Abstract
Background
Certain salivary proteins of phlebotomine sand flies injected into the host skin during blood-feeding are highly antigenic and elicit strong antibody-mediated immune responses in repeatedly-exposed hosts. These antibodies can be measured by enzyme-linked immuno sorbent assays (ELISAs) using salivary gland homogenates (SGHs) as the source of antigens and serve as a markers for exposure to biting sand flies. Large-scale screening for anti-sand fly saliva antibodies requires replacement of SGH with recombinant salivary proteins. In East Africa, Phlebotomus orientalis is the main vector of Leishmania donovani, a trypanosomatid parasite causing visceral leishmaniasis. We tested recombinant salivary proteins derived from Ph. orientalis saliva to study exposure of domestic animals to this sand fly species.
Methodology/Principal Findings
Antigenic salivary proteins from Ph. orientalis were identified by immunoblot and mass spectrometry. Recombinant apyrase rPorSP15, yellow-related protein rPorSP24, ParSP25-like protein rPorSP65, D7-related protein rPorSP67, and antigen 5-related protein rPorSP76 were tested using ELISA with sera of domestic animals from L. donovani foci in Ethiopia where Ph. orientalis is present. Our results highlighted recombinant yellow-related protein rPorSP24 as the most promising antigen, displaying a high positive correlation coefficient as well as good sensitivity and specificity when compared to SGH. This recombinant protein was the most suitable one for testing sera of dogs, sheep, and goats. In addition, a different antigen, rPorSP65 was found efficacious for testing canine sera.
Conclusions/Significance
Recombinant salivary proteins of Ph. orientalis, specifically rPorSP24, were shown to successfully substitute SGH in serological experiments to measure exposure of domestic animals to Ph. orientalis, the vector of L. donovani. The results suggest that rPorSP24 might be a suitable antigen for detecting anti-Ph. orientalis antibody-mediated reactions also in other host species.
Author Summary
The sand fly Phlebotomus orientalis is the main vector of Leishmania donovani, the causative agent of visceral leishmaniasis in East Africa. During bloodfeeding, sand flies inject saliva into the host skin and repeated bites result in a specific antibody response in the bitten hosts. Antibody responses are directed against sand fly salivary proteins and the levels of these antibodies reflect the intensity of exposure to biting sand flies. The antibody reactions can be measured using salivary gland homogenates (SGHs), but for large-scale testing its use is impractical because of the amount of work required to obtain sufficient quantities of SGH. Recombinant proteins prepared based on the antigens in the sand fly saliva can substitute whole SGH in large-scale studies. We tested five recombinant proteins from Ph. orientalis saliva expressed in Escherichia coli and demonstrated that the yellow-related protein rPorSP24 can replace the SGH in estimating exposure to sand flies of dogs, goats, and sheep in Ethiopia. Immune reactions to vector saliva in endemic areas, provides useful information on levels of exposure and, thereby, on the effectiveness of vector control programs.
Introduction
Phlebotomine sand flies are the vectors of Leishmania parasites causing leishmaniasis, the disease responsible for an estimated 1.3 million new human cases and 20 000 to 30 000 deaths annually [1]. During blood-feeding, sand fly females inoculate saliva into the host skin. Over the last three decades, various research groups have investigated the composition and biological activities of saliva, as well the potential use of salivary antigens in an anti-Leishmania vaccine (reviewed in [2]).
Sand fly salivary molecules are also highly antigenic and elicit strong antibody-mediated response in repeatedly exposed hosts. This response can be utilized as a marker for exposure to biting sand flies. In animals experimentally-exposed to sand fly bites the production of specific anti-saliva IgG antibodies is positively correlated with the number of blood-fed sand flies [3,4]. The elevated antibody levels persisted in bitten hosts for weeks or even months [3–6] but decreased after the last exposure to sand flies, suggesting that screening for anti-saliva antibodies can be used also for estimating the timing of exposure [7,8]. As a reliable epidemiological tool, anti-sand fly saliva antibodies have already been successfully employed to evaluate the effectiveness of vector control interventions [4,9], to estimate the risk of Leishmania transmission [4, 10–12], and to indicate the feeding preferences of sand flies [13–15].
Screening for anti-sand fly saliva antibodies in large populations is impractical due to the amount of work required to obtain sufficient quantities of salivary gland homogenate (SGH). However, the use of recombinant salivary proteins enables to circumvent the necessity for sand fly colony maintenance, laborious dissections of salivary glands and potential cross-reactivity with non-vector species [8]. The main salivary antigens in several sand fly species have already been characterized [4,12,16–18], however, recombinant salivary proteins from only three species—Lutzomyia longipalpis, Ph. perniciosus, and Ph. papatasi—have been tested so far in seroepidemiological studies [13,19–25].
Here, we focus on Ph. orientalis, the most important vector of human visceral leishmaniasis (VL) in East Africa [reviewed in [26]]. In Ethiopia, the main endemic areas of VL are located in the lowlands of southwestern Ethiopia and in the Metema-Humera plains in the northwest [27], where Ph. orientalis was found to be an abundant sand fly species [28]. This opportunistic sand fly feeds on different mammals, depending on the host availability [29–31]. Indeed, anti-Ph. orientalis antibodies have recently been detected in several domestic animal species in Ethiopia—dogs, donkeys, sheep, goats, and cows using SGH as antigen [15]. In the present study, five proteins from saliva of Ph. orientalis were expressed in Esherichia coli and evaluated as markers for exposure using sera of domestic animals, namely dogs, sheep, and goats, from L. donovani endemic foci in northern Ethiopia.
Methods
Ethical statement
BALB/c mice were maintained and handled in the animal facility of Charles University in Prague in accordance with institutional guidelines and the Czech legislation (Act No. 246/ 1992 coll. on Protection of Animals against Cruelty in present statutes at large), which complies with all relevant European Union and international guidelines for experimental animals. The experiments were approved by the Committee on the Ethics of Animal Experiments of the Charles University in Prague (Permit Number: 24773/2008-10001) and were performed under the Certificate of Competency (Registration Number: CZ 02439, CZ 02457) in accordance with the Examination Order approved by Central Commission for Animal Welfare of the Czech Republic. Sera of domestic animals were collected within the study by [15]. Their collection was approved by the Ethiopian National Research Ethics Review Committee (NRERC) under approval no. 3.10/3398/04. For more details see [15].
