3 | 3 | 2 | |||
| Absent | 60 | 75 | 45 | 90 | 60 | 75 | |||
| Hematuresis | 0.389 | 0.974 | 0.254 | ||||||
| Present | 46 | 61 | 36 | 71 | 51 | 56 | |||
| Absent | 17 | 16 | 11 | 22 | 12 | 21 | |||
| Hydronephrosis | 0.285 | 0.593 | 0.756 | ||||||
| Present | 40 | 42 | 29 | 53 | 36 | 46 | |||
| Absent | 23 | 35 | 18 | 40 | 27 | 31 | |||
| Bladder cancer | 0.317 | 0.347a | 0.115 | ||||||
| Yes | 7 | 5 | 6 | 6 | 8 | 4 | |||
| No | 55 | 72 | 41 | 87 | 55 | 73 | |||
| Tumor grade | 0.284 | 0.372 | 0.696 | ||||||
| 1/2 | 27 | 40 | 20 | 47 | 29 | 38 | |||
| 3 | 36 | 37 | 27 | 46 | 34 | 39 | |||
| Pathological T stage | 0.000 | 0.002 | 0.000 | ||||||
| a-2 | 28 | 60 | 21 | 67 | 27 | 61 | |||
| 3/4 | 35 | 17 | 26 | 26 | 36 | 16 | |||
| LVI | 0.502a | 0.028a | 0.131a | ||||||
| Present | 4 | 2 | 5 | 1 | 5 | 1 | |||
| Absent | 59 | 75 | 42 | 92 | 58 | 76 | |||
Note:
Continuity correction χ2 test.
Abbreviations: LMR, lymphocyte–monocyte ratio; LVI, lymphovascular invasion; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio.
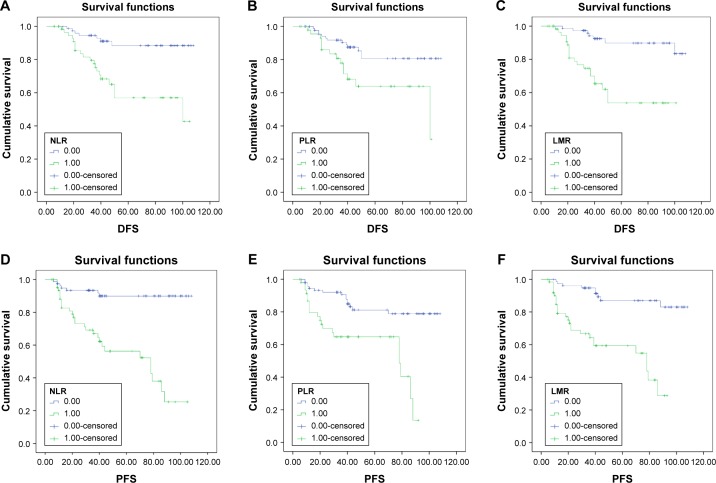
The 5-year DFS rate in the high-NLR (≥2.2) group was significantly lower than in the low-NLR (<2.2) group (54.3% vs 88.2%; Figure 2A). The 5-year DFS rate in the high-PLR (≥128) group was significantly lower than in the low-PLR (<128) group (63.9% vs 80.6%; Figure 2B). The 5-year DFS rate in the low-LMR (<3.6) group was significantly lower than in the high-LMR (≥3.6) group (53.4% vs 89.8%; Figure 2C). Similarly, the 5-year PFS rate was 55.3% for the high-NLR (≥2.2) group and 89.9% for the low-NLR (<2.2) group (Figure 2D). The 5-year PFS rate was 65.1% for the high-PLR (≥128) group and 81.2% for the low PLR (<128) group (Figure 2E). The 5-year PFS rate was 59.1% for the low-LMR (<3.6) group and 87.0% for the high-LMR (≥3.6) group (Figure 2F).
Figure 2.
Kaplan–Meier curves for DFS and PFS of patients with UUTUC.
Notes: (A) DFS curves of patients with UUTUC in the low-NLR group (<2.2) vs the high-NLR group (≥2.2), P<0.001; (B) DFS curves of patients with UUTUC in the low-PLR group (<128) vs the high-PLR group (≥128), P=0.01; (C) DFS curves of patients with UUTUC in the high-LMR group (≥3.6) vs the low-LMR group (<3.6), P<0.001. (D) PFS curves of patients with UUTUC in the low-NLR group (<2.2) vs the high-NLR group (≥2.2), P<0.001; (E) PFS curves of patients with UUTUC in the low-PLR group (<128) vs the high-PLR group (≥128), P<0.001; (F) PFS curves of patients with UUTUC in the high-LMR group (≥3.6) vs the low-LMR group (,3.6), P<0.001.
