Abstract
Whites have an increased risk of atrial fibrillation (AF) compared to Blacks. The mechanism underlying this association is unknown. Left atrial (LA) size is an important AF risk factor, and studies in older adults suggest Whites have larger LA diameters. However, because AF itself causes LA dilation, LA size differences may be due to greater subclinical AF among older Whites. We therefore assessed for racial differences in LA size among young adults at low AF risk. The Coronary Artery Risk Development in Young Adults (CARDIA) study enrolled White and Black participants between 18 and 30 years of age. LA diameter was measured in a subset of participants using echocardiography at Year 5 (n = 4,201) and Year 25 (n = 3,373) of follow up. LA volume was also assessed at Year 5 (n = 2,489). Multivariate linear regression models were used to determine the adjusted association between race and LA size. In unadjusted analyses, mean LA diameter was significantly larger among Blacks compared to Whites both at Year 5 (35.5 ± 4.8 mm versus 35.1 ± 4.5 mm, p = 0.01) and Year 25 (37.4 ± 5.1 mm versus 36.8 ± 4.9 mm, p = 0.002). After adjusting for demographics, comorbidities, and echocardiographic parameters, Whites demonstrated an increased LA diameter (0.7 mm larger at Year 5, 95% CI 0.3–1.1, p<0.001; 0.6 mm larger at Year 25, 95% CI 0.3–1.0, p<0.001). There was no significant association between race and adjusted Year 5 LA volume. In conclusion, in a young, well-characterized cohort, the larger adjusted LA diameter among White participants suggests inherent differences in atrial structure may partially explain the higher risk of AF in Whites. The incongruent associations between race, LA diameter, and LA volume suggest that LA geometry, rather than size alone, may have implications for AF risk.
Introduction
Although atrial fibrillation (AF) is the most commonly encountered clinical arrhythmia, the factors responsible for its induction and perpetuation remain incompletely understood [1]. Compared to Whites, Blacks have more established AF risk factors yet paradoxically exhibit a 25–40% reduced risk of the disease [2,3]. Indeed, both White race and European ancestry are independently associated with AF risk [2–4]. While the strength of the race-AF association suggests the pathways that mediate this relationship play an important role in AF pathogenesis, these mechanisms remain entirely unknown.
In addition to race, increased left atrial (LA) size imparts substantial AF risk [5–7]. Previous studies have found that Whites may have significantly larger LA diameters compared to Blacks [8,9], raising the possibility that the race-AF association could be mediated by inherent racial differences in atrial size. However, prior investigations have enrolled predominantly elderly patients at heightened risk of AF. Since AF itself may also cause LA enlargement [10–12], it remains unclear whether these observed differences in atrial size by race are instead an artifact of a greater subclinical AF burden among older Whites compared to Blacks.
Comparison of LA size between Black and White individuals without AF could clarify whether subclinical AF contributes to atrial enlargement in these previously studied populations; the presence of LA size differences by race in a young population at low risk for AF would suggest that subclinical AF cannot explain these measured differences in atrial size. We therefore used the Coronary Artery Risk Development in Young Adults (CARDIA) study to characterize the association between race, LA diameter, and LA volume among a population-based cohort of young adults with a low burden of cardiovascular disease. To further explore this association, we also sought to determine whether percent European ancestry within Blacks is predictive of LA size. We hypothesized that LA diameter and volume measurements would be larger among Whites and increase with percent European ancestry in this young cohort at low risk for AF.
Methods
CARDIA is a prospective, community-based cohort study sponsored by the National Heart, Lung, and Blood Institute. Eligibility, enrollment, and follow-up protocols have been previously published [13]. Briefly, 5,115 Black and White individuals 18 to 30 years of age were recruited between 1985 and 1986 from four medical centers (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). At baseline, all participants underwent a medical history, physical exam, and laboratory testing. Participants were then prospectively followed and measurements were repeated at Years 2, 5, 7, 10, 15, 20, and 25. In addition, participants underwent transthoracic echocardiography at Year 5 and Year 25.
All measurements were made according to a protocol standardized across the four enrollment centers. Race was self-reported by study participants. Resting heart rate in beats/min was assessed by measuring the pulse over 30 seconds. Blood pressure was determined by obtaining three successive measurements at one-minute intervals with the participant in a seated position; recorded values were derived from the average of the second and third measurements. Height and weight were obtained in light clothing. Participants were considered active smokers if they smoked more than 5 cigarettes per week. Alcohol consumption was derived from the participant’s self-reported intake of beer, wine, and liquor and expressed in drinks/week as previously described [14].