Host sera
Murine sera were obtained from animals exposed at least ten-times to about 150 insectary-bred sand fly females of a single species in two-week interval. Ten mice were exposed to Ph. orientalis, four to Ph. papatasi, and four to Sergentomyia schwetzi. Four mice served as non-exposed controls. Serum samples of Ethiopian domestic animals, obtained during the previous study by [15] included 179 sheep, 36 dog, and 233 goat sera. Sera from 30 sheep, 14 dogs, and 15 goats non-exposed to sand flies were used as negative controls. More details about samples from domestic animals (both of Ethiopian origin and controls) are provided in [15].
Sand flies and salivary gland dissection
The colony of Ph. orientalis (originating from Ethiopia, Melka Werer) was established in 2008 [32] and reared under standard conditions as described in [33]. Salivary glands were dissected from 4–6 day old female sand flies in 20 mM Tris buffer with 150 mM NaCl and stored at -20°C. Before use, salivary glands were disrupted by freeze-thawing three times in liquid nitrogen [34].
Immunoblot
Antigenic proteins were selected based on the reactivity of two pools of canine sera (five sera each) from endemic area in Ethiopia with SGH using one pool (five sera) of non-exposed dogs as control. Salivary proteins (equivalent to 20 glands per well) were separated by SDS-PAGE on 12% polyacrylamide gel under non-reducing conditions. Proteins were transferred from the gel to nitrocellulose membranes using an iBLOT dry system (Invitrogen). Membranes were cut into strips and blocked overnight with 5% nonfat dry milk in Tris buffer with 0.05% Tween (Tris-Tw) and incubated for 1 hour with dog sera diluted 1:50 in Tris-Tw. Then, the strips were incubated with peroxidase conjugated anti-dog IgG (Bethyl Laboratories) diluted 1:3000 in Tris-Tw. Antigenic protein bands were visualized using the substrate solution with diaminobenzidine. The protein profile was compared with Ph. orientalis SGH studied by [35] and the identity of antigenic bands was confirmed by proteome analysis and mass spectrometry; according to the protocol described in [35].
Recombinant proteins preparation
Five proteins from Ph. orientalis salivary glands were chosen for expression in E. coli: PorSP15, PorSP24, PorSP65, PorSP67, and PorSP76 (in [35] marked as PorASP15, PorMSP24, PorMSP65, and PorMSP67, and PorASP76, respectively) (Table 1). The PCR products from a previously constructed cDNA library [35] were used as the starting material. Products were amplified by PCR under the following conditions: initial incubation (3 minutes in 94°C), then 30 cycles of denaturation (30 seconds in 94°C), annealing (1 minute in 57°C), and elongation (1 minute in 72°C). The whole reaction was terminated by heating to 72°C for 10 minutes. Specific primers were synthesized according to the sequences of the mature protein (without the signal peptide) (Table 1). Thereafter, we followed the procedure described in [5]. Briefly, PCR products were ligated into E. coli pGEM-T Easy Vector (Promega) using TA cloning and the ligation products were transfected into E. coli competent cells TOP10 (Invitrogen). Vectors were replicated in bacteria and after that, the gene for yellow-related protein was restricted using Spe I and Xho I and the genes for the remaining four proteins were restricted using Nde I and Xho I enzymes. Restricted E. coli pET-42 Expression Vectors (Novagen) were ligated and ligation products were transformed into E. coli competent cells TOP10 (Invitrogen) again. Plasmids were isolated from the bacteria, and transfected into E. coli BL21 (DE3) gold (Agilent) for expression. E. coli lysates were prepared under denaturing conditions and His-tagged proteins (with six histidins) were purified under denaturing conditions with 8M urea in Ni-NTA column (731–1550: Bio-Rad, USA). Purity of the recombinant proteins was verified on immunoblot using the monoclonal anti-polyHistidine-peroxidase (A7058-1VL: Sigma Aldrich, UK) and protein concentration was measured by the Lowry method (Bio-Rad) following the manufacturer’s protocol.
Table 1. Phlebotomus orientalis salivary proteins expressed in Escherichia coli.
| Sequence name | Protein family | GenBank ACCN | Forward primer | Reverse primer |
|---|---|---|---|---|
| PorSP15 | Apyrase | AGT96431 | CATATGGCTCCTAGAGCAACAAAAT | CTCGAGCTTAATGCCTTTGGGAT |
| PorSP24 | Yellow-related protein | AGT96428 | ACTAGTTTTCACGTTGAAAGAGAAT | CTCGAGCTTTGTCTTGGGATCATA |
| PorSP65 | ParSP25-like protein | AGT96466 | CATATGGATCGGGGAGTGGATGG | CTCGAGGTGCAATCGGTTGTTTATG |
| PorSP67 | D7-related protein | AGT96467 | CATATGCTGCAATTCCCTCGTGAT | CTCGAGTTTTGCCGATATCTCATCC |
| PorSP76 | Antigen 5-related protein | AGT96441 | CATATGGCTAACGACTATTGCCAGC | CTCGAGTGTCCTGGGCTTCTTGAG |
Sequence name, the protein family, GenBank accession number, and forward and reverse primers for PCR amplification are provided for each protein.
ELISA experiments
The ELISA protocol described in [15] was used with the following modification: The ELISA plates Immulon 4HBX (96w flat bottomed plate, 735–0465: VWR, USA) were coated in concentrations of 5 μg/ml (0.5 μg/well) for recombinant proteins and 28 ng/well for SGH (corresponding to 0.2 of salivary gland/well). In all ELISA tests, serum samples were tested individually.