Abbreviations: DFS, disease-free survival; LMR, lymphocyte–monocyte ratio; NLR, neutrophil–lymphocyte ratio; PFS, progression-free survival; PLR, platelet–lymphocyte ratio; UUTUC, upper urinary tract urothelial carcinoma.
As shown in Table 2, univariate analysis revealed that age (P=0.001 and P=0.009), smoking (P=0.007 and P=0.015), LVI (P=0.001 and P<0.001), pathological stage (P<0.001 and P<0.001), NLR (P<0.001 and P<0.001), PLR (P=0.01 and P<0.001), and LMR (P<0.001 and P<0.001) were significantly associated with DFS and PFS, respectively. Differential grade was significantly associated with PFS (P=0.018). Multivariate analysis indicated that pathological stage, NLR, and LMR were identified as independent prognostic factors for DFS (P<0.001, P=0.035, and P=0.002) and PFS (P<0.001, P=0.005, and P=0.002; Table 3). In addition, smoking was identified as an independent prognostic factor for DFS (P=0.024).
Table 2.
Univariate analysis of DFS and PFS in 140 patients with upper urinary tract urothelial carcinoma
| Variable | n | DFS
|
PFS
|
||
|---|---|---|---|---|---|
| χ2 | P-value | χ2 | P-value | ||
| Age (years) | |||||
| <70 | 81 | 10.734 | 0.001 | 6.828 | 0.009 |
| ≥70 | 59 | ||||
| Sex | |||||
| Male | 86 | 0.09 | 0.765 | 0.226 | 0.635 |
| Female | 54 | ||||
| Smoking status | |||||
| Yes | 40 | 7.297 | 0.007 | 5.924 | 0.015 |
| No | 100 | ||||
| Hypertension | |||||
| Yes | 53 | 0.011 | 0.917 | 0.277 | 0.599 |
| No | 87 | ||||
| Diabetes | |||||
| Yes | 21 | 0.011 | 0.915 | 0.655 | 0.418 |
| No | 119 | ||||
| Tumor location | |||||
| Left | 65 | 2.106 | 0.147 | 0.222 | 0.637 |
| Right | 75 | ||||
| Tumor necrosis | |||||
| Yes | 5 | 1.351 | 0.245 | 0.766 | 0.382 |
| No | 135 | ||||
| Hematuresis | |||||
| Yes | 107 | 0.05 | 0.822 | 0.054 | 0.816 |
| No | 33 | ||||
| Hydronephrosis | |||||
| Yes | 82 | 0.099 | 0.753 | 0.038 | 0.846 |
| No | 58 | ||||
| Bladder cancer | |||||
| Yes | 12 | 0.595 | 0.441 | 2.27 | 0.132 |
| No | 128 | ||||
| Tumor grade | |||||
| 1/2 | 67 | 2.689 | 0.101 | 5.64 | 0.018 |
| 3 | 73 | ||||
| Pathological T stage | |||||
| a-2 | 88 | 44.272 | 0.000 | 60.893 | 0.000 |
| 3/4 | 52 | ||||
| LVI | |||||
| Yes | 6 | 11.551 | 0.001 | 12.549 | 0.000 |
| No | 134 | ||||
| NLR | |||||
| ≥2.2 | 63 | 14.473 | 0.000 | 28.806 | 0.000 |
| <2.2 | 77 | ||||
| PLR | |||||
| ≥128 | 47 | 6.7 | 0.01 | 17.071 | 0.000 |
| <128 | 93 | ||||
| LMR | |||||
| ≥3.6 | 77 | 17.841 | 0.000 | 26.635 | 0.000 |
| <3.6 | 63 | ||||
Abbreviations: DFS, disease-free survival; LMR, lymphocyte–monocyte ratio; NLR, neutrophil–lymphocyte ratio; PFS, progression-free survival; PLR, platelet–lymphocyte ratio; LVI, lymphovascular invasion.
Table 3.
