Abstract
A strategy for the efficient two-step synthesis of triazole derivatives of oxindoles and spirooxindoles is presented. Using a common set of N-propargylated isatins, a series of mechanistically-distinct stereoselective reactions with different combinations of nucleophiles and catalysts provide access to diverse hydroxy-oxindoles, spiroindolones, and spirocyclic oxazoline structures. The resulting N-propargylated oxindoles are then converted to triazoles using copper-catalyzed azide-alkyne cycloaddition (CuAAC) reactions. Overall, this strategy affords a 64-member pilot-scale library of diverse oxindoles and spirooxindoles.
Keywords: heterocycles, isatins, oxindoles, spirooxindoles, spiroindolones, oxazolines, triazoles
Introduction
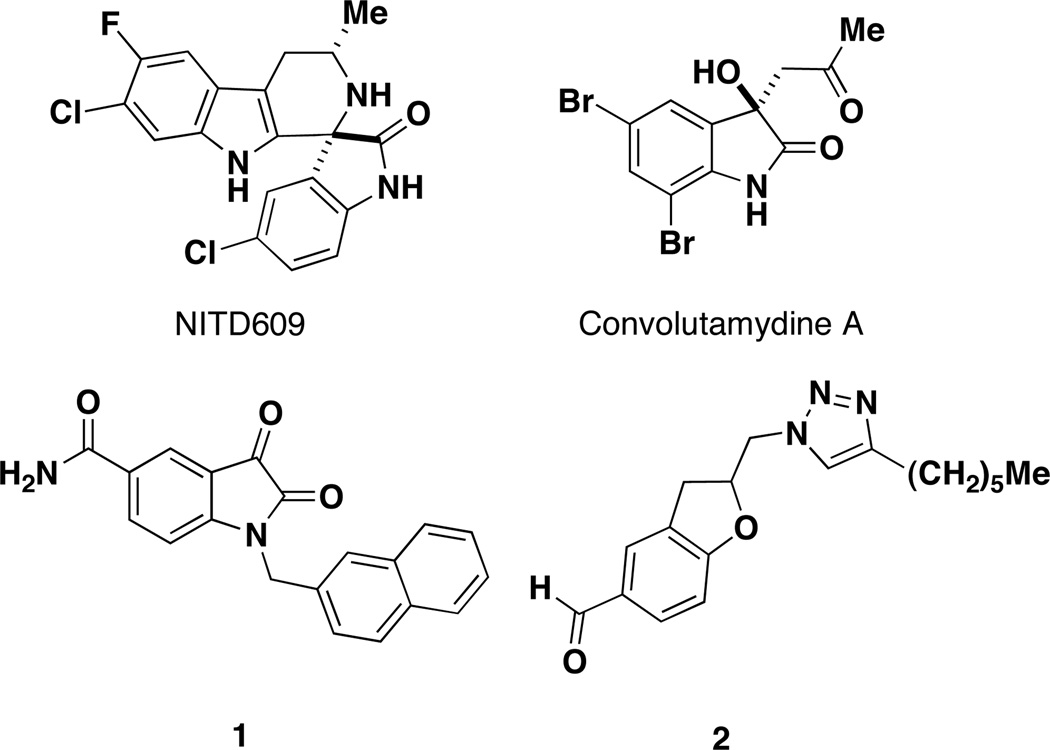
Complex functionalized molecules are important compounds of interest for biological probes and as new molecules for pharmaceutical lead discovery. Oxindole and spirooxindole scaffolds have generated considerable synthetic interest due to their occurrence in diverse natural products and notable biological activity.1 In a recent discovery, spiroindolone NITD609 demonstrated nanomolar activity as a therapeutic agent that kills the blood stage of Plasmodium falciparum and has single-dose efficacy in a rodent malaria model (Figure 1).2 Various hydroxy-oxindoles scaffolds also demonstrate important biological activity, such as Convolutamydine A, a natural product with potent activity against leukemia cells.3 Substituted isatin (indole-2,3-diones) scaffolds have also shown promising examples of biological activity.4 For example, isatin 1 is a potent inhibitor of SARS CoV 3C-like proteases.5 Initially driven by efficient synthetic methods, the 1,2,3-triazole has now emerged as a heterocycle of biological interest in drug discovery and medicinal chemistry programs.7 For example, triazole 2 shows activity against tuberculosis strain H37RV.6 The significant biological activities observed for oxindoles and triazoles emphasizes the need to develop efficient synthetic strategies to access these scaffolds and increase structural diversity for drug discovery and medicinal chemistry programs.
Figure 1.
Biologically active oxindoles and triazoles
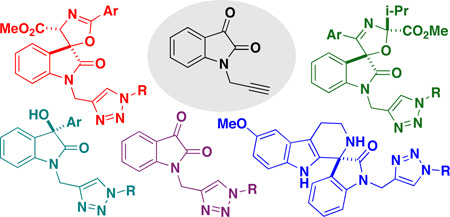
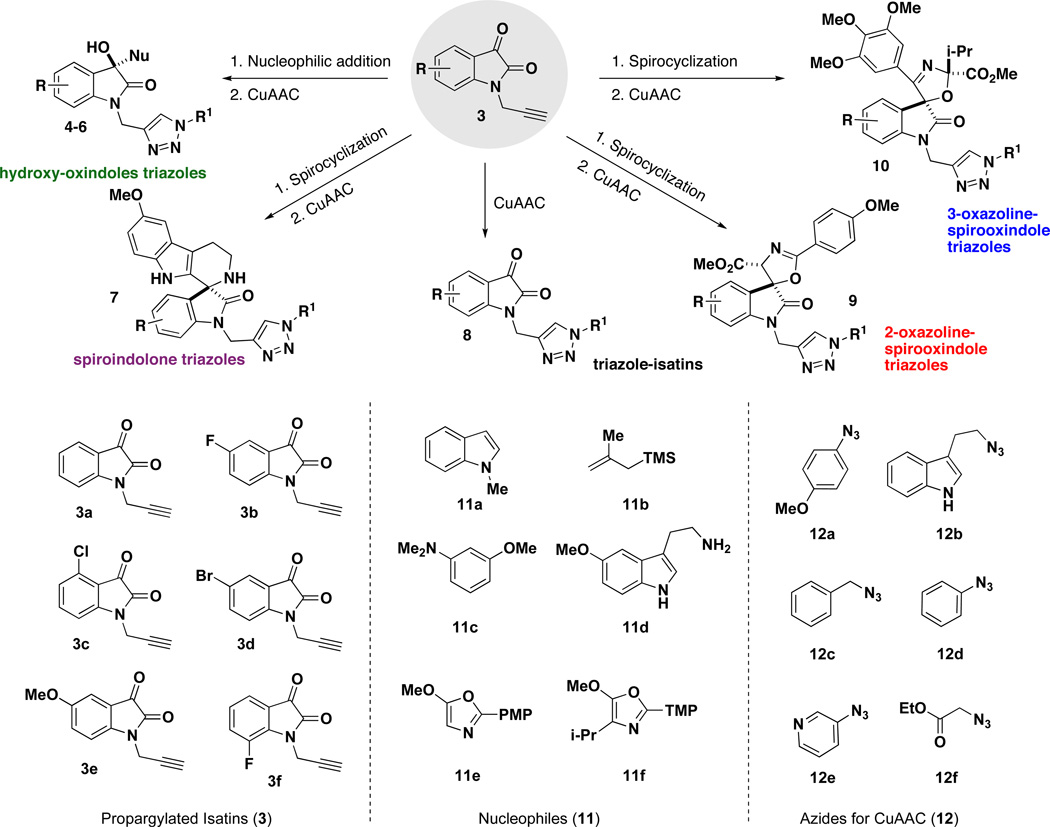
Previous work from our laboratory has demonstrated several methods of catalytic activation of the isatin dicarbonyl for efficient and selective nucleophilic additions and spirocyclizations at the 3-position.8 The strategy we envisioned utilizes a common set of N-propargylated isatins 3 to access diverse oxindole scaffolds through a series of mechanistically-distinct nucleophilic addition pathways. Each resulting oxindole scaffold contains an alkyne group that provides further opportunities for structural diversification with triazole heterocycles using the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction. We also envisioned that the N-propargylated isatins 3 would be directly utilized in the CuAAC reaction as a fifth scaffold because triazole-containing isatins have not previously been reported or evaluated for biological activity. Here we describe the realization of this strategy for rapid access (two synthetic steps) to a