In the first series of experiments (evaluation step), sera from ten experimentally-bitten mice and sera of 10 dogs, 10 goats, and 35 sheep with the highest anti-Ph. orientalis SGH titer values found by [15] were used to evaluate the antigenicity of the five recombinant proteins with anti-Ph. orientalis saliva IgG. Sera from non-exposed animals (3 mouse, 3 dogs, 3 goats, and 8 sheep) were used as negative controls.
In the second series of experiments, 16 murine sera (4 exposed to Ph. orientalis, 4 to Ph. papatasi, 4 to Se. schwetzi, and 4 non-exposed controls) were used to verify specificity of selected recombinant proteins. Based on the results of evaluation experiments with murine sera, three recombinant proteins with significant correlation with SGH (rPorSP24, rPorSP67, and rPorSP76) were selected.
In the third series of experiments (validation step), selected recombinant proteins with correlation coefficient higher than 0.7 from evaluation experiments (rPorSP15, rPorSP24, rPorSP65, and rPorSP67) were tested with the whole set of serum samples from Ethiopia (179 sheep, 36 dogs, and 233 goats) and an appropriate number of non-exposed controls (30 sheep, 14 dogs, and 15 goats).
Statistical analysis
The non-parametric Spearman test was used to assess correlations between total anti-SGH and anti-recombinant protein IgG levels using GraphPad Prism version 6 (GraphPad Software, Inc., San Diego, CA). For evaluating the possible cross-reactivity with other sand fly species non-parametric Wilcoxon Rank-Sum test in GraphPad Prism version 6 (GraphPad Software, Inc., San Diego, CA) was used. Statistical significance was considered when the p-value was<0.05. Cut-off values were calculated from the mean optical density of control sera plus 3 standard deviations. The optical density values of anti-SGH antibodies were used as the gold standard to validate recombinant proteins in ELISA tests using positive and negative predictive values, sensitivity, and specificity.
Accesion numbers
Accession numbers of proteins used in this study: AGT96431, AGT96428, AGT96466, AGT96467, AGT96441. Accession numbers of proteins discussed in this study: AAL16051, AHA49643, AAL11049, AAL11048, AFY13224, ABI20147, AHF48995, AHF48996, AAD32198, AAS05318, AHF49000
Results
Identification of Ph. orientalis salivary antigens by immunoblot
To identify antigenic proteins in Ph. orientalis salivary glands, two pools of canine sera (five sera each) from naturally-exposed dogs were tested with SGH of Ph. orientalis. Individual bands were identified based on the proteomic analysis, immunoblot, and mass spectrometry of Ph. orientalis SGH. Canine sera reacted with at least 10 protein bands (Fig 1); five of them were identified as ParSP25-like protein PorSP65, yellow-related protein PorSP24, apyrase PorSP15, antigen 5-related protein PorSP76, and D7-related protein PorSP67 (for GenBank ACCN refer to Table 1). These five antigenic proteins were chosen for expression in E. coli. Very weak reaction was observed between SGH and negative control sera around 30, 40, and 90 kDa (Fig 1).
Fig 1. Identification of Phlebotomus orientalis salivary antigens in dogs.
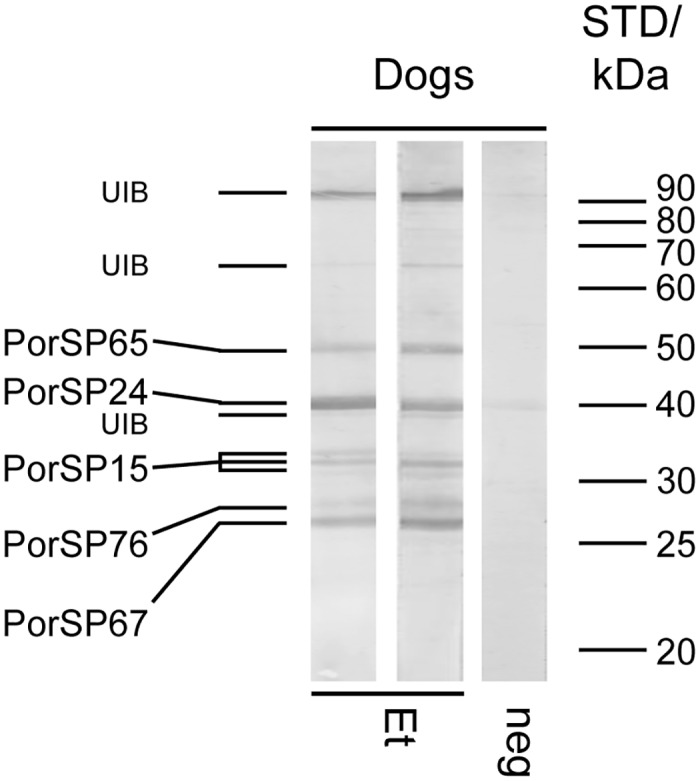
Ph. orientalis salivary proteins were separated under non-reducing conditions by SDS-PAGE on a 12% gel and incubated with two different pools of sera from naturally-exposed Ethiopian dogs (Et), and one pooled sera from non-exposed control dogs (neg). Each pool was a mixture of 5 individual samples. Five antigenic proteins (PorSP65, PorSP24, PorSP15, PorSP76, and PorSP67) were identified by successive proteome analysis and mass spectrometry. Molecular weights of standard (STD) are indicated. UIB means unidentified bands.
Evaluation of recombinant proteins with anti-Ph. orientalis IgG
To evaluate the reactivity of anti-Ph. orientalis saliva IgG with five recombinant proteins, we screened them first with selected sera of naturally-exposed domestic animals from Ethiopia (dogs, sheep, and goats), mice experimentally-bitten by Ph. orientalis, and non-exposed controls. The antigenicity of recombinant proteins was evaluated based on the correlation of antibody reactions with SGH for each of the twenty combinations between recombinant proteins and animal species (Table 2).