Multivariate analysis of DFS and PFS in 140 patients with upper urinary tract urothelial carcinoma
| Variables | DFS
|
PFS
|
||||
|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | |
| Sex | 0.061 | 0.312 | 0.092–1.055 | 0.356 | 0.616 | 0.220–1.723 |
| Age | 0.197 | 1.865 | 0.724–4.802 | 0.548 | 1.259 | 0.594–2.665 |
| Smoking | 0.024 | 4.193 | 1.208–14.555 | 0.112 | 2.231 | 0.829–6.001 |
| Hypertension | 0.598 | 0.771 | 0.293–2.026 | 0.602 | 0.798 | 0.342–1.863 |
| Diabetes | 0.861 | 0.890 | 0.241–3.282 | 0.712 | 1.198 | 0.459–3.129 |
| Pathological T stage | 0.000 | 9.279 | 2.776–31.017 | 0.000 | 10.110 | 3.582–28.529 |
| Tumor grade | 0.924 | 1.049 | 0.389–2.828 | 0.535 | 1.313 | 0.556–3.101 |
| LVI | 0.150 | 4.898 | 0.563–42.626 | 0.228 | 3.165 | 0.487–20.565 |
| Bladder cancer | 0.450 | 0.547 | 0.114–2.619 | 0.866 | 1.111 | 0.328–3.760 |
| Tumor necrosis | 0.551 | 0.392 | 0.018–8.488 | 0.700 | 1.613 | 0.141–18.413 |
| Tumor location | 0.727 | 0.838 | 0.311–2.261 | 0.406 | 1.461 | 0.598–3.570 |
| Hematuresis | 0.182 | 2.384 | 0.665–8.540 | 0.084 | 2.514 | 0.885–7.139 |
| Hydronephrosis | 0.659 | 1.258 | 0.455–3.476 | 0.562 | 1.304 | 0.532–3.195 |
| NLR | 0.035 | 0.326 | 0.115–0.924 | 0.005 | 3.819 | 1.494–9.761 |
| PLR | 0.431 | 1.441 | 0.580–3.582 | 0.053 | 2.234 | 0.990–5.042 |
| LMR | 0.002 | 6.307 | 1.938–20.530 | 0.002 | 4.909 | 1.804–13.358 |
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; LMR, lymphocyte–monocyte ratio; LVI, lymphovascular invasion; NLR, neutrophil–lymphocyte ratio; PFS, progression-free survival; PLR, platelet–lymphocyte ratio.
Discussion
Since Coussen and Werb proposed that tumor formation is derived from chronic inflammation, many studies have shown that inflammation is related to the development and progression of cancer.12,13,32
As critical cellular components of human immunity, peripheral leukocytes, and platelets play an important role in carcinogenesis. Neutrophilia is associated with malignancy, because neutrophils produce circulating vascular endothelial cell growth factor; the overexpression of which promotes the formation of tumor blood vessels. Cytokines, including interleukin-1, interleukin-6, and tumor necrosis factor, may cause elevation of neutrophilia.33,34 Lymphocytes are the main components of antitumor immunity, and their reduction may cause abnormal immune function and reduce antitumor immunity. With the help of CD4+ T-cells, CD8+ T-cells can control tumor growth by cytotoxic activity and inducing apoptosis of tumor cells.34 The low level of lymphocyte infiltration in cancer-adjacent tissues is conducive to the proliferation and metastasis of cancer cells, thus affecting the prognosis of tumor patients.35 As major cellular components, platelets also have an important role in cancer progression and metastasis. Platelets can facilitate amplification of cancer-related coagulation by providing a procoagulant surface or working synergistically with elevated fibrinogen and can accumulate to protect tumor cells from immune responses.36,37 Some researchers believe that platelets function as dynamic reservoirs of proangiogenic and antiangiogenic proteins.38 Monocytes can initiate tumorigenesis by producing high levels of reactive oxygen and nitrogen species, which can react with DNA and promote tumor progression and metastasis by releasing a large number of cytokines.39
Some novel inflammatory indicators can be established by combining peripheral blood cell counts, such as NLR, LMR, and PLR, which have been investigated for their predictive prognostic value in malignant tumors. The prognostic values of NLR, LMR, and PLR have also been studied in UUTUC. Dalpiaz et al40 reported that high NLR (≥2.7) was significantly associated with shorter cancer-specific survival (CSS) and overall survival (OS) rates in univariate analysis, and NLR was an independent maker for CSS and OS. Azuma et al41 revealed that recurrence-free survival and CSS rates of patients in the high-NLR group (≥2.5) were lower than those in the low-NLR group (<2.5). They also showed that NLR is an independent risk factor for recurrence-free survival and CSS. Using a cutoff value of 3.0, NLR was also an independent risk factor for CSS.42,43 Consistent with the aforemntioned results, we validated preoperative NLR as a prognostic marker in UUTUC. Using a cutoff value of 2.2, the patients in the high-NLR group had poorer DFS and PFS than those in the low-NLR group. In the multivariate analysis, NLR was also an independent risk factor for DFS and PFS.
