Abstract
Aims
This study looked at whether the inverse association of circulating N-terminal pro-B-type natriuretic peptide (NT-proBNP) with incident diabetes is modified by changes in NT-proBNP (ΔNT-proBNP) levels.
Methods
lasma NT-proBNP was assayed at baseline and 3.2 years later (visit 3) in the Multi-Ethnic Study of Atherosclerosis (MESA).ΔNT-proBNP was calculated as NT-proBNPvisit3 − NT-proBNPbaseline. A Poisson distribution was fitted to determine the incidence density of diabetes, adjusted for age, race, gender, educational attainment, antihypertensive medication, total intentional exercise and plasma IL-6 levels. In the primary analysis (n = 3236 without diabetes up to visit 3, followed for a mean of 6.3 years), incidence density was regressed for the following categories of baseline NT-proBNP: (1) <54.4 pg/mL; (2) 54.4–85.9 pg/mL; and (3) 86–54.2 pg/mL. This was crossed with categories of ΔNT-proBNP as medians (ranges): (1) −6.2 (−131–11.7) pg/mL; (2) 19.8 (11.8–30.1) pg/mL; (3) 44.0 (30.2–67.9) pg/mL; and (4) 111.2 (68.0–3749.9) pg/mL.
Results
The incidence density of diabetes followed a U-shaped association across categories of ΔNT-proBNP within categories of baseline NT-proBNP after adjusting for other covariates (P = 0.02). At each level of baseline NT-proBNP, the incidence density of diabetes was lowest for small-to-moderate increases in NT-proBNP.
Conclusion
This analysis suggests that NT-proBNP has a biphasic association with diabetes in which the risk of incident diabetes decreases within a ‘physiological range’ of ΔNT-proBNP, and plateaus or increases as NT-proBNP concentrations increase, probably in response to pathophysiological conditions leading to high levels of NT-proBNP.
Keywords: Change, Diabetes, Incidence, MESA study, Natriuretic peptides, NT-proBNP
1. Introduction
Although prospective case-cohort [1,2] and longitudinal studies [3,4] have shown that plasma concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP) [1,2,4] and atrial natriuretic peptide (ANP) [3] are inversely associated with incident diabetes, cross-sectional data have reported that the associations between NT-proBNP and blood glucose and insulin resistance plateau at levels of NT-proBNP > 100 pg/mL [5]. These findings are consistent with the hypothesis that any of the possible antidiabetic effects of BNP may be non-linear, plateauing at higher NT-proBNP levels [4].
NT-proBNP can change substantially over time (ΔNT-proBNP) in response to physiological and pathological factors, even in individuals with low baseline values [5–7]. It is currently not known whether such changes can influence the association between baseline NT-proBNP and incident diabetes. The Multi-Ethnic Study of Atherosclerosis (MESA) offers the opportunity to address this question because it has measured NT-proBNP at two time points 3.2 years apart, with a subsequent long-term follow-up.
Our hypothesis was that the associations between baseline NT-proBNP and ΔNT-proBNP with incident diabetes may be better represented using non-linear instead of linear models, such that baseline values of NT-proBNP are inversely associated with incident diabetes, but plateau for levels >100 pg/mL. An additional hypothesis was that diabetes incidence is higher in individuals with low baseline levels of NT-proBNP that decreased over 3.2 years compared with those whose NT-proBNP levels increased.
2. Design and methods
2.1. Study subjects
At baseline during 2000–2002, the MESA recruited 6814 men and women, ages 45–84 years, of white, black, Chinese and Hispanic race/ethnicity who were initially free of self-reported cardiovascular disease (CVD); further details of MESA recruitment and design have been published elsewhere [8]. The institutional review boards at all participating centers approved the study, and written informed consent was obtained from every participant prior to data collection. Analysis of the overall incident diabetes was restricted to 4872 MESA participants who were free of diabetes at baseline and had NT-proBNP measured during 2000–2002, among whom 239 were lost to follow-up and 189 died without diabetes (Fig. 1). At the third MESA visit, 4057 of these participants had NT-proBNP measured a second time, which revealed that 228 had developed diabetes since their baseline visit and thus were excluded from the analysis of ΔNT-proBNP. Participants were further classified by baseline NT-proBNP values into < or ≥ 154.2 pg/mL groups and followed for 6.3 years (Fig. 2). None of these subjects were lost to follow-up during the 6.3 years.
Fig. 1.
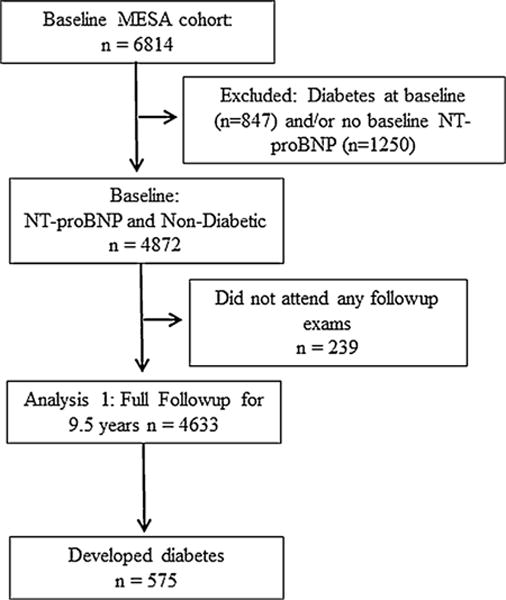
Study algorithm showing number of individuals available for analysis 1: follow-up from MESA baseline.
Fig. 2.
