Abstract
Chronic failure to suppress intake during states of positive energy balance leads to weight gain and obesity. The ability to use context – including interoceptive satiety states – to inhibit responding to previously rewarded cues appears to depend on the functional integrity of the hippocampus. Recent evidence implicates energy dense Western diets in several types of hippocampal dysfunction, including reduced expression of neurotrophins and nutrient transporters, increased inflammation, microglial activation, and blood brain barrier permeability. The functional consequences of such insults include impairments in an animal’s ability to modulate responding to a previously reinforced cues. We propose that such deficits promote overeating, which can further exacerbate hippocampal dysfunction and thus initiate a vicious cycle of both obesity and progressive cognitive decline.
Introduction
There is mounting neuroanatomical and behavioral evidence that the hippocampus is a primary brain substrate for the control of food intake (for reviews see [1, 2]). For example, the hippocampus receives neural input from brain areas involved with the detection of metabolic signals, the perception of internal cues, taste, and reward. It is also the site of receptors for a multitude of neurochemical signals (e.g., cholecystokinin, leptin, insulin, glucose, ghrelin) that are known to contribute to energy intake and body weight regulation. In addition, hippocampal neurons project to multiple brain areas that are important substrates for energy balance and ingestive behavior [3, 4]. Furthermore, the hippocampus is critically involved with cognitive processes (e.g., memory, decision-making) that animals may use to determine when to eat [5, 6]. Accordingly, it should not be surprising that interference with hippocampal functioning would have adverse consequences for the control of eating and appetitive behavior.
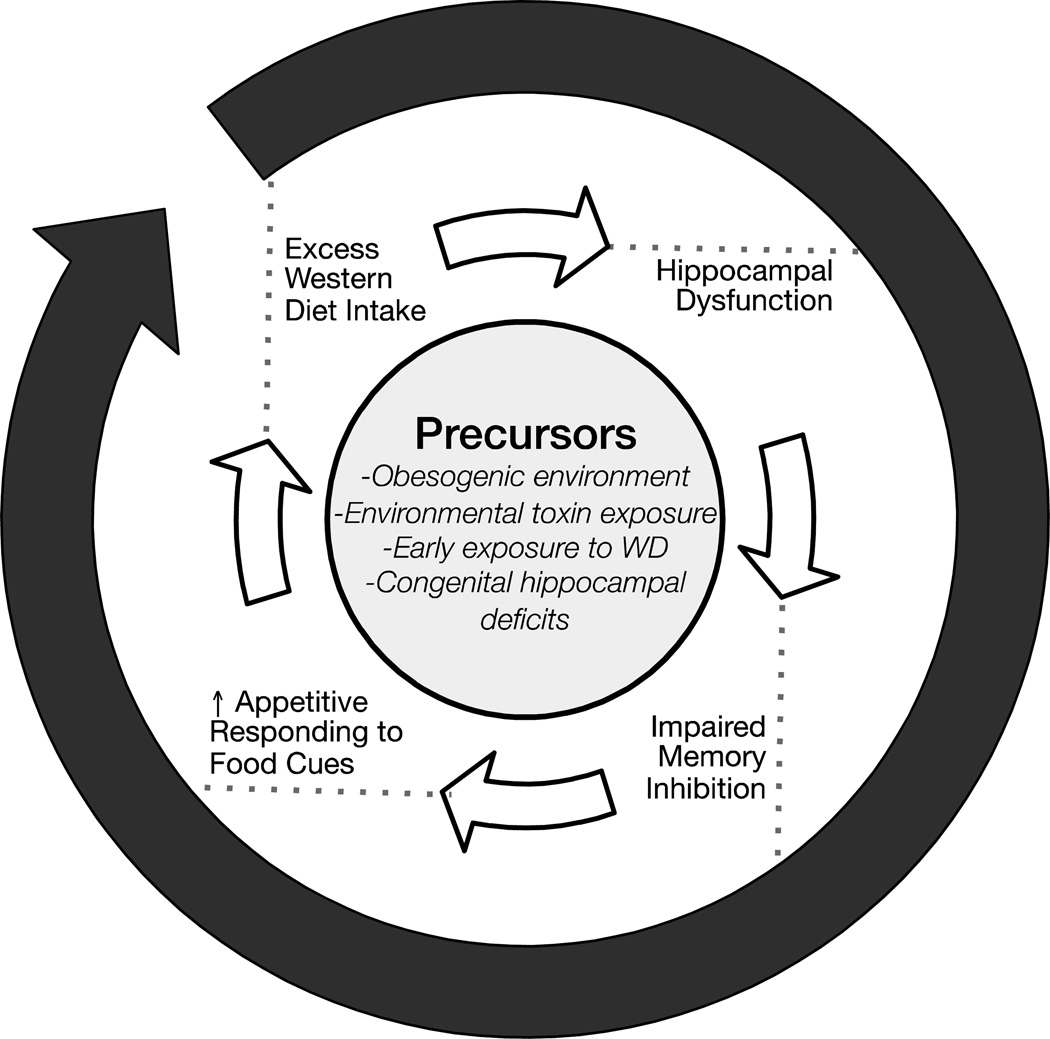
Recent evidence shows that consuming energy dense “western” diets has harmful effects on the hippocampus [7–9]. We and others have proposed that intake of such diets could invoke a vicious cycle (see F.1) of hippocampal dysfunction and impaired inhibitory cognitive control of responding to environmental food cues, resulting in excess intake, obesity, and further hippocampal dysfunction [2, 10–12]. In this review we describe the associative underpinnings of this vicious cycle and highlight recent findings pointing to the neurobehavioral mechanisms that may initiate and perpetuate it.
