Abstract
Purpose
To study age-related changes of intraocular pressure (IOP) and assess the cohort effect in both cross-sectional and longitudinal settings among elderly Chinese adults.
Methods
Participants were enrolled from the Lingtou Eye Cohort Study with Chinese government officials aged 40 years and older at baseline and received physical check-up and ocular examinations from 2010 to 2012. IOP was measured using a non-contact tonometer according to standardized protocols, as well as systolic blood pressure (SBP), diastolic blood pressure (DBP) and body mass index (BMI). Participants who had attended IOP measurements in both 2010 and 2012 were included in this study. Cross-sectional association of IOP with age was assessed using multivariate liner regression analyses and based on the data of 2010. Longitudinal changes in IOP were assessed by paired t-test.
Results
A total of 3372 subjects were enrolled in the current analysis (2010 mean [SD] age, 61.9 [7.1] years; 60.2% men). The mean IOP in 2010 was 15.4±2.3 mmHg for women and 15.2±2.3 mmHg for men with an intersex difference (P = 0.029). Cross-sectional analysis showed that IOP was negatively associated with age (P = 0.003, β = -0.033 for women and P<0.001, β = -0.061 for men) adjusted for baseline SBP, DBP and BMI. Paired t-test suggested that IOP was higher in the year 2012 than 2010 in women (P = 0.006) but did not change significantly in men within 2 years (P = 0.345). In addition, the 2-year changes of IOP were not associated with age adjusted for baseline IOP in 2010 (P = 0.249).
Conclusion
Cross-sectional data suggests that IOP is lower in people with older age. Longitudinal data does not support such findings and thus the identified decreasing pattern with age in cross-sectional analysis is likely caused by cohort effects.
Introduction
Elevated intraocular pressure (IOP) is a major, and currently the only modifiable risk factor for glaucoma, a common disease and leading cause of irreversible blindness worldwide.[1] Age has also been established as a significant contributing factor to glaucoma.[2] The relationship between IOP and age has been previously investigated in many cross-sectional studies. Studies based on European or American populations mostly reported an increase of IOP with age, such as in the Beaver Dam Eye Study and the Barbados Eye Study. [3, 4] On the other hand, a decreasing trend of IOP with age in Asian people has been reported in a majority of studies. The Shihpai Eye Study in Taiwan, the Tajimi Eye Study in Japan and the Healthy Twin and the GENDISCAN Study of Korean and Mongolian populations all reported a negative association between IOP and age.[5–7] This discrepancy was explained as secondary to ethnic and environmental influences.[6]
Cross-sectional studies are susceptible to cohort effects when investigating for age effects; that is, an essential selection bias exists in different birth cohorts of the study population due to different environmental and social exposures. Therefore longitudinal studies may present an advantage in illustrating any true underlying associations. However, longitudinal studies of IOP change are rare and show varying results.[8–12] Further research and data, especially from longitudinal studies, are needed to assess the relationship between changes in IOP and age.
A variety of factors have previously been proposed and demonstrated to be associated with IOP. Body mass index (BMI) and systolic blood pressure (SBP) were the most frequently reported factors from previous studies all over the world.[13–16] These should be taken into consideration when investigating the relationship between IOP and age as they are potential confounders. In this paper, we aimed to investigate age-related changes of IOP in both cross-sectional and longitudinal settings and to identify the impact of cohort effect on current cross-sectional analysis.
Materials and Methods
Study population
The study participants were enrolled from the Lingtou Eye Cohort Study, which has been described in detail elsewhere.[17] In brief, government employees aged 40 years and older without history of major cardiovascular events were recruited through the Guangzhou Government Servant Physical Check-up Center in 2008 for long-term follow-up study on account of their high retention rates for annual check-up. The study was conducted under the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Zhongshan Ophthalmic Center, Sun Yat-Sen University, Guangzhou. Written informed consent was obtained from all participants.