pilot-scale library of diverse triazole-containing hydroxy-oxindoles 4–6, spiroindolones 7, isatin-triazoles 8, and spirooxazolines 9–10 emanating from a common set of propargylated isatins 3 (Scheme 1). Overall, our two-step strategy affords a library of 64 oxindole and spirooxindole compounds, including 15 core scaffolds and 49 triazole derivatives. The efficient stereoselective syntheses of complex heterocycles combining both oxindole and triazole motifs have not been described previously. Based on the breadth of biological activity known for isatins, oxindoles and spirooxindoles, these densely functionalized heterocycles should serve as important biological probes for chemical biology.
Scheme 1.
Outline of Synthetic Strategy towards Substituted Triazole-containing Oxindoles
Results and Discussion
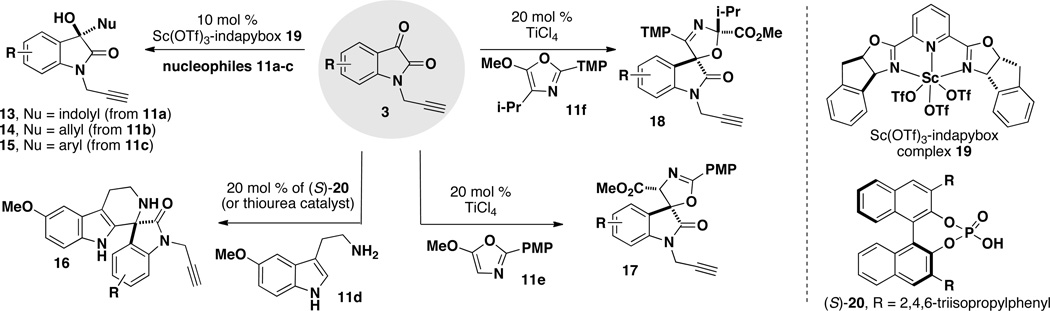
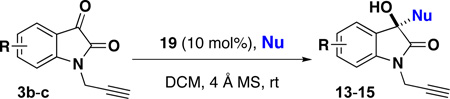
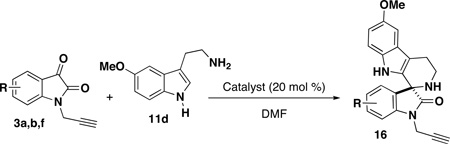
A key feature in this strategy is the regio-, diastereo- and enantioselective synthesis of the oxindole and spirooxindole scaffolds (Scheme 2). Based on the nucleophile component (11) utilized (Scheme 1), four oxindole scaffolds were selected for this library: hydroxy-oxindoles 13–15 are prepared enantioselectively using a chiral Lewis acid catalyst (19);8b spiroindolones 16 are prepared enantioselectively using a chiral Brönsted acid catalyst (20);8c and the 2-oxazoline and 3-oxazoline spirocycles 17 and 18 are each prepared diastereo- and regioselectively using a titanium(IV) Lewis acid catalyst (Scheme 2).8a For some scaffolds, isatins (3) are selected based on substitution patterns that ensure high selectivity.
Scheme 2.
Nucleophilic Addition Reactions to Access Oxindoles and Spirooxindoles.
First, a series of 3-substituted-3-hydroxy-oxindole scaffolds 13–15 were accessed using scandium(III)-catalyzed enantioselective additions with representative π-nucleophiles: N-methylindole (11a), 2-methallylsilane (11b), and N,N-dimethyl-m-anisidine (11c).8b Scandium(III) complexes formed with the 2,6-bis[(3aS,8aR)-3a,8a-dihydro-8H-indeno[1,2-d]oxazolin-2yl]pyridine ligand (e.g. 19) are known to be effective chiral Lewis acid catalysts with good chelating potential.9 As outlined in Table 1, isatins 3b–c were utilized to afford hydroxy oxindoles 13–15 with high yields (78–97%) and enantioselectivity (85–99% ee). All reactions were performed at room temperature, with the exception of entries 1 and 4, which were performed at −20 °C due to the high reactivity of the nucleophile. In the case of the methallylsilane (11b, entries 2 and 5), the reaction is run in acetonitrile with TMSCl and NaSbF6 as additives to increase the efficiency of the reaction and promote in situ deprotection of any resulting OTMS product so that the hydroxy-oxindole products 13–15 are obtained exclusively.10,11
Table 1.
Enantioselective Synthesis of Substituted Hydroxy-Oxindole Scaffolds
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | isatin | nucleophile | product | yielda | %eeb |
| 1c | 5-F | 3b | 11a | 13b | 97 | 98 |
| 2d | 5-F | 3b | 11b | 14b | 79 | 87 |
| 3 | 5-F | 3b | 11c | 15b | 97 | 96 |
| 4c | 4-Cl | 3c | 11a | 13c | 78 | 86 |
| 5d | 4-Cl | 3c | 11b | 14c | 90 | 94 |
| 6 | 4-Cl | 3c | 11c | 15c | 97 | 99 |
Isolated yields.