F.1.
A vicious cycle model of obesity and cognitive decline.
The Model
Functions of the hippocampus
The hippocampus is recognized as an important substrate for the encoding and retrieval of both spatial and several nonspatial forms of memory [13]. Recent data indicate that the hippocampus is also needed for decision-making processes, especially those involved with response selection in the face of conflicting or ambiguous information [14]. For example, the hippocampus has been implicated in resolving approach-avoidance discrepancies in situations in which two opposing response tendencies are experienced simultaneously [15, 16]. Other findings suggest adaptive response selection is based on the contribution of the hippocampus to the context-dependent inhibition of approach tendencies [14, 17, 18]. The retrieval of situationally-appropriate memories by contextual cues and the reduction of interference by memories formed in other contexts are also considered functions of the hippocampus [19, 20].
Contextual control of conflict resolution in ingestive behavior
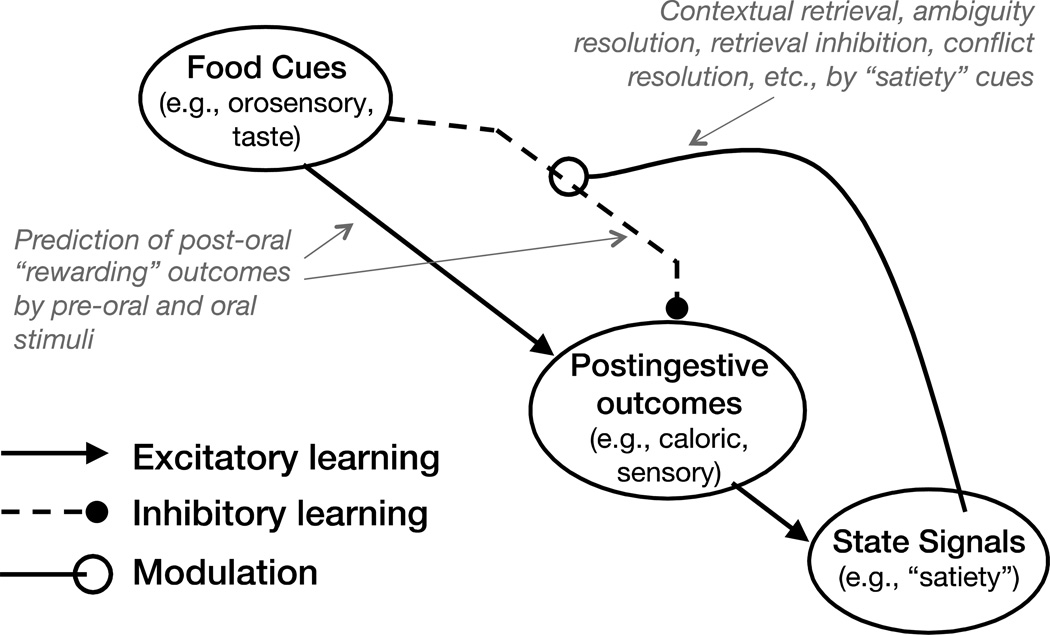
The model diagrammed in F.2 (adapted from [11]) shows how interoceptive “satiety” states may serve as contextual stimuli that animals can use to resolve ambiguities associated with whether or not to perform appetitive responses. Specifically, that diagram indicates that food and food-associated environmental stimuli are embedded concurrently in two conflicting associations. The excitatory association enables food cues to retrieve the memory (formed when animals eat food when they are not food sated) of hedonically-positive post-ingestive consequences. The retrieval of this memory promotes appetitive responding to food-related external cues.
F.2.
An associative model of energy intake and body weight regulation.