The study was initiated in 2008 and included physical and ophthalmologic examinations, as well as questionnaire administered by face-to-face interview. Height, weight, SBP and diastolic blood pressure (DBP) were measured according to standardized protocols by trained nurses and detailed medical histories including ocular, systemic and surgical history (confirmed by medical records) were collected. All participants of the baseline survey were invited to attend the annual follow-up examinations. Follow-up examinations were the same as baseline and performed according to the standardized protocols.
Our study is an exploratory perspective study and included 3770 participants from the Lingtou Eye cohort study who had attended IOP measurement in both 2010 and 2012. Cross-sectional analysis was based on the IOP data initially measured in 2010 and longitudinal analysis was based on the data in 2010 and 2012 of six birth cohorts ranging from the 1930s to 1960s. We further excluded 150 (4.0%) who received IOP lowering treatment or had undergone corneal or intraocular surgery in at least one eye, and 241 (6.4%) whose IOP values were out of the normal range (10-21mmHg). Subjects in the birth cohorts of the 1920s and the 1970s were excluded due to a small sample size of 7 (0.2%) participants. Thus, 3372 (89.4%, 1342 women and 2030 men) were available for the present analysis.
Measurement of intraocular pressure
IOP was measured in both eyes by non-contact tonometer (CT-80A computerized tonometer, Topcon Ltd., Japan) prior to pupil dilation. The data was saved as the mean of 3 continuous measurements. If the 3 consecutive measurements could not achieve SE < 5% or if the subject could not cooperate, the IOP was considered unreliable and further tested for two more times by a trained nurse to get a reliable value. If a reliable value couldn’t be reached at retest, the IOP value was then deemed as missing and not included in the analysis. One final reading was recorded for each eye.
Measurement of blood pressure and body mass index
Blood pressure was measured following protocol using an automatic upper arm blood pressure monitor by trained nurses. Height and weight were measured with subjects wearing light clothes without shoes in the standing position using an automatic height and weight tester. Height was measured to the closest 0.5 cm and weight was measured to the closest 0.5 kg. BMI was calculated as weight in kilograms divided by height in meters squared.
Statistical analysis
All data analysis was performed using Stata Package (Stata 8.0; Stata Corp, College Station, Texas, USA). Measurements from the right eye were selected for analysis because of the high correlation between the two eyes and were summarized using means and standard deviations (SD). Univariate and Multivariate liner regression models were used to investigate the cross-sectional associations between IOP and age. BMI, SBP and DBP were also included in the regression model as confounding factors. In the longitudinal analysis, participants were divided into six age groups by five-year intervals ranging from 50–54 years to 75–79 years. Paired t-test was used to compare the intraocular pressure of the same subject between 2010 and 2012 and a P-value of equal or less than 0.05 was considered statistically significant.
Results
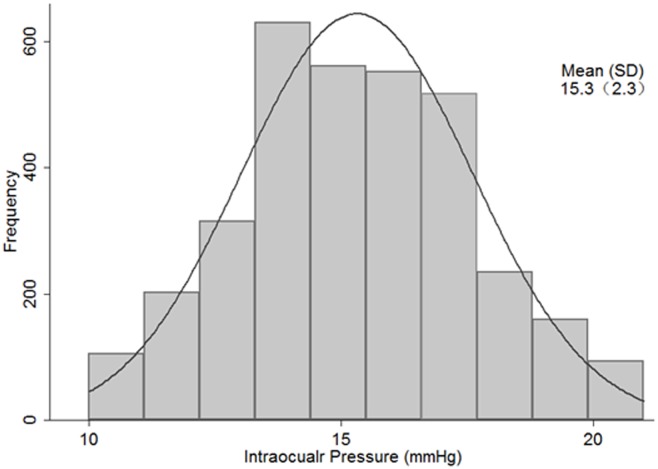
Data of 3372 people were used for analysis of which 60.2% were men. Table 1 summarizes the baseline characteristics of the participants by birth cohorts. The mean IOP was 15.4±2.3 mmHg for women and 15.2±2.3 mmHg for men. Student’s t-test showed that women had higher mean IOP values than men in general (P = 0.029). Fig 1 illustrates the distribution of IOP for the population. IOP was nearly normally distributed with a peak at 14 to 15 mmHg.
