Abstract
Objective
Recent evidence has shown that, in heart failure (HF), clinically relevant concentrations of angiotensin-(1-7) [Ang-(1-7)] counteracts angiotensin II induced cardiac depression and produces positive inotropic effects in both left ventricle (LV) and myocytes. However, the underlying electrophysiological mechanism is unclear. We investigated the role and mechanism of Ang-(1-7) on LV myocyte L-type calcium current (ICa,L) responses in normal state and in HF.
Method
We compared the effect of Ang-(1-7) (10−5 M) on ICa,L responses in isolated LV myocytes obtained from 11 rats with isoproterenol (ISO) induced HF (3 months after 170 mg/kg subcutaneous for 2 days) and from 8 age-matched normal control rats by patch clamp technique.
Results
In normal myocytes, compared with baseline, superfusion of Ang-(1-7) caused no significant changes in ICa,L (8.2 ± 0.2 versus 8.0 ± 0.3 pA/pF, p= not significant). In HF myocytes, the baseline ICa,L was significantly reduced (5.3 ± 0.1 versus 8.0 ± 0.3 pA/pF, p < 0.01). Ang-(1-7) produced a 21% increase in ICa,L (6.4±0.1 versus 5.3±0.1 pA/pF, p < 0.01). Pretreatment of HF myocytes with a nitric oxide (NO) synthase inhibitor (L-NAME, 10−5 M) resulted in a significantly greater increase in ICa,L (28%, 8.4 ± 0.1 versus 6.5 ± 0.1 pA/pF, p < 0.01) during Ang-(1-7) superfusion. In contrast, during incubation with the bradykinin (BK) inhibitor HOE 140 (10−6 M), Ang-(1-7) induced increase in ICa,L was significantly decreased. The Ang-(1-7) induced increase in ICa,L was abolished by [D-Ala7]-Ang-(1-7) (A-779, 10−5 M).
Conclusions
HF alters the response of ICa,L to Ang-(1-7). In normal myocytes, Ang-(1-7) has no significant effect on ICa,L. However, in HF myocytes, Ang-(1-7) increases ICa,L. These effects are mediated by the Ang-(1-7) Mas receptors and involve activation of NO/BK pathways.
Keywords: calcium, heart failure, ion currents, receptors, vasodilator agents
Introduction
The renin–angiotensin system (RAS) is a cascade of enzymatic reactions resulting ultimately in the formation of angiotensin II (Ang II). Recent research has expanded knowledge about RAS by adding new components to the pathways: the heptapeptide angiotensin-(1–7) [Ang-(1–7)]; an angiotensin converting enzyme (ACE) homologous enzyme, ACE2, the Ang-(1–7) forming enzyme; and the G-protein coupled Mas receptor as a molecular receptor for Ang-(1–7) [Donoghue et al. 2000; Ferrario, 2011; Ferrario and Varagic, 2010; Santos et al. 2003].
While Ang II is a key contributor to the progression of heart failure (HF), accumulating evidence suggests that Ang-(1-7) may play an important role in counteracting the pressor, proliferative and profibrotic actions of Ang II in the heart [Ferrario et al. 2010; Stewart et al. 2008]. Ang-(1–7) contributes to the beneficial effects of ACE inhibitors (ACEI) and AT1-receptor blockers (ARBs) both in experimental conditions [Ferrario et al. 1991; Iyer et al. 1998] and in humans [Ferrario et al. 1998, 2002; Luque et al. 1996; Schindler et al. 2007; Zisman et al. 2003]. We previously demonstrated that in normal conscious dogs, Ang II produced no marked changes in intact left ventricle (LV) contractile function. In contrast, after HF, Ang II reduced LV contractility [Cheng et al. 1996). More recently, we found that, in HF, clinically relevant concentrations of Ang-(1-7) counteracted Ang II induced cardiac depression and produced positive inotropic effects in the LV and in cardiac myocytes [Cheng et al. 2008] by mechanisms that remained to be studied. Experimental studies suggest that, in the heart, Ang-(1-7) counteracts Ang II actions through re-establishing impulse propagation [De Mello, 2004], activating the sodium pump, hyperpolarizing the cell membrane, and increasing the conduction velocity [De Mello et al. 2007].
Since voltage-gated Ca2+ channels play a fundamental role in the regulation of cardiac function by various neurotransmitters, the beneficial effects of Ang-(1-7) on cardiac contractile response in HF may be due to Ang-(1-7) induced alteration in the regulation of the Ca2+ channel. No previous studies have specifically examined Ang-(1-7) induced changes in calcium current (ICa,L) in normal or in pathological states. The intracellular pathway coupling Ang-(1-7) stimulation is still incompletely characterized. Accordingly, we evaluated the hypothesis that, in HF, Ang-(1-7) produces positive modulation on L-type calcium channel activity, which is coupled with Ang-(1-7) Mas receptors, acting through a nitric oxide (NO)/bradykinin (BK) mediated mechanism.
The rat model of isoproterenol (ISO) induced HF has been studied by many investigators [Rona, 1985; Suzuki et al. 1998; Teerlink et al. 1994], including our laboratory [Zhang et al. 2005]. It has been demonstrated that the pathological changes in ISO-treated rats resemble those of myocardial infarction [Teerlink et al. 1994]. Therefore, in the present study, we used this rat model to: (1) assess the response of ICa,L to Ang-(1-7) in normal and HF myocytes; and (2) evaluate the potential mechanism of Ang-(1-7) induced changes on ICa,L in relation to the activation of Ang-(1-7) Mas receptor, NO syntheses and BK.
Methods
Experimental HF model
This investigation was approved by the Wake Forest University Animal Care and Use Committee, and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985).
As previously described [Suzuki et al. 1998; Teerlink et al. 1994], HF in the rat model was induced by ISO injections with some modification [3 months after 170 mg/kg, subcutaneous (sc) for 2 days]. Briefly, male Sprague-Dawley rats (200–250 g) received two sc injections of 170 mg/kg ISO in HCl at 24-hour intervals. The mortality rate was approximately 20–30% within 48 hours. A control group of rats received the same amount of sterile saline. Animals were housed and fed under the same conditions.
A period of 3 months after the ISO injection, survivors (n = 11) and sham-injected rats (n = 8) were lightly anesthetized with intraperitoneal ketamine HCl (50 mg/kg) and xylazine (10 mg/kg). Then, hemodynamic measurements were obtained using 1.4 F, miniaturized, combined catheter transducers (Model SPR-774; Millar Instruments, Houston, TX) inserted into the LV through the apex to verify the presence of HF in the ISO-treated rats. Body, heart and lung weights were obtained at the completion of every study.