Genome-wide data was obtained among Black participants using a microarray platform (Genome-Wide Human SNP Array 6.0, Affymetrix, Santa Clara, CA) as part of the National Heart, Lung, and Blood Institute's Candidate Gene Association Resource (CARe) study [15]. HapMap CEU (Utah residents with northern and western European ancestry) and YRI (Yoruba ethnic group, Ibadan, Nigeria) populations served as European and African ancestral reference populations, respectively. The mean locus-specific percent European ancestry from 601,241 autosomal SNPs was calculated with LAMP-LD [16]. To determine the mean percent European ancestry for each participant, locus-specific ancestry was averaged across the entire genome.
Study participants underwent two-dimensional echocardiography at Year 5 (Acuson, Siemens Healthcare, Erlangen, Germany) and Year 25 (Artida, Toshiba Medical Systems, Otawara, Japan) [17]. Echocardiograms were analyzed in core laboratories at the University of California, Irvine (Year 5) and the Johns Hopkins University (Year 25). LA diameter was measured from the leading edge of the posterior aortic wall to the leading edge of the posterior LA wall using two-dimensional guided M-mode echocardiography in a standard parasternal long-axis view. LA volume was calculated by multiplying the M-mode LA diameter by the two-dimensional area of the LA in the apical four-chamber view by π/6 [18]. Left ventricular (LV) mass was calculated using the Devereux formula as previously described [17]. LV ejection fraction (EF) was assessed at Year 5 with the Teichholz technique using M-mode echocardiography in a parasternal window [19], while LVEF was quantified at Year 25 using two-dimensional four-chamber apical views [20].
Continuous variables with normal distributions are presented as means ± standard deviations (SD) and were compared using t tests. Non-normally distributed continuous variables are presented as medians with interquartile ranges (IQR) and were compared using Wilcoxon rank-sum tests. The association between categorical variables was determined using Chi-squared tests. Linear regression models were used to determine the association between race and LA size both before and after controlling for clinical variables likely to differ by race and known to be associated with LA size [21], including age [22], gender [23], smoking status [22], alcohol consumption [24], body mass index [23], heart rate [25], systolic blood pressure [22], antihypertensive treatment[26], ejection fraction [23], and left ventricular mass [27].
Confounding variables were grouped and added to successive regression models to better understand the iterative effects of certain classes of covariates on the measured outcomes (Fig 1). Linear regression models were also used to determine the association between percent European ancestry and LA size. Because the distribution of European ancestry was right skewed, we also assessed the associations between ancestry, LA diameter, and LA volume after both log-transformation and categorization (by quartile) of this predictor.
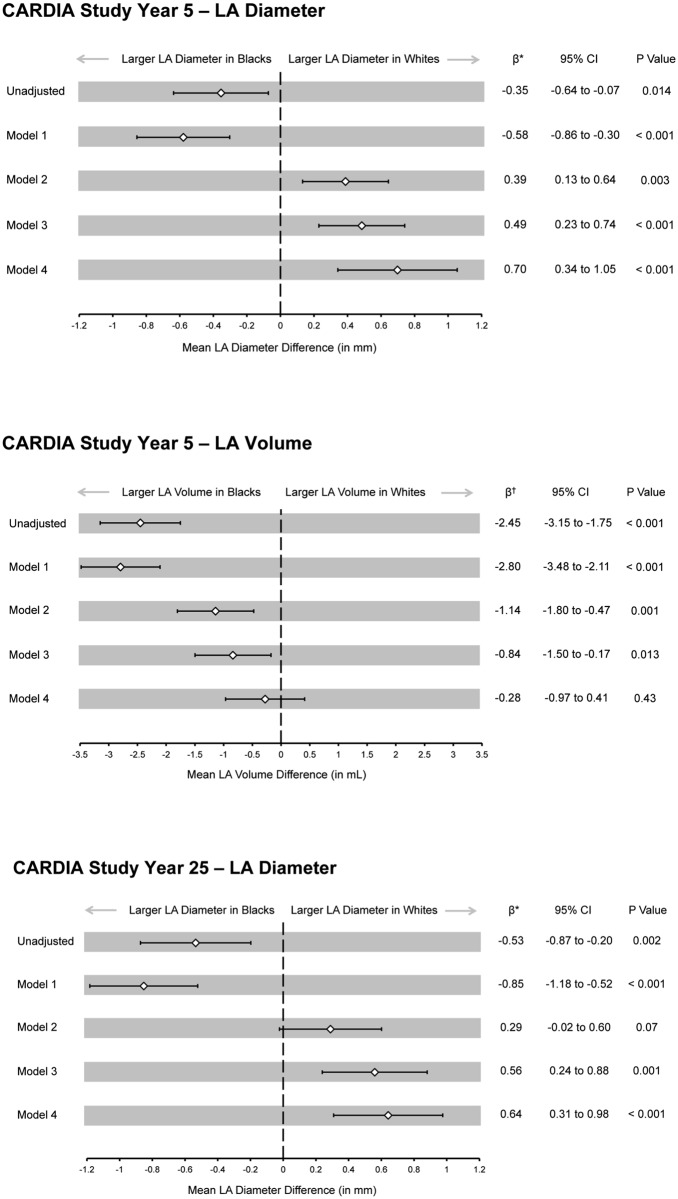
Fig 1. Association Between Race and Left Atrial Size.