Table 1. Baseline Characteristics of the Participants (mean ± standard deviation).
| Birth Cohorts (years) | No. Of Subjects | Mean ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IOP (mmHg) | SBP (mmHg) | DBP (mmHg) | BMI (kg/m2) | |||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| 50–54 | 358 | 270 | 15.7±2.4 | 15.6±2.3 | 125.7±15.0 | 117.5±15.0 | 76.8±10.5 | 69.3±10.1 | 25.1±3.0 | 23.8±2.9 |
| 55–59 | 476 | 462 | 15.6±2.2 | 15.4±2.3 | 127.6±15.9 | 122.2±15.8 | 76.1±10.3 | 69.6±10.6 | 25.1±2.8 | 23.9±3.2 |
| 60–64 | 434 | 290 | 15.2±2.2 | 15.4±2.3 | 132.3±15.1 | 126.3±16.4 | 75.9±9.5 | 70.1±10.3 | 24.8±2.7 | 24.4±3.3 |
| 65–69 | 327 | 152 | 14.9±2.3 | 15.5±2.4 | 135.2±14.7 | 131.0±15.4 | 74.2±9.3 | 69.1±9.5 | 24.9±3.0 | 24.1±3.6 |
| 70–74 | 294 | 118 | 14.9±2.2 | 14.8±2.4 | 137.0±16.3 | 136.9±18.0 | 72.1±9.7 | 68.0±10.7 | 24.0±3.2 | 23.7±2.9 |
| 75–79 | 141 | 50 | 14.6±2.2 | 15.5±2.3 | 136.6±15.4 | 143.5±17.9 | 69.1±10.2 | 71.2±8.3 | 24.2±3.3 | 24.7±3.8 |
| Total | 2030 | 1342 | 15.2±2.3 | 15.4±2.3* | 131.5±16.0 | 125.3±17.3** | 74.8±10.2 | 69.5±10.3** | 24.8±3.0 | 24.0±3.2** |
IOP: intraocular pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: blood mass index.
P values for difference between males and females using group t-test:
* p<0.05;
** p<0.001
Fig 1. Distribution of intraocular pressure among Chinese adults in Lingtou, China (2010).
Histogram of Intraocular pressure for the population under study at baseline. Right eye data was used and the total number is 3372. The dark grey curve represents the normal distribution.
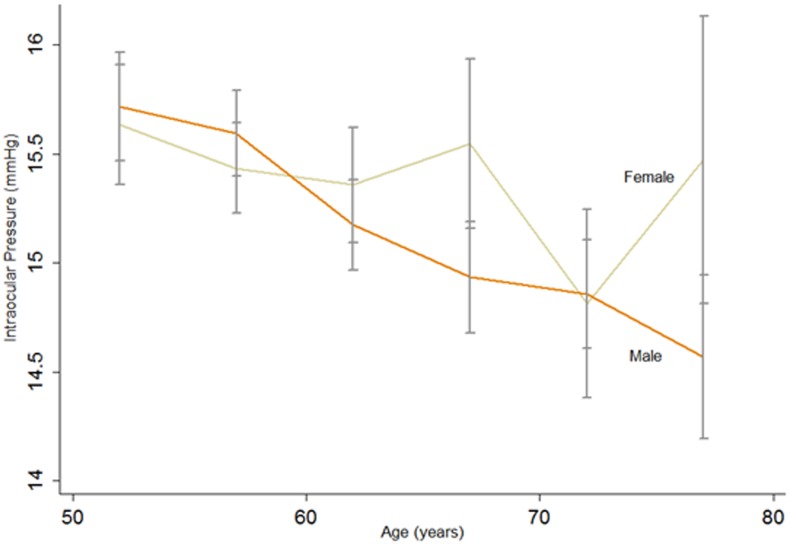
Cross-sectional analysis showed that IOP decreased significantly with age in men of all birth cohorts and women with the exception of the 65–69 birth cohort (Fig 2). Table 2 showed the cross-sectional associations of related risk factors of the year 2010 with IOP. Multiple liner regression showed IOP was negatively related with age after adjusting for SBP, DBP and BMI (P = 0.003, β = -0.033 for women and P<0.001, β = -0.061 for men).