Determined using HPLC analysis with chiral stationary phase.
Reaction performed at −20 °C.
Reaction was performed using 3.0 equiv of TMSCl and 0.1 equiv of NaSbF6 in MeCN.
Using an asymmetric Pictet-Spengler-type spirocyclization reaction,12 three spiroindolone scaffolds were prepared upon acid-catalyzed spirocyclization of 5-methoxytryptamine (11d) with isatins (Table 2).8c,13 We have previously shown that this reaction is efficiently catalyzed using Lewis acidic metal salts, thioureas, or BINOL-derived chiral phosphoric acids. The 5-fluorospiroindolone 16b was prepared using a BINOL-derived phosphoric acid catalyst to demonstrate the enantioselective synthesis and evaluate the retention of enantiomeric excess in the CuAAC reaction. Using (S)-3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate (20) as the catalyst, fluorospiroindolone 16b was attained in excellent yield (86%) and high enantioselectivity (84% ee) (Table 2, entry 2).11 Using either Sc(OTf)3 or 1,3-bis(3,5-bis(trifluoromethyl)phenyl)thiourea provides an efficient (and less expensive) catalyst for the preparation of racemic spiroindolones (Table 2, entries 1 and 3). Isatins 3b and 3f were selected due to the therapeutic relevance of fluorinated molecules as well as the efficient performance of these isatins in the Pictet-Spengler reaction.2b, 14
Table 2.
Enantioselective Synthesis of Spiroindolone Scaffolds
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | isatin | product | catalyst | yield (%)a | %eeb |
| 1 | H | 3a | 16a | Sc(OTf)3 | 89 | -- |
| 2 | 5-F | 3b | 16b | 20 | 86 | 84 |
| 3 | 7-F | 3f | 16f | Thiourea | 92c | -- |
Isolated yield.
Determined using HPLC analysis with chiral stationary phase.
Reaction was performed in DCM solvent.
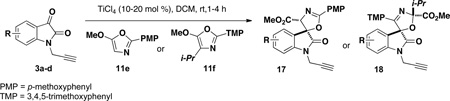
A series of spirooxindole oxazoline scaffolds were prepared using the titanium(IV)-catalyzed regio- and diastereoselective addition and spirocyclization of 5-methoxyoxazoles to isatins.8a, 15 Substitution at the 4-position of the oxazole controls the regiochemistry, with the 4-isopropyloxazole (11f) giving rise to the 3-oxazoline scaffold 18 and the 4-H oxazole (11e) leading to the 2-oxazoline scaffold 17.8a, 11, 16 In general, the oxazole addition to propargyl isatins 3a–d proceeded with high regioand diastereoselectivity (>99% rr, up to 99% dr) and in high yields (up to 95%); however, in two cases (17b and 18c) the isolated yield was low due to the presence of by-products that proved to be difficult to separate by column chromatography. All reactions proceeds with high diastereoselectivity, but the 4-chloroisatin 3c is particularly effective for dictating high diastereoselectivity in the formation of 2-oxazolines, such as 17c.
Triazole Synthesis
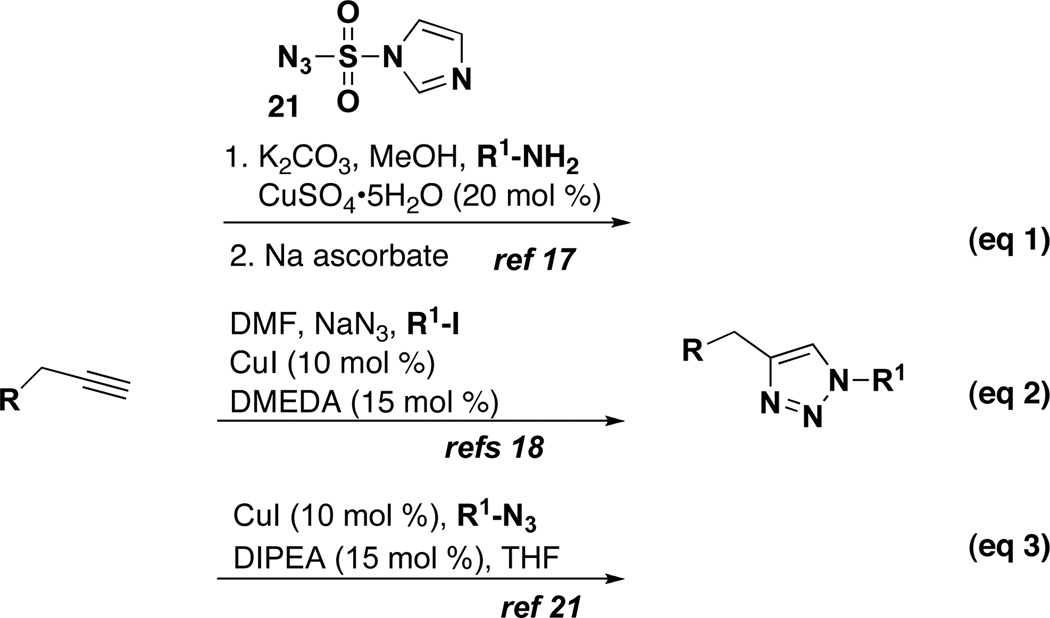
This collection of diverse oxindole and spirooxindole scaffolds contains a common feature through the N-propargyl group of each scaffold, which can be further diversified using azide-alkyne cycloaddition chemistry. We set out to compare several variations of CuAAC reactions for this collection of N-propargylated oxindoles. We began by exploring one-pot reaction conditions that utilize in situ generated azides, prepared from the corresponding amine using the shelf-stable diazotransfer reagent 1H-imidazole-1-sulfonyl azide (21) (Scheme 3, eq 1).17 Unfortunately, this method did not work for these oxindole substrates due to side reactions and decomposition of starting material under the diazo transfer conditions. Several solvent combinations (THF, MeOH/THF, DCM), copper reagents, and conditions (i.e. Cu(I) vs. Cu(II) reduction by sodium ascorbate in situ) were explored, but only minimal triazole formation was observed for the one-pot procedure. Next, we investigated a route utilizing in situ generated aryl azides prepared from commercially-available aryl iodides with sodium azide in the presence of copper iodide (Scheme 3, eq 2).18 This method was also considered to be attractive because it provides access to diverse aryl triazoles, including amino-substituted triazoles that are not available when preparing azides from amines. This method has previously only been reported for less-functionalized substrates, but here the conditions generally afforded low yields (33–40%) for the more complex oxindole substrates. Low yields are attributed to the formation of undesired polymericside products.
Scheme 3.
