Abstract
Purpose
To assess the incidence of clinical allergy and end-Induction anti-asparaginase antibodies in children with high-risk acute lymphoblastic leukemia treated with pegylated E. coli asparaginase (PEG ASNase) and determine if they carry any prognostic significance.
Patients and Methods
Of 2057 eligible patients, 1155 patients were allocated to “augmented” arms where PEG ASNase replaced native ASNase post-Induction. Erwinia ASNase could replace native ASNase after allergy, if available. Allergy and survival data were complete for 990 patients. End-Induction antibody titers were available for 600 patients.
Results
During Consolidation, 29.2% (289/990 patients) had an allergic reaction. There were less allergic reactions to Erwinia ASNase than native ASNase (OR=4.33; p<0.0001) or PEG ASNase (OR=3.08; p<0.0001) during Interim Maintenance #1 only. There was no significant difference in 5-year EFS between patients who received PEG ASNase throughout the entire study post-Induction versus those who developed an allergic reaction to PEG ASNase during Consolidation and received Erwinia ASNase subsequently (80.8 ± 2.8% and 81.6 ± 3.8% (p=0.66), respectively). Patients with positive antibody titers post-Induction were more likely to have an allergic reaction to PEG ASNase (OR=2.4; p<0.001). Neither the 5-year EFS between patients with a negative versus positive antibody titer (80 ± 2.6% and 77.7 ± 4.3%, respectively, p=0.68) nor patients who did not receive any asparaginase post-Consolidation and patients who received PEG ASNase throughout the study (p=0.22) were significantly different.
Conclusion
We demonstrate differences in the incidence rates of toxicity between asparaginase preparations, but not in EFS. The presence of anti-asparaginase antibodies did not affect EFS.
Keywords: childhood acute lymphoblastic leukemia, asparaginase, drug hypersensitivity, survival
Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer in children. Currently, cure rates surpass 85%.(1) Asparaginase (ASNase) is an enzyme that catalyzes the hydrolysis of the amino acid asparagine into aspartic acid and ammonia and has been a key chemotherapeutic agent in the treatment of ALL for over 40 years. The first use of asparaginase in treating children with ALL was reported in 1966 and with asparaginase derived from Escherichia coli in 1967.(2-4) It has been a mainstay of therapy ever since.
Despite the vast experience with ASNase, questions remain regarding the optimal preparation, dose, and dosing schedule. L-asparaginase comes from two bacterial sources, Escherichia coli (native ASNase, Elspar, Lundbeck, Deerfield, IL) and Erwinia chrysanthemi (Erwinia ASNase, Erwinase, Speywood Laboratories, Maidenhead, United Kingdom). Pegylation of native ASNase (PEG ASNase, Oncaspar, Sigma-Tau, Gaithersburg, MD) decreases proteolysis, increases drug half-life, and decreases immunogenicity with at least equivalent efficacy at an appropriate dose and schedule.(5, 6) Erwinia ASNase has been found to have less toxicity but inferior efficacy when used with the same dose and schedule as native ASNase in two large multi-institutional cooperative group trials, which is believed to be at least partly due to the shorter half-life of Erwinia ASNase.(7, 8)
We report herein the experience for patients treated on arms containing PEG ASNase on CCG-1961 (Treatment of Patients with Acute Lymphoblastic Leukemia with Unfavorable Features). We describe associations of allergic reactions and anti-asparaginase antibodies as well as different preparations of asparaginase with event-free survival (EFS). This represents the largest cohort of PEG ASNase-treated patients reported to date.
Patients and Methods
Between November 1996 and May 2002, 2057 eligible patients with newly diagnosed high-risk ALL (HR-ALL) were enrolled onto CCG-1961. The patient characteristics of this cohort and treatment details have been previously published.(9) High-risk status was determined by standard National Cancer Institute (NCI) criteria and included patients who were either over 10 years old or had a white blood cell count greater than 50,000 cells/μL. All patients were treated with a standard four-drug Induction that included native ASNase given intramuscularly (IM). Patients with <25% blasts on Day 7 bone marrow aspirate were considered rapid early responders (RER) and randomized to either standard intensity and standard duration (Arm A), standard intensity and increased duration (Arm B), increased intensity and standard duration (Arm C), and increased intensity and increased duration (Arm D) of post-Induction therapy. All slow early responders (SER, Day 7 marrow with ≥25% blasts) received increased intensity and increased duration post-Induction intensification and were additionally randomized to receive cyclophosphamide and either doxorubicin or idarubicin. Patients randomized to increased intensity arms were respectively assigned 6 or 10 doses of PEG ASNase post-Induction (n=1155) given IM. The NCI and the Institutional Review Boards of the participating institutions approved this protocol. Informed consent was obtained from the patients, their parents, or both prior to starting therapy.