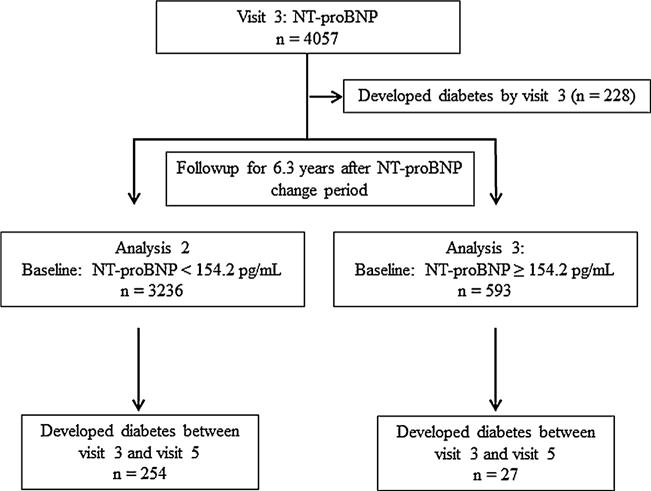
Study algorithm showing number of individuals available for analyses 2 and 3: follow-up from MESA visit 3.
2.2. Blood and anthropomorphic measurements, and other covariates
During visit 1, demographic and anthropometric data, blood lipids, insulin, glucose and interleukin (IL)-6 were measured in venous samples drawn following a 12-h fast at the Collaborative Studies Clinical Laboratory at Fairview University Medical Center in Minneapolis, MN, USA. Serum glucose was measured using a VITROS analyzer (Ortho Clinical Diagnostics, Rochester, NY, USA). Insulin was determined by a radioimmunoassay method using a Human Insulin Specific RIA kit (Linco Research, St. Charles, MO, USA). Homoeostasis model assessment of insulin resistance (HOMA-IR) was calculated as insulin * glucose [9]. Triglyceride (TG), total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) concentrations were measured, using the cholesterol oxidase method (Roche Diagnostics, Indianapolis, IN, USA), and low-density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald equation [10]. Plasma NT-proBNP was measured at baseline and then at visit 3 (3.2 years apart) by the Veterans Affairs San Diego Healthcare System, using an Elecsys 2010 analyzer (Roche Diagnostics); intra- and interassay coefficients of variation were 1.3 and 4.8%, respectively [11], and the reported day-to-day variations were 5.5 and 2.6% when mean concentrations were 6.4 pmol/L and 113.6 pmol/L, respectively [12]. IL-6 concentrations (pg/mL) were measured by ultrasensitive enzyme-linked immunosorbent assay (ELISA; Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN, USA) [13–15].
Resting seated heart rates and blood pressures were measured three times, using a Dinamap PRO 100 automated oscillometric sphygmomanometer (Critikon Inc., Tampa, FL, USA), with the average of the last two measurements used for data analyses. Age, race/ethnicity, gender, body mass index (BMI) computed as weight (kg) divided by square of height (m), waist circumference (cm) determined over light clothing at the level of the umbilicus, total intentional exercise as the metabolic equivalent per min per week (MET-min/week), statin use and presence of diabetes were also recorded.
2.3. Ascertainment of incident diabetes and other clinical variables
Incident diabetes was ascertained on the basis of new fasting glucose levels ≥ 126 mg/dL or new use of insulin or oral hypoglycaemic medications, assessed during study clinic examinations at visits 2, 3, 4 and 5 after median follow-up durations of 1.6, 3.2, 4.8 and 9.5 years, respectively. Hypertension was defined as seated systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, or the self-reported use of antihypertensive medication.
2.4. Definition of subclinical cardiovascular disease
Subclinical CVD was defined as the presence of carotid plaque, left ventricular hypertrophy (LVH) or coronary artery calcium (CAC) > 0. Carotid plaque was defined as the presence of > 25% diameter narrowing, estimated from Doppler recordings of peak systolic velocities [16]. CAC was determined by electron-beam or multidetector computed tomography (CT) [17]. Left ventricular ejection fraction (LVEF) and LV mass were determined by cardiac magnetic resonance imaging (MRI) [18], and LVH was defined as the LV mass corrected by height above the 95th percentile by gender: 52 g/m and 49 g/m for males and females, respectively.
2.5. Statistical analysis
Continuous variables with normal distributions are presented as means ± SD, and categorical variables as frequencies and percentages. The association between baseline NT-proBNP and diabetes incidence density was calculated in individuals free of diabetes from baseline to visit 5 (9.5 years of follow-up). In addition, the association between ΔNT-proBNP by categories of baseline NT-proBNP and diabetes incidence density was also determined in those free of diabetes at visit 3 and from visit 3 to visit 5 (6.3 years of follow-up).
2.5.1. Analysis 1: overall incident diabetes
Cox proportional hazards models were used to estimate hazard ratios (HRs) for incident diabetes regressed on baseline NT-proBNP, with time at risk of first diagnosis, death, MESA dropout or administrative censoring at the latest date of follow-up during 2010–2012 (9.5 years). Baseline NT-proBNP levels were divided into four categories: <54.4 pg/mL; 54.4–85.9 pg/mL; 86.0–154.2 pg/mL; and >154.2 pg/mL. These categories were chosen because, on dividing baseline NT-proBNP by sextiles, it was noted that HRs for incident diabetes did not decrease until NT-proBNP values were > 54.4 pg/mL, the highest range for the 3rd sextile (considered low values vs the overall median). Thus, the chosen ranges for the 4th, 5th and 6th sextiles were the cutoff values used to evaluate the progression of incident diabetes by categories of NT-proBNP. Values above the 6th sextile (>154.2 pg/mL) were likely to reflect pathology [5,19] and were chosen to address the hypothesis of a plateau in the association between NT-proBNP and incident diabetes. To evaluate the level of NT-proBNP at which there were changes in slope, a series of single-knot linear spline models was used, with knots placed between 30 and 300 at 5-pg/mL intervals. This method is described in detail elsewhere [20].