Methods Compared for the Synthesis of Triazoles with Propargylated Isatins
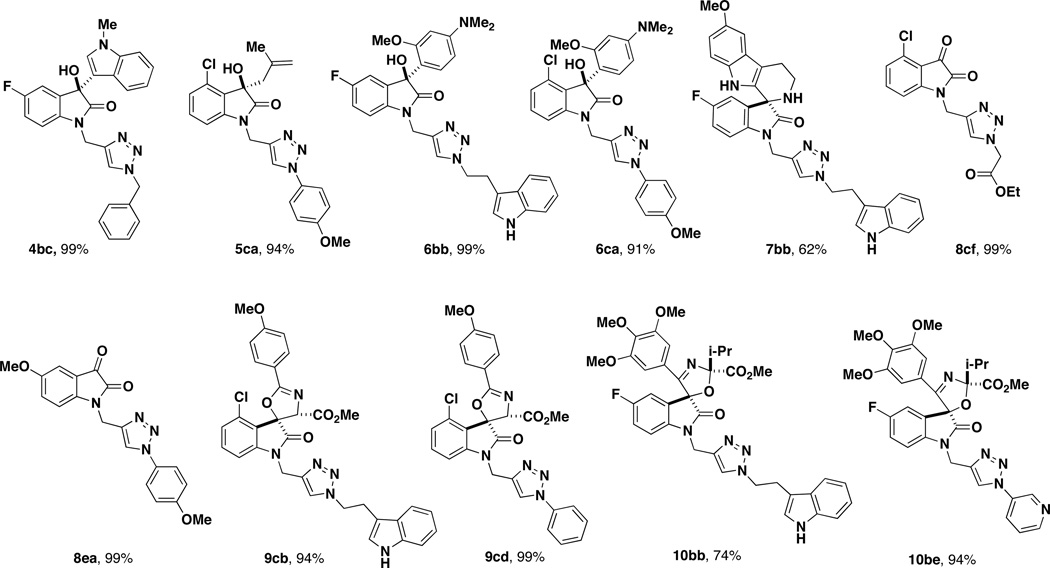
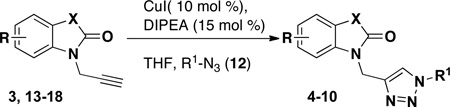
Ultimately, we utilized a method applicable for the synthesis of both alkyl- and aryl-substituted triazoles using purified azides that were prepared in one-step from the corresponding amine diazotransfer reagent 21 (Table 4).19 Azides 12a–f were selected to include indolyl, pyridyl, ester, and phenyl derivatives in order to provide a variation in molecular weights, hydrogen bonding donor and acceptor properties, and capabilities for π-interactions. Azides 12a–e were each prepared from the amine using diazotransfer reagent 21, and azide 12f was prepared by direct displacement of a chloride with sodium azide.20 After purification and isolation, azides 12a–f were then allowed to react directly with the N-propargyl-oxindoles or spirooxindoles in the presence of catalytic Cu(I) iodide (10 mol %) and di-isopropylethylamine (15 mol %) in THF to afford triazoles 4–10.21 A representative combination of N-propargyl oxindole scaffolds and azide components were evaluated to ascertain the scope of compounds accessible using this strategy (Figure 2). Overall, these reactions proceeded with high yields (> 70%) for 36 of 45 triazoles prepared, and only 8 of the 45 reactions afforded less than 66% yield (Table 4). These low yields are attributed to lower conversion and unreacted starting material for the given reaction time. Of practical consideration, the CuAAC reactions of isatins (entries 20–27) proceeded with excellent yields and were particularly clean and easy to purify.
Table 4.
Synthesis of Triazole-functionalized Isatins, Oxindoles, and Spirooxindoles
 | |||||
|---|---|---|---|---|---|
| entry | R | isatin/oxindole | azide (R1) | producta | yield (%)b |
| 1 | 5-F | 13b | 12a | 4ba | 78 |
| 2 | 5-F | 13b | 12b | 4bb | 80 |
| 3 | 5-F | 13b | 12c | 4bc | 99 |
| 4 | 5-F | 14b | 12a | 5ba | 64 |
| 5 | 5-F | 14b | 12b | 5bb | 71 |
| 6 | 5-F | 15b | 12a | 6ba | 56 |
| 7 | 5-F | 15b | 12b | 6bb | 99 |
| 8 | 4-Cl | 13c | 12a | 4ca | 40 |
| 9 | 4-Cl | 13c | 12b | 4cb | 70 |
| 10 | 4-Cl | 14c | 12a | 5ca | 94 |
| 11 | 4-Cl | 14c | 12b | 5cb | 58 |
| 12 | 4-Cl | 15c | 12a | 6ca | 91 |
| 13 | 4-Cl | 15c | 12b | 6cb | 94 |
| 14 | 4-Cl | 15c | 12c | 6cc | 58 |
| 15 | H | 16a | 12c | 7ac | 95 |
| 16 | H | 16a | 12f | 7af | 95 |
| 17 | 5-F | 16b | 12b | 7bb | 62 |
| 18 | 5-F | 16b | 12c | 7bc | 97 |
| 19 | 7-F | 16f | 12a | 7fa | 70 |
| 20 | H | 3a | 12d | 8ad | 69 |
| 21 | H | 3a | 12a | 8aa | 99 |
| 22 | H | 3a | 12e | 8ae | 95 |
| 23 | 4-Cl | 3c | 12a | 8ca | 55 |
| 24 | 4-Cl | 3c | 12f | 8cf | 99 |
| 25 | 4-Cl | 3c | 12c | 8cc | 97 |
| 26 | 5-Br | 3d | 12a | 8da | 74 |
| 27 | 5-OMe | 3e | 12a | 8ea | 99 |
| 28 | 5-F | 17b | 12b | 9bb | 74 |
| 29 | 4-Cl | 17c | 12a | 9ca | 88 |
| 30 | 4-Cl | 17c | 12b | 9cb | 94 |
| 31 | 4-Cl | 17c | 12c | 9cc | 88 |
| 32 | 4-Cl | 17c | 12f | 9cf | 87 |
| 33 | 4-Cl | 17c | 12d | 9cd | 99 |
| 34 | H | 18a | 12a | 10aa | 92 |
| 35 | H | 18a | 12b | 10ab | 76 |
| 36 | H | 18a | 12f | 10af | 70 |
| 37 | H | 18a | 12d | 10ad | 90 |
| 38 | 5-F | 18b | 12a | 10ba | 80 |
| 39 | 5-F | 18b | 12b | 10bb | 74 |
| 40 | 5-F | 18b | 12c | 10bc | 70 |
| 41 | 5-F | 18b | 12e | 10be | 94 |
| 42 | 5-F | 18b | 12d | 10bd | 74 |
| 43 | 4-Cl | 18c | 12b | 10cb | 66 |
| 44 | 4-Cl | 18c | 12d | 10cd | 93 |
| 45 | 5-Br | 18d | 12a | 10da | 99 |
All reactions performed with CuI (10 mol %) and DIPEA (15 mol %) in THF from 12–24 h.