The prognostic value of LMR has been identified in many malignant tumors, such as renal cell carcinoma, esophageal squamous cell carcinoma, and lung cancer,27,30,44 but only rarely in UUTUC. To the best of our knowledge, only one study has examined the prognostic value of LMR in patients with UUTUC.28 LMR, age, and pathological T-stage were independent predictors of OS in patients with UUTUC.28 Similarly, we found that LMR could be an independent predictor of DFS and PFS in patients with UUTUC.
PLR is another index of systemic inflammation that has been validated as a prognostic predictor in some tumors, including colorectal and ovarian cancer and pancreatic ductal adenocarcinoma.45–47 However, to date, no study has specifically investigated the significance of PLR in UUTUC. Our study is believed to be the first to show that elevated PLR (≥128) was significantly associated with LVI, which is considered to be an independent factor for the prognosis in UUTUC.5,6 This indicates that PLR may be associated with prognosis of UUTUC. However, in multivariate analysis, we could not prove that PLR was an independent factor in UUTUC.
We compared NLR, PLR, and LMR to evaluate survival outcomes in patients with UUTUC simultaneously. All three makers had a significant association with pathological T stage; LMR was significantly associated with age, and PLR was significantly associated with LVI. European guidelines on UUTUC state that tumor stage and grade, age, LVI, extensive tumor necrosis, tumor architecture (eg, papillary vs sessile), concomitant carcinoma in situ, and some molecular markers such as microsatellite instability are closely related to the prognosis of UUTUC.31 With good association with pathological T stage, LVI, or age, NLR, PLR, and LMR may have a good relationship with prognosis of UUTUC. In multivariate analysis, we concluded that both NLR and LMR were independent predictors of DFS and PFS. Kim et al48 studied the prognostic value of systemic inflammatory responses in patients with UUTUC. They found that both NLR and PLR had no relationship with prognosis of UUTUC. However, they demonstrated that derived NLR (neutrophil count/white cell count–neutrophil count) was an independent prognostic factor, which was consistent with the findings of Proctor et al.49 Our results differed from those of Kim et al. This was partly because of the difference in the study design and ethnic diversity. Kim et al selected 5.0 and 150 as cutoff values of NLR and PLR empirically, which differed from 2.2 to 128 that were calculated by the ROC curve in our study. The patients in our study were Chinese, whose tumor characteristics were different from those of foreign patients.
It is crucial to select suitable cutoff values of NLR, PLR, and LMR. Almost all studies determined their cutoff values empirically, which differed from each other significantly. Unlike previous studies, we used the ROC curve to determine the ideal cutoff values of NLR, PLR, and LMR. We concluded that NLR was superior to PLR and LMR as a predictive factor in patients with UUTUC undergoing RNU; the AUC of NLR was 0.759, which was superior to 0.659 of PLR and 0.717 of LMR.
There were some limitations to our study. First, this was a retrospective study, and because the patients were not treated by the same doctor it was difficult to ensure the consistency of the clinicopathological data. Second, even if we excluded the effects of inflammation and other factors on the inflammatory cell counts, we could not guarantee that all the interference factors were excluded. Third, OS was another important indicator of prognosis but was not taken into account. Fourth, in our survival analysis, we could not adjust for confounding variables such as the association between clinical factors (age, stage, or LVI) and LMR, NLR, or PLR. Therefore, the results of our survival analysis may be less accurate than that of the survival analysis that could adjust for the association. Additionally, some other clinicopathological parameters, such as tumor architecture (eg, papillary vs sessile), and concomitant carcinoma in situ, were not included in our study. In addition, all patients enrolled in our study were Chinese, so we cannot eliminate the influence of ethnic diversity. Finally, our study sample was small, and the significance of NLR, PLR, and LMR needs to be verified by a large sample.