Model 1: Adjusted for age and gender. Model 2: Adjusted for Model 1 variables, smoking status, alcohol consumption, and body mass index. Model 3: Adjusted for Model 2 variables, heart rate, systolic blood pressure, and antihypertensive treatment. Model 4: Adjusted for Model 3 variables, left ventricular mass, and left ventricular ejection fraction. * Mean millimeter difference in left atrial diameter between Black and White participants. † Mean milliliter difference in left atrial volume between Black and White participants. CI, confidence interval; LA, left atrium; mL, milliliter; mm, millimeter.
All clinical variables were assessed at both Year 5 and Year 25. Separate analyses were performed at each study time point using covariate and echo data obtained during the same study visit. Individuals without a LA diameter measurement on the Year 5 or Year 25 echocardiogram were excluded from the diameter analysis for the respective year. Due to protocol differences, two-dimensional LA area was not measured in the apical four-chamber view on the Year 25 echocardiogram. As a result, volume analyses were limited to participants with both LA diameter and LA area measurements on the Year 5 examination only. Because clinical evidence [5] and current models of AF pathophysiology identify absolute LA diameter or volume (versus LA dimension corrected for body surface area) as the primary atrial-based determinant of AF risk, our outcome was a non-indexed LA diameter or volume.
Data were analyzed using Stata 12.0 (StataCorp, College Station, TX, USA). A two-tailed p < 0.05 was considered statistically significant. Certification to use deidentified CARDIA data was obtained from the University of California, San Francisco Committee on Human Research.
Results
Study Groups
Of the 4,352 participants examined at the Year 5 visit, 4,243 underwent echocardiography and 4,201 Black and White participants had adequate assessment of LA diameter. Of the 3,498 participants attending the Year 25 examination, 3,474 underwent echocardiography and 3,373 had adequate assessment of LA diameter. Mean age at the Year 5 and Year 25 visits was 30 ± 4 and 50 ± 4 years, respectively. Maximal participant age at the Year 5 and Year 25 visits was 36 and 56 years, respectively. Compared to Whites, Blacks demonstrated a significantly higher mean BMI, had an increased mean systolic blood pressure, were more likely to receive pharmacologic antihypertensive therapy, more frequently smoked cigarettes, and had a higher LV mass (Table 1).
Table 1. Baseline Characteristics of CARDIA Participants by Race*.
| Black (n = 2,038) | White (n = 2,163) | P value† | |
|---|---|---|---|
| Age, years, mean ± SD | 29 (4) | 30 (3) | < 0.001 |
| Male Gender, n (%) | 872 (43) | 1,023 (47) | 0.003 |
| Active Smoker, n (%) | 699 (34) | 498 (23) | < 0.001 |
| Alcohol Consumption, drinks/week, median (IQR) | 0 (0–5) | 2 (0–6) | < 0.001 |
| Body Mass Index, kg/m2, mean ± SD | 27.4 (6.7) | 24.9 (4.7) | < 0.001 |
| Heart Rate, beats/minute, mean ± SD | 69 (10) | 68 (10) | 0.03 |
| Systolic Blood Pressure, mm Hg, mean ± SD | 110 (12) | 106 (11) | < 0.001 |
| Antihypertensive Treatment, n (%) | 52 (3) | 16 (1) | < 0.001 |
| Ejection Fraction, %, mean ± SD | 63 (7) | 63 (6) | 0.32 |
| Left Ventricular Mass, grams, mean ± SD | 153 (46) | 146 (43) | < 0.001 |
* Data describes the 4,201 participants who underwent the Year 5 echocardiographic examination with analyzable LA diameter data.
