Abstract
Fecal microbiota transplant has become more acceptable as a therapeutic for recurrent Clostridium difficile infection. The FDA has an enforcement discretion policy for practitioner's performing this therapy, which includes informed consent for this experimental treatment. This manuscript describes a typical procedure that can be followed that includes the important aspects of this preparation and treatment.
Keywords: FMT, fecal microbiota transplant, Clostridium difficile, CDI, stool
In a fecal microbiota transplant (FMT), donor stool is infused into a recipient's gastrointestinal tract.1, 2 FMT, which is classified by the United States Food and Drug Administration (FDA) as an investigational new drug (IND), is currently under enforcement discretion by that organization to treat recurrent Clostridium difficile infection (CDI) not responsive to standard therapies.3 Thus, a formal IND application is currently advisable but not required before undertaking FMT as a treatment for CDI that has not improved after standard therapies.
The biologic plausibility that supports FMT is that normal nonpathogenic bacteria from donor stool specimens can repopulate the intestinal tract of the recipient and supplant an overgrowth of C. difficile.4 The FDA guidelines indicate that the FDA will exercise enforcement discretion regarding the new IND requirements for the use of fecal microbiota for transplantation to treat CDI not responsive to standard therapies. The enforcement policy dictates that the treating, licensed health care provider obtain adequate informed consent from the patient (or his or her legally authorized representative) for use of the FMT products. It is also required that the stool specimen is obtained from a donor known to the patient or known to the licensed health care provider treating the patient. In addition, the donor and stool must be qualified by screening and testing performed under the direction of the licensed health care provider for providing the FMT product that will be used to treat his or her patient.3
Our institution, Emory University, has performed more than 125 FMT procedures, with a greater than 90% success rate, as defined by cessation of diarrhea at 4 weeks after final treatment (which may include more than one FMT). All of our patients received 3 or more courses of anti-CDI antibiotics without resolution of CDI before they were referred for FMT. Herein, we summarize the laboratory tests and processes that our institution has developed to safely and efficiently perform FMT in a variety of clinical circumstances.
When patients with CDI arrive at the gastroenterology clinic for FMT, a physician or midlevel practitioner (nurse practitioner or physician's assistant) reviews their medical histories and explains the FMT process. Patients are asked about food and/or medication allergies; they are informed of the known and unknown risks of FMT. The practitioner warns patients that bloating, cramping, and/or constipation are commonly reported for a few days following the procedure. It is imperative that patients are told that there are rare reports of autoimmune diseases, such as rheumatoid arthritis and idiopathic thrombocytopenia, occurring after FMT. Also, they must be reminded of possible as-yet-unreported adverse effects from FMT due to its investigational status.
FMTs performed via colonoscopy carry the risks associated with colonoscopy, such as adverse sedative reaction and bowel perforation; other methods of FMT administration carry different risks. Appropriate documentation of informed consent should be entered into the patient's medical record, including mention of the investigational status of FMT and the known and unknown risks of the procedure.2
If the prospective recipient consents, the recipient is given the choice of using a donor stool specimen from a friend or family member. Alternatively, the recipient may use stool provided by standard donors known to the institution. Standard donors are selected for their health, availability for donation, and reliability. Donors should be confident in their ability to reliably produce a stool specimen on the morning of the procedure; having approved backup donors available may be a wise precaution.
All propective donors are assigned an anonymous identification number before they fill out a modified donor questionnaire provided by the AABB (formerly known as the American Association of Blood Banks), (Figure 1); this form inquires about their health, diet, and potential risk factors for infectious diseases. Donors each void a stool specimen for analysis, and a phlebotomist performs a blood draw. Donors at our institution are tested for the following pathogens using the specified tests.5
Figure 1.
Donor questionnaire modified from the AABB questionnaire (formerly known as the American Association of Blood Banks).
Human immunodeficiency virus (HIV) types 1 and 2: antibody
Hepatitis A: immunoglobulin (lg)M, IgG
Hepatitis B: hepatitis B surface antigen (HBsAg)
Hepatitis C: antibody
Salmonella, Shigella, and Campylobacter: routine bacterial stool culture
Treponema pallidum: rapid plasma reagin test (RPR; if results are positive, those findings are confirmed by antibody testing)
Ova and parasite: fecal screen
Carbapenem resistant Enterobacteriacae: screening culture
Vancomycin resistant Enterococcus: screening culture
Helicobacter pylori: stool antigen
Clostridium difficile: polymerase chain reaction (PCR)
The questionnaire and test results from each prospective donor are reviewed to determine his or her suitability as a donor. Donors who test positive for the presence of potential pathogens are informed confidentially and referred for treatment, as appropriate. In such cases, alternative donors should be requested of or presented to the prospective FMT recipient. Donors who report significant exposure to 1 or more risk factors on their questionnaires are also deemed to be unsuitable donors. Standard donors must recomplete the questionnaire, and their blood and feces must be recollected for culturing and testing at least every 3 to 6 months to ensure that they remain suitable as candidates.
Once suitable donor(s) are identified, an appointment is made for colonoscopy. Although it is possible to perform FMT in the inpatient setting, we find it is most convenient when it is performed on an outpatient basis. We recommend that patients who are hospitalized with CDI have their diarrheal symptoms controlled using anti-CDI antibiotics and undergo FMT after they are discharged. FMT can have a role in the inpatient treatment of fulminant refractory CDI; however, we do not regularly practice FMT in this setting.