However, food cues do not always signal that the post-ingestive consequences of intake will be positive. For example, when an animal’s energy needs have been satisfied (i.e., they are food sated), the post-ingestive consequences may be neutral, or even aversive. This circumstance results in the formation of an inhibitory association that opposes the capacity of the excitatory association to excite the memory of the post-ingestive reward. Thus, when encountering external food cues animals must resolve the ambiguity produced by these conflicting associations to determine whether or not to engage in appetitive behavior. According to F.2, animals can solve this problem because their satiety signals act as contextual stimuli [21] that retrieve the memory of the inhibitory association, thereby antagonizing the ability of the excitatory association to retrieve the reward memory. The conflict is thus resolved in favor of refraining from engaging in appetitive and consummatory behaviors. As discussed above, this role for satiety signals describes a hippocampal-dependent function.
Supporting data
Hippocampal insult disrupts the inhibitory control of feeding
The case of Henry Moliason (a.k.a. H.M.) provided early evidence for hippocampal involvement in the control of intake [22] Following bilateral medial temporal lobectomy that damaged his hippocampus, H.M. not only exhibited anterograde amnesia so severe that he was unable to recall a meal he consumed a few minutes earlier, he would consume a second full meal minutes later and rate himself no more satiated after, compared to before, eating. Subsequent studies showed that rats with hippocampal damage more selective than H.M.’s increase their meal frequency [23], exhibit greater response perseveration on progressive ratio schedules [24] and show impairments in their ability to discriminate among their deprivation states [25, 26].
Recent findings also substantiate hippocampal involvement in feeding behavior. For example, temporary inactivation of the dorsal hippocampus in rats decreases intermeal intervals [27], suggesting a diminished capacity for satiety signals to inhibit intake during the period following a meal. Furthermore, the conversion of circulating triglycerides to fatty acids increases following food intake, providing a source of satiety signals detectable in the brain [28]. Interference with this conversion in the dorsal hippocampus increases weight gain in rodents [29]. In the ventral hippocampus, activation of receptors for the anorectic peptides leptin and GLP-1 decreases food intake and appetitive behavior based on food rewards [30, 31], whereas direct administration of the orexigenic hormone ghrelin (which presumably antagonizes satiety signaling) increases meal frequency and the ability of environmental food-related cues to stimulate eating [32]. These results suggest that interference with the hippocampal processing of either anorectic or orexigenic promotes positive energy balance.
Imaging studies in humans also provide evidence that the hippocampus is included among a group of feeding-related brain substrates that are influenced by both interoceptive satiety signals and external food cues. For example, consistent with the rodent findings noted above, a recent fMRI study with humans showed that the effect of satiation on the hippocampal response to palatable and energy-dense food was specifically associated with the meal’s ability to increase triglyceride, and reduce ghrelin, levels [33]. Hippocampal activation is also modulated as a function of obesity and feeding state by interoceptive signals of gastric distension [34] and by visual images of foods [35].