Of 2057 eligible patients enrolled on CCG-1961, 1067 patients were excluded from analyses for the following reasons: Philadelphia chromosome positive, missing reaction status data, or randomized to standard intensity arms. Data were complete for 990 patients treated on PEG ASNase containing arms, who received single monthly doses of PEG ASNase (2500 IU/m2/dose) during post-Induction courses prior to Maintenance therapy. Native ASNase (6000 IU/m2/dose × 6 per single dose PEG ASNase) was given when PEG ASNase was unavailable. Patients with adverse reactions to PEG or native ASNase received Erwinia ASNase (10,000 IU/m2/dose every other day × 6 doses per dose of PEG ASNase).
Institutions were required to submit details for all patients experiencing a reaction to any form of ASNase as defined by the Children's Cancer Group Toxicity and Complications Criteria, which is based on the NCI Common Toxicity Criteria. Per protocol, patient serum samples were collected at the end of Induction, and prior to Delayed Intensification (DI) #1 and Maintenance for anti-ASNase antibody determination. The methodology and definitions of positive and negative antibody titers have been previously published.(10)
First, we analyzed the allergic reaction incidence rates to the three different forms of ASNase for patients enrolled on PEG ASNase containing arms (Aim 1). Then, allergic reactions to PEG ASNase were correlated with the presence or absence of end-Induction anti-ASNase antibodies (Aim 2). Since patients with a high anti-ASNase antibody titer were thought to be more likely to react to PEG ASNase exposure during Consolidation, a sub-analysis was performed to examine patients with positive end-Induction antibodies and allergic reactions to PEG ASNase limited to Consolidation only. Next, end-Induction anti-ASNase antibodies were correlated with EFS (Aim 3). In order to determine whether patients who developed a severe allergic reaction to PEG ASNase could be substituted with Erwinia ASNase, we compared the EFS of patients receiving PEG ASNase throughout the study without an allergic reaction to patients who developed an allergic reaction to PEG ASNase during Consolidation and were subsequently substituted with Erwinia ASNase (Aim 4). Last, since a significant minority of patients did not receive any ASNase after Consolidation due to a severe adverse event, EFS of patients receiving PEG ASNase throughout the study was compared to patients who received PEG ASNase during Consolidation only and did not receive any form of ASNase thereafter (Aim 5).
Statistical methods
Study data for outcome analyses were frozen March 28, 2008. All analyses were restricted to patients enrolled on arms containing PEG ASNase on CCG-1961. Event-free survival was defined as the time from randomization to first event (failure to achieve remission, relapse, second malignancy, or death) or last contact. Estimates for EFS were calculated using the Kaplan-Meier method and standard errors of the estimates were obtained by the method of Peto and Peto.(11) The log-rank test was used to compare survival curves among groups. Odds ratios and their 95% CI were estimated. Chi-square tests were used to analyze categorical variables. Statistical significance was defined as p<0.05. All analyses were performed using SAS® software. All graphics were generated using R (http://www.R-project.org, version 2.8.1).
Results
Incidence of Allergic Reactions by Phase
The total number of patients who received three different forms of ASNase and the incidence of allergic reactions during each phase is summarized in Table 1. The likelihood of allergic reactions was similar with PEG ASNase, native ASNase, and Erwinia ASNase during all phases of therapy with the exception of Interim Maintenance (IM) #1, where the incidence of an allergic reaction to Erwinia ASNase was less likely than that for both native ASNase (OR=0.23; p<0.0001) and PEG ASNase (OR=0.32; p<0.0001) (See Table 2).