† P values are for the comparison of the indicated characteristic in Black versus White participants.
IQR, interquartile range; SD, standard deviation.
Baseline Characteristics and LA Diameter
In bivariate analyses, nearly all of the traditional risk factors for atrial enlargement were significantly associated with LA diameter at both Year 5 and Year 25 (Table 2).
Table 2. Unadjusted Association Between Traditional Risk Factors and Left Atrial Diameter.
| CARDIA Study Year 5 | CARDIA Study Year 25 | |||||
|---|---|---|---|---|---|---|
| Risk Factor | β* | 95% CI | P value | β* | 95% CI | P value |
| Age (per 10 years) | 0.9 | 0.5 to 1.3 | < 0.001 | 1.0 | 0.5 to 1.4 | < 0.001 |
| Male Gender | 2.4 | 2.1 to 2.6 | < 0.001 | 2.5 | 2.2 to 2.9 | < 0.001 |
| Active Smoker | 0.4 | 0.1 to 0.7 | < 0.001 | 0.01 | -0.4 to 0.5 | 0.97 |
| Alcohol Consumption (per 5 drinks/week) | 0.2 | 0.1 to 0.2 | < 0.001 | 0.1 | -0.3 to 0.2 | 0.172 |
| Body Mass Index (per 5 kg/m2) | 1.7 | 1.6 to 1.8 | < 0.001 | 3.1 | 2.9 to 3.4 | < 0.001 |
| Heart Rate (per 10 beats/min) | -0.6 | -0.8 to -0.5 | < 0.001 | -0.4 | -0.5 to -0.2 | < 0.001 |
| Systolic Blood Pressure (per 10 mmHg) | 0.9 | 0.8 to 1.1 | < 0.001 | 0.7 | 0.6 to 0.8 | < 0.001 |
| Antihypertensive Treatment | 3.2 | 2.0 to 4.3 | < 0.001 | 2.0 | 1.6 to 2.3 | < 0.001 |
| Ejection Fraction (per 10%) | 0.3 | -0.02 to 0.6 | 0.07 | -0.4 | -0.6 to -0.2 | 0.001 |
| Left Ventricular Mass (per 10 grams) | 0.6 | 0.5 to 0.6 | < 0.001 | 0.5 | 0.4 to 0.5 | < 0.001 |
| Black Race | 0.4 | 0.1 to 0.6 | 0.01 | 0.5 | 0.2 to 0.9 | 0.002 |
* Mean millimeter increase in left atrial diameter per unit change in baseline characteristic.
Race and LA Diameter
In unadjusted analyses, mean LA diameter was significantly larger among Blacks compared to Whites both at Year 5 (35.5 ± 4.8 mm versus 35.1 ± 4.5 mm, p = 0.01) and Year 25 (37.4 ± 5.1 mm versus 36.8 ± 4.9 mm, p = 0.002). After adjusting for the demographic, medical comorbidity, and echocardiographic variables listed in Table 2, Whites demonstrated a significantly increased LA diameter at both Year 5 and Year 25 (Fig 1). The mean difference in LA diameter by race increased after controlling for alcohol and tobacco consumption, body mass index, hemodynamic data, and echocardiographic parameters. The same pattern was observed for the Year 25 LA diameter data. When the race-LA diameter association was adjusted only for LV mass, there was no significant difference in atrial size between races (Year 5: Whites 0.04 mm larger, 95% CI -0.21 to 0.27, p = 0.78, Year 25: Whites 0.17 mm larger, 95% CI -0.14 to 0.48, p = 0.28).
On average, LA diameter increased by 1.9 ± 5.0 mm between the Year 5 and Year 25 examinations. Year 5 LA diameter was significantly associated with Year 25 diameter; for each 1 millimeter increase in Year 5 diameter the Year 25 diameter increased by 0.4 mm (95% CI 0.4 to 0.5, p < 0.001). The unadjusted mean change in LA diameter over 20 years of follow up was not significantly different between races (1.8 ± 4.8 mm in Whites versus 2.0 ± 5.4 mm in Blacks, p = 0.25).