Table 2. Evaluation of recombinant proteins by correlation analysis.
| HOST | SGH | rPorSP15 | rPorSP24 | rPorSP65 | rPorSP67 | rPorSP76 | |
|---|---|---|---|---|---|---|---|
| Dog | controls (n = 3) | 0.396 ± 0.049 | 0.329 ± 0.037 | 0.289 ± 0.008 | 0.278 ± 0.038 | 0.341± 0.050 | 0.332 ± 0.053 |
| exposed (n = 10) | 1.129 ± 0.336 | 0.709 ± 0.181 | 0.734 ± 0.232 | 1.008 ± 0.304 | 0.742 ± 0.227 | 0.755 ± 0.230 | |
| ρ | N.A. | 0.830 *** | 0.868 *** | 0.852 *** | 0.687 ** | 0.599 * | |
| Goat | Controls (n = 3) | 0.143 ± 0.002 | 0.215 ± 0.021 | 0.150 ± 0.023 | 0.161 ± 0.030 | 0.213 ± 0.031 | 0.354 ± 0.058 |
| exposed (n = 10) | 0.265 ± 0.224 | 0.559 ± 0.244 | 0.329 ± 0.234 | 0.411 ± 0.234 | 0.422 ± 0.202 | 0.737 ± 0.230 | |
| ρ | N.A. | 0.797 *** | 0.835 *** | 0.412 | 0.802 *** | 0.643* | |
| Sheep | controls (n = 8) | 0.133 ± 0.014 | 0.294 ± 0.041 | 0.213 ± 0.048 | 0.166 ± 0.027 | 0.288 ± 0.047 | 0.520 ± 0.113 |
| exposed (n = 35) | 0.218 ± 0.141 | 0.486 ± 0.138 | 0.238 ± 0.165 | 0.313 ± 0.180 | 0.353 ± 0.084 | 0.573 ± 0.091 | |
| ρ | N.A. | 0.629 *** | 0.806 *** | 0.777 *** | 0.373 * | 0.349 * | |
| Mouse | controls (n = 3) | 0.086 ± 0.006 | 0.158 ± 0.022 | 0.142 ± 0.037 | 0.154 ± 0.034 | 0.136 ± 0.029 | 0.214 ± 0.059 |
| exposed (n = 10) | 1.395 ± 0.562 | 0.245 ± 0.186 | 0.394 ± 0.329 | 0.753 ± 0.761 | 0.334 ± 0.314 | 0.391 ± 0.136 | |
| ρ | N.A. | 0.308 | 0.857 *** | 0.456 | 0.560 * | 0.802 *** |
Spearman-Rank Correlation Matrix test for optical densities between sera tested against Ph. orientalis SGH and against each salivary recombinant protein. Mean values of exposed animals and non-exposed controls ± standard deviation, correlation coefficient (ρ) are indicated. N.A. = not applicable, asterisk (*) indicates significant correlations: *p<0.05, **p<0.01, ***p<0.001. Combinations of significant correlation and ρ>0.8 are in bold.
In canine sera, a significant correlation was achieved for all tested proteins; the highest correlation coefficient was found for rPorSP24 (ρ = 0.868), followed by rPorSP65, and rPorSP15. Similarly, in sheep and goat sera the best correlation (above 0.8) was found for rPorSP24, other recombinant proteins with correlation coefficient above 0.75 were rPorSP67 and rPorSP15 for goats and rPorSP65 for sheep (Table 2). Sera from experimentally-bitten mice showed significant correlation with three out of five proteins tested; the highest correlation coefficient was achieved for rPorSP24 (ρ = 0.857).
Specificity of recombinant proteins
Specificity of recombinant proteins was tested only with murine sera due to the absence of positive control samples from other host species. Three recombinant proteins with significant correlation to SGH in evaluation experiments with murine sera (Table 2) were selected to verify their specific reaction with anti-Ph. orientalis IgG. Fig 2 shows the strong reactions of sera from mice exposed to Ph. orientalis bites with SGH and with all tested recombinant proteins (rPorSP67, rPorSP76, and rPorSP24), while the recognition of these antigens by sera from mice exposed to solely to Ph. papatasi or Se. schwetzi were similar to the negative controls (sera of unexposed mice).
Fig 2. Specificity of recombinant proteins.
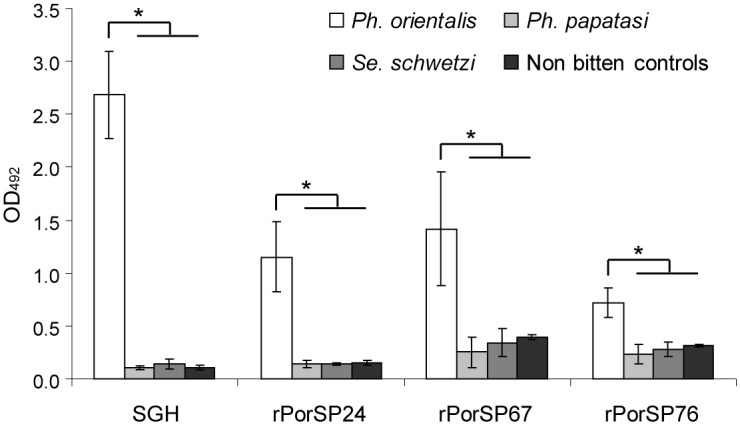
Results from ELISA are presented as mean optical densities ± standard deviation of IgG antibody reaction with P. orientalis salivary gland homogenate (SGH) and three recombinant proteins (rPorSP24, rPorSP67, and rPorSP76) in mice experimentally bitten by Ph. orientalis, Ph. papatasi, or Se. schwetzi, and non-exposed control mice. Four mice per sand fly colony and four non-exposed controls were used. Asterisks (*) indicate significant differences (p<0.05, calculated with non-parametric Wilcoxon Rank-Sum Test) of IgG levels between mice bitten by Ph. orientalis and mice bitten by other sand fly species or non-bitten controls.
Validation of selected recombinant proteins
Four recombinant proteins with the highest correlation from evaluation experiments (rPorSP15, rPorSP24 rPorSP65, and rPorSP67) were chosen for further validation using the whole set of Ethiopian serum samples (179 sheep, 36 dogs, and 233 goats) and non-exposed controls.