Fig 2. Effect of aging on intraocular pressure (IOP) in cross-sectional analysis.
Cross-sectional change of IOP with increasing age for both genders. Each line was simply connected by 6 points which were the mean IOP value of the right eye of the six birth cohorts (50–54;55–59;60–64;65–69;70–74;75–79) from left to right, respectively. The error bars for each point show the upper and lower 95% confidence limits.
Table 2. Cross-sectional Associations of Related Risk Factors with IOP in 2010 according to Univariate and multivariate Regression Analyses.
| Factors | Univariate regression | Multivariate regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Regression coefficients | P Value | Regression coefficients | P Value | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Age(yrs) | -0.050(-0.063~-0.037) | -0.022(-0.041~-0.004) | <0.001 | 0.019 | -0.061(-0.077~-0.046) | -0.033(-0.055~-0.109) | <0.001 | 0.003 |
| SBP(mmHg) | 0.016(0.010~0.022) | 0.016(0.009~0.023) | <0.001 | <0.001 | 0.022(0.013~0.031) | 0.013(0.002~0.024) | <0.001 | 0.025 |
| DBP(mmHg) | 0.032(0.022~0.042) | 0.033(0.021~0.045) | <0.001 | <0.001 | 0.002(-0.012~0.015) | 0.016(-0.000~0.033) | 0.805 | 0.061 |
| BMI(kg/m2) | 0.075(0.041~0.110) | 0.053(0.014~0.093) | <0.001 | 0.008 | 0.030(-0.005~0.065) | 0.023(-0.018~0.064) | 0.096 | 0.279 |
IOP: intraocular pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: blood mass index.
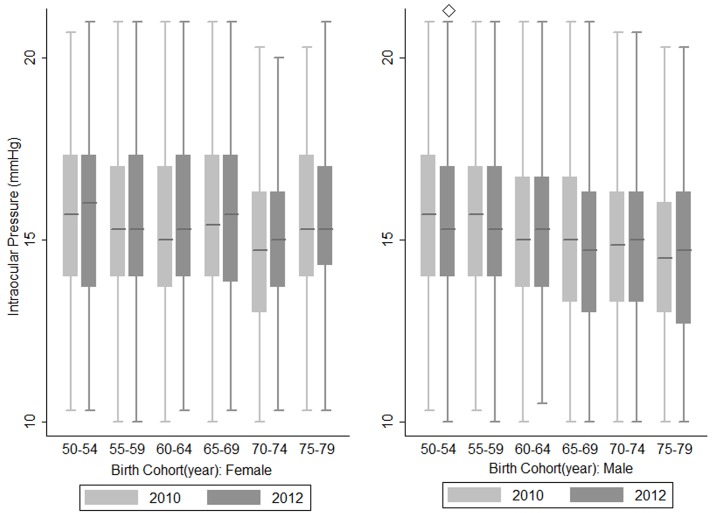
Fig 3 shows the 2-year longitudinal changes of IOP by sex specific birth cohorts. Paired t-test showed there were no statistically significant differences in IOP of the study population between 2010 and 2012 of all birth cohorts, except for the 50–54 birth cohort in men which showed a lower IOP in 2012 (P = 0.006). Regarding sex, IOP increased significantly with age in women (P = 0.006) but remained relatively steady in men (P = 0.345). Furthermore, the 2-year changes of IOP were not associated with age after adjusting for baseline IOP (P = 0.249).