HRs were adjusted for potential confounders associated with the development of type 2 diabetes (T2D). The minimal model included age, race and gender. A more extended model (model 2) included model-1 variables plus the highest educational level achieved, use of antihypertensive medication, total intentional exercise (MET-min/week) and IL-6 concentrations. Because BMI, insulin resistance and blood glucose are presumably on the causal pathway between NT-proBNP and the development of T2D, they were not included as confounders in the extended model [5,21–23]. However, an additional model (model 3) including BMI or waist circumference and fasting blood glucose (FBG) was used to determine if the association between NT-proBNP and incident diabetes was independent of these variables. Sensitivity analysis was also performed to determine whether the association between NT-proBNP and incident diabetes differed by gender, age (< or ≥65 years), state of insulin resistance, presence of CVD and death during follow-up.
2.6. Analyses 2 and 3: incident diabetes after visit 3
It was hypothesized that physiological and pathological mechanisms can affect concentrations of NT-proBNP and influence the level of ΔNT-proBNP [5]. For that reason, categories of ΔNT-proBNP (Fig. 3) were established in relation to incident diabetes, stratified by categories of baseline NT-proBNP as described above. In addition, analysis 2 was restricted to individuals with baseline NT-proBNP < 154.2 pg/mL to avoid those with values in the pathological range and a higher risk of CVD [5,19] while still maintaining adequate sample size. ΔNT-proBNP was initially divided into sextiles, but because the first three sextiles were not substantially different for incident diabetes, they were grouped into one category. Thus, the resulting four categories (range) of NT-proBNP represent: decrease or slight increase (from −131 to 11.7 pg/mL); small increase (11.8 to 30.1 pg/mL); moderate increase (30.2 to 67.9 pg/mL); and large increase (>68 pg/mL). Also examined was the curvature over categories of ΔNT-proBNP (quadratic form over category codes 1–4) estimated by Poisson models (rather than Cox models, for convenience in estimating incidence density), adjusted as in model 2 plus categories of baseline NT-proBNP (codes 1–3). Individuals with baseline NT-proBNP ≥ 154.2 pg/mL were analyzed separately (analysis 3). According to our hypothesis, individuals at highest risk of incident diabetes would have baseline NT-proBNP values < 54.4 pg/mL which, when crossed with categories of ΔNT-proBNP, would represent a decrease or slight increase (from −131 to 11.7 pg/mL). These analyses were performed using SAS PROC GENMOD, version 9.3 (SAS Institute Inc., Cary, NC, USA) with a Poisson distribution. Significance was set at P < 0.05.
Fig. 3.
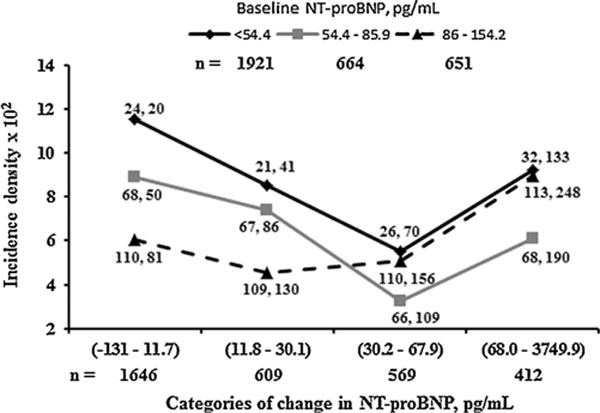
Graph showing adjusted diabetes incidence density from MESA visits 3 to 5 (6.3 years of follow-up) by categories of ΔNT-proBNP at different levels of baseline NT-proBNP in 3236 individuals with baseline NT-proBNP < 154.2 pg/mL and free of diabetes at or before visit 3. Diabetes incidence density: diabetes cases per 100 people followed for 6.3 years; n: number of individuals in each category. Categories of baseline NT-proBNP were low (<54.4 pg/mL), moderate (54.4–85.9 pg/mL) and high (86–154.2 pg/mL). Baseline NT-proBNP values > 154.2 pg/mL (n = 593) were deemed to represent predominantly cardiac pathophysiology (see main text). ΔNT-proBNP categories (range) on the x axis represent decrease/slight (−131–11.7 pg/mL), small (11.8–30.1 pg/mL), moderate (30.2–67.9 pg/mL) and large (>68 pg/mL) increases in NT-proBNP. Values are NT-proBNP medians at visits 1 and 3. The association between incidence density and categories of ΔNT-proBNP was adjusted for gender, race, age, highest educational level achieved, use of anti-hypertensive medication, total intentional exercise (MET-min/week) and IL-6. The association between incidence density and categories of change in NT-proBNP, adjusted for NT-proBNP categories at baseline, had a U-shaped pattern (P = 0.02).
3. Results
3.1. Demographics and other general characteristics at MESA baseline
At baseline, individuals in the highest category of NT-proBNP were 13 years older and mostly women compared with those in the lowest category (Table 1). The proportion of whites was almost doubled in the highest category compared with the lowest category of NT-proBNP, while the proportion of Chinese was half that, and African Americans slightly lower, in the highest category. The proportion of Hispanics was about the same (∼20%) across the different categories of NT-proBNP. The proportion of obese individuals was 31.8% vs 25.0% in the lowest vs highest categories of NT-proBNP. Given these strong associations, all other continuous variables were adjusted for age, race and gender (Table 1). Individuals in the highest category of NT-proBNP had lower values of FBG, TC, LDL-C, TG and HOMA-IR (P < 0.001). In contrast, SBP, IL-6 and HDL-C were higher in the highest category of NT-proBNP compared with the lowest category (P ≤ 0.0001). The percentage of individuals with LVEF < 50% and subclinical CVD progressively rose across categories of NT-proBNP. There was no difference in total intentional exercise and DBP among categories of baseline NT-proBNP. Also, NT-proBNP concentrations were much more variable in those with values ≥100 (SD: 316.4) pg/mL than in those with values < 100 (SD: 26.5) pg/mL. Time to development of T2D was one-third of a year shorter in the highest category of baseline NT-proBNP than in the lowest category.