Conclusion
This is believed to be the first study to compare the prognostic value of NLR, LMR, and PLR in patients with UUTUC who underwent RNU. We confirmed that NLR and LMR could be independent predictors of DFS and PFS. According to the ROC curves, NLR was a superior predictive factor to LMR. These findings need prospective studies to be validated.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey MS, Badalato GM, Hruby GW, Razmjoo M, McKiernan JM. Prognostic indicators for upper tract urothelial carcinoma after radical nephroureterectomy: the impact of lymphovascular invasion. BJU Int. 2012;110(6):798–803. doi: 10.1111/j.1464-410X.2011.10893.x. [DOI] [PubMed] [Google Scholar]
- 3.Margulis V, Shariat SF, Matin SF, et al. Upper Tract Urothelial Carcinoma Collaboration The Upper Tract Urothelial Carcinoma Collaboration Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 4.Fajkovic H, Cha EK, Xylinas E, et al. Disease-free survival as a surrogate for overall survival in upper tract urothelial carcinoma. World J Urol. 2013;31(1):5–11. doi: 10.1007/s00345-012-0939-5. [DOI] [PubMed] [Google Scholar]
- 5.Hong B, Park S, Hong JH, Kim CS, Ro JY, Ahn H. Prognostic value of lymphovascular invasion in transitional cell carcinoma of upper urinary tract. Urology. 2005;65(4):692–696. doi: 10.1016/j.urology.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Bolenz C, Fernandez MI, Trojan L, et al. Lymphangiogenesis occurs in upper tract urothelial carcinoma and correlates with lymphatic tumour dissemination and poor prognosis. BJU Int. 2009;103(8):1040–1046. doi: 10.1111/j.1464-410X.2008.08135.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda K, Asakuma J, Horiguchi A, et al. Prognostic factors for upper urinary tract urothelial carcinoma after nephroureterectomy. Urol Int. 2012;88(2):225–231. doi: 10.1159/000335274. [DOI] [PubMed] [Google Scholar]
- 8.Chromecki TF, Bensalah K, Remzi M, et al. Prognostic factors for upper urinary tract urothelial carcinoma. Nat Rev Urol. 2011;8(8):440–447. doi: 10.1038/nrurol.2011.96. [DOI] [PubMed] [Google Scholar]
- 9.Morizane S, Iwamoto H, Masago T, et al. Preoperative prognostic factors after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Int Urol Nephrol. 2013;45(1):99–106. doi: 10.1007/s11255-012-0347-1. [DOI] [PubMed] [Google Scholar]
- 10.Schiavoni G, Gabriele L, Mattei F. The tumor microenvironment: a pitch for multiple players. Front Oncol. 2013;3:90. doi: 10.3389/fonc.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 12.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103(33):12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maltoni M, Caraceni A, Brunelli C, et al. Steering Committee of the European Association for Palliative Care Prognostic factors in advanced cancer patients: evidence-based clinical recommendations – a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23(25):6240–6248. doi: 10.1200/JCO.2005.06.866. [DOI] [PubMed] [Google Scholar]
- 15.Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. Japanese. [PubMed] [Google Scholar]
- 17.Cedres S, Torrejon D, Martinez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14(11):864–869. doi: 10.1007/s12094-012-0872-5. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir Y, Akin ML, Sucullu I, Balta AZ, Yucel E. Pretreatment neutrophil/lymphocyte ratio as a prognostic aid in colorectal cancer. Asian Pac J Cancer Prev. 2014;15(6):2647–2650. doi: 10.7314/apjcp.2014.15.6.2647. [DOI] [PubMed] [Google Scholar]
- 19.Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30(1):432. doi: 10.1007/s12032-012-0432-4. [DOI] [PubMed] [Google Scholar]
- 20.Liao W, Zhang J, Zhu Q, et al. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl Oncol. 2014;7(2):248–255. doi: 10.1016/j.tranon.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: a meta-analysis. PLoS One. 2014;9(3):e92079. doi: 10.1371/journal.pone.0092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cetinkunar S, Guzel H, Emre Gokce I, et al. High levels of platelet/lymphocyte ratio are associated with metastatic gastric cancer. J BUON. 2015;20(1):78–83. [PubMed] [Google Scholar]
- 23.Chen Q, Dai Z, Yin D, et al. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine. 2015;94(13):e574. doi: 10.1097/MD.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asari S, Matsumoto I, Toyama H, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today. 2015 doi: 10.1007/s00595-015-1206-3. Epub 2015 Jun 25. [DOI] [PubMed] [Google Scholar]