Fig 3. Comparison of intraocular pressure by sex and birth cohorts in 2010 and 2012.
Box plots showing the IOP of the right eye between the year 2010 and 2012 in each birth cohort of the population, ◇ represents a P value <0.05 calculated by paired t-test comparing the mean IOP of the year 2010 and 2012.
Discussion
Our study is the first study to combine both cross-sectional and longitudinal analysis in a large Southern Chinese adult population, suggesting for the first time that the decreasing trend of IOP with age reported by most established cross-sectional studies in Asia is likely caused by cohort effects.
The mean IOP reported in our study was similar to the Liwan Eye Study which was also based on a Southern Chinese adult population (15.4±3.1 mmHg for women; 15.0±3.2 mmHg for men), and the Beijing Eye Study which was based on a Northern Chinese elderly population (15.6±3.0 mmHg),[18] but higher than the reported mean IOP in Japanese studies. Fukuoka et al. reported a mean IOP of 14.1±2.3 mmHg based on the Tajimi Eye Study while Nomura reported a lower IOP value in a larger Japanese study population (11.5±2.4 mmHg for women; 11.9±2.5 mmHg for men).[6, 9] Regarding sex, most studies reported that women had a higher IOP than men,[5, 8] while others found no difference between the sexes.[6] Our study reported a higher IOP in women, and although the underlying mechanisms are unknown, one possible explanation relates to the changing aqueous production with hormonal differences and the onset of menopause.[19]
Most American and European studies, both cross-sectional and longitudinal in nature, conclude that IOP increases with age. One study suggested that age-related structural changes in the trabecular meshwork substantially counteracts the reduced production of aqueous humor with age.[8, 15, 20] While there have been fewer studies based on African populations, a study in West Cameroon and the well-known Barbados Eye study also reported this association.[21, 22] The majority of studies in Asia however, have reported the opposite trend.[5, 7, 18] The Handan Eye Study in China reported a reversed U like course interaction between IOP and age.[13] This was discussed in other studies as being due to a “survival effect”. A smaller proportion of obese and hypertensive subjects exist in the elderly groups due to the increased mortality of cardiovascular disease, and therefore IOP would seem to be lower with age in the absence of these risk factors, forming the rear decline of this curve.[23]
The inconsistency of Asian study findings with other, similar studies was noted by Yoshida and Fukuoka and attributed to ethnic and environmental effects. They proposed that the hypertensive effects caused by high BP and BMI in Europeans and Americans outweighed the hypotensive effects caused by age, causing IOP to seemingly increase with age. Conversely, as the prevalence of obesity and hypertension is lower in Japan, the hypotensive effects of age might predominate, resulting in an apparent decrease in IOP with age. [6, 23] Our cross-sectional analysis supports this and validates the negative correlation of IOP with age reported by most Asia studies. This finding persisted after adjusting for both blood pressure and BMI.
Few longitudinal studies of IOP and age exist in the literature, and even fewer of Asian populations, and no consensus has been reached at present. Nomura reported a significant increase in IOP with age in a 9-year longitudinal follow-up, while Nakano reported a decreasing trend of IOP with age in all age groups over a 10-year follow-up.[9, 11]
In our longitudinal analysis, IOP was found to increase with age in women over the two-year follow up between 2010 and 2012, but not found to change in men, which is inconsistent with the cross-sectional results. This inconsistency was supported by another Japanese cross-sectional and longitudinal study.[9] It is challenging for cross-sectional studies to separate the effects of developmental influences from cohort effects when examining across a wide range of ages. Cohort effects may influence population data, as people who are born at similar times are exposed to intrinsically similar events and demographic trends in life, making the study group unique and different from other population groups. In particular, this manifests as different lifestyle and environmental factors affecting educational levels, nutritional intake and exercise habit.