Table 1.
Demographic, anthropometric, physiological and metabolic characteristics of 4872 individuals without diabetes at baseline according to baseline category of NT-proBNP in the Multi-Ethnic Study of Atherosclerosis.
| n | Categories NT-proBNP (pg/mL)
|
P value | |||
|---|---|---|---|---|---|
| 24 (4.9–54.3) | 67.4 (54.4–85.9) | 112.2 (86–154.2) | 233.3 (154.3–6657) | ||
|
| |||||
| 2783 | 926 | 928 | 927 | ||
| Males (%) | 60.6 | 41.2 | 33 | 32.9 | <0.0001 |
| Age (years) | 58.1 (0.2) | 63.2 (0.3) | 66.2 (0.3) | 71.1 (0.3) | <0.0001 |
| Race | <0.0001 | ||||
| Whites | 36.0 | 43.0 | 50.6 | 54.1 | |
| African American | 26.2 | 21.8 | 17.6 | 19.4 | |
| Hispanics | 22.3 | 23.1 | 20.2 | 18.1 | |
| Chinese | 15.5 | 12.1 | 11.6 | 8.4 | |
| Obese (%) | 31.8 | 27.7 | 25.6 | 25.0 | 0.0008 |
| Anti HTN medication (%) | 22.9 | 30.7 | 30.4 | 46.9 | <0.0001 |
| Education | 85.4 | 82.15 | 81.5 | 78.4 | <0.0001 |
| Total intentional exercise (MET/min/week) | 1558 (50) | 1639 (79) | 1655 (81) | 1510 (87) | 0.80000 |
| Triglycerides (mg/dL) | 135.3 (1.7) | 125.7 (2.7) | 119.9 (2.7) | 120.6 (2.9) | <0.0001 |
| HDL-C (mg/dL) | 50.2 (0.3) | 51.5 (0.5) | 53.4 (0.5) | 53.8 (0.5) | <0.0001 |
| Glucose (mg/dL) | 90.9 (0.2) | 89.6 (0.4) | 88.3 (0.4) | 87.8 (0.4) | <0.0001 |
| HOMA-IR | 2.39 (0.03) | 2.11 (0.05) | 1.86 (0.05) | 1.79 (0.05) | <0.0001 |
| SBP (mmHg) | 129.3 (0.5) | 131.7 (0.7) | 132.8 (0.8) | 136.4 (0.9) | <0.0001 |
| DBP (mmHg) | 71.8 (0.2) | 71.8 (0.3) | 71.4 (0.3) | 72 (0.4) | <0.0001 |
| IL-6 (pg/mL) | 1.42 (0.03) | 1.45 (0.04) | 1.59 (0.04) | 1.71 (0.05) | <0.0001 |
| Low LVEF, % | 1.02 | 1.22 | 0.52 | 3.51 | <0.0001 |
| Subclinical CVD, % | 19.9 | 26.3 | 31.0 | 46.7 | <0.0001 |
| Time to develop diabetes, days | 2815 (21) | 2870 (34) | 2888 (35) | 2695 (38) | 0.0005 |
Values are shown as mean (SE) adjusted for age, race and gender, except when age was the dependent variable. Obese was based on BMI ≥ 30 kg/m2, anti HTN medication = percent of individuals on anti-hypertensive medications. HDL-C: high-density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment of insulin resistance; SBP: systolic blood pressure; DBP: diastolic blood pressure; IL-6: interleukin-6; low LVEF: left ventricular ejection fraction < 50%. Subclinical cardiovascular disease (CVD) includes all individuals with at least 2 of the following: left ventricular hypertrophy, carotid plaque and coronary artery calcium.
3.2. Analysis 1: adjusted HRs for incident diabetes by categories of NT-proBNP
During an average of 9.5 years of follow-up, 575 (12.4%) participants developed T2D. Table 2 shows the incidence density for diabetes by categories of baseline NT-proBNP to be 10.5, 7.8, 7.1 and 6.3 cases of diabetes per 100 people followed for 9.5 years. HRs adjusted for age, race and gender (model 1), and further adjusted for level of education achieved, use of BP-lowering medications, IL-6 and total intentional exercise (model 2) across baseline NT-proBNP categories showed an inverse association between NT-proBNP categories and HRs of incident diabetes. A similar inverse association was also observed between NT-proBNP at visit 3 with incident diabetes. However, the U-shaped association found with baseline NT-proBNP was not seen with the linear spline model (data not shown).
Table 2.
Adjusted hazard ratios for incident diabetes during 9.5 years of follow-up across categories of baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) in 4633 individuals without diabetes at baseline in the Multi-Ethnic Study of Atherosclerosis (MESA).
| Categories of NT-proBNP [pg/mL, median (range)]
|
||||
|---|---|---|---|---|
| 24.4 (4.9–54.3) | 67.6 (54.4–85.9) | 112.2 (86–154.2) | 231.5 (154.3–6657) | |
| Cases/total at risk (n) | 320/2172 | 127/1174 | 75/805 | 53/482 |
| Person-years, sum total | 17,166 | 9162 | 6191 | 3231 |
| Unadjusted incidence densitya | 10.5 | 7.8 | 7.1 | 6.3 |
| Model 1 | 1 | 0.73 (0.57–0.93) | 0.66 (0.5–0.86) | 0.72 (0.54–0.95) |
| Model 2 | 1 | 0.71 (0.55–0.90) | 0.61 (0.46–0.80) | 0.59 (0.44–0.79) |
| Model 3 | 1 | 0.75 (0.58–0.95) | 0.67 (0.51–0.88) | 0.68 (0.51–0.90) |
Model 1: age, race and gender; model 2: model 1 + educational attainment, use of antihypertensive medication, plasma interleukin-6 and total intentional exercise (MET-min/week); model 3: model 2 + body mass index.