On the day of the FMT, donors collect and submit a stool specimen using a sterile collection kit provided in advance. They also submit a brief form (Figure 2) describing any recent changes to their health, diet, or bowel habits. Donors must attest that they have personally produced the stool specimen provided and report the time that they voided it. On receipt, donor stool is processed in a biosafety cabinet after donning appropriate personal protective equipment (gloves and fluid impervious gown). Using a sterile wooden blade and a plastic fecal concentrator kit (Fisher Scientific, Hanover Park, IL), approximately 50 grams of donor stool is emulsified in 250 mL of nonbuffered sterile saline. Particles should be eliminated or reduced to 1 to 2 mm in width to prevent clogging in the infusion tube during colonoscopy. The resulting solution is transported to the endoscopy suite in a sterile plastic flask (from which the saline was retrieved) with an airtight seal, within a sealed, opaque biohazard bag. The solution is stored at room temperature until the FMT is performed; the FMT infusion solution is infused within 6 hours of donor voiding. Donor specimens are discarded if they were voided more than 6 hours before FMT. Some institutions prefer to emulsify the donor stool specimen in the endoscopy suite using gauze as a filter.
Figure 2.
Fecal transplant donor submission form.
Patients must meet clinical indications for colonoscopy before undergoing FMT delivered in this manner. Any suspicious lesions detected may be biopsied as usual. After appropriate anesthesia is administered, the colonoscope is advanced to the most proximal portion of the colon of the patient (typically, the cecum). This positioning ensures adequate distribution of the FMT solution throughout the colon. The donor stool solution is then instilled into the proximal colon.
Patients with CDI who are not suitable candidates for colonoscopy may receive FMT via an upper-gastrointestinal approach. We have performed FMT via sterile tubes advanced through the nares, mouth, or percutaneous endoscopic gastrostomy tube. Whatever means of access is used, the distal end of the infusion tube should preferably be advanced beyond the pyloric sphincter into the distal duodenum or jejunum. Postpyloric positioning should be confirmed via imaging before infusion of the stool solution.
Patients are instructed to stop taking any antibiotics or probiotics 24 to 48 hours before FMT. They are additionally advised not to restart taking those medications after the procedure and to try to avoid unnecessary antibiotic therapy in the future. Regardless of approach, all patients should undergo colonic purgation by ingesting polyethylene glycol the night before the procedure; a prescription is provided for this in advance.
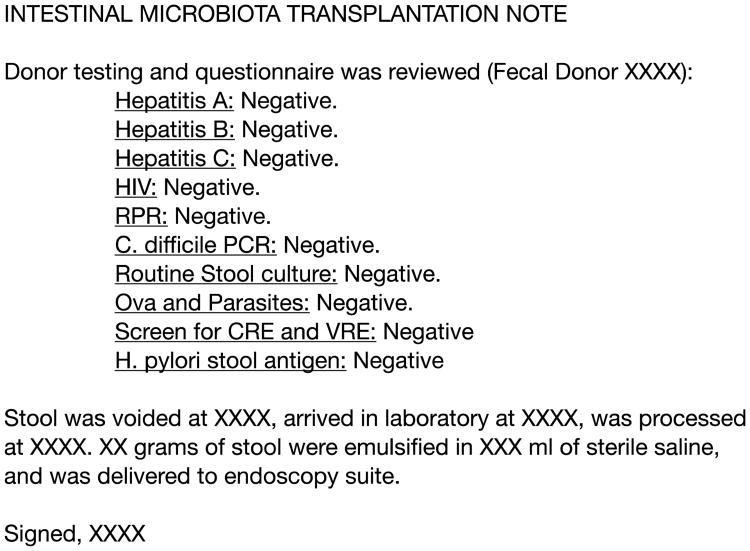
If laboratory personnel are involved, they should document, via a transplantation preparation note in the medical records of the FMT recipient, their involvement in the process. The note should include the anonymous identification number of the fecal donor; the results and date of the most recent screening tests for the donor; and the times when the donor stool was voided, when it was delivered to the laboratory, when it was processed, and when it was delivered to the endoscopy suite (Figure 3).
Figure 3.
Intestinal microbiota transplantation preparation procedure note. HIV indicates human immunodeficiency virus; RPR, rapid plasma reagin; C. difficile, Clostridium difficile; CRE, carbapenem resistant Enterobacteriacae; VRE, vancomycin resistant enterococci; H. pylori, Helicobacter pylori.
Patients should be scheduled for clinical follow-up within 4 to 8 weeks after receiving FMT. If patients are feeling well, they often choose to skip the follow up appointment, but follow up is essential given the importance of making sure that there are no adverse events after this therapy. It is common for patients to experience some irregular bowel habits after FMT; these irregularities usually resolve within a few weeks. When present, constipation and diarrhea generally respond to over-the-counter medications. If continued symptoms of diarrhea persist 1 week after the procedure, patients should be tested for C. difficile via PCR; patients may be considered for repeat FMT if their results are positive.
Abbreviations
- FMT
fecal microbiota transplant
- FDA
United States Food and Drug Administration
- IND
investigational new drug
- CDI
Clostridium difficile infection
- HIV
human immunodeficiency virus
- Ig
immunoglobulin
- HBsAg
hepatitis B surface antigen
- RPR
rapid plasma reagin
- PCR
polymerase chain reaction
- HIV
human immunodeficiency virus
- C. difficile
Clostridium difficile
- KPC, CRE
carbepenem resistant Enterobacteriacae
- VRE
vancomycin resistant enterococci
References
- 1.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46(2):145–149. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 2.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 3.United States Government DoHaHS, Food and Drug, Administration, Center for Biologics Evaluation and Research. Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium Difficile Infection Not Responsive to Standard Therapies. [Accessed on February 13, 2015];2013 Jul; Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatorylnformation/Guidances/Vaccines/ucm361379.htm.
- 4.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathogens. 2012;8(10):e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]