Consistent with previous reports, the hearts of ISO-injected rats displayed large areas of infarct-like necrosis involving about one-third to one-half of the LV and extending to the adjacent area of the ventricular septum and right ventricle [Teerlink et al. 1994; Zhang et al. 2005]. Diffuse transmural and subendocardial necrosis were also observed. In order to obtain a high yield of viable isolated myocytes, as previously described, and with some modification [Pfeffer et al. 1979], the gross lesions of infarct-like necrosis area were measured and discarded from evaluation.
Isolation of LV myocytes
Myocytes were enzymatically dissociated by Langendorff perfusion as previously described [Suzuki et al. 1998]. With our well-established technique, more than 80% yield of viable myocytes was obtained from both control and ISO-treated rats. The cells were used within 12 hours.
Electrophysiological measurement
Membrane calcium current was recorded at 20–22°C using a whole cell patch-clamp technique as previously described [Hamill et al. 1981]. An Axopatch 200A amplifier (Axon Instruments, Forster City, CA) was interfaced with a 16-bit A/D-D/A converter (Digidata 1322 A, Axon Instruments, Sunnyvale, CA). PClamp software was used for data acquisition and analysis (PClamp8.02, Axon Instruments, Sunnyvale, CA). Data were filtered with a 5 kHz low-pass filter and digitized at 5 kHz.
After stabilization, a drop of cell pellet containing the isolated myocytes was placed in a perfusion chamber (0.5 ml volume) mounted on the stage of an inverted microscope (IMT 2-F3, Olympus, Herndon, VA) and continuously superfused at a constant rate of 2 ml/min. Only quiescent rod-shaped cells with clear cross striations were studied. Borosilicate glass micropipettes (OD.1.5 mm) were pulled with a puller (Model P-97, Flaming/Brown Micropipette Puller, Sutter Instrument, Novato, CA). The tip resistances were 1.5–3.0 MΩ when filled with pipette (internal) solution. Liquid junction potentials (<5 mV) were corrected before the pipette touched the cell. After formation of the GΩ-seal, the electrode capacitance was compensated electronically. Then the cell membrane was ruptured by gentle suction to establish the whole cell configuration. In a subgroup of cells, a perforated patch recording technique was used in which nystatin stock was added to the internal solution (final nystatin concentration of 100–200 μg/ml) [Horn and Marty, 1988]. The development of electrical access was monitored by the appearance of a capacitive current. The access resistance was <20 MΩ.
The membrane capacitance and series resistance were compensated to minimize the duration of the capacitive transient. The membrane capacitance was measured before compensation with a 10 mV depolarizing step from a holding potential of −80 mV to −70 mV, and the integrated area under the current transient calculated with the following formula: Cm = τcI0/ΔVm[1−(I∞/I0)], where Cm is the membrane capacitance, τc is the decay time constant of membrane capacitance, I0 is the maximum capacitance current value, ΔVm is the amplitude of the voltage step, and I∞ is the amplitude of steady-state current. The membrane capacitance was used as an index to normalize ICa,L for cell size. The series resistance was calculated as Rs = ΔVm/I0, where Rs is the series resistance, compensated at about 80%.
Myocytes were voltage clamped at −80 mV. Ca2+ currents were elicited by stepping up the membrane voltage from a holding potential to 0 mV resting potential for 200 ms at 10 s intervals. To avoid contamination by fast sodium channel activation and to reduce the rundown of ICa,L, a pre-pulse (−45 mV, 60 ms duration) was applied before approaching the test potential. The average peak ICa,L rundown was about 10–20% for 30 min after initial measurement. Most (80%) of the rundown occurred within the initial 8–10 min. Thus, the window of time between 10 and 30 min after the initial recording was chosen to measure ICa,L with respect to drug effects [Zhang et al. 2005]. ICa,L was measured by the standard method as the difference between peak inward current and the current at the end of a 200 ms pulse. For current–voltage (I-V) relationships, test potentials were from −40 to +60 mV at 5 mV step increments and 0.1 Hz.
Solutions
The compositions of the pipette solution and recording bath solution were chosen to allow isolation of ion flow through the Ca2+ channel by blocking other ionic currents. Initially, the myocytes were superfused with a modified Tyrode's solution containing NaCl 137 mM, KCl 5.4 mM, MgSO4 1.2 mM, glucose 15 mM, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10 mM, CaCl2 1.5 mM. The pH was adjusted to 7.4 with NaOH at 20–22°C. After formation of the GΩ seal, super-fusion buffer was changed to a patch recording bath solution, i.e. Na+- K+ free Tyrode's solution, in which NaCl was substituted by tetraethylammonium chloride and KCl replaced by CsCl l mM and 3 mM 4-aminopyridine (4-AP) in order to abort the potassium current. The solution was gassed with 100% O2. The internal solution for the pipette contained Cs aspartate 140 mM, MgCl2 1.0 mM, di-sodium adenosine triphosphate (Na2ATP) 3 mM, guanidine triphosphate (GTP) 0.4 mM, ethylene glycol tetraacetic acid (EGTA) 10 mM and HEPES 5 mM. The pH was adjusted to 7.2 (with titrated CsOH). For perforated patch recording, nystatin stock solution (10 mg/ml in acidified methanol) was freshly prepared each day and added to the internal solution at a final concentration of 100–200 μg/ml. The pipette was dipped in nystatin-free internal solution for 2 s and then backfilled with nystatin internal solution [Horn and Marty, 1988].
Drugs
Ang-(1-7), the Ang-(1-7) Mas receptor antagonist [D-Ala7]-Ang-(1-7) (A-779), ISO, L-NGnitro-L-arginine methyl ester (L-NAME) and HOE 140 were obtained from Sigma Chemical Co. (St Louis, MO).
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical comparisons were performed with Student's t-test or analysis of variance (ANOVA). A p value of <0.05 was considered significant. When a significant overall effect was present, intergroup comparisons were performed using a Bonferroni correction for multiple comparisons. Two-tailed unpaired Student's t-tests were used to evaluate mean differences in hemodynamic parameters and ICa,L between the control and ISO-treated groups.
Results
Verification of experimental HF
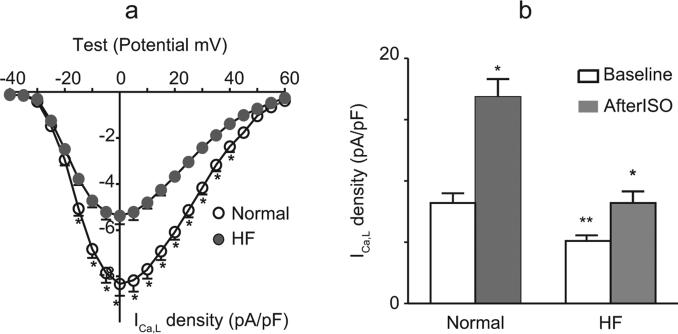
Of the 15 rats in the HF group, 11 survived after ISO injection. Characteristics of the general hemodynamic and ICa,L alterations in ISO-treated rats are summarized in Table 1. The basal and β-adrenergic response of peak ICa,L in normal and HF myocytes are exhibited in Figure 1.