Diabetes cases per 100 people followed for 9.5 years.
The linear spline model adjusted for variables in model 2 showed a 15% decrease in HR for every SD (25.8 pg/mL) increase in NT-proBNP (P = 0.002) among those whose values were < 55 pg/mL. In contrast, it showed a 22% increase in HR for each SD (319.7 pg/mL) increase in NT-proBNP among those with values ≥ 150 pg/mL (P = 0.04). Differences in slopes of the association between HRs for incident diabetes and NT-proBNP differed with values < and ≥ 55 pg/mL (P = 0.001) and also with values < and ≥ 150 pg/mL (P < 0.0001; Table 3).
Table 3.
Linear spline regression coefficients above and below a single knot per SD of baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) on hazard ratios of incident diabetes during 9.5 years of follow-up of 4872 individuals without diabetes at baseline in the Multi-Ethnic Study of Atherosclerosis (MESA).
| NT-proBNP cutoff (pg/mL) | Coefficient (SE) | NT-proBNP SD < knot (pg/mL) | P | Coefficient (SE) | NT-proBNP SD ≥ knot (pg/mL) | P | P for interaction |
|---|---|---|---|---|---|---|---|
| 35 | −0.07 (0.08) | 17.2 | 0.4 | −0.04 (0.06) | 138.8 | 0.5 | 0.5 |
| 45 | −0.11 (0.07) | 21.8 | 0.1 | 0.02 (0.06) | 153.8 | 0.8 | 0.1 |
|
| |||||||
| 55 | −0.15 (0.07) | 25.8 | 0.02 | 0 (0.07) | 168.6 | 1.0 | 0.03 |
|
| |||||||
| 80 | −0.21 (0.06) | 34.8 | 0.0006 | 0.06 (0.08) | 209.4 | 0.4 | 0.0007 |
| 100 | −0.24 (0.06) | 40.5 | <0.0001 | 0.12 (0.08) | 240.2 | 0.2 | <0.0001 |
| 120 | −0.23 (0.06) | 45.4 | <0.0001 | 0.14 (0.09) | 271.7 | 0.09 | <0.0001 |
| 140 | −0.25 (0.06) | 49.7 | <0.0001 | 0.18 (0.10) | 302.8 | 0.05 | <0.0001 |
|
| |||||||
| 150 | −0.20 (0.05) | 49.7 | <0.0001 | 0.22 (0.10) | 319.7 | 0.04 | <0.0001 |
|
| |||||||
| 200 | −0.18 (0.05) | 60.0 | 0.0002 | 0.28 (0.13) | 400.0 | 0.02 | <0.0001 |
| 300 | −0.14 (0.05) | 63.9 | 0.002 | 0.48 (0.2) | 589.1 | 0.02 | 0.005 |
Values were regressed on baseline NT-proBNP multiplied by SD of baseline NT-proBNP at each cutoff value; coefficients were adjusted for age, race, gender, antihypertensive medication, statin use, educational attainment, plasma interleukin-6 and total intentional exercise (MET-min/week); upper grey row: lowest knot below which the slope of association between incident diabetes and baseline NT-proBNP is inversed but, above the knot, plateaus; lower grey row: knot below which there is an inverse association between incident diabetes and baseline NT-proBNP but is, above the knot, positive.
Model 3, which was further adjusted for BMI or waist circumference as potential mediators, did not substantially modify the association between categories of NT-proBNP and incident diabetes (Table 2). Adjusting separately for baseline FBG and HOMA-IR weakened the association, rendering it non-significant (data not shown). Further adjustments for categories of LVEF < or ≥50% did not change the association between incident diabetes and categories of baseline NT-proBNP.
In the sensitivity analysis, HRs for incident diabetes as adjusted in model 2 showed a gender × categories of NT-proBNP interaction (P = 0.0002; Table 4). In men, the association between categories of NT-proBNP and incident diabetes followed a quadratic association (P = 0.004) whereas, in women, it followed an inverse association (P < 0.0001). There was no interaction effect between NT-proBNP at baseline and categories of HOMA-IR values ≤ or > 2.7 mU/L × mmol/dL/22.5 (P = 0.20) or of age < or ≥65 years (P = 0.30). There was also no NT-proBNP effect by race interaction (P = 0.40). Sensitivity analysis also showed that excluding individuals who developed CVD or died during the 9.5-year follow-up did not substantially change the association between baseline NT-proBNP and incident diabetes.
Table 4.
Hazard ratios (HRs) for incident diabetes by categories of baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) in non-diabetic men and women at baseline in the Multi-Ethnic Study of Atherosclerosis (MESA).
| Categories of NT-proBNP [pg/mL, median (range)]
|
|||||||
|---|---|---|---|---|---|---|---|
| 24.4 (4.9–54.3) | 67.6 (54.4–85.9) | 112.2 (86–154.2) | 231.5 (154.3–6657) | P for linear trend | P for quadratic trend | P for interaction | |
| HRa for men | 1 | 0.66 (0.44–0.96) | 0.85 (0.55–1.25) | 0.89 (0.57–1.35) | 0.07 | 0.004 | 0.0002 |
| HRa for women | 1 | 0.7 (0.51–0.95) | 0.49 (0.34–0.69) | 0.46 (0.31–0.67) | <0.0001 | 0.3 | |
Adjusted for age, race, gender, antihypertensive medication, statin use, educational attainment, plasma interleukin-6 and total intentional exercise (MET-min/week).