Isolated yield.
Figure 2.
A) Representative final oxindole and spirooxindole products.
Because hydroxy-oxindoles 13–15 were prepared as enantiomerically-enriched compounds using asymmetric catalysis, it is especially important to demonstrate that the enantomeric excess of these compounds was maintained during the CuAAC reaction since these compounds are known to form stabilized carbocations under metal- and acid-catalyzed conditions.22 The enantiomerically-enriched N-propargylated 3-hydroxy-oxindoles were subjected to the CuAAC conditions with azides 12a–c, e (Table 4, entries 1–14). The enantiomeric excess of the triazole-containing 3-hydroxy3-indolyl-oxindole 4ba was confirmed by HPLC analysis using chiral stationary phase by comparison to the triazoles produced by racemic synthesis.23 Due to the similarity of hydroxy-oxindoles 13–15, the retention of enantiomeric excess for 4ba was used as a model for retention of enantioselectivity for all triazole products. Enantiomerically-enriched spiroindolone 16b was used to demonstrate that the enantiomeric excess for this class of spirooxindoles is retained under CuAAC reaction conditions (entry 17).23b
The spirocyclic oxazolines 17 and 18 afforded spirocyclic triazoles 9 and 10 in excellent yields (Table 4, entries 28–45); however, the reversed order of the reaction sequence was also investigated. For spirooxazoline-triazole 9, the titanium(IV)-catalyzed addition and spirocyclization of 5-methoxyoxazole 11e can also be performed using a triazole-containing isatin (derived from azide 12a) in 71% yield and maintaining high diastereoselectivity (97:3 dr). However, the success of the spirocyclization is dependent on the nature of the triazole substrate (see Supporting Information). Although the reaction proceeded successfully with the isatin triazole derived from p-methoxy phenyl azide 12a, the use of an isatin triazole derived from azide 12b did not undergo spirocyclization, presumably due to interactions between the indole ring and the titanium catalyst.
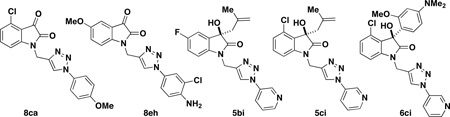
Although our earlier investigations had shown that the one-pot reaction using aryl iodides proceeded with low yield, we briefly explored this approach to incorporate additional triazole diversity (Table 5). For example, this one-pot method allows the synthesis of triazole isatin 8eh containing an amino group, which cannot be accessed with the previous route described above (Table 4). This CuAAC strategy uses 10 mol % of CuI and 15 mol % of DMEDA with one equivalent of aryl-iodide and one equivalent of the alkyne in DMF.18 Triazoles 8cg and 8eh were obtained when isatins 3c,e were subjected to the reaction with aryl-iodides; however, low yields were observed (Table 5, entries 1–2). Similarly, with hydroxy-oxindoles 14 and 15, the reaction was sluggish with catalytic amounts of copper, also affording low yields of the triazole products. When the amount of copper was increased to stoichiometric amounts, several unidentified side-products were formed and the increase in yield was negligible. In order to reduce the amount of side products and increase yields, conditions were modified to use a stoichiometric amount of copper(II) reagent with sodium ascorbate to generate the catalytic Cu(I) species in situ. Even with these optimized conditions, the yield of pyridinyl triazoles remained low (Table 5, entries 3–5). This result is in contrast to the reaction of pyridyl azide 12e, which was successfully utilized and afforded triazole products with excellent yields (Table 4, entries 22 and 41). However, this method can provide additional interesting compounds for biological screening.
Table 5.
Synthesis of Triazole-functionalized Oxindoles and Isatins from Aryl-iodides
 | |||||
|---|---|---|---|---|---|
| entry | R1 | isatin/oxindole | Ar-N3a | product | yield (%)b |
| 1c | 4-Cl | 3c | 12g | 8ca | 23 |
| 2c | 5-OMe | 3e | 12h | 8eh | 35 |
| 3d | 5-F | 14b | 12i | 5bi | 28 |
| 4d | 4-Cl | 14c | 12i | 5ci | 40 |
| 5d | 4-Cl | 15c | 12i | 6ci | 19 |
Azide generated in situ from the corresponding aryl-iodide.
Isolated yield.
Reactions performed using CuI (10 mol %), and DMEDA (15 mol %) according to Scheme 3, eq 3.
Reaction performed with 1 equiv of CuSO4•5H2O, and 1.1 equiv. of sodium ascorbate.