Table 1. Number of patients for each course by reaction status, n(%).
| Course of Therapy | Erwinia ASNase | PEG ASNase | Native ASNase | Total | |||
|---|---|---|---|---|---|---|---|
| No Reaction | Allergic Reaction | No Reaction | Allergic Reaction | No Reaction | Allergic Reaction | ||
| Consolidation | 2 (66.7) | 1 (33.3) | 642 (71.4) | 257 (28.6) | 57 (64.8) | 31 (35.2) | 990 |
| IM1 | 216 (91.9) | 19 (8.1) | 487 (78.7) | 132 (21.3) | 63 (72.4) | 24 (27.6) | 941 |
| DI1 | 301 (94.7) | 17 (5.4) | 457 (95.2) | 23 (4.8) | 61 (96.8) | 2 (3.2) | 861 |
| IM2 | 195 (92.4) | 16 (7.6) | 277 (89.6) | 32 (10.4) | 37 (100) | 0 (0) | 557 |
| DI2 | 189 (97.4) | 5 (2.6) | 270 (98.2) | 5 (1.8) | 32 (94.1) | 2 (5.9) | 503 |
Note: ASNase=asparaginase, IM=interim maintenance, DI=delayed intensification
Table 2. Sample size (n), odds ratios, and confidence intervals (CI) for each comparison of allergic reactions by preparation and course.
| Course of Therapy | Erwinia ASNase:Native ASNase | Erwinia ASNase:PEG ASNase | PEG ASNase:Native ASNase | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | n | Odds Ratio (95% CI) | N | Odds Ratio (95% CI) | n | |
| Consolidation | 0.92 (0.08, 11.11) | 91 | 1.25 (0.11, 14.29) | 902 | 0.74 (0.46, 1.17) | 987 |
| IM #1 | 0.23 (0.12, 0.45) | 322 | 0.32 (0.2, 0.54) | 854 | 0.71 (0.43, 1.18) | 706 |
| DI #1 | 1.72 (0.39, 7.69) | 381 | 1.12 (0.59, 2.13) | 798 | 1.54 (0.35, 6.67) | 543 |
| IM #2 | ---- | 248 | 0.71 (0.38, 1.33) | 520 | ---- | 346 |
| DI #2 | 0.42 (0.08, 2.27) | 228 | 1.43 (0.41, 5) | 469 | 0.30 (0.06, 1.59) | 309 |
Note: ASNase=asparaginase, IM=interim maintenance, DI=delayed intensification
End-Induction Anti-ASNase Antibody Status and Allergic Reactions to PEG ASNase
Of 600 patients with antibody data at the end of Induction, 340 received PEG ASNase during subsequent phases. There were 97 (28.5%) patients with a positive antibody (titer > 1.1) and 243 (71.5%) with a negative antibody (titer ≤ 1.1). Patients with a positive antibody were 2.41 times more likely to have an allergic reaction to PEG ASNase post-Induction than patients who had a negative antibody (OR=2.41; 95% CI=1.49, 3.89; p<0.001). Of note, there were no significant demographic differences between patients who had antibody data and those who did not (See Supplemental Tables 1-7) and this was consistent with patient demographic information reported on the whole population of the study. (9)
Sub-analysis of Antibody Status and Allergic Reactions
There were 298 patients who had antibody titer measurements and received PEG ASNase during Consolidation. Of these, 89 (29.9%) had an allergic reaction to PEG ASNase and 88 (29.5%) had a positive antibody. Among those with a positive antibody, 34.1% (30/88) had an allergic reaction to PEG ASNase when limiting the timeframe to Consolidation only (OR=1.3; 95% CI=0.78, 2.3; p=0.33). There was no statistically significant increase in risk for patients who had a positive antibody titer and who had an allergic reaction when limiting the timeframe to Consolidation alone.