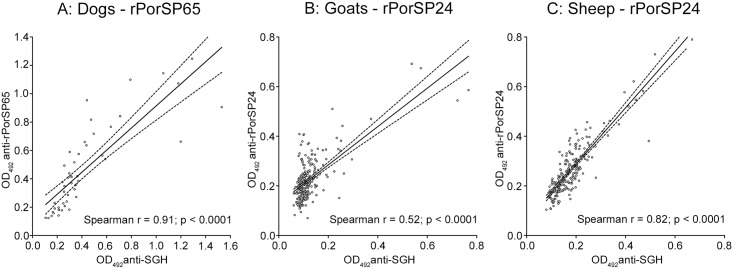
For canine sera, the highest correlation coefficient was achieved with rPorSP65 (ρ = 0.906) followed by rPorSP24 and PorSP15. For sheep as well as for goats, the highest correlation coefficient was detected with rPorSP24 (ρ = 0.818 and ρ = 0.522, respectively) followed with rPorSP65 for sheep and with rPorSP15 and rPorSP67 for goats (Fig 3). All results from correlation analyses between SGH and four recombinant proteins were highly significant and cut-off values for individual recombinant proteins were the lowest for the proteins with the highest correlation coefficient (Fig 3). Additionally, rPorSP24 reached the highest values of positive and negative predictive values (PPV and NPV) in all host species as well as the sensitivity in dogs and the specificity in goats and sheep. The specificity in dogs was the best with rPorSP15 and the sensitivity in goats with rPorSP67. The best combinations (the lowest cut-off value, the highest correlation coefficient, PPV, NPV, specificity, and sensitivity values) between SGH and recombinant protein for each animal species tested are shown in Fig 4.
Fig 3. Validation of recombinant proteins.
Selected recombinant proteins were validated in ELISA tests with sera of indicated domestic animals naturally-exposed to Phlebotomus orientalis. The analysis was based on comparison with anti-Ph. orientalis SGH IgG as a standard. The table provides cut-off values, mean values of optical density ± standard deviation of IgG levels in animals exposed to Ph. orientalis, correlation coefficients between IgG levels against SGH and a recombinant protein (ρ), positive predictive values (PPV), negative predictive values (NPV), sensitivity, and specificity. Asterisks (*) indicate significant correlations: *p<0.05, **p<0.01, ***p<0.001. Combinations with the correlation coefficient lower than 0.7 in evaluation experiments (Table 2) were excluded from the validation experiments. For each combination, the lowest cut-off value, the highest correlation coefficient, and the highest PPV, NPV, sensitivity, and specificity values are shaded grey. N.A. means not applicable.
Fig 4. Correlation analyzes between IgG antibodies against SGH and recombinants rPorSP65 and rPorSP24 in ELISA.
For each animal species from validation experiments (Fig 3), the protein with the highest positive correlation was displayed: A: rPorSP65 (ParSP25-like protein) tested with canine sera (n = 50), B: rPorSP24 (yellow-related protein) tested with goat sera (n = 248), C: rPorSP24 (yellow-related protein) tested with sheep sera (n = 209). Sera from naturally-exposed Ethiopian animals together with sera from non-exposed controls were included in this analysis. Correlation coefficients and p-values from Spearman-Rank analysis are indicated.
Discussion
We have studied antigenic salivary proteins of Ph. orientalis, the most important vector of VL in Ethiopia and Sudan, using sera of naturally-exposed hosts. Antigenic proteins were identified on immunoblot based on their recognition by canine sera. These were: D7-related protein (PorSP67), antigen 5-related protein (PorSP76), apyrase (PorSP15), yellow-related protein (PorSP24), and ParSP25-like protein (PorSP65). The antigenicity of their recombinant counterparts expressed in E. coli was validated in large-scale tests using sera from naturally-exposed dogs, sheep, and goats. The utilization of recombinant proteins as markers for exposure may help to highlight the specificity of the reaction and to evade nonspecific binding as observed when SGH was recognized by negative control. sera.
Sand fly salivary proteins from the D7-related family are well known antigens; they were recognized by sera of mice bitten by Ph. papatasi [10], dogs bitten by Lu. longipalpis and Ph. perniciosus [4,36], and humans bitten by Ph. papatasi [12]. As far as we are aware, five recombinant D7-related (rD7) proteins from sand fly saliva have already been tested as exposure markers; one from Lu. longipalpis (AAL16051, also known as LJL13) [13], another from Ph. perniciosus (AHA49643) [21], and three from Ph. papatasi (AAL11049, AAL11048, and AFY13224) [5,20]. However, only some of them bound anti-saliva IgG and their antigenicity was host-specific. The rD7 protein (AAL11049) was specifically recognized by sera from mice bitten by Ph. papatasi [5], but the same protein was not recognized by human sera from Tunisia [20]. Another rD7 protein (AAL11048) from Ph. papatasi did not react with sera from mice immunized by sand flies [5]. The rD7 protein LJL13 was recognized by dogs naturally-exposed to Lu. longipalpis but not by sera from foxes and humans from endemic focus of L. infantum [13]. In the present study, rPorSP67 showed promising results with limited number of goat sera during the evaluation test but was not validated in a broader test, when medium or low correlation coefficient, NPV, PPV, sensitivity, and specificity with values ranging between 0.28 and 0.6 were observed. This suggests that this recombinant protein would not be useful as an exposure marker to Ph. orientalis bites.
Proteins of the antigen 5-related family from various sand fly species were repeatedly shown to be potent salivary antigens, being recognized by sera of mice bitten by Ph. papatasi and Ph. arabicus [5,16], dogs bitten by Ph. perniciosus [4], rabbits exposed to Ph. tobbi [18], and hamsters bitten by Ph. argentipes [37]. In our study, rPorSP76 showed high correlation with SGH only with sera from experimentally-bitten mice (ρ = 0.8), thus its use in field studies with domestic animals is not justified.