Table 1.
Characteristics of general features of ISO-induced HF in adult rats.
| Normal |
HF |
|
|---|---|---|
| (N = 8) | (N = 11) | |
| Heart rate (beats/min) | 266 ± 8 | 270 ± 10 |
| Heart/body weight, g/kg | 3.37 ± 0.06 | 3.85 ± 0.09* |
| Lung/body weight, g/kg | 3.48 ± 0.09 | 4.21 ± 0.16* |
| LV SP (mmHg) | 113 ± 3 | 81 ± 4* |
| LV EDP (mmHg) | 5.7 ± 0.62 | 17.5 ± 1.1* |
| LV dp/dtmax (mmHg/s) | 7418 ± 411 | 4558 ± 261* |
| LV dp/dtmin (mmHg/s) | −6645 ± 359 | −3484 ± 325* |
| SV (μl) | 184 ± 16 | 99 ± 9* |
| LV EF, % | 62 ± 2 | 36 ± 2* |
| τ (ms) | 11.7 ± 0.53 | 21.4 ± 2.4* |
| Cm (pF) | 164 ± 13 (n = 31) | 278 ± 21* (n = 38) |
| ICa,L density (pA/pF) | 8.1 ± 0.3 (n = 31) | 5.0 ± 0.2* (n = 38) |
Values are mean ± SEM.
p < 0.01, HF versus normal rats (myocytes).
Cm, membrane capacitance; HF, ISO-induced heart failure model; LVSP, left ventricular systolic pressure; ICa,L, L-type calcium current; LV EDP, LV end diastolic pressure; LV dp/dtmax and LV dp/dtmin, maximum and minimum time derivative of LVP; LV EF, LV ejection fraction; N, number of animals; n, number of cells; SV, stroke volume; τ, time constant of LV pressure decay.
Figure 1.
Basal and β-adrenergic response of the peak ICa,L in normal and ISO-induced HF myocytes. (a) Current–voltage (I-V) relationships of normal and ISO-induced HF myocyte groups with −80 mV holding potential, 5 mV, 200 ms depolarization steps from −40 mV to 60 mV (with a prepulse of −45 mV for 60 ms) every 10 s. Values are mean ± standard error of the mean (SEM) (n = 8 of normal and n = 8 of HF); *p < 0.05, compared with normal. (b) Group means (± SEM) of baseline peak ICa,L and ISO (10−7 M) responses of peak ICa,L in normal and HF myocytes (n = 8 of normal and n = 8 of HF); p < 0.01, baseline versus ISO; **p < 0.01, the difference between normal and HF myocytes.
HF, heart failure; ICa,L, L-type calcium current; ISO, isoproterenol.
Compared with normal controls, LV end-diastolic pressure increased about 3-fold, and LV dp/dtmax and LV dp/dtmin were significantly decreased 3 months after ISO injection. The rate of LV relaxation slowed as indicated by a significant increase in the time constant of isovolumic LV pressure decay (τ, 183%; p <0.01).
All ISO-treated animals had clear evidence of HF (anorexia, edema and pulmonary congestion). In the ISO-injected rats, the total infarction area averaged 38 ± 11%. Diffuse subendocardial necrosis was also observed. There was no significant change in body weight (539 ± 22 g versus 531 ± 16 g, p > 0.05), whereas the heart weight (2.07 ± 0.09 g versus 1.26 ± 0.05 g, p < 0.01), calculated ratio of heart-to-body weight (3.85 ± 0.09 g/kg versus 3.37 ± 0.06 g/kg, p < 0.01) and calculated ratio of wet-lung-to-body weight (4.21 ± 0.16 g/kg versus 3.48 ± 0.09 g/kg, p < 0.01) were all significantly increased in ISO-injected rats. These findings demonstrated the existence of established HF in this model.
ICa,L electrophysiological characteristic in ISO-induced rat HF LV myocytes
In our present study, both in normal and HF groups, the recorded current was increased by Bay K 8644 (10−6 M) and blocked by nifedipine (5 × 10−6 M), consistent with the characteristics of ICa,L (data not shown). As shown in Figure 1 and Table 1, compared with that of normal rat myocyte groups, the I-V relationship did not shift in the HF group. However, in ISO-induced rat HF LV myocytes, the membrane capacitance was significantly increased and the current density was significantly lower than that of the normal myocytes (5.0 ± 0.2 versus 8.1 ± 0.3 pA/pF, p < 0.01), indicating an absolute reduction of ICa,L in HF. In addition, the response of ICa,L to β-adrenergic response stimulation in HF myocytes was significantly attenuated. In the normal myocytes, in response to the exposure to ISO (10−7M), ICa,L increased by about 116 ± 11%; p < 0.01, n = 8). However, in HF myocytes, ISO only caused an increase of about 59 ± 7% in ICa,L (p < 0.01, n = 8). These results demonstrated the existence of electrophysiological remodeling in our established ISO-induced HF models.
Effect of Ang-(1-7) on ICa,L in normal and HF myocytes
To determine the direct effects of Ang-(1-7) on ICa,L, myocytes were exposed to Ang-(1-7) (10−5 M). The dosing protocol of Ang-(1-7] (10−5 M) used in this study was chosen from our initial dosage response study, which showed a more than a 20-fold increase in plasma Ang-(1-7) levels, mimicking the elevations caused by ACEI in the established phase of hypertension in spontaneously hypertensive rats (SHR) [Iyer et al. 1998]. We reported that exposure to Ang-(1-7) (10−5 M) was well tolerated by both normal and HF myocytes, and caused nearly maximum increase in myocyte contractility of HF myocytes [Cheng et al. 2008]. Thus, the effects of Ang-(1-7) (10−5 M) were assessed in the following series of experiments.