- 25.Zhang WW, Liu KJ, Hu GL, Liang WJ. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol. 2015;36(11):8831–8837. doi: 10.1007/s13277-015-3533-9. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Zhang F, Sheng XG, Zhang SQ. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1-IIa cervical cancer patients who undergo radical surgery. Onco Targets Ther. 2015;8:1355–1362. doi: 10.2147/OTT.S82174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutterer GC, Stoeckigt C, Stojakovic T, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol. 2014;32(7):1041–1048. doi: 10.1016/j.urolonc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Hutterer GC, Sobolev N, Ehrlich GC, et al. Pretreatment lymphocyte-monocyte ratio as a potential prognostic factor in a cohort of patients with upper tract urothelial carcinoma. J Clin Pathol. 2015;68(5):351–355. doi: 10.1136/jclinpath-2014-202658. [DOI] [PubMed] [Google Scholar]
- 29.Lin B, Chen C, Qian Y, Feng J. Prognostic role of peripheral blood lymphocyte/monocyte ratio at diagnosis in diffuse large B-cell lymphoma: a meta-analysis. Leuk Lymphoma. 2015;56(9):2563–2568. doi: 10.3109/10428194.2015.1014367. [DOI] [PubMed] [Google Scholar]
- 30.Han LH, Jia YB, Song QX, Wang JB, Wang NN, Cheng YF. Prognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2015;16(6):2245–2250. doi: 10.7314/apjcp.2015.16.6.2245. [DOI] [PubMed] [Google Scholar]
- 31.Roupret M, Babjuk M, Comperat E, et al. European Association of Urology European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63(6):1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90(3):222–231. doi: 10.1111/j.1365-2613.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szkandera J, Stotz M, Eisner F, et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS One. 2013;8(11):e78225. doi: 10.1371/journal.pone.0078225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 36.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 37.Pichler M, Dalpiaz O, Ehrlich GC, et al. Validation of the preoperative plasma fibrinogen level as a prognostic factor in a European cohort of patients with localized upper tract urothelial carcinoma. J Urol. 2014;191(4):920–925. doi: 10.1016/j.juro.2013.10.073. [DOI] [PubMed] [Google Scholar]
- 38.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 40.Dalpiaz O, Ehrlich GC, Mannweiler S, et al. Validation of pretreatment neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. BJU Int. 2014;114(3):334–339. doi: 10.1111/bju.12441. [DOI] [PubMed] [Google Scholar]
- 41.Azuma T, Matayoshi Y, Odani K, et al. Preoperative neutrophil-lymphocyte ratio as an independent prognostic marker for patients with upper urinary tract urothelial carcinoma. Clin Genitourin Cancer. 2013;11(3):337–341. doi: 10.1016/j.clgc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Luo HL, Chen YT, Chuang YC, et al. Subclassification of upper urinary tract urothelial carcinoma by the neutrophil-to-lymphocyte ratio (NLR) improves prediction of oncological outcome. BJU Int. 2014;113(5b):E144–E149. doi: 10.1111/bju.12582. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka N, Kikuchi E, Kanao K, et al. A multi-institutional validation of the prognostic value of the neutrophil-to-lymphocyte ratio for upper tract urothelial carcinoma treated with radical nephroureterectomy. Ann Surg Oncol. 2014;21(12):4041–4048. doi: 10.1245/s10434-014-3830-3. [DOI] [PubMed] [Google Scholar]
- 44.Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9(9):e108062. doi: 10.1371/journal.pone.0108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17(3):216–222. doi: 10.3109/1354750X.2012.656705. [DOI] [PubMed] [Google Scholar]
- 46.Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13(7):499–503. doi: 10.1007/s12094-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 47.Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197(4):466–472. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 48.Kim M, Moon KC, Choi WS, et al. Prognostic value of systemic inflammatory responses in patients with upper urinary tract urothelial carcinoma. World J Urol. 2015;33(10):1439–1457. doi: 10.1007/s00345-015-1484-9. [DOI] [PubMed] [Google Scholar]
- 49.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107(4):695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]