3.3. Analyses 2 and 3: adjusted incidence density of diabetes by categories of ΔNT-proBNP at different levels of baseline NT-proBNP
Between visits 3 and 5, 254/3236 (7.9%) individuals developed diabetes (incidence density of 9.0 per 100 people followed for 6.3 years). The incidence density of diabetes was determined at each crossed category of baseline NT-proBNP and ΔNT-proBNP for those with baseline NT-proBNP < 154.2 pg/mL (Fig. 3). On Poisson regression analysis, parameter estimates were similar between models 1 and 2; thus, only values for model 2 are provided here. Incidence density of diabetes decreased by 38% between the lowest NT-proBNP category (< 54.4 pg/mL) and the 85.9–154.2-pg/mL category of baseline NT-proBNP after adjusting for categories of ΔNT-proBNP (P = 0.01). The association between incidence density of diabetes and ΔNT-proBNP, adjusted as in model 2 plus for baseline NT-proBNP category, was U-shaped (P = 0.02). This U-shaped association was observed at all three levels of baseline NT-proBNP and was confirmed by the lack of baseline NT-proBNP × ΔNT-proBNP interaction (P = 0.80). The U-shaped association persisted after further adjustment for changes in BMI or waist circumference between baseline and visit 3, and on excluding individuals who developed CVD during follow-up. It was noted that diabetes risk was higher for both decrease/slight and large increases in ΔNT-proBNP, and the turning point in the risk of incident diabetes appeared to be an NT-proBNP value of 130 pg/mL. This value was an approximation determined by averaging the median NT-proBNP values at visit 3 across ΔNT-proBNP categories in which diabetes incidence first started to rise (Fig. 3). Thus, for the category of baseline NT-proBNP < 54.4 pg/mL, this was 133 + 70/2 = 102 pg/mL and, similarly, 156 + 130/2 = 143 pg/mL for baseline NT-proBNP in the range of 54–84 pg/mL, and 190 + 109/2 = 140 pg/mL in the range of 85–154 pg/mL.
There were 593 participants with baseline NT-proBNP ≥ 154.2 pg/mL, and changes through to visit 3 were substantial, with 43% with decreases (median: −101 pg/mL) and 42% with increases (median: +160 pg/mL), with only 15% stable (changes of −23 to +29 pg/mL). The adjusted incidence density for T2D was 2.9, 1.0 and 5.5 per 100 people followed for 6.3 years in each category of NT-proBNP, respectively. Although the T2D risk was nominally highest in those who decreased or increased, the association did not reach statistical significance.
4. Discussion
Our present analysis has demonstrated that baseline NT-proBNP is inversely associated with incident diabetes over 9.5 years. However, this inverse association does not persist throughout the whole range of baseline values, but only becomes positive at levels of NT-proBNP ≥ 150 pg/mL. Furthermore, the association between incident diabetes and baseline NT-proBNP can be modified by ΔNT-proBNP. At each level of baseline NT-proBNP, the risk of incident diabetes substantially decreases among those who, over a 3.2-year period, had an increase in NT-proBNP of 11.8–67.9 pg/mL compared with those with a decrease/slight increase (−131 to +11.7 pg/mL) or a large increase (≥68.0 pg/mL).
Three of the previous studies evaluating the association between natriuretic peptides and incident diabetes [1–3] were performed in predominantly white populations. Only the study by Lazo et al. [4] included African Americans, and their findings extended previous observations of the inverse association between NT-proBNP and incident diabetes to an ethnically diverse population. Similar to the Lazo et al. study, our present analysis also reports a gender-based NT-proBNP interaction explained by an inverse association between incident diabetes and NT-proBNP in women, and a quadratic association between NT-proBNP and incident diabetes in men. The reasons for these differing patterns of association are not known. Our findings suggest that factors related to glucose metabolism are on the causal pathway between NT-proBNP and incident diabetes, and are partially independent of BMI and waist circumference.
Collectively, our analysis also showed that low levels of NT-proBNP (<54.4 pg/mL) are associated with an increased incidence of T2D, but the incidence could be lowered if NT-proBNP increases over time, provided that its absolute value does not rise beyond a certain pathological value. Although the exact cutoff value above which incidence diabetes rises is difficult to determine, our present data suggest it may be a value of approximately 130 pg/mL.
The finding of a non-linear association between baseline NT-proBNP and incident diabetes is in agreement with Lazo et al. [4], although they reported that the change in slope occurred at a different value (76.2 pg/mL). Nevertheless, these data support our hypothesis of a cutoff value below which levels of NT-proBNP vary primarily in response to physiological factors and above which levels of NT-proBNP are predominantly influenced by pathological conditions [5]. The exact NT-proBNP value at which changes in slopes occur signalling a change from physiological to pathological conditions is not known, and may depend on gender, race and a combination of comorbid conditions, and may occur at values ≤54.4 or ≥130 pg/mL of NT-proBNP. Further research assessing the dose–response relationship between natriuretic peptides and fat storage, blood glucose and insulin sensitivity would help to discern any cause-and-effect relationships.
As regards glucose metabolism, levels of NT-proBNP < 54.4 pg/mL may represent a state of insufficient production of natriuretic peptides. At the other end of the spectrum, values of NT-proBNP ≥ 130 pg/mL are likely to be induced by the concurrent presence of subclinical CVD and inflammation [5]. It has been shown that consistently elevated levels of NT-proBNP are associated with a state of resistance to the action of natriuretic peptides and also with the synthesis of biologically inactive natriuretic peptides [24,25]. Therefore, the increased risk of incident diabetes associated with low NT-proBNP values and loss of protective effect of NT-proBNP at high levels may be induced by distinct biological mechanisms. A description of the potential biological mechanisms linking the development of T2D with absolutely or relatively low levels of natriuretic peptides is presented in our supplementary material (Fig. S1; see supplementary material associated with this article online).