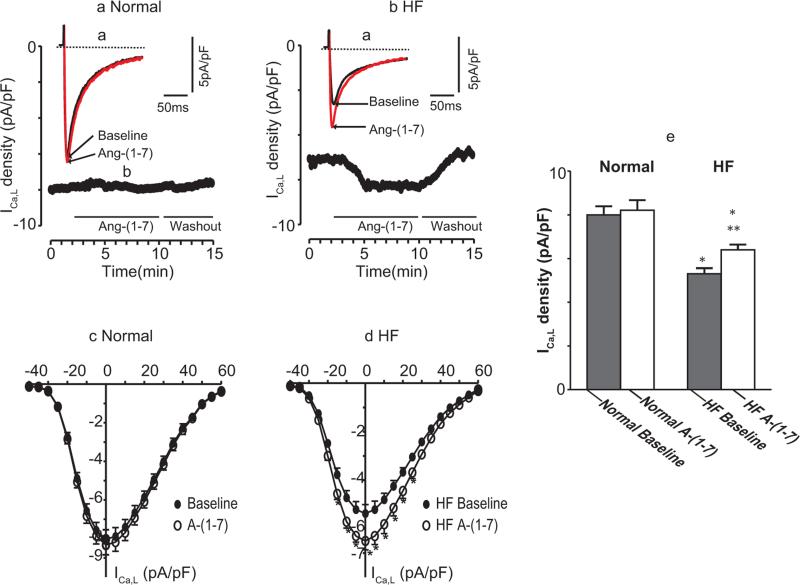
The effects of Ang-(1-7) on ICa,L in normal and ISO-induced HF myocytes are summarized in Table 2 and illustrated in Figure 2. Compared with normal myocyte baseline values, the membrane capacitance in HF myocytes was significantly increased while the baseline current density was significantly decreased (5.0 ± 0.1 versus 8.2 ± 0.2 pA/pF, p < 0.01). Superfusion of Ang-(1-7) (10−5 M) had no effect on peak ICa,L in normal myocytes [2.5%, 8.2 ± 0.2 versus 8.0 ± 0.3 pA/pF, p = not significant (NS), n = 18]. However, in HF myocytes, Ang-(1-7) caused significant increases in peak ICa,L (28%, 6.4 ± 0.1 versus 5.0 ± 0.1 pA/pF, p < 0.01, n = 32) (Figure 2A, B and E).
Table 2.
Effect of Ang-(1-7) on ICa,L of cardiomyocytes: normal versus HF.
| ICa,L density (pA/pF) |
% Changes of ICa,L |
||
|---|---|---|---|
| Baseline | Ang-(1-7) | % | |
| Normal (N = 8; n = 18) | 8.0 ± 0.3 | 8.2 ± 0.2 | 2.5 |
| HF (N = 11; n = 32) | 5.0 ± 0.1$ | 6.4 ± 0.1* | 28.2$ |
Values are mean ± SEM.
p < 0.01, Ang-(1-7) versus baseline.
p < 0.01, HF versus normal.
HF, heart failure; ICa,L, L-type calcium current; N, number of animals; n, number of cells.
Figure 2.
Effects of Ang-(1-7) on ICa,L in normal and ISO-induced HF myocytes. (a) Examples of original current traces were elicited by depolarization pulses from a holding potential of −80 mV to 0 mV for 200 ms (with a brief prepulse to −45 mV, 60 ms). Ventricular myocytes were initially superfused with external solution. After a stable steady peak ICa,L was recorded, Ang-(1-7) 10−5 M was superfused. The superimposed current tracings were recorded before and after exposure to Ang-(1-7). (b) The time course of peak ICa,L measured every 10 s and plotted as a function of time (in min). Horizontal bars below the graph indicate the time and duration of the application of Ang-(1-7). The voltage protocol was the same as in (a). (c) Current–voltage (I-V) relationship of ICa,L in the absence and presence of Ang-(1-7) in 8 normal myocytes. The cells were depolarized from a holding potential of −80 mV to a test potential from −40 to +60 mV in 5 mV step increments. (d) I-V relationship for 8 HF cells, measured under the same experimental conditions as in (c) * indicates that the difference between before and after drug is statistically significant (p < 0.05). (e). Group means of ICa,L responses to Ang-(1-7) in normal and HF myocytes (n = 10 and 26, respectively). Values are mean (± SEM); *p < 0.01 versus baseline; **p < 0.05, Ang-(1-7) versus baseline. The results showed that, in normal myocytes, Ang-(1-7) has no significant effect on ICa,L. In contrast, in HF myocytes, Ang-(1-7) caused significant increases in ICa,L.
HF, heart failure; ICa,L, L-type calcium current; SEM, standard error of the mean.
As shown in Figure 2B, after Ang-(1-7) superfusion, ICa,L of HF myocyte increased gradually and reached a steady peak level for about 2 min. This Ang-(1-7) stimulation, lasting for about 3–5 min, was reversed to baseline values after Ang-(1-7) washout. Figure 2C and Figure 2D show the I-V relationships of ICa,L at baseline and after Ang-(1-7) in normal and HF myocytes, respectively, at different depolarization potential levels. Ang-(1-7) caused no change in the voltage dependence of peak ICa,L amplitude in normal and HF myocytes. The stimulative effects of Ang-(1-7) did not persist after washout. To eliminate the influence of ‘runup’ or ‘rundown’, we used nystatin perforated patch recordings in a subgroup to examine the effect of A-(1-7) in separate experiments. A similar result was observed (data not shown), consistent with previous findings [Zhang et al. 2005].
Effects of the activation of NO synthase, BK and Ang-(1-7) Mas receptor on ICa,L responses to Ang-(1-7) of HF myocytes
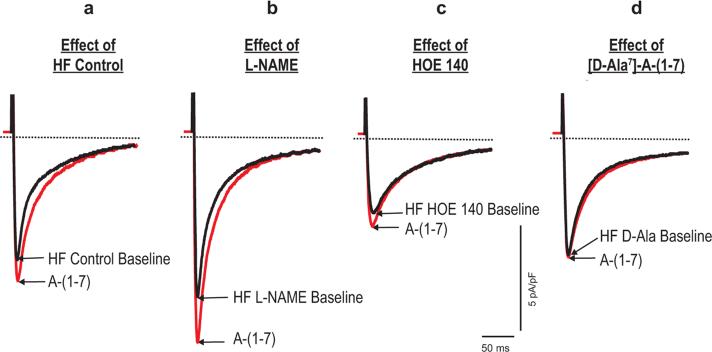
To determine the potential mechanism of Ang-(1-7) induced altered ICa,L response in HF, 3 subsets of HF myocytes from these animals were pretreated with the NO synthase inhibitor (L-NAME, 10−5 M), a BK receptor antagonist (HOE 140,10−6 M) or the Ang-(1-7) Mas receptor antagonist (A-779, 10−5 M) [Maia et al. 2004], respectively, followed by Ang-(1-7) (10−5 M) superfusion. Figures 3 to 5 display the example and group means of the effects of L-NAME, HOE 140 and A-779 on Ang-(1-7) induced changes of ICa,L in HF myocytes.
Figure 3.