End-Induction Antibody Status and EFS
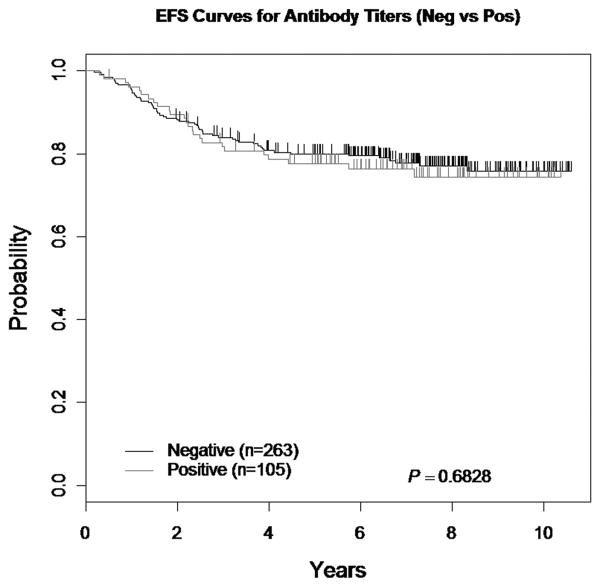
Of 600 patients with end-Induction antibody data, 368 patients were enrolled on PEG ASNase containing arms (though not all received PEG ASNase). Of the 368 patients, 105 (28.5%) had a positive antibody titer and 263 (71.5%) had a negative antibody titer. The 5-year EFS for patients randomized to a PEG ASNase containing regimen with a negative antibody titer was 80 ± 2.6% versus 77.7 ± 4.3% for patients with a positive antibody titer (p=0.68) (See Figure 1).
Figure 1. EFS comparing Antibody Status (Negative versus Positive).
Sub-analysis Antibody Status and EFS
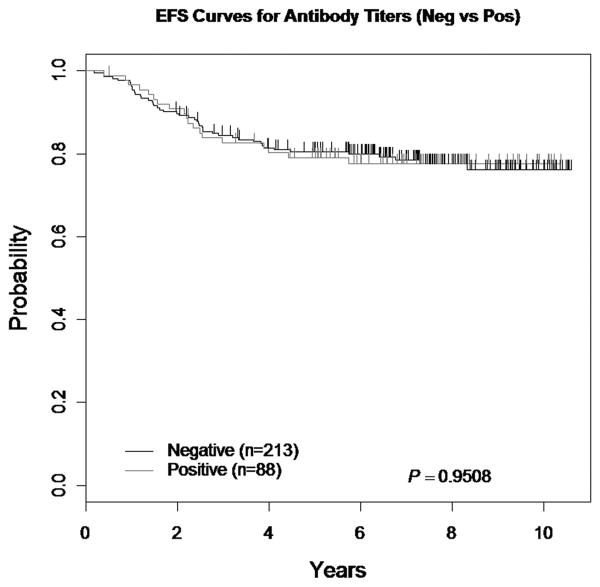
Among 368 patients with complete antibody titer data, 67 did not receive PEG ASNase during Consolidation. Of the remaining 301 patients, 88 (29.2%) had a positive antibody and 213 (70.8%) had a negative antibody. The 5-year EFS for patients who received only PEG ASNase throughout therapy was 80.4±2.9% and 79.1±4.6% (p=0.95) for patients with a negative and positive antibody, respectively (See Figure 2). Of the 65 patients who had an event, 46 (70.8%) had a negative antibody.
Figure 2. EFS comparing Antibody Status (sub-analysis).
Allergic Reactions and EFS
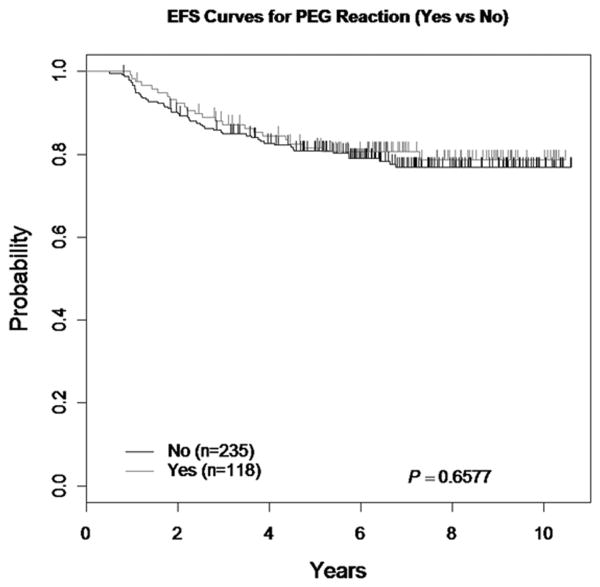
When the EFS of patients who received PEG ASNase throughout the study without an allergic reaction was compared to patients who had an allergic reaction to PEG ASNase during Consolidation and were switched to Erwinia ASNase in subsequent phases without an allergic reaction, the 5-year EFS was 80.8 ± 2.8% and 81.6 ± 3.8%, respectively (p=0.66) (Figure 3). There were no demographic differences between patients who had allergic reactions and those who did not.