Because the infusion of insulin can decrease levels of NT-proBNP in patients with heart failure [26], reverse causality could be an explanation for the association between NT-proBNP and incident diabetes. However, a Mendelian randomization study by Pfister et al. [2] supports causality by documenting that a genetic variant of the BNP gene associated with increased levels of NT-proBNP is inversely associated with incident diabetes. Furthermore, the lowering of blood glucose during an oral glucose tolerance test (OGTT) associated with an intravenous infusion of BNP [27] favours the notion that natriuretic peptides may be on the causal pathway in the development of T2D. Future research should be aimed at elucidating whether either stimulating an increase in synthesis and release or administering natriuretic peptides in individuals with low baseline levels could initially improve insulin sensitivity and, thus, prevent the further development of obesity and T2D compared with those who maintain low levels of natriuretic peptide.
4.1. Strengths and limitations
The main strengths of our present analysis were its large cohort of individuals free of CVD and T2D who were followed for an extended period of time (9.5 years), and the low intra- and interassay coefficients of variation for NT-proBNP. Also unique to our study was that NT-proBNP was measured on two occasions 3.2 years apart. This provided an opportunity to evaluate whether rising NT-proBNP levels in individuals initially at high risk of developing T2D because of low baseline values would lower their risk of future diabetes.
However, our lack of measurement of day-to-day and week-to-week variations in NT-proBNP, reported to range between 26 and 47% in healthy individuals, diminishes the ability of this study to determine a cutoff value to distinguish between physiological and pathological levels of ΔNT-proBNP [7,28]. Another limitation was the lack of measurement of BNP and ANP. Although both participate in the metabolism of glucose and lipids [29], it is not known which of these peptides has a more significant association with incident diabetes. Unfortunately, the MESA study was not designed to evaluate the development of diabetes; consequently, a family history of diabetes, a risk factor, was not ascertained. Nevertheless, despite the lack of this important risk factor, our results were similar to those of other longitudinal studies that have assessed the association between different forms of natriuretic peptides and incident diabetes [1,3,4].
Our analysis also relied on fasting glucose levels and/or the use of antidiabetic medications for diagnosing diabetes, and did not perform OGTTs or measure HbA1c values in participants. Moreover, although our study design was strong in sample size, event number and sorting out temporal sequences of events, all observational studies are subject to unknown residual confounders, which prevent firm inferences of causality.
5. Conclusion
This study supports previous observations of an inverse association between baseline NT-proBNP and incident diabetes, and further extends the association to include other racial and ethnic groups. In addition, it shows that the association does not follow a linear pattern throughout the whole range of values, and that those at high risk of developing T2D because of low baseline levels of NT-proBNP (<54.4 pg/mL) could lower their risk by increasing their NT-proBNP to values no greater than around 130 pg/mL. These findings demonstrate a biphasic association between incident diabetes and NT-proBNP, and suggest that two distinct biological mechanisms, one at physiological and another at pathological levels of NT-proBNP, contribute to the association with incident diabetes.
Supplementary Material
Acknowledgments
This research was supported by contracts N01-HC-95159 to N01-HC-95169 from the US National Heart, Lung, and Blood Institute, grants UL1-TR-000040 and UL1-TR-001079 from the US National Center for Research Resources (NCRR), and Roche Diagnostics. The authors also thank the investigators and staff of the Multi-Ethnic Study of Atherosclerosis (MESA) for their valuable contributions. A full list of MESA investigators and institutions can be found at www.mesa-nhlbi.org/.
Abbreviations
- ANP
atrial natriuretic peptide
- BMI
Body Mass Index
- CVD
cardiovascular disease
- CT
computed tomography
- CAC
coronary artery calcium
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- IL-6
interleukin-6
- LVH
left ventricular hypertrophy
- LDL-C
low-density lipoprotein cholesterol
- MESA
Multi-Ethnic Study of Atherosclerosis
- NP
natriuretic peptides
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- PGC1A
peroxisome proliferator-activated receptor-γ coactivator 1α
- TC
total cholesterol
- TG
triglycerides
- BNP
B-type natriuretic peptide
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.diabet.2015.04.005.
Footnotes
Disclosure of interest
Otto A. Sanchez declares that he has no conflicts of interest concerning this article. Daniel Duprez MD, PhD, consultant to Novartis and AstraZeneca. Hossein Bahrami, no disclosures. Lori B. Daniels, consultant to Singulex and Alere, Inc., speaking fees from Critical Diagnostics. Aaron R. Folsom, no disclosures. Joao A. Lima, consultant to Toshiba Medical Systems/Bracco Diagnostics Inc. Alan Maisel, consultant to Alere, Inc., BG Medicine, Brahms, Critical Diagnostics, EFG Diagnostics, Novartis, Abbott. Carmen A. Peralta and David R Jacobs declare that they has no conflicts of interest concerning this article.