Examples of the effects of L-NAME, HOE 140 and Ang-(1-7) blockade on Ang-(1-7) induced changes of ICa,L in HF myocytes. (a) Effect of HF control. A HF myocyte was initially superfused with external solution. After stable steady peak ICa,L recorded, then Ang-(1-7) 10−5 M was continuously superfused. ICa,L was elicited by depolarization pulses from a holding potential of −80 mV to 0 mV for 200 ms (with a brief prepulse to −45 mV, 60 ms). The superimposed current tracings were recorded before and 4 min after exposure to Ang-(1-7). (b) Effect of L-NAME. Different HF myocytes were pre-incubated with L-NAME (10−5 M) for 30 min. The voltage protocol was the same as in (a). Superimposed current tracings recorded before and 4 min after exposure to Ang-(1-7). (c) Effect of HOE 140. Other subsets of HF myocytes were pre-incubated with HOE 140 (10−6 M) for 20 min. Then Ang-(1-7) voltage protocol was repeated, and superimposed current tracings were recorded before and 4 min after exposure to Ang-(1-7). (d) Effect of Ang-(1-7) blockade. HF myocytes were pre-incubated with A-779 (10−5 M) for 20 min. The same voltage protocol as in (a) was used. Superimposed current tracings were recorded before and 4 min after exposure to A-(1-7).
HF, heart failure; ICa,L, L-type calcium current.
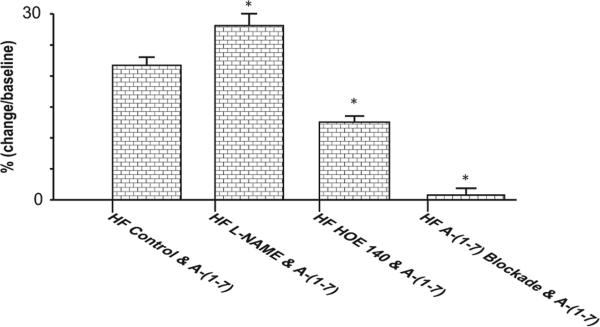
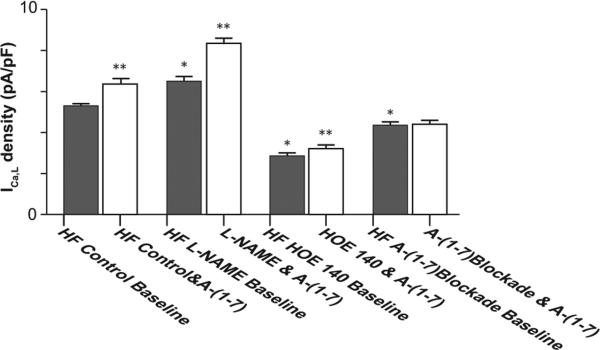
Figure 5.
Group means of Ang-(1-7) induced percentage changes in ICa,L of HF myocytes with and without pretreatment with L-NAME, HOE 140 or A-779, respectively; L-NAME increased, HOE 140 decreased, and A-779 prevented A-(1-7) induced percentage increases in ICa,L.
* p<0.01 versus HF control and Ang-(1-7).
HF, heart failure; ICa,L, L-type calcium current.
Effect of NOS blockade
In the first series of studies, myocytes were pre-incubated with L-NAME (10−5 M) for 30 min, followed by Ang-(1-7) (10−5 M) superfusion. As summarized in Figure 3, pretreatment of myocytes with L-NAME increased the baseline ICa,L (6.5 ± 0.2 versus 5.1 ± 0.1 pA/pF, p < 0.01, n = 16) and significantly altered myocyte ICa,L response to Ang-(1-7) (Figure 4). In untreated HF myocytes, Ang-(1-7) caused a 21% increases of ICa,L (Table 2 and Figure 4). In HF myocytes pretreated with L-NAME, Ang-(1-7) caused a 28% significantly greater increase in ICa,L (8.4 ± 0.1 versus 6.5 ± 0.1 pA/pF, p < 0.01) (Figure 5).
Figure 4.
Group means of the effects of L-NAME, HOE 140 and A-779 on Ang-(1-7) induced changes of ICa,L in HF myocytes. In HF myocytes pretreated with L-NAME, the baseline ICa,L and A-(1-7) induced increase in ICa,L were increased. In contrast, with incubation of HOE 140, the baseline ICa,L and Ang-(1-7) induced increase in ICa,L were significantly decreased. Ang-(1-7) blockade after myocyte incubation with A-779: the baseline ICa,L was decreased and Ang-(1-7) induced increase in ICa,L was abolished.
*p <0.01, drug incubation baseline versus HF control baseline; **p < 0.01, Ang-(1-7) versus corresponding baseline.
HF, heart failure; ICa,L, L-type calcium current.
Effect of HOE 140
In the second series of studies, HF myocytes were pre-incubated with a BK inhibitor, HOE 140 (10−6 M) for 20 min and then exposed to 10−5 M Ang-(1-7). As shown in Figures 3 to 5, pre-incubation of HF myocytes with HOE 140 elicited a significant decrease in baseline ICa,L (2.9 ± 0.1 pA/pF in HF myocytes versus 5.3 ± 0.1 pA/pF in vehicle-treated myocytes, p < 0.01, n = 12 and 26, respectively) as well as the Ang-(1-7) induced increase in ICa,L (12.2%, 2.9 ± 0.1 versus 3.3 ± 0.2 pA/pF, p < 0.05, n = 12).
Effect of Ang-(1-7) Mas receptor blockade
In the third series of studies, HF myocytes were pre-incubated with an Ang-(1-7) Mas receptor antagonist, A-779 (10−5 M) for 20 min, followed by Ang-(1-7) (10−5 M) superfusion. As shown in Figures 3 to 5, compared with unincubated cells, pre-incubation with A-779 decreased baseline values of peak ICa,L of HF myocytes (4.3 ± 0.1 versus 5.3 ± 0.1 pA/pF, p < 0.05, n = 15 and 26, respectively). Moreover, Ang-(1-7) induced increase in ICa,L of HF myocytes was abolished by the incubation of myocytes with A-779.
Discussion
Our study, for the first time, demonstrates that HF alters the response of ICa,L to Ang-(1-7). In normal myocytes, Ang-(1-7) has no significant effect on ICa,L. However, in HF myocytes, Ang-(1-7) increases ICa,L. These effects are mediated through the Mas receptors and involve activation of NO/BK pathways. This study provides new insights into the underlying electrophysiological mechanism of the beneficial function effects of Ang-(1-7) in HF.
ICa,L electrophysiological remodeling in ISO-induced rat HF LV myocytes
To further characterize the mechanisms of ISO-induced HF, we compared the membrane capacitance, I-V relationship and the ICa,L response to β-adrenergic stimulation. The membrane capacitance of myocytes from HF rats was increased due to myocyte hypertrophy [Ming et al. 1994; Zhang et al. 2005]. The ICa,L current density of ISO-treated rat myocytes was significantly lower than that of normal control rat myocytes, indicating an absolute reduction of ICa,L in the ISO-induced HF model. Our finding of a reduced ICa,L density of HF in the present study is in agreement with past reports [Ming et al. 1994; Santos et al. 1995]. In agreement with another study [Rossner, 1991], the I-V relationship did not shift significantly in ISO-induced HF myocytes. In addition, in ISO-treated rat myocytes, ISO (10−7M) super-fusion caused less increase in ICa,L, indicating an attenuated response to β-adrenergic stimulation. These observations are consistent with previous findings in congestive HF hamster, rat and pig models [Hatem et al. 1994]. The abnormalities of ICa,L we observed in the ISO-treated rat myocytes were also similar to those reported in myocytes from patients with HF [Ouadid et al. 1995]. These findings demonstrate cardiac ICa,L electro-physiological remodeling in ISO-induced HF.