Figure 3. EFS comparing PEG ASNase Reaction Status (Yes versus No).
PEG ASNase versus no PEG ASNase post-Consolidation and EFS
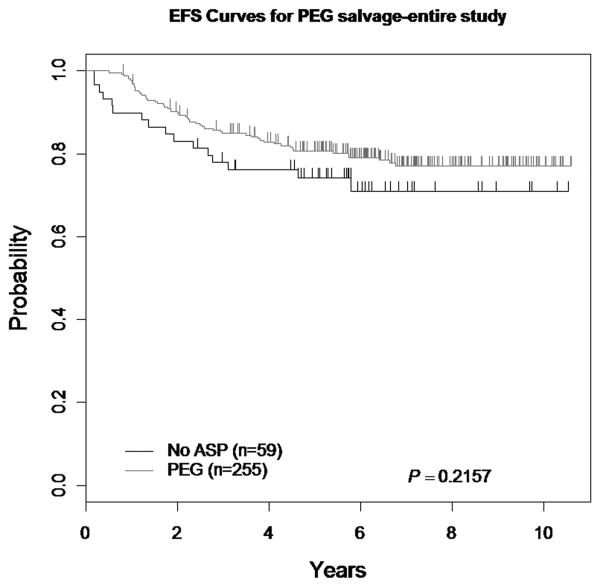
There was no statistically significant difference in the 5-year EFS in patients who received PEG ASNase during Consolidation only as compared to patients who received PEG ASNase during all post-Induction courses (74.2 ± 6.5% and 80 ± 2.7%, respectively; p=0.22) (Figure 4). There were no demographic differences between those who received PEG ASNase throughout treatment and those who did not.
Figure 4. EFS comparing PEG ASNase v. No PEG ASNase post-Consolidation.
Discussion
Asparaginase has been an integral component of multi-agent chemotherapeutic regimens for the treatment of ALL for over 40 years. To the best of our knowledge, CCG 1961 has been one of the largest studies conducted using PEG ASNase. We conducted a secondary analysis of allergic reactions to three different ASNase preparations used in the study and outcomes by antibody status, allergic reaction to PEG ASNase, and post-Consolidation receipt of PEG ASNase.
Incidence rates of hypersensitivity reactions to ASNase during Consolidation in this study were consistent with reported rates and ranged from 2-45%.(10, 12-16) Erwinia ASNase was associated with less allergic reactions when compared to both PEG ASNase and native ASNase during IM #1 only. In all other phases of therapy, there was no significant difference in incidence of allergic reactions. Possible explanations for these findings could be that too few patients received Erwinia ASNase or native ASNase for a meaningful comparison during Consolidation, not many patients had allergic reactions to any form of ASNase during DI #1 and DI #2 since dexamethasone was administered concurrently, and in IM #2, there were small numbers of reactions as the majority of allergic reactions occurred early during the course of treatment. The decreased incidence of reactions to Erwinia ASNase may also be related to its shorter half-life and different antigenic structures. We do know that reports indicate that antibodies to native ASNase measured by ELISA do not cross-react to Erwinia ASNase, so it is unlikely that the antibodies measured also react to Erwinia ASNase.(17, 18) It is likely that there is no significant difference in hypersensitivity reactions between forms of asparaginase when treated on this protocol.