Race and LA Volume
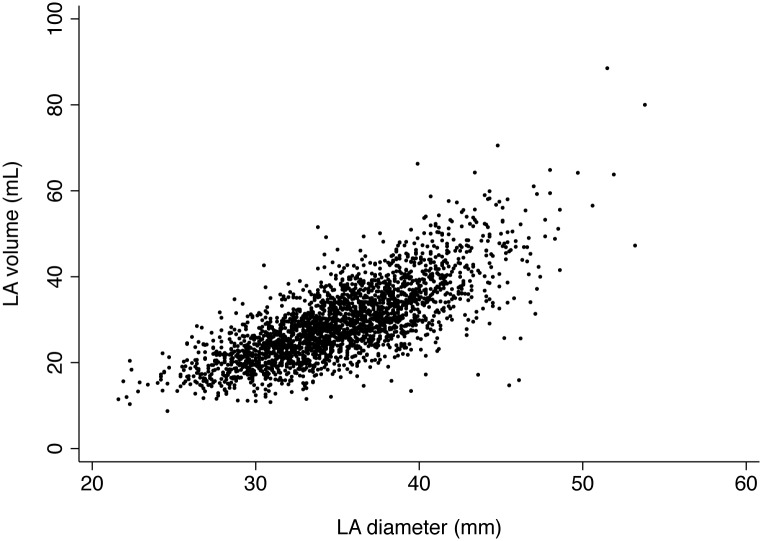
A total of 2,489 participants (1,243 Black, 50%) underwent LA volume assessment on the Year 5 echocardiogram. There was a strong and significant correlation between the Year 5 LA diameter and LA volume measurements (r = 0.75, p < 0.001, Fig 2). In unadjusted analyses, LA volume was significantly larger among Blacks compared to Whites (30.7 ± 9.5 mL versus 28.2 ± 8.2 mL, p < 0.001). After controlling for LV mass alone, mean LA volume remained, on average, 1.4 mL larger in Blacks (95% CI 0.9 to 2.0 mL, p < 0.001). With full adjustment for the clinical and echocardiographic variables in Table 2, the larger mean LA volume among Blacks was no longer statistically significant (Fig 1).
Fig 2. Correlation Between LA Volume and LA Diameter on the Year 5 Echocardiogram.
Correlation between LA volume and LA diameter among 2,489 White and Black participants (r = 0.75, p < 0.001). Both measurements were obtained on the Year 5 echocardiogram. LA, left atrium; mL, milliliter; mm, millimeter.
European Ancestry and LA Size
European ancestry data was available for 767 of the 2,038 Black participants (38%) with available Year 5 LA diameter data, 669 of the 1,580 Black participants (42%) with Year 25 LA diameter data, and 443 of the 1,243 Black participants (36%) with Year 5 LA volume data. Median percent European ancestry among Black participants was 17% (IQR 12–24%). We did not observe a significant association between percent European ancestry and LA diameter at either study time point; at Year 5, each 10 percent absolute increase in European ancestry was associated with a non-significant 0.1 mm decrease in LA diameter (-0.10, 95% CI -0.38 to 0.18, p = 0.50), while at Year 25 each 10 percent absolute increase in European ancestry was associated with a non-significant 0.02 mm increase in LA diameter (0.02, 95% CI -0.32 to 0.37, p = 0.90). Similarly, there was no significant association between European ancestry and LA volume; each 10 percent absolute increase in European ancestry was associated with a 0.08 mL decrease in LA volume (-0.08, 95% CI -0.75 to 0.59, p = 0.82). These associations were not meaningfully changed after adjustment for the variables listed in Table 2 or when percent European ancestry estimates were either log-transformed or categorized by quartile (S1 Table).
Discussion
In a large, population-based cohort of young adults, we observed a larger unadjusted LA diameter among Black versus White participants. After controlling for several demographic variables and clinical comorbidities, however, adjusted LA diameter was significantly greater among Whites. Although racial differences in atrial diameter were consistent across two serial echocardiographic assessments separated by 20 years, they were notably modest in magnitude. Furthermore, we did not identify a significant association between race and adjusted LA volume, nor was there a discernable relationship between percent European ancestry and LA size (diameter or volume) within Black participants.