To the best of our knowledge, this is the first longitudinal study to report on the age-related changes of IOP in Southern China and the strengths include the relatively large population size. Nevertheless, some limitations of this study should be taken into consideration. The Goldmann Applanation Tonometer (GAT), which is widely believed to be the gold-standard measurement tool, was not used in this study as NCT does not require corneal contact or anesthesia and is more efficient at measuring large populations. Though reported to be reliable for values within the normal IOP range, the test-retest repeatability was ± 2.8 mmHg for CT80 non-contact tonometers from previous studies and may affect the validity of IOP results compared to other studies. [5],[24–26] Central corneal thickness (CCT) has a significant association with IOP but unfortunately was not measured in our study due to restrictions of the hospital.[27, 28] Lastly, as the IOP measurements in our study were not performed at the same time of day for different visits due to logistical difficulty, the comparability of recordings may be affected by diurnal fluctuations. The circadian fluctuations of IOP can be substantial in healthy elderly adults and also independent of mean CCT or CCT fluctuations in glaucoma patients.[29, 30] However the largest fluctuation mostly occurred during the night whereas all the examinations in our study was taken place at office hours (8:30–12:00 am and 2:30–5:00 pm) and the majority of participants came to the physical check-up center at a routine time, thus enhances comparability.[30, 31]
As this study is of a community cohort with the majority of subjects having IOPs within a normal range, the study conclusions may not be directly inferable to the general population. Besides, the two-year observational time is relatively short and we can not draw a definite conclusion of the age related changes of IOP. We are following this group of participants and further findings from longitudinal observations will be reported. However, the combined cross-sectional and longitudinal studies on this large population cohort offers substantial evidence and an important illustration of the size of cohort effects.
In summary, the distribution of IOP in our study was found to be similar to that of most studies based in East Asian populations. The discrepancy between cross-sectional and longitudinal analysis suggests the decreasing trend of IOP with age reported in many studies is likely due to cohort effects. Cohort effects should be taken into consideration while investigating time-related variables such as age. Future population-based studies with prolonged follow-up would aim to illustrate the relationship between IOP and age to best address the resource demands of glaucoma in an aging society.
Supporting Information
(XLS)
Acknowledgments
This study was supported by the Fundamental Research Funds of the State Key Laboratory in Ophthalmology, National Natural Science Foundation of China (81125007) and a research grant from the Brien Holden Vision Institute. The authors alone are responsible for the content writing of the paper.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by the Fundamental Research Funds of the State Key Laboratory in Ophthalmology, and the National Natural Science Foundation of China (81125007) and a research grant from the Brien Holden Vision Institute. The sponsors had no role in study design, data collection, analysis or decision to publish or prepare of the manuscript.