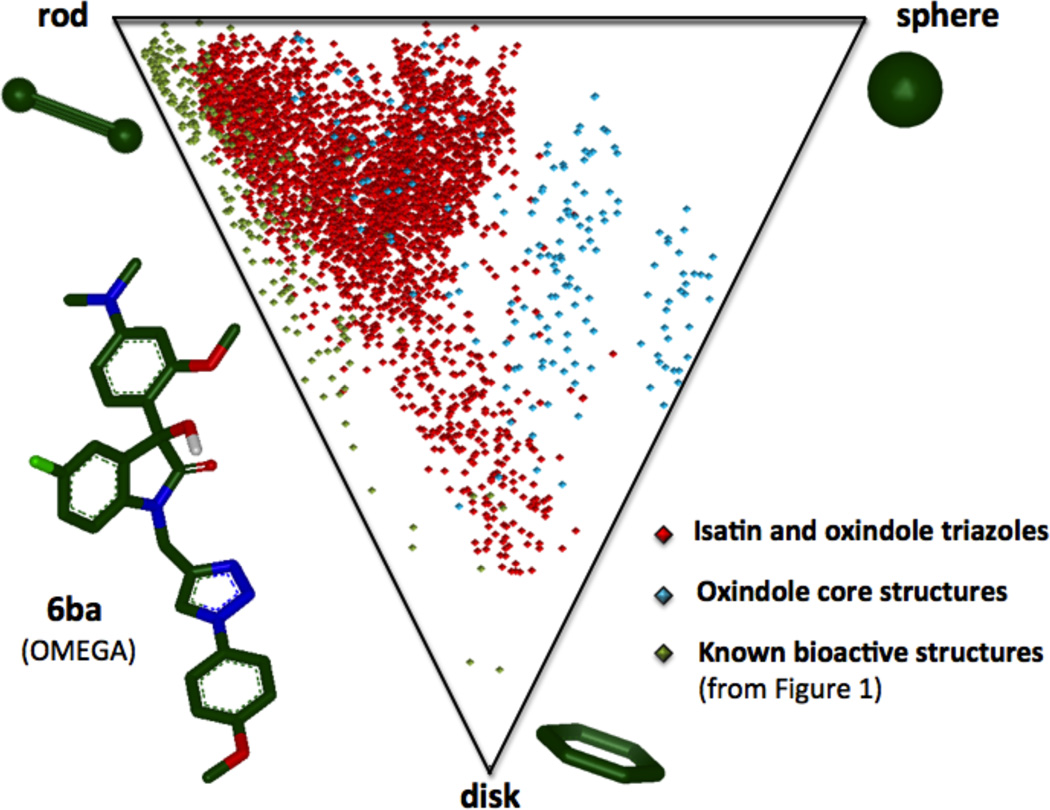
An analysis of the molecular properties and shape for this collection of oxindole compounds indicates desirable properties and diversity for high-throughput screening and the discovery of pharmaceutical leads or biological probes. Molecular properties for all compounds were calculated (see Supporting Information) and a summary of the average values are provided in Table 6. The majority of compounds and average values are within accepted ranges for the development of lead compounds.24 The nucleophile and azide building blocks selected here afford a collection of compounds with molecular weights ranging from 259–721, and calculated partition coefficients (cLogP) values with a range of 0.56 to 5.38, based on the XLogP method of Wang.25 The molecular weights span a range that includes molecular weights appropriate for lead-like molecules, as well as access to higher molecular weight compounds, which could prove useful as biological probes for the disruption of protein-protein interactions.26 In order to visualize the molecular shape diversity, we generated a scatter plot based on the principal moments of inertia (PMI) ratios (Figure 3), a method developed by Sauer and Schwarz.27 This method classifies the molecular shape into three categories: rod (acetylene), disk (benzene), or spherical (adamantine). Since several conformations of a compound are capable of binding to a biological target, a collection of 3D-conformations ≤3 kcal/mol from the minimum energy conformer are represented. This shape analysis also includes the structures of the known biologically active compounds in Figure 1 for comparison.
Table 6.
Average values of Molecular Properties
Figure 3.
Scatter plot with principal moments of inertia (PMI) ratios plotted to compare the molecular shape diversity of oxindole core structures (blue), triazole-containing isatins and oxindoles (red), and known biologically-active compounds from Figure 1 (green). For each compound, PMI ratios were calculated for all minimum energy conformers ≤3 kcal/mol from the global minimum.
In conclusion, we have developed an efficient enantio- and diastereoselective synthetic strategy to access a diverse 64-compound pilot-scale library including 15 oxindole scaffolds and 49 triazole containing-oxindoles and isatins. We demonstrate that enantiomeric excess resulting from the catalytic asymmetric synthesis of oxindoles and spirooxindoles is retained upon further functionalization with the CuAAC reaction, thus providing efficient methods to prepare libraries of enantiomerically-enriched spirocyclic compounds. The nucleophile and azide building blocks selected here afford a collection of compounds with diversity that is appropriate for high-throughput screening and the discovery of pharmaceutical leads or biological probes. All of the compounds in this report have been submitted to the NIH Molecular Libraries Small Molecule Repository for biological screening.
Supplementary Material
Table 3.
Regio- and Stereoselective Spirocyclization to Afford 2- and 3-spirooxazoline Scaffolds
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | isatin | oxazole | product | yield (%)a | drb |
| 1 | 5-F | 3b | 11e | 17b | 55c | 90:10 |
| 2 | 4-Cl | 3c | 11e | 17c | 74 | 99:1 |
| 3 | H | 3a | 11f | 18a | 77c | 90:10 |
| 4 | 5-F | 3b | 11f | 18b | 87 | 93:7 |
| 5 | 4-Cl | 3c | 11f | 18c | 51c | 95:5 |
| 6 | 5-Br | 3d | 11f | 18d | 95 | 99:1 |
Isolated yield of major diastereomer.
Determined by analysis of 1H NMR spectroscopy of crude reaction mixture.
Yields sacrificed for purity due to the presence of by-products which proved to be difficult to separate by column chromatography (conversion ≥80% by TLC).
ACKNOWLEDGMENT
This work was supported by NIH/NIGMS (P41-GM0891583). A.K.F. acknowledges 3M Corporation for a Nontenured Faculty Award. J.J.B. acknowledges support from the National Science Foundation in the form of a graduate research fellowship; A.S. would like to thank Bristol-Myers Squibb for an undergraduate research fellowship.
Footnotes
SUPPORTING INFORMATION
Complete characterization data for 27 compounds, including HPLC data for enantioenriched compounds; 1H NMR spectra and mass spectrometry data available for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
AUTHOR CONTRIBUTIONS
A.K.F. conceived the strategy and experiments; J.P.M., J.J.B., G.E.A. and A.S. designed and performed the experiments; A.K.F. and J.P.M. co-wrote the manuscript; J.P.M. and J.J.B. co-wrote the Supporting Information.