Differences in LA diameter between races have been observed in cohorts of older adults (mean age > 65 years) with a high burden of cardiovascular disease. Data from both the Cardiovascular Health Study [8] and the Heart and Soul Study [9] indicate that older Whites exhibit an approximate 2 mm larger left atrial diameter compared to Blacks. As a 5 mm larger LA diameter has been associated with a 40% heightened AF risk [5], these prior findings might explain the substantially elevated risk of AF among Whites compared to non-Whites. AF itself, however, is a known cause of LA dilation, and arrhythmia detection can be difficult when episodes are paroxysmal and asymptomatic. Therefore, in these previously studied populations at high risk for the development of AF, it is unclear if subclinical disease can explain the observed racial differences in atrial diameter.
In the present investigation, we observed a statistically significant larger adjusted LA diameter among Whites on both the Year 5 and Year 25 exams. It is notable that the absolute adjusted difference in LA diameter between races was similar on both the Year 5 and Year 25 exams, suggesting that racial differences in LA size are present at a young age and remain stable over time. Recent evidence indicates that racial differences in AF risk diminish in the setting of comorbid diseases [4], suggesting that race may play an especially important role in driving risk among patients with lone AF. These LA diameter results support this observation, as differences in atrial diameter between races were largest after controlling for a variety of pathologic factors known to increase LA diameter and diminished or disappeared in the presence of AF risk factors. Furthermore, the present study provides compelling evidence that previously described racial differences in LA diameter [8,9] are not entirely explained by poor AF ascertainment. However, it should be recognized that the absolute difference in mean adjusted atrial diameter at each time point (0.70 mm larger in Whites at Year 5, 0.64 mm at Year 25) in the current investigation was smaller than previously reported in cohorts of older patients [8,9]. In light of prior data relating absolute atrial size and AF risk, the differences in diameter observed in the present study are unlikely to fully explain the approximate 25–40% reduction in AF risk previously described among Blacks compared to Whites [2,3]. While it remains possible that atrial size disparities by race become more evident and clinically relevant with advanced age, it is clear that there is not a large racial difference in LA diameter among young adults.
Contrary to previous investigations, Black participants in our study had a significantly larger unadjusted LA diameter on both the Year 5 and Year 25 echocardiograms. This finding could be attributed to racial and age-related differences in LV mass. Ventricular hypertrophy, which is quantified by measurement of LV mass, influences LA size via its contribution to diastolic dysfunction. Blacks had a significantly increased LV mass on both the Year 5 and Year 25 echocardiograms, and after adjustment for this single variable there was no longer a significant difference in LA diameter by race at either time point. It is likely that LV mass represents a readily quantifiable “downstream” measurement that is effected by a variety of pathologic pathways, including hypertension. Notably, prior studies among older populations have not identified significant differences in LV mass by race [9,28]. It is therefore possible that racial differences in LV mass among younger individuals explains the larger unadjusted mean atrial diameter among Blacks in our study, while equalization of ventricular mass at advanced age accounts for the unadjusted findings from previous investigations performed in older patients. Alternatively, increased mortality among younger Blacks with left ventricular hypertrophy [29] (and LA enlargement) could account for differences in LV mass and unadjusted atrial size between the CARDIA cohort and these more elderly study populations.
In addition to atrial diameter, we also measured LA volume on the Year 5 echocardiogram. While prior investigations have established LA diameter as a significant, independent risk factor for incident AF [5,6], more recent data suggests LA volume may be superior for predicting a composite cardiovascular endpoint of AF, heart failure, stroke, transient ischemic attack, myocardial infarction, coronary revascularization, or cardiovascular death [25]. In the present study we did not identify a significant difference in adjusted LA volume between young White and Black participants on the Year 5 exam, and the 95% confidence intervals surrounding this estimate exclude a large, clinically meaningful difference in LA volume between races in early adulthood. It is presently unclear why the comparison of adjusted LA diameter and volume between races did not yield qualitatively consistent results, although race-specific differences in LA geometry could be postulated. Because LA volume requires two separate echocardiographic measurements for its calculation (LA diameter in the parasternal short axis view and LA area in the apical four-chamber view), this parameter may be more prone to bias than LA diameter, especially in obese or otherwise difficult to image patients. Although LA diameter was previously found to be significantly larger among Whites versus Blacks enrolled in the Heart and Soul Study, there was similarly no observed difference in LA volume between races [9]. As LA diameter has been reproducibly associated with incident AF [5,6], these findings suggest that diameter may specifically capture an important aspect of atrial geometry that mediates racial differences in AF risk. In addition, this could imply that LA morphology, rather than volume (or size) alone, is particularly important in imparting AF risk for all susceptible patients.