Apyrases are well-known salivary enzymes with anti-haemostatic properties [38]. Antigenic properties of apyrases were described in SGHs of various vector-host models such as Ph. perniciosus and dogs [4, 17] or Ph. argentipes and hamsters [37]. Recombinant apyrase (ABI20147) was recognised by sera of mice immunized with Ph. duboscqi saliva [39]. Two apyrases in a recombinant form (AHF48995, AHF48996) were used as exposure markers for dogs, hares, and rabbits bitten by Ph. perniciosus [21,22], however, the most recent work by Kostalova et al.[23] revealed that neither of these recombinant proteins gave optimal ELISA results in large-scale tests with naturally-exposed dogs. Our results showed a significant correlation between antibody response against SGH of Ph. orientalis and recombinant apyrase rPorSP15 in sera of all domestic animals tested but not in mice sera. The best correlation (ρ = 0.7) was observed with canine sera with medium values of NPV, PPV, sensitivity, and specificity (ranging between 0.59 and 0.68) suggesting necessity of further validation before its utilization as antigen for detecting dog exposure to Ph. orientalis.
The most promising and universal candidate for an exposure marker to sand fly bites belongs to the family of yellow-related proteins. Strong antibody responses to these proteins were previously demonstrated for various sand fly and host species, including dogs and humans [3–7,10,12–14,17,36,40]. Ph. perniciosus recombinant yellow-related protein AHF49000 was successfully used as an antigen both in ELISA and immunoblot reacting well with sera of mice and dogs experimentally-bitten by Ph. perniciosus [21]. Yellow-related recombinant proteins were also validated as exposure markers to sand fly bites in endemic areas; Lu. longipalpis AAD32198 and AAS05318 for humans [19,13] and Ph. perniciosus AHF49000 for dogs, rabbits, and hares [22,23]. In the present study, rPorSP24 from Ph. orientalis confirmed the high reactivity of yellow-related protein family with antibodies from sera of bitten hosts and this advocates for its use as an exposure marker in large-scale field studies. It reached high correlation with SGH in mice (ρ = 0.9), sheep (ρ = 0.8), and dogs (ρ = 0.8) and the best in goats (ρ = 0.5). Similarly, high correlation between SGH and recombinant yellow-related protein was previously attained with dogs (ρ > 0.7 [21–23]), hares (ρ = 0.9 [22]), and rabbits (ρ = 0.7 [22]). Moreover, rPorSP24 achieved the highest values of specificity, sensitivity, PPV and NPV with majority of the host species tested. In dogs, this recombinant protein showed 100% NPV and sensitivity but lower values of PPV and specificity (0.66 and 0.41, respectively), indicating higher probability of false positivity among non-exposed dogs. On the other hand, in sheep, very high values of PPV and specificity were achieved (both 0.9) but medium values of NPV and sensitivity (0.58 and 0.68, respectively) could indicate possible false negative results. However, this statistical analysis is based on data from naturally-exposed hosts and negative controls; further validation is needed using sera of experimentally-bitten animals as a positive control.
The fifth recombinant protein tested belongs to the ParSP25-like family. Antigenicity of this protein family was previously demonstrated in Ph. perniciosus [4,17]. So far as we are aware, no recombinant protein from this group was prepared and used for measuring the antibody reaction with sera of bitten hosts. Our results suggest significant correlation between rPorSP65 and antibody response against SGH of Ph. orientalis. The highest correlation coefficient was observed with canine sera (ρ = 0.9), accompanied by high degree of sensitivity (0.95). Nevertheless, the specificity of the test with rPorSP65 was very low (0.1) suggesting high probability of false positivity among non-exposed dogs.
Antigenic specificity of recombinant proteins was confirmed by using murine sera experimentally-exposed to Sergentomyia schwetzi or Phlebotomus papatasi. These two sand fly species are present in Ethiopia, in some places sympatrically with Ph. orientalis [27]. Antibodies from sera of mice bitten by Ph. papatasi or Se. schwetzi did not react with the recombinant proteins with significant correlation from evaluation experiments (rPorSP24, rPorSP67, and rPorSP76) and confirmed that they are species-specific.
The reactivity of recombinant proteins might be affected by the expression system or conditions of protein purification. Antibodies from bitten hosts can be targeted also to the glycosylated parts of the antigen that are lacking in proteins from the E. coli expression system, therefore some authors prefer to express recombinant proteins in mammalian cells [20,24]. Nevertheless, some of the recombinant proteins without posttranslational modifications proved to be as efficient markers of exposure as native antigens [5,21–23]. Similarly, several proteins prepared in this study were found as suitable antigens, despite their being expressed in E. coli.
In conclusion, our study suggests rPorSP24, the recombinant protein from the yellow-related family, as the most reliable and universally efficacious antigen for measuring exposure of dogs, sheep and goats to Ph. orientalis bites. In addition, the recombinant protein rPorSP65 from ParSP25-like group was found as a good antigen to screen for canine exposure but its low specificity suggests possible false positivity in some specimens. Serological tests using these proteins could be a highly practical and economical tool for screening of domestic animals for exposure to the main vector of L. donovani in East Africa. Moreover, the promising characteristics of rPorSP24 suggest a potential use of this antigen for screening sera of other hosts, including humans. The availability of recombinant salivary proteins should enable to measure anti-Ph. orientalis antibodies in large-scale experiments to evaluate vector control programs in areas affected by VL in East Africa. However, further studies are needed to validate such recombinant protein-based test for routine use.
Acknowledgments
We are grateful to Aysheshm Kassahun, Carla Maia, Gad Baneth and Jan Votypka for participating in field sample collection and would like to express special thanks to professor Asrat Hailu (Addis Ababa University) for his valuable help during the field work. We appreciate Helena Kulikova, Lenka Zitkova and Kristina Simkova for excellent technical and administrative support, Tatiana Kostalova for the maintenance of the Ph. orientalis colony and for help with statistical analysis, and Eva Nyvltova for help with recombinant protein preparation. Sera of control domestic animals (non-exposed to sand flies) were generously provided by prof. David Modry (Faculty of Veterinary Medicine, University of Veterinary and Pharmaceutical Sciences Brno, the Czech Republic) and Dr. Kamil Sedlak (State Veterinary Institute Prague, the Czech Republic).