References
- 1.Ozel AB, Moroi SE, Reed DM, Nika M, Schmidt CM, Akbari S, et al. Genome-wide association study and meta-analysis of intraocular pressure. Human genetics. 2013. 10.1007/s00439-013-1349-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guedes G, Tsai JC, Loewen NA. Glaucoma and aging. Current aging science. 2011;4(2):110–7. . [DOI] [PubMed] [Google Scholar]
- 3.Wu SY, Leske MC. Associations with intraocular pressure in the Barbados Eye Study. Archives of ophthalmology. 1997;115(12):1572–6. Epub 1997/12/24. . [DOI] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Investigative ophthalmology & visual science. 1992;33(7):2224–8. . [PubMed] [Google Scholar]
- 5.Lin HY, Hsu WM, Chou P, Liu CJ, Chou JC, Tsai SY, et al. Intraocular pressure measured with a noncontact tonometer in an elderly Chinese population: the Shihpai Eye Study. Archives of ophthalmology. 2005;123(3):381–6. Epub 2005/03/16. 10.1001/archopht.123.3.381 . [DOI] [PubMed] [Google Scholar]
- 6.Fukuoka S, Aihara M, Iwase A, Araie M. Intraocular pressure in an ophthalmologically normal Japanese population. Acta ophthalmologica. 2008;86(4):434–9. 10.1111/j.1600-0420.2007.01068.x . [DOI] [PubMed] [Google Scholar]
- 7.Lee MK, Cho SI, Kim H, Song YM, Lee K, Kim JI, et al. Epidemiologic characteristics of intraocular pressure in the Korean and Mongolian populations: the Healthy Twin and the GENDISCAN study. Ophthalmology. 2012;119(3):450–7. 10.1016/j.ophtha.2011.09.016 . [DOI] [PubMed] [Google Scholar]
- 8.Astrom S, Stenlund H, Linden C. Intraocular pressure changes over 21 years—a longitudinal age-cohort study in northern Sweden. Acta ophthalmologica. 2013. 10.1111/aos.12232 . [DOI] [PubMed] [Google Scholar]
- 9.Nomura H, Shimokata H, Ando F, Miyake Y, Kuzuya F. Age-related changes in intraocular pressure in a large japanese population. Ophthalmology. 1999;106(10):2016–22. 10.1016/s0161-6420(99)90417-7 [DOI] [PubMed] [Google Scholar]
- 10.Baek SU, Kee C, Suh W. Longitudinal analysis of age-related changes in intraocular pressure in South Korea. Eye. 2015. 10.1038/eye.2015.11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano T, Tatemichi M, Miura Y, Sugita M, Kitahara K. Long-term physiologic changes of intraocular pressure: a 10-year longitudinal analysis in young and middle-aged Japanese men. Ophthalmology. 2005;112(4):609–16. 10.1016/j.ophtha.2004.10.046 . [DOI] [PubMed] [Google Scholar]
- 12.Zhao D, Kim MH, Pastor-Barriuso R, Chang Y, Ryu S, Zhang Y, et al. A longitudinal study of age-related changes in intraocular pressure: the Kangbuk Samsung Health Study. Investigative ophthalmology & visual science. 2014;55(10):6244–50. 10.1167/iovs.14-14151 . [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Liang YB, Wong TY, Yang XH, Lian L, Zhu D, et al. Intraocular pressure and its relationship to ocular and systemic factors in a healthy Chinese rural population: the Handan Eye Study. Ophthalmic epidemiology. 2012;19(5):278–84. Epub 2012/09/18. 10.3109/09286586.2012.708084 . [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Ishikawa M, Karita K, Kokaze A, Harada M, Take S, et al. Association of blood pressure and body mass index with intraocular pressure in middle-aged and older Japanese residents: a cross-sectional and longitudinal study. Acta medica Okayama. 2014;68(1):27–34. . [DOI] [PubMed] [Google Scholar]
- 15.Rochtchina Elena, Mitchell P, Wang Jie Jin. Relationship between age and intraocular pressure:the Blue Mountain Eye Study. Clinical and Experimental Ophthalmology. 2002;2002(30):173–5. Epub 175. [DOI] [PubMed] [Google Scholar]