References
- 1.(a) Galliford CV, Scheidt KA. Pyrrolidinyl-Spirooxindole Natural Products as Inspirations for the Development of Potential Therapeutic Agents. Angew. Chem. Int. Ed. 2007;46:8748–8758. doi: 10.1002/anie.200701342. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Brennan MK. Asymmetric Syntheses of Oxindole and Indole Spirocyclic Alkaloid Natural Products. Synthesis. 2009;2009:3003–3025. [Google Scholar]; (c) Badillo JJ, Hanhan NV, Franz AK. Enantioselective synthesis of substituted oxindoles and spirooxindoles with applications in drug discovery. Curr. Opin. Drug Discovery Devel. 2010;13:758–776. [PubMed] [Google Scholar]; (d) Russel J. In: Oxindoles and Spirocyclic Variations: Strategies for C3 Functionalization Heterocyclic Scaffolds II. Gribble GW, editor. Vol. 26. Berlin / Heidelberg: Springer; 2011. pp. 397–431. [Google Scholar]
- 2.(a) Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck H-P, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. Spiroindolones, a Potent Compound Class for the Treatment of Malaria. Science. 2010;329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yeung BKS, Zou B, Rottmann M, Lakshminarayana SB, Ang SH, Leong SY, Tan J, Wong J, Keller-Maerki S, Fischli C, Goh A, Schmitt EK, Krastel P, Francotte E, Kuhen K, Plouffe D, Henson K, Wagner T, Winzeler EA, Petersen F, Brun R, Dartois V, Diagana TT, Keller TH. Spirotetrahydro β-Carbolines (Spiroindolones): A New Class of Potent and Orally Efficacious Compounds for the Treatment of Malaria. J. Med. Chem. 2010;53:5155–5164. doi: 10.1021/jm100410f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng K, Yin C, Liu X, Lin L, Feng X. Catalytic Asymmetric Addition of Alkyl Enol Ethers to 1,2-Dicarbonyl Compounds: Highly Enantioselective Synthesis of Substituted 3-Alkyl-3-Hydroxyoxindoles. Angew. Chem. Int. Ed. 2011;50:2573–2577. doi: 10.1002/anie.201007145. [DOI] [PubMed] [Google Scholar]
- 4.(a) Lee D, Long SA, Murray JH, Adams JL, Nuttall ME, Nadeau DP, Kikly K, Winkler JD, Sung C-M, Ryan MD, Levy MA, Keller PM, DeWolf WE. Potent and Selective Nonpeptide Inhibitors of Caspases 3 and 7. J. Med. Chem. 2001;44:2015–2026. doi: 10.1021/jm0100537. [DOI] [PubMed] [Google Scholar]; (b) Abdel-Rahman AH, Keshk EM, Hanna MA, El-Bady SM. Synthesis and evaluation of some new spiroindoline-based heterocycles as potentially active antimicrobial agents. Biorg. Med. Chem. 2004;12:2483–2488. doi: 10.1016/j.bmc.2003.10.063. [DOI] [PubMed] [Google Scholar]; (c) Millemaggi A, Taylor RJK. 3-Alkenyl-oxindoles: Natural Products, Pharmaceuticals, and Recent Synthetic Advances in Tandem/Telescoped Approaches. Eur. J. Org. Chem. 2010:4527–4547. [Google Scholar]; (d) Zhou F, Liu Y-L, Zhou J. Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position. Adv. Synth. Catal. 2010;352:1381–1407. [Google Scholar]
- 5.Zhou L, Liu Y, Zhang W, Wei P, Huang C, Pei J, Yuan Y, Lai L. Isatin Compounds as Noncovalent SARS Coronavirus 3C-like Protease Inhibitors. J. Med. Chem. 2006;49:3440–3443. doi: 10.1021/jm0602357. [DOI] [PubMed] [Google Scholar]
- 6.(a) Pibiri I, Buscemi S. A Recent Portrait of Bioactive Triazoles. Current Bioactive Compounds. 2010;6:208–242. [Google Scholar]; (b) Tripathi RP, Yadav AK, Ajay A, Bisht SS, Chaturvedi V, Sinha SK. Application of Huisgen (3+2) cycloaddition reaction: Synthesis of 1-(2,3-dihydrobenzofuran-2-yl-methyl [1,2,3]-triazoles and their antitubercular evaluations. Eur. J. Med. Chem. 2010;45:142–148. doi: 10.1016/j.ejmech.2009.09.036. [DOI] [PubMed] [Google Scholar]; (c) Siddiqui N, Ahsan W, Alam MS, Ali R, Jain S, Azad B, Akhtar J. Triazoles: as potential bioactive agents. Int. J. Pharm. Sci. Rev. Res. 2011;8:161–169. [Google Scholar]
- 7.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]; (c) Meldal M, Tornøe CW. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]; (d) Finn MG, Fokin VV. Click chemistry: function follows form. Chem. Soc. Rev. 2010;39:1231–1232. doi: 10.1039/c003740k. [DOI] [PubMed] [Google Scholar]
- 8.(a) Badillo JJ, Arevalo GE, Fettinger JC, Franz AK. Titanium-Catalyzed Stereoselective Synthesis of Spirooxindole Oxazolines. Org. Lett. 2010;13:418–421. doi: 10.1021/ol1027305. [DOI] [PubMed] [Google Scholar]; (b) Hanhan NV, Sahin AH, Chang TW, Fettinger JC, Franz AK. Catalytic Asymmetric Synthesis of Substituted 3-Hydroxy-2-Oxindoles. Angew. Chem. Int. Ed. 2010;49:744–747. doi: 10.1002/anie.200904393. [DOI] [PubMed] [Google Scholar]; (c) Badillo JJ, Silva-Garcia A, Shupe BH, Fettinger JC, Franz AK. Enantioselective Pictet-Spengler reactions of isatins for the synthesis of spiroindolones. Tetrahedron Lett. 2011;52(43):5550–5553. doi: 10.1016/j.tetlet.2011.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Evans DA, Sweeney ZK, Rovis T, Tedrow JS. Highly Enantioselective Syntheses of Homopropargylic Alcohols and Dihydrofurans Catalyzed by a Bis(oxazolinyl)pyridine-Scandium Triflate Complex. J. Am. Chem. Soc. 2001;123:12095–12096. doi: 10.1021/ja011983i. [DOI] [PubMed] [Google Scholar]; (b) Evans DA, Fandrick KR, Song H-J, Scheidt KA, Xu R. Enantioselective Friedel-Crafts Alkylations Catalyzed by Bis(oxazolinyl)pyridine-Scandium(III) Triflate Complexes. J. Am. Chem. Soc. 2007;129:10029–10041. doi: 10.1021/ja072976i. [DOI] [PubMed] [Google Scholar]
- 10.(a) Hanhan NV, Ball-Jones NR, Tran NT, Franz AK. Catalytic Asymmetric [3+2] Annulation of Allylsilanes with Isatins: Synthesis of Spirooxindoles. Angew. Chem. Int. Ed. 2012;51:989–992. doi: 10.1002/anie.201105739. [DOI] [PubMed] [Google Scholar]; (b) Hanhan NV, Tang YC, Franz AK. Scandium(III)-Catalyzed Enantioselective Allylation of Isatins Using Allylsilanes. Org. Lett. 2012 doi: 10.1021/ol300496v. Submitted. [DOI] [PubMed] [Google Scholar]
- 11.The absolute configuration of these molecules was determined by analogy to previously published x-ray crystal data. See references 8a–c for x-ray crystal data.
- 12.For recent examples and reviews of the Pictet-Spengler reaction, see: Taylor MS, Jacobsen EN. Highly Enantioselective Catalytic Acyl-Pictet-Spengler Reactions. J. Am. Chem. Soc. 2004;126:10558–10559. doi: 10.1021/ja046259p. Seayad J, Seayad AM, List B. Catalytic Asymmetric Pictet-Spengler Reaction. J. Am. Chem. Soc. 2006;128:1086–1087. doi: 10.1021/ja057444l. Stöckigt J, Antonchick AP, Wu F, Waldmann H. The Pictet–Spengler Reaction in Nature and in Organic Chemistry. Angew. Chem. Int. Ed. 2011;50:8538–8564. doi: 10.1002/anie.201008071. For a review of chiral phosphoric acids, see: Terada M. Chiral Phosphoric Acids as Versatile Catalysts for Enantioselective Transformations. Synthesis. 2010:1929–1982.