Data Availability
All relevant data are within the paper.
Funding Statement
This project was funded by Czech Science Foundation (project no. 13-05292S, http://gacr.cz/en/, PV), the Bill and Melinda Gates Foundation, Global Health Program (project no. OPPGH5336, http://www.gatesfoundation.org/, AW), and Charles University (GAUK project no. 675012/B-BIO, http://www.cuni.cz/UKEN-1.html, MS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO 2015. http://www.who.int/mediacentre/factsheets/fs375/en/. Retrieved on November 4th 2015.
- 2.Abdeladhim M, Kamhawi S, Valenzuela JG. What's behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect Genet Evol. 2014; 28: 691–703. 10.1016/j.meegid.2014.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hostomska J, Rohousova I, Volfova V, Stanneck D, Mencke N, Volf P. Kinetics of canine antibody response to saliva of the sand fly Lutzomyia longipalpis. Vector Borne Zoonotic Dis. 2008; 8: 443–450. 10.1089/vbz.2007.0214 [DOI] [PubMed] [Google Scholar]
- 4.Vlkova M, Rohousova I, Drahota J, Stanneck D, Kruedewagen EM, Mencke N, et al. Canine antibody response to Phlebotomus perniciosus bites negatively correlates with the risk of Leishmania infantum transmission. PLoS Negl Trop Dis. 2011; 5: e1344 10.1371/journal.pntd.0001344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlkova M, Rohousova I, Hostomska J, Pohankova L, Zidkova L, Drahota J, et al. Kinetics of antibody response in BALB/c and C57BL/6 mice bitten by Phlebotomus papatasi. PLoS Negl Trop Dis. 2012; 6: e1719 10.1371/journal.pntd.0001719 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Martin-Martin I, Molina R, Jimenez M. Kinetics of Anti-Phlebotomus perniciosus Saliva Antibodies in Experimentally Bitten Mice and Rabbits. PLoS ONE. 2015; 10: e0140722 10.1371/journal.pone.0140722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinhas V, Andrade BB, Paes F, Bomura A, Clarencio J, Miranda JC, et al. Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis. Eur J Immunol. 2007; 37: 3111–3121. [DOI] [PubMed] [Google Scholar]
- 8.Clements MF, Gidwani K, Kumar R, Hostomska J, Dinesh DS, Kumar V, et al. Measurement of recent exposure to Phlebotomus argentipes, the vector of Indian visceral leishmaniasis, by using human antibody responses to sand fly saliva. Am J Trop Med Hyg. 2010; 82: 801–807. 10.4269/ajtmh.2010.09-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gidwani K, Picado A, Rijal S, Singh SP, Roy L, Volf P, et al. Antibody response to sand fly saliva to evaluate human visceral leishmaniasis vector exposure in India and Nepal: effect of long-lasting insecticidal nets. Trop Med Int Health. 2011; 16: 66–66 [Google Scholar]
- 10.Rohousova I, Ozensoy S, Ozbel Y, Volf P. Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology. 2005; 130: 493–499. [DOI] [PubMed] [Google Scholar]
- 11.de Moura TR, Oliveira F, Novais FO, Miranda JC, Clarêncio J, Follador I, et al. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl Trop Dis. 2007; 1: e84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzouki S, Ben Ahmed M, Boussoffara T, Abdeladhim M, Ben Aleya-Bouafif N, Namane A, et al. Characterization of the antibody response to the saliva of Phlebotomus papatasi in people living in endemic areas of cutaneous leishmaniasis. Am J Trop Med Hyg. 2011; 84: 653–661. 10.4269/ajtmh.2011.10-0598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teixeira C, Gomes R, Collin N, Reynoso D, Jochim R, Oliveira F, et al. Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Negl Trop Dis. 2010; 4: e638 10.1371/journal.pntd.0000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes RB, Mendonça IL, Silva VC, Ruas J, Silva MB, Cruz MS, et al. Antibodies against Lutzomyia longipalpis saliva in the fox Cerdocyon thous and the sylvatic cycle of Leishmania chagasi. Trans R Soc Trop Med Hyg. 2007; 101: 127–133. [DOI] [PubMed] [Google Scholar]
- 15.Rohousova I, Talmi-Frank D, Kostalova T, Polanska N, Lestinova T, Kassahun A, et al. Exposure to Leishmania spp. and sand flies in domestic animals in northwestern Ethiopia. Parasit Vectors. 2015; 8: 360 10.1186/s13071-015-0976-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hostomska J, Volfova V, Mu JB, Garfield M, Rohousova I, Volf P, et al. Analysis of salivary transcripts and antigens of the sand fly Phlebotomus arabicus. BMC Genomics. 2009; 10: 282 10.1186/1471-2164-10-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Martin I, Molina R, Jimenez M. An insight into the Phlebotomus perniciosus saliva by a proteomic approach. Acta Trop. 2012; 123: 22–30. 10.1016/j.actatropica.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Rohousova I, Volfova V, Nova S, Volf P. Individual variability of salivary gland proteins in three Phlebotomus species. Acta Trop. 2012; 122: 80–86. 10.1016/j.actatropica.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 19.Souza AP, Andrade BB, Aquino D, Entringer P, Miranda JC, Alcántara R, et al. Using recombinant proteins from Lutzomyia longipalpis saliva to estimate human vector exposure in visceral leishmaniasis endemic areas. PLoS Negl Trop Dis. 2010; 4: e649 10.1371/journal.pntd.0000649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzouki S, Abdeladhim M, Abdessalem CB, Oliveira F, Ferjani B, Gilmore D, et al. Salivary antigen SP32 is the immunodominant target of the antibody response to Phlebotomus papatasi bites in humans. PLoS Negl Trop Dis. 2012; 6: e1911 10.1371/journal.pntd.0001911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drahota J, Martin-Martin I, Sumova P, Rohousova I, Jimenez M, Molina R, et al. Recombinant antigens from Phlebotomus perniciosus saliva as markers of canine exposure to visceral leishmaniases vector. PLoS Negl Trop Dis. 2014; 8: e2597 10.1371/journal.pntd.0002597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Martin I, Molina R, Rohousova I, Drahota J, Volf P, Jimenez M. High levels of anti-Phlebotomus perniciosus saliva antibodies in different vertebrate hosts from the re-emerging leishmaniosis focus in Madrid, Spain. Vet Parasitol. 2014; 202: 207–216. 10.1016/j.vetpar.2014.02.045 [DOI] [PubMed] [Google Scholar]