- 16.Nemesure B, Wu SY, Hennis A, Leske MC. Factors related to the 4-year risk of high intraocular pressure: the Barbados Eye Studies. Archives of ophthalmology. 2003;121(6):856–62. Epub 2003/06/11. 10.1001/archopht.121.6.856 . [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Niu Y, Wang D, Wang Y, Holden BA, He M. The association of longitudinal trend of fasting plasma glucose with retinal microvasculature in people without established diabetes. Investigative ophthalmology & visual science. 2015;56(2):842–8. 10.1167/iovs.14-15943 . [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Huang W, Li Y, Zheng Y, Foster PJ, Congdon N, et al. Intraocular pressure, central corneal thickness, and glaucoma in chinese adults: the liwan eye study. American journal of ophthalmology. 2011;152(3):454–62.e1. 10.1016/j.ajo.2011.03.005 . [DOI] [PubMed] [Google Scholar]
- 19.Qureshi IA. Intraocular pressure: a comparative analysis in two sexes. Clinical physiology. 1997;17(3):247–55. . [DOI] [PubMed] [Google Scholar]
- 20.Memarzadeh F, Ying-Lai M, Azen SP, Varma R, Los Angeles Latino Eye Study G. Associations with intraocular pressure in Latinos: the Los Angeles Latino Eye Study. American journal of ophthalmology. 2008;146(1):69–76. 10.1016/j.ajo.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennis A, Wu SY, Nemesure B, Leske MC, Barbados Eye Studies G. Hypertension, diabetes, and longitudinal changes in intraocular pressure. Ophthalmology. 2003;110(5):908–14. 10.1016/S0161-6420(03)00075-7 . [DOI] [PubMed] [Google Scholar]
- 22.Preussner PR, Grossmann A, Ngounou F, Kouogan G, Tamon J. Glaucoma screening in Western Cameroon. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2009;247(12):1671–5. 10.1007/s00417-009-1166-7 . [DOI] [PubMed] [Google Scholar]
- 23.Yoshida M, Take S, Ishikawa M, Kokaze A, Karita K, Harada M, et al. Association of smoking with intraocular pressure in middle-aged and older Japanese residents. Environmental health and preventive medicine. 2013. 10.1007/s12199-013-0359-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbonaro F, Andrew T, Mackey DA, Spector TD, Hammond CJ. Comparison of three methods of intraocular pressure measurement and their relation to central corneal thickness. Eye. 2010;24(7):1165–70. 10.1038/eye.2010.11 . [DOI] [PubMed] [Google Scholar]
- 25.Smedowski A, Weglarz B, Tarnawska D, Kaarniranta K, Wylegala E. Comparison of three intraocular pressure measurement methods including biomechanical properties of the cornea. Investigative ophthalmology & visual science. 2014;55(2):666–73. 10.1167/iovs.13-13172 . [DOI] [PubMed] [Google Scholar]
- 26.AlMubrad TM, Ogbuehi KC. The effect of repeated applanation on subsequent IOP measurements. Clinical & experimental optometry. 2008;91(6):524–9. 10.1111/j.1444-0938.2008.00298.x . [DOI] [PubMed] [Google Scholar]
- 27.Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Central corneal thickness and intraocular pressure in a Mongolian population. Ophthalmology. 1998;105(6):969–73. 10.1016/S0161-6420(98)96021-3 . [DOI] [PubMed] [Google Scholar]
- 28.Foster PJ, Broadway DC, Garway-Heath DF, Yip JL, Luben R, Hayat S, et al. Intraocular pressure and corneal biomechanics in an adult British population: the EPIC-Norfolk eye study. Investigative ophthalmology & visual science. 2011;52(11):8179–85. 10.1167/iovs.11-7853 . [DOI] [PubMed] [Google Scholar]
- 29.Fogagnolo P, Capizzi F, Orzalesi N, Figus M, Ferreras A, Rossetti L. Can mean central corneal thickness and its 24-hour fluctuation influence fluctuation of intraocular pressure? Journal of glaucoma. 2010;19(6):418–23. 10.1097/IJG.0b013e3181aff432 . [DOI] [PubMed] [Google Scholar]
- 30.Fogagnolo P, Orzalesi N, Ferreras A, Rossetti L. The circadian curve of intraocular pressure: can we estimate its characteristics during office hours? Investigative ophthalmology & visual science. 2009;50(5):2209–15. 10.1167/iovs.08-2889 . [DOI] [PubMed] [Google Scholar]
- 31.Liu JH, Kripke DF, Hoffman RE, Twa MD, Loving RT, Rex KM, et al. Nocturnal elevation of intraocular pressure in young adults. Investigative ophthalmology & visual science. 1998;39(13):2707–12. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.