Direct effects and possible mechanism of Ang-(1-7) on ICa,L in normal and HF
It is increasingly recognized that Ang-(1-7) is an important component of the functional cardiac RAS. ACE2 and Ang-(1-7) Mas receptor coexist in cardiomyocytes. The heart is an important target for the formation and action of Ang-(1-7), and both ACE and Ang-(1-7) are present in the heart tissue. Recent evidence indicates that Ang-(1-7)/ACE2/Mas axis plays a key role in the maintenance of the structure and function of the heart [Averill et al. 2003; De Mello et al. 2007; De Mello, 2009; Ferreira et al. 2001; Loot et al. 2002; Marques et al. 2011; Santos et al. 2006; Stewart et al. 2008]. However, the exact means by which Ang-(1-7) affords cardioprotection are unclear. Potential mechanisms include increased Ang II degradation by ACE2 and increased Ang-(1-7) formation or activity. The relative contribution of decreased Ang II levels versus increased Ang-(1-7) is difficult to decipher when ACE2 levels are manipulated in diseased states [Ferrario, 2011] or when RAS is pharmacologically blocked [Zisman et al. 2003]. To circumvent this issue, many studies have used chronic Ang-(1-7) treatment or infusion. For example, Santos and colleagues [Santos et al. 2006] first showed that increases in circulating Ang-(1-7) levels in transgenic rats protected against ISO induced HF. Ang-(1-7) treatment improves myocardial performance and survival in SHR rats following ischemia/reperfusion injury [Averill et al. 2003]. Ang-(1-7) preserved cardiac function, coronary perfusion and aortic endothelial function in a rat model for HF [Loot et al. 2002]. In line with these results, ACE2 knockout mice showed severe cardiac contractile dysfunction associated with ventricular dilatation [Crackower et al. 2002]. Isolated hearts of Mas-deficient mice presented a severely impaired heart function with lower systolic tension, increased dimensions of LV, and a slower postischemic cardiac recovery [Santos et al. 2006]. An oral formulation of Ang-(1-7) produced beneficial effects in infarcted and ISO-treated rats [Marques et al. 2011]. It must be recognized, however, that although these studies established a cardioprotective role for Ang-(1-7), they did not demonstrate the direct effects of this peptide on the heart.
To obviate the limitations from previous investigations, we assessed in the present study the direct effect of Ang-(1-7) in freshly-isolated cardiomyocytes obtained from normal control and HF rats. We clearly show that superfusion of Ang-(1-7) (10−5 M) caused no significant changes in peak ICa,L in normal myocytes. In contrast, Ang-(1-7) caused a significantly greater increase in ICa,L in HF myocytes. This effect was completely abolished by Ang-(1-7) Mas receptor antagonist, A-779, indicating that the positive modulation of ICa,L following Ang-(1-7) superfusion was mediated by the specific G protein-coupled receptor, Mas. This observation is in keeping with several previous studies suggesting the participation of the Mas receptor in Ang-(1-7) cardioprotection [Marques et al. 2011; Santos et al. 2006; Tallant et al. 2005]. This finding correlated well with our past report demonstrating that, compared with normal controls, only after HF, Ang-(1-7) (10−5 M) produce positive inotropic effects in both LV and myocytes [Cheng et al. 2008].
As yet, only few groups have studied the effect of Ang-(1-7)/ACE2/Mas on ICa,L response of cardiomyocyte. However, our finding of the direct effect of Ang-(1-7) stimulated ICa,L responses in normal and in HF should be compared to previous studies. Santos and colleagues [Santos et al. 2006] studied age-matched wildtype and Mas receptor knockout C57BL/6 mice. They reported no significant differences between ICa,L current density, but found that the time to peak ICa,L current was statistically different between the two mice groups [Santos et al. 2006]. Their failure to observe significant differences of ICa,L current density between wildtype control and Mas receptor knockout mice may be attributed to the fact that in the normal heart the local expression level of Mas receptor and Ang-(1-7) are much lower than those under the pathological condition [Averill et al. 2003]. By using a rapid atrial pacing canine HF model, Liu and colleagues found that, after 14 days of atrial pacing, the atrial myocyte ICa,L in paced + Ang-(1-7) group was significantly higher than that of the paced group and there was no significant difference between the paced + Ang-(1-7) and the sham groups [Liu et al. 2011]. Although these previous studies did not address the direct cardiac effect of Ang-(1-7) on ICa,L, both our study and other studies demonstrate that Ang-(1-7) plays an important modulatory role in cardiac tissue calcium homeostasis.
A novel finding in the present study is that the positive modulation of ICa,L with Ang-(1-7) was enhanced after HF. In HF myocytes, the basal ICa,L was significantly decreased, and its response to β-adrenergic response stimulation (ISO, 10−7 M) was also markedly blunted (59% versus 116% increment; p < 0.01). However, the stimulatory response to Ang-(1-7) (10−5 M) was significantly enhanced (21% versus 3%; p < 0.01).
The mechanism(s) for the Ang-(1-7) induced enhanced increase in ICa,L for HF cells versus normal cells is unclear. We speculate that an increase in Ang-(1-7) Mas receptor density on the membrane of HF cells may contribute to our current findings. It has been demonstrated that upregulation of ACE2 with increased A-(1-7) forming activity occurred in failing human myocardium [Zisman et al. 2003]. Averill and colleagues [Averill et al. 2003] showed that development of HF subsequent to coronary artery ligation leads to increased expression of Ang-(1-7), which was restricted to myocytes. Although the Ang-(1-7) receptor density per myocyte has not been measured in HF previously, it is possible that the enhanced increased ICa,L in LV myocytes of rats with HF may reflect the presence of an increase in the number of Ang-(1-7) Mas receptor per cell. Further studies are currently underway to elucidate this point.