Allergic reactions to ASNase are thought to occur as a result of antibodies causing significant toxicity and neutralizing the enzyme's activity.(19, 20) Although different methods exist to measure the presence of anti-ASNase antibodies, the current standard is enzyme-linked immunosorbent assays.(15, 21) In this study, end-Induction antibodies were associated with an increased incidence of clinical allergy to PEG ASNase during subsequent phases, but did not affect 5-year EFS. In a sub-analysis of patients with positive end-Induction antibody titers and a subsequent allergic reaction, there was no statistically significant increased risk of an allergic reaction during Consolidation versus later phases of therapy. We expected that patients with positive antibody titers would have a higher incidence of allergic reactions during Consolidation than later phases of therapy as the overall presence of anti-ASNase antibodies had a statistically significant development of allergic reactions. However, this was not the case. It is possible that the incidence of allergic reactions when limited to Consolidation was not statistically significant because of the prednisone taper that continued into Consolidation, though the steroids were stopped over a week prior to additional ASNase. Furthermore, repeated exposure to PEG ASNase may have increased the likelihood of an allergic reaction occurring when patients have anti-ASNase antibodies. Additionally, the presence of anti-ASNase antibodies did not result in inferior 5-year EFS as has been suggested in the literature.(13, 15)
There have been various studies investigating the clinical efficacy of different preparations of ASNase in the treatment of childhood ALL. In a pair of studies comparing native ASNase to PEG ASNase (DFCI 91-01 and CCG 1962), there was no difference in survival between the two groups, but the studies were not powered to examine this question.(14, 15) In a trio of studies comparing native ASNase to Erwinia ASNase (DFCI 95-01, EORTC-CLG 58881, and NUH/MA-SPORE), native ASNase was shown to improve survival compared to Erwinia ASNase when given on the same schedule and at the same doses.(7, 8, 22) However, it is known that Erwinia ASNase has a shorter half-life and it is possible that the difference in pharmacokinetics and relative under-dosing of the Erwinia ASNase was the cause of inferior survival in DFCI and EORTC studies. Vrooman et al. examined the outcomes of patients on DFCI ALL Consortium 00-01 who developed allergies to native ASNase and were switched to Erwinia ASNase. The dose of native ASNase in that study was 25,000 IU/m2/dose given weekly, which was substituted with Erwinia ASNase 25,000 IU/m2/dose given twice weekly. They found neither an increase in toxicity nor a difference in EFS.(23) Willer et al. performed a retrospective review of patients initially treated with native ASNase who developed high anti-asparaginase antibody levels (>200 AU/mL) and discovered they did not have an increase in asparaginase activity as measured by the indo-oxin method after switching to PEG ASNase, although there was a significant increase in asparaginase activity when patients were switched to Erwinia ASNase. Due to potentially decreased efficacy with Erwinia ASNase based on the DFCI and EORTC study results, we analyzed the survival data for patients who were switched to Erwinia ASNase post-consolidation due to an allergic reaction to PEG ASNase and found no difference in 5-year EFS when compared to patients who received PEG ASNase throughout the study. Previously published data on patients enrolled on CCG-1961 with neutralizing antibodies to native ASNase indicated patients can be treated with Erwinia ASNase and still retain good activity of the enzyme.(24) These data suggest that six doses of Erwinia ASNase 10,000 IU/m2/dose IM may be an adequate substitute for PEG ASNase 2500 IU/m2 IM and can replace the PEG ASNase in those that develop antibodies and/or clinical allergy to native ASNase.
Since a small but significant number of patients did not receive any asparaginase post-Consolidation due to a serious adverse event (e.g. anaphylaxis, thrombosis, pancreatitis, etc.), we compared the 5-year EFS of these patients to those who received PEG ASNase throughout the study and found no difference. Although we are tempted to speculate that this may indicate that ASNase is unnecessary after Consolidation, the numbers of patients are small and this finding should be investigated in the future. More recently, reviews of patients who could not receive further asparaginase on frontline studies (UKALL 2003-Samarasinghe et al.) and in relapsed patients (ALLR3-Masurekar et al.) showed no difference in EFS or OS when comparing those who could not continue to receive ASNase versus those who did.(25, 26) Of course these reflect different populations of patients on different protocols, but as more evidence emerges, we believe that this should be further investigated.
Here, we demonstrate: (1) Erwinia ASNase is associated with less hypersensitivity when compared to PEG ASNase and native ASNase during IM #1 only; (2) the presence of end-Induction anti-ASNase antibodies is associated with an increased risk of allergic reaction in subsequent phases, (3) the presence of end-Induction anti-ASNase antibodies does not affect EFS; (4) patients with an allergy to PEG ASNase do not have poorer EFS when substituted with Erwinia ASNase, and (5) there may be no difference in EFS between patients who did not receive any form of ASNase post-Consolidation and those who received PEG ASNase throughout the study.