References
- 1.Everett BM, Cook NR, Chasman DI, Magnone MC, Bobadilla M, Rifai N, et al. Prospective evaluation of B-type natriuretic peptide concentrations and the risk of type 2 diabetes in women. Clin Chem. 2013;59:557–65. doi: 10.1373/clinchem.2012.194167. [Epub 2013/01/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister R, Sharp S, Luben R, Welsh P, Barroso I, Salomaa V, et al. Mendelian randomization study of B-type natriuretic peptide and type 2 diabetes: evidence of causal association from population studies. PLoS Med. 2011;8:e1001112. doi: 10.1371/journal.pmed.1001112. [Epub 2011/11/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnusson M, Jujic A, Hedblad B, Engstrom G, Persson M, Struck J, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer Study. J Clin Endocrinol Metab. 2012;97:638–45. doi: 10.1210/jc.2011-2425. [Epub 2011/11/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazo M, Young JH, Brancati FL, Coresh J, Whelton S, Ndumele CE, et al. NH2-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes. 2013;62:3189–93. doi: 10.2337/db13-0478. [Epub 2013/06/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, et al. The associations between metabolic variables and NT-proBNP are blunted at pathological ranges: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2014;63:475–83. doi: 10.1016/j.metabol.2013.11.017. [Epub 2014/01/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggers KM, Venge P, Lind L. Prognostic usefulness of the change in N-terminal pro B-type natriuretic peptide levels to predict mortality in a single community cohort aged ≥70 years. Am J Cardiol. 2013;111:131–6. doi: 10.1016/j.amjcard.2012.08.058. [Epub 2012/10/09] [DOI] [PubMed] [Google Scholar]
- 7.Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152:828–34. doi: 10.1016/j.ahj.2006.08.021. [Epub 2006/10/31] [DOI] [PubMed] [Google Scholar]
- 8.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [Epub 2002/10/25] [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [Epub 1985/07/01] [DOI] [PubMed] [Google Scholar]
- 10.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [Epub 1972/06/01] [PubMed] [Google Scholar]
- 11.Karl J, Borgya A, Gallusser A, Huber E, Krueger K, Rollinger W, et al. Development of a novel. N-terminal-proBNP (NT-proBNP) assay with a low detection limit. Scand J Clin Lab Invest Suppl. 1999;230:177–81. [Epub 1999/07/02] [PubMed] [Google Scholar]
- 12.Mueller T, Gegenhuber A, Poelz W, Haltmayer M. Comparison of the Biomedica NT-proBNP enzyme immunoassay and the Roche NT-proBNP chemiluminescence immunoassay: implications for the prediction of symptomatic and asymptomatic structural heart disease. Clin Chem. 2003;49(6 Pt 1):976–9. doi: 10.1373/49.6.976. [Epub 2003/05/27] [DOI] [PubMed] [Google Scholar]
- 13.Peralta CA, Katz R, Shlipak M, Dubin R, DeBoer I, Jenny N, et al. Kidney function decline in the elderly: impact of lipoprotein-associated phospholipase A(2) Am J Nephrol. 2011;34:512–8. doi: 10.1159/000333045. [Epub 2011/11/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peralta CA, Jacobs DR, Jr, Katz R, Ix JH, Madero M, Duprez DA, et al. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012;59:41–9. doi: 10.1053/j.ajkd.2011.08.015. [Epub 2011/10/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–54. doi: 10.1093/aje/kwn350. [Epub 2008/12/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharrett AR, Ding J, Criqui MH, Saad MF, Liu K, Polak JF, et al. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: the Multiethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006;186:441–7. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [Epub 1990/03/15] [DOI] [PubMed] [Google Scholar]
- 18.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 Suppl. 2):S357–65. doi: 10.2214/AJR.04.1868. [Epub 2006/05/23] [DOI] [PubMed] [Google Scholar]
- 19.Marz W, Tiran B, Seelhorst U, Wellnitz B, Bauersachs J, Winkelmann BR, et al. N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem. 2007;53:1075–83. doi: 10.1373/clinchem.2006.075929. [Epub 2007/04/21] [DOI] [PubMed] [Google Scholar]
- 20.Sanchez OA, Jacob DR, Jr, Bahrami H, Peralta CA, Daniels LB, Lima JA, et al. Increasing aminoterminal-pro-B-type natriuretic peptide precedes the development of arterial hypertension: themultiethnic study of atherosclerosis. J Hypertens. 2015;33 doi: 10.1097/HJH.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng S, Fox CS, Larson MG, Massaro JM, McCabe EL, Khan AM, et al. Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am J Cardiol. 2011;108:979–84. doi: 10.1016/j.amjcard.2011.05.033. [Epub 2011/08/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90. doi: 10.1016/j.jacc.2005.08.050. [Epub 2006/01/03] [DOI] [PubMed] [Google Scholar]
- 23.Neeland IJ, Winders BR, Ayers CR, Das SR, Chang AY, Berry JD, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol. 2013;62:752–60. doi: 10.1016/j.jacc.2013.03.038. [Epub 2013/04/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clerico A, Vittorini S, Passino C. Circulating forms of the B-type natriuretic peptide prohormone: pathophysiologic and clinical considerations. Adv Clin Chem. 2012;58:31–44. doi: 10.1016/b978-0-12-394383-5.00008-4. [DOI] [PubMed] [Google Scholar]
- 25.Potter LR, Garbers DL. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J Biol Chem. 1992;267:14531–4. [Epub 1992/07/25] [PubMed] [Google Scholar]
- 26.Halbirk M, Norrelund H, Moller N, Schmitz O, Botker HE, Wiggers H. Short-term changes in circulating insulin and free fatty acids affect Nt-pro-BNP levels in heart failure patients. Int J Cardiol. 2010;144(1):140–2. doi: 10.1016/j.ijcard.2008.12.152. [Epub 2009/01/30] [DOI] [PubMed] [Google Scholar]
- 27.Heinisch BB, Vila G, Resl M, Riedl M, Dieplinger B, Mueller T, et al. B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: a randomised placebo-controlled cross-over study in healthy men. Diabetologia. 2012;55:1400–5. doi: 10.1007/s00125-011-2392-1. [Epub 2011/12/14] [DOI] [PubMed] [Google Scholar]
- 28.O’Hanlon R, O’Shea P, Ledwidge M, O’Loughlin C, Lange S, Conlon C, et al. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13:50–5. doi: 10.1016/j.cardfail.2006.09.003. [Epub 2007/03/07] [DOI] [PubMed] [Google Scholar]
- 29.Dessi-Fulgheri P, Sarzani R, Rappelli A. Role of the natriuretic peptide system in lipogenesis/lipolysis. Nutr Metab Cardiovasc Dis. 2003;13:244–9. doi: 10.1016/s0939-4753(03)80018-2. [Epub 2003/12/03] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