When our analysis was restricted to Black participants, we did not observe a significant association between percent European ancestry and either of the LA size measurements. The confidence intervals surrounding these point estimates suggest a clinically important association between European ancestry and LA size is very unlikely. We notably used a two-population model to calculate European ancestry; in this setting, European ancestry and African ancestry are perfectly inversely correlated. The association between African ancestry and atrial size among Blacks would therefore yield identical results (but with opposite beta coefficients). In light of prior data linking relatively small increases in European ancestry to AF risk, these results lend further support to the notion that atrial size differences do not adequately explain the substantially heightened AF risk among Whites. Since only a modest proportion of Blacks had percent European ancestry calculations, the resultant loss of power may have diminished our ability to identify significant relationships (i.e. type II error). Alternatively, racial differences in LA diameter may be primarily determined by environmental exposures that are not proportional to genetic ancestry.
Potential limitations of our analysis should be recognized. We sought to characterize racial differences in LA size in a young cohort with a low prevalence of cardiovascular comorbidities to eliminate the potential bias introduced by subclinical AF. However, AF has not been systematically ascertained in the CARDIA cohort and it was not possible to identify the likely small number of patients with this diagnosis. Because the prevalence of AF among individuals < 55 years old is exceedingly low (0.1% for women, 0.2% for men) [30] and the oldest participants for the Year 5 and Year 25 echocardiograms were 36 and 56 years old, respectively, we believe it is unlikely that this potential source of bias could substantially impact our results. Due to slight differences in echocardiogram protocols between study visits, LA volume could only be calculated on the Year 5 examination. As noted above, it remains possible that differences in LA volume by race may become more manifest in older adults. We did not control for diastolic function in our multivariate models, although the prevalence of this abnormality in the CARDIA cohort is low and LV mass may have provided a sufficient surrogate [18]. Finally, race was self-reported by the study participants. It should be noted that the vast majority of studies examining racial differences in AF have used this methodology and that inaccurate self-reporting of race would likely bias our results towards the null. In addition, our ancestry data confirmed that the majority of self-identified Black participants were of predominately African descent.
In a large, multicenter, population-based cohort study of young adults, we observed a larger unadjusted LA diameter among Black versus White participants. After adjusting for clinical comorbidities, however, adjusted LA diameter was significantly greater among Whites and this difference persisted over a 20-year follow up period. A similar adjusted difference in LA volume by race, however, was not observed. Although atrial diameter may partially mediate the association between race and AF, the magnitude of this atrial size difference likely does not adequately explain the substantially heightened AF risk among Whites. Incongruent LA diameter and volume findings by race validate previous findings and suggest that LA geometry, rather than size alone, may have important implications for AF risk.
Supporting Information
(DOCX)
Data Availability
Data are available from the CARDIA Coordinating Center: http://www.cardia.dopm.uab.edu/contact-cardia. A description of the NHLBI policies governing the data and describing access to the data can be found at the following website: http://www.cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data.
Funding Statement
This work was made possible by grant numbers 12POST11810036 (TAD) and 12GRNT11780061 (GMM) from the American Heart Association and by the Joseph Drown Foundation (GMM). The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content. First Cardiology Consultants provided support in the form of salaries for authors KOO, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104: 1534–1539. 10.1016/j.amjcard.2009.07.022 [DOI] [PubMed] [Google Scholar]
- 2.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. Lippincott Williams & Wilkins; 2010;122: 2009–2015. 10.1161/CIRCULATIONAHA.110.958306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158: 111–117. 10.1016/j.ahj.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, Blacks, and Whites. Circulation. Lippincott Williams & Wilkins; 2013;128: 2470–2477. 10.1161/CIRCULATIONAHA.113.002449 [DOI] [PubMed] [Google Scholar]
- 5.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89: 724–730. [DOI] [PubMed] [Google Scholar]
- 6.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96: 2455–2461. [DOI] [PubMed] [Google Scholar]