There are a few limitations to this study. First, these analyses were done retrospectively and were not the endpoints that were defined for analysis. Second, current protocols no longer use native ASNase during Induction, which may alter the rates of antibodies formed against asparaginase during Induction and allergic reactions to asparaginase in subsequent courses. Third, we were unable to ascertain if patients who had allergic reactions or other adverse effects to asparaginase that caused the asparaginase to be discontinued were given other therapies that might have obviated the need for asparaginase.
The current generation of Children's Oncology Group studies uses PEG ASNase. Although allergic reactions are likely to continue, the results from this study suggests patients would be able to receive Erwinia ASNase without affecting outcome. Although a substantial amount of information regarding asparaginase and its use in the treatment of childhood leukemia exists, there is still much to be learned. We acknowledge that these analyses were not the initial aims of the study, but they may help in asking specific questions about ASNase in future clinical trials.
Supplementary Material
Acknowledgments
Research Support: Chair's Grant U10 CA98543-08, COG NCTN Network Group Operations Center Grant U10 CA180886, Statistics and Data Center Grant U10 CA98413-08 and COG NCTN Statistics & Data Center U10 CA180899
Footnotes
We demonstrate differences in the incidence rates of toxicity between asparaginase preparations, but not in EFS. The presence of anti-asparaginase antibodies did not affect EFS.
Disclosures: Please note Gaynon PS is a consultant/advisor to Jazz Pharmaceuticals, Sigma Tau Pharmaceuticals, Incyte Corporation, and Novartis and has received honoraria and research funding from Sigma Tau Pharmaceuticals. All other authors have no conflicts of interest to disclose.
References
- 1.Smith MA, Gloeckler-Ries LA, Gurney JG, Ross JA. Leukemia - SEER Pediatric Monograph. National Cancer Institute; 2005. Available from: http://seer.cancer.gov/publications/childhood/leukemia.pdf. [Google Scholar]
- 2.Dolowy WC, Henson D, Cornet J, Sellin H. Toxic and antineoplastic effects of L-asparaginase. Study of mice with lymphoma and normal monkeys and report on a child with leukemia. Cancer. 1966 Dec;19(12):1813–9. doi: 10.1002/1097-0142(196612)19:12<1813::aid-cncr2820191208>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Oettgen HF, Old LJ, Boyse EA, et al. Inhibition of leukemias in man by L-asparaginase. Cancer Res. 1967 Dec;27(12):2619–31. [PubMed] [Google Scholar]
- 4.Hill JM, Roberts J, Loeb E, Khan A, MacLellan A, Hill RW. L-asparaginase therapy for leukemia and other malignant neoplasms. Remission in human leukemia. JAMA. 1967 Nov 27;202(9):882–8. [PubMed] [Google Scholar]
- 5.Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL) Oncologist. 2007 Aug;12(8):991–8. doi: 10.1634/theoncologist.12-8-991. [DOI] [PubMed] [Google Scholar]
- 6.Molineux G. Pegylation: engineering improved biopharmaceuticals for oncology. Pharmacotherapy. 2003 Aug;23(8 Pt 2):3S–8S. doi: 10.1592/phco.23.9.3s.32886. Epub 2003/08/19. eng. [DOI] [PubMed] [Google Scholar]
- 7.Duval M, Suciu S, Ferster A, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children's Leukemia Group phase 3 trial. Blood. 2002 Apr 15;99(8):2734–9. doi: 10.1182/blood.v99.8.2734. [DOI] [PubMed] [Google Scholar]
- 8.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007 Feb 1;109(3):896–904. doi: 10.1182/blood-2006-06-027714. Epub 2006/09/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008 Mar 1;111(5):2548–55. doi: 10.1182/blood-2007-02-070342. Epub 2007/11/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panosyan EH, Seibel NL, Martin-Aragon S, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children's Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004 Apr;26(4):217–26. doi: 10.1097/00043426-200404000-00002. Epub 2004/04/17. eng. [DOI] [PubMed] [Google Scholar]