- 23.Kostalova T, Lestinova T, Sumova P, Vlkova M, Rohousova I, Berriatua E, et al. Canine antibodies against salivary recombinant proteins of Phlebotomus perniciosus: A longitudinal study in an endemic focus of canine leishmaniasis. PLoS Negl Trop Dis. 2015; 9: e0003855 10.1371/journal.pntd.0003855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzouki S, Kammoun-Rebai W, Bettaieb J, Abdeladhim M, Hadj Kacem S, Abdelkader R, et al. Validation of recombinant salivary protein PpSP32 as a suitable marker of human exposure to Phlebotomus papatasi, the vector of Leishmania major in Tunisia. PLoS Negl Trop Dis. 2015; 9: e0003991 10.1371/journal.pntd.0003991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mondragon-Shem K, Al-Salem WS, Kelly-Hope L, Abdeladhim M, Al-Zahrani M, Valenzuela JG, et al. Severity of Old World Cutaneous Leishmaniasis Is Influenced by Previous Exposure to Sandfly Bites in Saudi Arabia. PLoS Negl Trop Dis. 2015; 9: e3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elnaiem DE. Ecology and control of the sand fly vectors of Leishmania donovani in East Africa, with special emphasis on Phlebotomus orientalis. J Vector Ecol. 2011; 36: S23–S31. 10.1111/j.1948-7134.2011.00109.x [DOI] [PubMed] [Google Scholar]
- 27.Elnaiem DA, Ward R, Hassan KH, Miles MA, Frame IA. Infection rates of Leishmania donovani in Phlebotomus orientalis from a focus of visceral leishmaniasis in eastern Sudan. Ann Trop Med Parasitol. 1998; 92: 229–232. [DOI] [PubMed] [Google Scholar]
- 28.Gebresilassie A, Kirstein OD, Yared S, Aklilu E, Moncaz A, Tekie H, et al. Species composition of phlebotomine sand flies and bionomics of Phlebotomus orientalis (Diptera: Psychodidae) in an endemic focus of visceral leishmaniasis in Tahtay Adiyabo district, Northern Ethiopia. Parasit Vectors. 2015; 8: 248 10.1186/s13071-015-0849-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebre-Michael T, Balkew M, Berhe N, Hailu A, Mekonnen Y. Further studies on the phlebotomine sandflies of the kala-azar endemic lowlands of Humera-Metema (north-west Ethiopia) with observations on their natural blood meal sources. Parasit Vectors. 2010; 3: 6 10.1186/1756-3305-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebresilassie A, Yared S, Aklilu E, Kirstein OD, Moncaz A, Tekie H, et al. Host choice of Phlebotomus orientalis (Diptera: Psychodidae) in animal baited experiments: a field study in Tahtay Adiyabo district, northern Ethiopia. Parasit Vectors. 2015; 8: 190 10.1186/s13071-015-0807-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebresilassie A, Abbasi I, Aklilu E, Yared S, Kirstein OD, Moncaz A, et al. Host-feeding preference of Phlebotomus orientalis (Diptera: Psychodidae) in an endemic focus of visceral leishmaniasis in northern Ethiopia. Parasit Vectors. 2015; 8: 270 10.1186/s13071-015-0883-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seblova V, Volfova V, Dvorak V, Pruzinova K, Votypka J, Kassahun A, et al. Phlebotomus orientalis Sand Flies from Two Geographically Distant Ethiopian Localities: Biology, Genetic Analyses and Susceptibility to Leishmania donovani. PLoS Negl Trop Dis. 2013; 7: e2187 10.1371/journal.pntd.0002187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J Vector Ecol. 2011; 36: S1–S9. 10.1111/j.1948-7134.2011.00106.x [DOI] [PubMed] [Google Scholar]
- 34.Volf P, Rohousova I. Species-specific antigens in salivary glands of phlebotomine sandflies. Parasitology. 2001; 122: 37–41 [DOI] [PubMed] [Google Scholar]
- 35.Vlkova M, Sima M, Rohousova I, Kostalova T, Sumova P, Volfova, et al. Comparative analysis of salivary gland transcriptomes of Phlebotomus orientalis sand flies from endemic and non-endemic foci of visceral leishmaniasis. PLoS Negl Trop Dis. 2014; 8: e2709 10.1371/journal.pntd.0002709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bahia D, Gontijo NF, Leon IR, Perales J, Pereira MH, Oliveira G, et al. Antibodies from dogs with canine visceral leishmaniasis recognise two proteins from the saliva of Lutzomyia longipalpis. Parasitol Res. 2007; 100: 449–454. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Martin I, Molina R, Jimenez M. Identifying salivary antigens of Phlebotomus argentipes by a 2DE approach. Acta Trop. 2013; 126: 229–239. 10.1016/j.actatropica.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro JMC, Arca B. From sialomes to the sialoverse: An insight into the salivary potion of blood feeding insects. Adv In Insect Phys. 2009; 37: 59–118. [Google Scholar]
- 39.Hamasaki R, Kato H, Terayama Y, Iwata H, Valenzuela JG. Functional characterization of a salivary apyrase from the sand fly, Phlebotomus duboscqi, a vector of Leishmania major. J Insect Physiol. 2009. 55: 1044–1049. 10.1016/j.jinsphys.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes RB, Brodskyn C, de Oliveira CI, Costa J, Miranda JC, Caldas A, et al. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis. 2002; 186: 1530–1534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.