- 7.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76: 467–475. 10.4065/76.5.467 [DOI] [PubMed] [Google Scholar]
- 8.Manolio TA, Gottdiener JS, Tsang TSM, Gardin JM, Cardiovascular Health Study Collaborative Research Group. Left atrial dimensions determined by M-mode echocardiography in black and white older (> or = 65 years) adults (The Cardiovascular Health Study). Am J Cardiol. 2002;90: 983–987. [DOI] [PubMed] [Google Scholar]
- 9.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, et al. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123: 375.e1–7. 10.1016/j.amjmed.2009.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wozakowska-Kapłon B. Changes in left atrial size in patients with persistent atrial fibrillation: a prospective echocardiographic study with a 5-year follow-up period. Int J Cardiol. 2005;101: 47–52. 10.1016/j.ijcard.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 11.Suarez GS, Lampert S, Ravid S, Lown B. Changes in left atrial size in patients with lone atrial fibrillation. Clin Cardiol. 1991;14: 652–656. [DOI] [PubMed] [Google Scholar]
- 12.Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. 1990;82: 792–797. [DOI] [PubMed] [Google Scholar]
- 13.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41: 1105–1116. [DOI] [PubMed] [Google Scholar]
- 14.Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. Oxford University Press; 2005;161: 423–433. 10.1093/aje/kwi062 [DOI] [PubMed] [Google Scholar]
- 15.Musunuru K, Lettre G, Young T, Farlow DN, Pirruccello JP, Ejebe KG, et al. Candidate gene association resource (CARe): design, methods, and proof of concept. Circ Cardiovasc Genet. 2010;3: 267–275. 10.1161/CIRCGENETICS.109.882696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baran Y, Pasaniuc B, Sankararaman S, Torgerson DG, Gignoux C, Eng C, et al. Fast and accurate inference of local ancestry in Latino populations. Bioinformatics. Oxford University Press; 2012;28: 1359–1367. 10.1093/bioinformatics/bts144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Kurosaki T, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92: 380–387. [DOI] [PubMed] [Google Scholar]
- 18.Desai CS, Colangelo LA, Liu K, Jacobs DR, Cook NL, Lloyd-Jones DM, et al. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults: the coronary artery risk development in young adults study. Am J Epidemiol. Oxford University Press; 2013;177: 20–32. 10.1093/aje/kws224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2005. pp. 1440–1463. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 20.Kishi S, Armstrong AC, Gidding SS, Jacobs DR, Sidney S, Lewis CE, et al. Relation of Left Ventricular Mass at Age 23 to 35 Years to Global Left Ventricular Systolic Function 20 Years Later (from the Coronary Artery Risk Development in Young Adults Study). Am J Cardiol. Elsevier Inc; 2014;113: 377–383. 10.1016/j.amjcard.2013.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong AC, Gidding SS, Colangelo LA, Kishi S, Liu K, Sidney S, et al. Association of early adult modifiable cardiovascular risk factors with left atrial size over a 20-year follow-up period: the CARDIA study. BMJ Open. British Medical Journal Publishing Group; 2014;4: e004001–e004001. 10.1136/bmjopen-2013-004001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92: 835–841. [DOI] [PubMed] [Google Scholar]
- 23.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 24.Singh KJ, Cohen BE, Na B, Regan M, Schiller NB, Whooley MA. Alcohol consumption and 5-year change in left atrial volume among patients with coronary heart disease: results from the Heart and Soul study. J Card Fail. 2013;19: 183–189. 10.1016/j.cardfail.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 25.Tsang TSM, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47: 1018–1023. 10.1016/j.jacc.2005.08.077 [DOI] [PubMed] [Google Scholar]
- 26.Gottdiener JS, Reda DJ, Williams DW, Materson BJ, Cushman W, Anderson RJ. Effect of single-drug therapy on reduction of left atrial size in mild to moderate hypertension: comparison of six antihypertensive agents. Circulation. 1998;98: 140–148. [DOI] [PubMed] [Google Scholar]
- 27.Cioffi G, Mureddu GF, Stefenelli C, de Simone G. Relationship between left ventricular geometry and left atrial size and function in patients with systemic hypertension. J Hypertens. 2004;22: 1589–1596. 10.1097/01.hjh.0000125454.28861.76 [DOI] [PubMed] [Google Scholar]
- 28.Gottdiener JS, Reda DJ, Materson BJ, Massie BM, Notargiacomo A, Hamburger RJ, et al. Importance of obesity, race and age to the cardiac structural and functional effects of hypertension. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1994;24: 1492–1498. [DOI] [PubMed] [Google Scholar]
- 29.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322: 1561–1566. 10.1056/NEJM199005313222203 [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285: 2370–2375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data are available from the CARDIA Coordinating Center: http://www.cardia.dopm.uab.edu/contact-cardia. A description of the NHLBI policies governing the data and describing access to the data can be found at the following website: http://www.cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data.