The enhanced ICa,L response to Ang-(1-7) stimulation in HF may also be related to an altered signal transduction. Although, the intracellular pathway coupling Ang-(1-7) stimulation is incompletely characterized, it has been reported that an important subset of cardiovascular actions of Ang-(1-7) is related to its BK potentiating activity and its facilitation of NO release [Brosnihan et al. 1996; Pinheiro et al. 2004; Wiemer et al. 2002; Yuill et al. 2010]. Mas receptors localized to cardiac myocytes may activate NO production or stimulate BK [Almeida et al. 2000; Carvalho et al. 2007; Ferreira et al. 2001]. Consistent with previous studies, we observed the involvement of the NO and BK pathways in the altered ICa,L response to Ang-(1-7) in HF. We found that, in HF myocytes after pretreatment with L-NAME, the Ang-(1-7) induced increase in ICa,L was significantly increased. In contrast, after incubation with HOE 140, Ang-(1-7) caused increase in ICa,L of HF myocyte was significantly decreased. These findings indicate that NOS activation attenuates, but BK activation potentiates Ang-(1-7) induced positive modulation on ICa,L in HF. Thus HF may change NOS or BK expression and function, contributing to altered ICa,L response to Ang-(1-7) in HF. It is likely that upregulation of cardiac Ang-(1-7)/ACE2/Mas-mediated pathways may be responsible for the enhanced Ang-(1-7) induced positive modulation of ICa,L in HF. However, the exact contributions of upregulation of cardiac Ang-(1-7) Mas receptor versus altered NO or BK mediated pathways are unclear. Furthermore, HF may modify receptor crosstalk among AT1, AT2, Mas and Mas receptor sub-types, thus changing Ang-(1-7) mediated cardiac functional response. For example, AT2 mediated actions often counteract those elicited by activation of AT1 receptors [Carey and Siragy, 2003] and possibly involve interaction with the Ang-(1-7) Mas receptor [Pinheiro et al. 2004; Silva et al. 2007]. Further studies are needed to characterize fully the intracellular pathway coupling Ang-(1-7) Mas receptor stimulation.
Conclusion
In conclusion, we found that HF alters the response of ICa,L to Ang-(1-7). In normal myocytes, Ang-(1-7) has no significant effect on ICa,L. However, in HF myocytes, Ang-(1-7) increases ICa,L. These effects are coupled with Ang-(1-7) Mas receptors and involve activation of NO/BK pathways. The current study provides new insight into the electrophysiological mechanism for Ang-(1-7) induced improvement in cardiac contractile performance in HF, and continues to support the original hypothesis by Ferrario and colleagues [Ferrario et al. 1997] that the counter-regulatory Ang-(1-7) actions will occur primarily in conditions in which Ang II activity is enhanced.
Acknowledgements
The authors acknowledge the administrative assistance of Stacey Belton.
Funding
This work was supported in part by the National Institutes of Health (HL074318) and American Heart Association Grant-in-Aid (11GRNT7240020).
Footnotes
αAbstract presented at American Heart Association Meeting
Conflict of interest statement
The authors declare no conflicts of interest in preparing this article.
Contributor Information
Peng Zhou, Section on Cardiovascular Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Che Ping Cheng, Section on Cardiovascular Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Tiankai Li, Section on Cardiovascular Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA; Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China.
Carlos M. Ferrario, Department of Surgery, Internal Medicine-Nephrology, and Physiology-Pharmacology, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Heng-Jie Cheng, Section on Cardiovascular Medicine, Wake Forest School of Medicine, Winston-Salem, NC 27157-1045, USA; Institute for Regenerative Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA.
References
- Almeida A, Frabregas B, Madureira M, Santos R, Campagnole-Santos M, Santos R. Angiotensin-(1–7) potentiates the coronary vasodilatatory effect of bradykinin in the isolated rat heart. Braz J Med Biol Res. 2000;33:709–713. doi: 10.1590/s0100-879x2000000600012. [DOI] [PubMed] [Google Scholar]
- Averill D, Ishiyama Y, Chappell M, Ferrario C. Cardiac angiotensin-(1–7) in ischemic cardiomyopathy. Circulation. 2003;108:2141–2146. doi: 10.1161/01.CIR.0000092888.63239.54. [DOI] [PubMed] [Google Scholar]
- Brosnihan K, Li P, Ferrario C. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- Carey R, Siragy H. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Duarte F, Faria-Silva R, Fauler B, da Mata Machado L, de Paula R, et al. Evidence for Mas-mediated bradykinin potentiation by the angiotensin-(1–7) nonpeptide mimic AVE 0991 in normotensive rats. Hypertension. 2007;50:762–767. doi: 10.1161/HYPERTENSIONAHA.107.094987. [DOI] [PubMed] [Google Scholar]
- Cheng C, Suzuki M, Ohte N, Ohno M, Wang Z, Little W. Altered ventricular and myocyte response to angiotensin II in pacing-induced heart failure. Circ Res. 1996;78:880–892. doi: 10.1161/01.res.78.5.880. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zhou P, Cheng H, Cross M, Little W. Direct cardiac effect and cellular mechanism of angiotensin-(1–7) in heart failure. Circulation. 2008;118(Suppl. 2):abstract S513. [Google Scholar]
- Crackower M, Sarao R, Oudit G, Yagil C, Kozieradzki I, Scanga S, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- De Mello W. Angiotensin (1–7) re-establishes impulse conduction in cardiac muscle during ischaemia-reperfusion. The role of the sodium pump. J Renin Angiotensin Aldosterone Syst. 2004;5:203–208. doi: 10.3317/jraas.2004.041. [DOI] [PubMed] [Google Scholar]
- De Mello W. Renin angiotensin system as a regulator of cell volume. Implications to myocardial ischemia. Curr Cardiol Rev. 2009;5:65–68. doi: 10.2174/157340309787048149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W, Ferrario C, Jessup J. Beneficial versus harmful effects of angiotensin (1–7) on impulse propagation and cardiac arrhythmias in the failing heart. J Renin Angiotensin Aldosterone Syst. 2007;8:74–80. doi: 10.3317/jraas.2007.015. [DOI] [PubMed] [Google Scholar]
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Ferrario C. ACE2: more of Ang-(1–7) or less Ang II? Curr Opin Nephrol Hypertens. 2011;20:1–6. doi: 10.1097/MNH.0b013e3283406f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C, Ahmad S, Joyner J, Varagic J. Advances in the renin angiotensin system focus on angiotensin-converting enzyme 2 and angiotensin-(1–7). Adv Pharmacol. 2010;59:197–233. doi: 10.1016/S1054-3589(10)59007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C, Chappell M, Tallant E, Brosnihan K, Diz D. Counterregulatory actions of angiotensin-(1–7). Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- Ferrario C, Jaiswal N, Yamamoto K, Diz D, Schiavone M. Hypertensive mechanisms and converting enzyme inhibitors. Clin Cardiol. 1991;14:IV56–IV62. doi: 10.1002/clc.4960141809. [DOI] [PubMed] [Google Scholar]