- 11.Peto R, Peto J. Asymptotically Efficient Rank Invariant Test Procedures. Journal of the Royal Statistical Society Series A (General) 1972;135(2):185–207. [Google Scholar]
- 12.Muller HJ, Beier R, Loning L, et al. Pharmacokinetics of native Escherichia coli asparaginase (Asparaginase medac) and hypersensitivity reactions in ALL-BFM 95 reinduction treatment. Br J Haematol. 2001 Sep;114(4):794–9. doi: 10.1046/j.1365-2141.2001.03009.x. Epub 2001/09/21. eng. [DOI] [PubMed] [Google Scholar]
- 13.Woo MH, Hak LJ, Storm MC, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000 Apr;18(7):1525–32. doi: 10.1200/JCO.2000.18.7.1525. Epub 2000/03/29. eng. [DOI] [PubMed] [Google Scholar]
- 14.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001 Mar 1;97(5):1211–8. doi: 10.1182/blood.v97.5.1211. Epub 2001/02/27. eng. [DOI] [PubMed] [Google Scholar]
- 15.Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2002 Mar 15;99(6):1986–94. doi: 10.1182/blood.v99.6.1986. eng. [DOI] [PubMed] [Google Scholar]
- 16.Strullu M, Corradini N, Audrain M, et al. Silent hypersensitivity to Escherichia coli asparaginase in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2010 Aug;51(8):1464–72. doi: 10.3109/10428194.2010.494316. Epub 2010/06/16. eng. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Relling MV, Storm MC, et al. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia. 2003 Aug;17(8):1583–8. doi: 10.1038/sj.leu.2403011. [DOI] [PubMed] [Google Scholar]
- 18.Zalewska-Szewczyk B, Gach A, Wyka K, Bodalski J, Mlynarski W. The cross-reactivity of anti-asparaginase antibodies against different L-asparaginase preparations. Clinical and experimental medicine. 2009 Jun;9(2):113–6. doi: 10.1007/s10238-008-0026-9. [DOI] [PubMed] [Google Scholar]
- 19.Schein PS, Rakieten N, Gordon BM, Davis RD, Rall DP. The toxicity of Escherichia coli L-asparaginase. Cancer Res. 1969 Feb;29(2):426–34. Epub 1969/02/01. eng. [PubMed] [Google Scholar]
- 20.Peterson RG, Handschumacher RE, Mitchell MS. Immunological responses to L-asparaginase. J Clin Invest. 1971 May;50(5):1080–90. doi: 10.1172/JCI106579. Epub 1971/05/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B, Hak LJ, Relling MV, Pui CH, Woo MH, Storm MC. ELISA to evaluate plasma anti-asparaginase IgG concentrations in patients with acute lymphoblastic leukemia. J Immunol Methods. 2000 May 26;239(1-2):75–83. doi: 10.1016/s0022-1759(00)00182-4. Epub 2000/05/24. eng. [DOI] [PubMed] [Google Scholar]
- 22.Kwok CS, Kham SK, Ariffin H, Lin HP, Quah TC, Yeoh AE. Minimal residual disease (MRD) measurement as a tool to compare the efficacy of chemotherapeutic drug regimens using Escherichia Coli-asparaginase or Erwinia-asparaginase in childhood acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2006 Sep;47(3):299–304. doi: 10.1002/pbc.20684. Epub 2005/11/23. eng. [DOI] [PubMed] [Google Scholar]
- 23.Vrooman LM, Supko JG, Neuberg DS, et al. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. Feb;54(2):199–205. doi: 10.1002/pbc.22225. Epub 2009/08/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panosyan EHGP, Sather H, et al. Asparaginase (ASNase) activity, anti-ASNase antibody (Ab), and amino acid deamination in children with higher-risk acute lymphoblastic leukemia (HR ALL) (CCG-1961) Proc Am Soc Clin Oncol. 2002;21:399a. [Google Scholar]
- 25.Samarasinghe S, Dhir S, Slack J, et al. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2013 Sep;162(5):710–3. doi: 10.1111/bjh.12407. [DOI] [PubMed] [Google Scholar]
- 26.Masurekar A, Fong C, Hussein A, et al. The optimal use of PEG-asparaginase in relapsed ALL--lessons from the ALLR3 Clinical Trial. Blood cancer journal. 2014;4:e203. doi: 10.1038/bcj.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.