- 13.Duce S, Pesciaioli F, Gramigna L, Bernardi L, Mazzanti A, Ricci A, Bartoli G, Bencivenni G. An Easy Entry to Optically Active Spiroindolinones: Chiral Brønsted Acid-Catalysed Pictet–Spengler Reactions of Isatins. Adv. Synth. Catal. 2011;353:860–864. [Google Scholar]
- 14.Kenneth LK. Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. J. Fluorine Chem. 2006;127:1013–1029. [Google Scholar]
- 15.(a) Suga H, Shi X, Fujieda H, Ibata T. Reactions of 5-alkoxyoxazoles with aldehydes in the presence of Lewis acid: regio- and stereoselective formation of 4-alkoxycarbonyl-2-oxazolines. Tetrahedron Lett. 1991;32:6911–6914. [Google Scholar]; (b) Suga H, Shi X, Ibata T. Regio-Control of Formal [3 + 2] Cycloadditions of 5-Alkoxyoxazoles with Diethyl Oxomalonate. Chem. Lett. 1994;23:1673–1676. [Google Scholar]; (c) Mitchell JM, Shaw JT. A Structurally Diverse Library of Polycyclic Lactams Resulting from Systematic Placement of Proximal Functional Groups. Angew. Chem. Int. Ed. 2006;45:1722–1726. doi: 10.1002/anie.200503341. [DOI] [PubMed] [Google Scholar]
- 16.(a) Yu Z-X, Wu Y-D. A Theoretical Study of the Mechanisms and Regiochemistry of the Reactions of 5-Alkoxyoxazole with Thioaldehydes, Nitroso Compounds, and Aldehydes. J. Org. Chem. 2002;68:412–420. doi: 10.1021/jo026330e. [DOI] [PubMed] [Google Scholar]; (b) Yu Z-X, Wu Y-D. A DFT Study of the Mechanisms and Regio- and Stereochemistry of the Lewis Acid-Catalyzed Reactions of 5-Alkoxyoxazoles with Aldehydes: Aryl Substitution at the 2-Position of 5-Alkoxyoxazole Is Critical to the Formation of 4-Alkoxycarbonyl-2-oxazoline. J. Org. Chem. 2002;68:421–432. doi: 10.1021/jo0263317. [DOI] [PubMed] [Google Scholar]
- 17.Conrad WE, Fukazawa R, Haddadin MJ, Kurth MJ. The Davis-Beirut Reaction: N1,N2-Disubstituted-1H-Indazolones via 1,6-Electrophilic Addition to 3-Alkoxy-2H-Indazoles. Org. Lett. 2011;13:3138–3141. doi: 10.1021/ol2010424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Feldman AK, Colasson B, Fokin VV. One-Pot Synthesis of 1,4-Disubstituted 1,2,3-Triazoles from In Situ Generated Azides. Org. Lett. 2004;6:3897–3899. doi: 10.1021/ol048859z. [DOI] [PubMed] [Google Scholar]; (b) Andersen J, Bolvig S, Liang X. Efficient one-pot synthesis of 1-aryl 1,2,3-triazoles from aryl halides and terminal alkynes in the presence of sodium azide. Synlett. 2005:2941–2947. [Google Scholar]; (c) Ackermann L, Potukuchi HK, Landsberg D, Vicente R. Copper-Catalyzed "Click" Reaction/Direct Arylation Sequence: Modular Syntheses of 1,2,3-Triazoles. Org. Lett. 2008;10:3081–3084. doi: 10.1021/ol801078r. [DOI] [PubMed] [Google Scholar]
- 19.Goddard-Borger ED, Stick RV. An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-sulfonyl Azide Hydrochloride. Org. Lett. 2007;9:3797–3800. doi: 10.1021/ol701581g. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen DM, Miles DH. Copper(I)-Catalyzed Cycloaddition of Methyl O-Propargylpodocarpate and Azides at Room Temperature. Synth. Commun. 2011;41:1759–1771. [Google Scholar]
- 21.Dales N, Zhang Z, Fonarev J, Fu J, Kamboj R, Kodumuru V, Pokrovskaia N, Sun S. WO2008074835A1. Preparation of thiazole derivatives as stearoyl-CoA desaturase (SCD) inhibitors. 2008
- 22.(a) Guo C, Song J, Huang J-Z, Chen P-H, Luo S-W, Gong L-Z. Core-Structure-Oriented Asymmetric Organocatalytic Substitution of 3-Hydroxyoxindoles: Application in the Enantioselective Total Synthesis of (+)-Folicanthine. Angew. Chem. Int. Ed. 2011 doi: 10.1002/anie.201107079. [DOI] [PubMed] [Google Scholar]; (b) Castaldi MP, Troast DM, Porco JA. Stereoselective Synthesis of Spirocyclic Oxindoles via Prins Cyclizations. Org. Lett. 2009;11:3362–3365. doi: 10.1021/ol901201k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell-Verduyn LS, Szymanski W, Postema CP, Dierckx RA, Elsinga PH, Janssen DB, Feringa BL. One-Pot 'Click' reactions: Tandem Enantioselective Biocatalytic Epoxide Ring-Opening and [3+2] Azide-alkyne Cycloaddition. Chem. Commun. 2010;46:898–900. doi: 10.1039/b919434g. [DOI] [PubMed] [Google Scholar]
- 24.(a) Lipinski CA. Lead- and Drug-like Compounds: The Rule-of-five Revolution. Drug Discovery Today: Technologies. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]; (b) Hann MM, Oprea TI. Pursuing the leadlikeness concept in pharmaceutical research. Curr. Opin. Chem. Biol. 2004;8:255–263. doi: 10.1016/j.cbpa.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Fu Y, Lai L. A New Atom-additive Method for Calculating Partition Coefficients. J. Chem. Inf. Comput. Sci. 1997;37:615–621. [Google Scholar]
- 26.(a) Arkin MR, Wells JA. Small-molecule Inhibitors of Protein-protein Interactions: Progressing Towards the Dream. Nat. Rev. Drug Discovery. 2004;3:301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]; (b) Yin H, Hamilton AD. Strategies for Targeting Protein-protein Interactions with Synthetic Agents. Angew. Chem. Int. Ed. 2005;44:4130–4163. doi: 10.1002/anie.200461786. [DOI] [PubMed] [Google Scholar]
- 27.Sauer WHB, Schwarz MK. Molecular Shape Diversity of Combinatorial Libraries: A Prerequisite for Broad Bioactivity. J. Chem. Inf. Comput. Sci. 2003;43:987–1003. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
- 28.Ertl P, Rohde B, Selzer P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment Based Contributions and its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.