- Ferrario C, Martell N, Yunis C, Flack J, Chappell M, Brosnihan K, et al. Characterization of angiotensin-(1–7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11:137–146. doi: 10.1016/s0895-7061(97)00400-7. [DOI] [PubMed] [Google Scholar]
- Ferrario C, Smith R, Brosnihan B, Chappell M, Campese V, Vesterqvist O, et al. Effects of omapatrilat on the renin-angiotensin system in salt-sensitive hypertension. Am J Hypertens. 2002;15:557–564. doi: 10.1016/s0895-7061(02)02268-9. [DOI] [PubMed] [Google Scholar]
- Ferrario C, Varagic J. The ANG-(1–7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Renal Physiol. 2010;298:F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Santos R, Almeida A. Angiotensin-(1–7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001;38:665–668. doi: 10.1161/01.hyp.38.3.665. [DOI] [PubMed] [Google Scholar]
- Hamill O, Marty A, Neher E, Sakmann B, Sigworth F. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hatem S, Sham J, Morad M. Enhanced Na(+)-Ca2+ exchange activity in cardiomyopathic Syrian hamster. Circ Res. 1994;74:253–261. doi: 10.1161/01.res.74.2.253. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Ferrario C, Chappell M. Angiotensin-(1–7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension. 1998;31:356–361. doi: 10.1161/01.hyp.31.1.356. [DOI] [PubMed] [Google Scholar]
- Liu E, Xu Z, Li J, Yang S, Yang W, Li G. Enalapril, irbesartan, and angiotensin-(1–7) prevent atrial tachycardia-induced ionic remodeling. Int J Cardiol. 2011;146:364–370. doi: 10.1016/j.ijcard.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Loot A, Roks A, Henning R, Tio R, Suurmeijer A, Boomsma F, et al. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- Luque M, Martin P, Martell N, Fernandez C, Brosnihan K, Ferrario C. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1–7) in essential hypertension. J Hypertens. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- Maia L, Ramos M, Fernandes L, de Carvalho M, Campagnole-Santos M, Souza dos Santos R. Angiotensin-(1–7) antagonist A-779 attenuates the potentiation of bradykinin by captopril in rats. J Cardiovasc Pharmacol. 2004;43:685–691. doi: 10.1097/00005344-200405000-00011. [DOI] [PubMed] [Google Scholar]
- Marques F, Ferreira A, Sinisterra R, Jacoby B, Sousa F, Caliari M, et al. An oral formulation of angiotensin-(1-7) produces cardioprotective effects in infarcted and isoproterenol-treated rats. Hypertension. 2011;57:477–483. doi: 10.1161/HYPERTENSIONAHA.110.167346. [DOI] [PubMed] [Google Scholar]
- Ming Z, Nordin C, Siri F, Aronson R. Reduced calcium current density in single myocytes isolated from hypertrophied failing guinea pig hearts. J Mol Cell Cardiol. 1994;26:1133–1143. doi: 10.1006/jmcc.1994.1132. [DOI] [PubMed] [Google Scholar]
- Ouadid H, Albat B, Nargeot J. Calcium currents in diseased human cardiac cells. J Cardiovasc Pharmacol. 1995;25:282–291. doi: 10.1097/00005344-199502000-00014. [DOI] [PubMed] [Google Scholar]
- Pfeffer M, Pfeffer J, Fishbein M, Fletcher P, Spadaro J, Kloner R, et al. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- Pinheiro S, Simoes e Silva A, Sampaio W, de Paula R, Mendes E, Bontempo E, et al. Nonpeptide AVE 0991 is an angiotensin-(1–7) receptor Mas agonist in the mouse kidney. Hypertension. 2004;44:490–496. doi: 10.1161/01.HYP.0000141438.64887.42. [DOI] [PubMed] [Google Scholar]
- Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985;17:291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- Rossner K. Calcium current in congestive heart failure of hamster cardiomyopathy. Am J Physiol. 1991;260:H1179–H1186. doi: 10.1152/ajpheart.1991.260.4.H1179. [DOI] [PubMed] [Google Scholar]
- Santos P, Barcellos L, Mill J, Masuda M. Ventricular action potential and L-type calcium channel in infarct-induced hypertrophy in rats. J Cardiovasc Electrophysiol. 1995;6:1004–1014. doi: 10.1111/j.1540-8167.1995.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Santos R, Castro C, Gava E, Pinheiro S, Almeida A, Paula R, et al. Impairment of in vitro and in vivo heart function in angiotensin-(1–7) receptor MAS knockout mice. Hypertension. 2006;47:996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- Santos R, Simoes e Silva A, Maric C, Silva D, Machado R, de Buhr I, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Bramlage P, Kirch W, Ferrario C. Role of the vasodilator peptide angiotensin-(1–7) in cardiovascular drug therapy. Vasc Health Risk Manag. 2007;3:125–137. [PMC free article] [PubMed] [Google Scholar]
- Silva D, Vianna H, Cortes S, Campagnole-Santos M, Santos R, Lemos V. Evidence for a new angiotensin-(1–7) receptor subtype in the aorta of Sprague-Dawley rats. Peptides. 2007;28:702–707. doi: 10.1016/j.peptides.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Stewart J, Jr., Lazartigues E, Lucchesi P. The angiotensin converting enzyme 2/Ang-(1–7) axis in the heart: a role for MAS communication? Circ Res. 2008;103:1197–1199. doi: 10.1161/CIRCRESAHA.108.189068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Ohte N, Wang Z, Williams D, Jr., Little W, Cheng C. Altered inotropic response of endothelin-1 in cardiomyocytes from rats with isoproterenol-induced cardiomyopathy. Cardiovasc Res. 1998;39:589–599. doi: 10.1016/s0008-6363(98)00166-7. [DOI] [PubMed] [Google Scholar]
- Tallant E, Ferrario C, Gallagher P. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the Mas receptor. Am J Physiol Heart Circ Physiol. 2005;289:H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- Teerlink J, Pfeffer J, Pfeffer M. Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circ Res. 1994;75:105–113. doi: 10.1161/01.res.75.1.105. [DOI] [PubMed] [Google Scholar]
- Wiemer G, Dobrucki L, Louka F, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1–7) on the endothelium. Hypertension. 2002;40:847–852. doi: 10.1161/01.hyp.0000037979.53963.8f. [DOI] [PubMed] [Google Scholar]
- Yuill K, McNeish A, Kansui Y, Garland C, Dora K. Nitric oxide suppresses cerebral vasomotion by sGC-independent effects on ryanodine receptors and voltage-gated calcium channels. J Vasc Res. 2010;47:93–107. doi: 10.1159/000235964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cheng H, Onishi K, Ohte N, Wannenburg T, Cheng C. Enhanced inhibition of L-type Ca2+ current by beta3-adrenergic stimulation in failing rat heart. J Pharmacol Exp Ther. 2005;315:1203–1211. doi: 10.1124/jpet.105.089672. [DOI] [PubMed] [Google Scholar]
- Zisman L, Keller R, Weaver B, Lin Q, Speth R, Bristow M, et al. Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]