Such findings supports the associative model depicted in F.2, by providing evidence that (a) the hippocampus is sensitive to physiological signals that are informative about energy state; (b) appetitive behavior is altered by manipulations that influence the detection of those signals hippocampal receptors; (c) that hippocampal activation in responses to food-related cues is modulated by those signals; and (d) that disruption of hippocampal function has adverse effects on the ability to detect or use energy state cues to control the power of food cues to evoke eating and appetitive behavior after energy homeostasis has been achieved.
Links between diet, obesity and hippocampal dysfunction
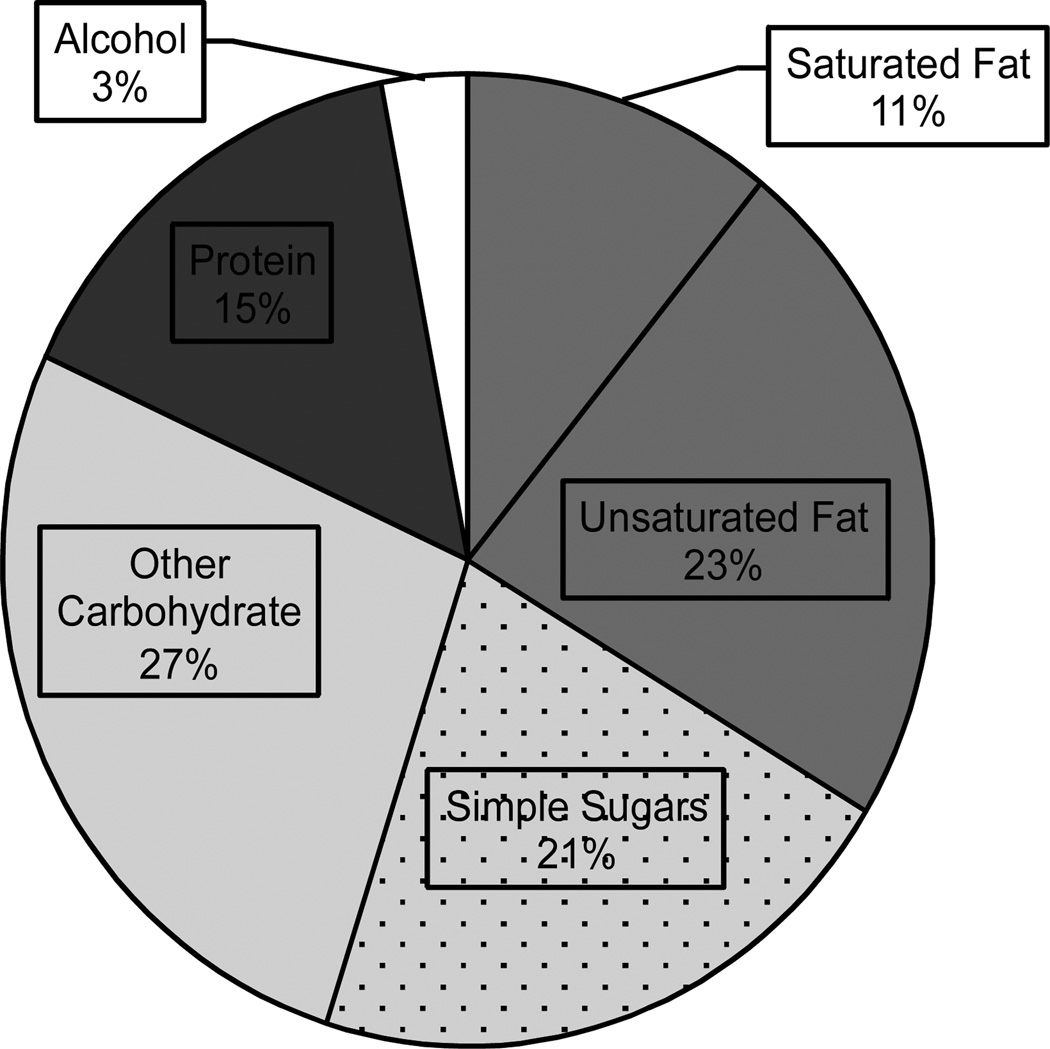
Saturated fat and simple sugar intake is strongly associated with weight gain, and diets high in these macronutrients are common throughout the United States [36, see F.3], Europe [37], and other Western and Westernized societies with high obesity rates. There is increasing evidence that consumption of these “Western Diets” (WD) can induce cognitive deficits and perturb hippocampal function. Recent evidence indicates that even relatively short-term consumption of a WD is associated with signs of hippocampal pathology. For example, 10 days maintenance on a WD led to reduced hippocampal and hypothalamic mRNA expression of GLUT1 and MCT1, genes involved in the transport of glucose and monocarboxylates (e.g., ketones, lactate, pyruvate), respectively [38]. Elevated levels of cell death in the hippocampus have also been reported after three days of WD exposure [39].
F.3.
Average macronutrient consumption for US adults, 2011–2012
Extended maintenance on a WD leads to more pronounced neurological consequences. It has been known for some time that WD can lead to neuroinflammation [40–45] and reduced hippocampal and hypothalamic levels of brain-derived neurotrophic factor (BDNF) [46–48], a protein that serves to promote neurogenesis, synaptic transmission, and memory performance [46, 49–51]. Current research has demonstrated that sustained WD access can also impair long-term potentiation (a potential cellular mechanism for learning and memory) in the hippocampus and greater hippocampal formation [52, 53]. Further, WD consumption induces microglial activation in the hippocampus, which is improved following roux-en-y gastric bypass surgery and caloric restriction [54, 55]. Contemporary research by our laboratory has established that WD maintenance can alter the blood-brain barrier (BBB), a critical interface between the cirulatory and nervous systems. This BBB damage is indicated by reduced expression of the tight-junction proteins that comprise the BBB [56], and increases in BBB permeability to molecular tracers such as sodium fluorescein. These pathologies are most pronounced in the hippocampal formation [56, 57], which is believed to be especially vulnerable to insult as a result of its high nutrient demands and pronounced cellular plasticity [58].
It is currently unclear whether these pathological events influence each other, or occur independently. Neuroinflammation, for instance, has been shown to induce neurodegeneration [41, 59, 60] and BBB remodelling [61–63] under some circumstances. However, BBB damage (and the subsequent infiltration of blood-borne toxins) can also promote inflammation, microglial activation, and cell death; for this reason, BBB breakdown is hypothesized to contribute to the progression of neurodegenerative conditions such as Alzheimer’s disease and multiple sclerosis [64].
Maintenance on a WD impairs performance on hippocampal-dependent tasks
Consistent with evidence of hippocampal injury, WD-fed rodents are selectively impaired at numerous hippocampal-dependent tasks. The earliest of these reports indicated that diet could alter spatial performance in the Morris Water Maze [47, 65]. Subsequent reports confirmed and expanded these findings, first indicating that WD maintenance led to increased spatial reference and working memory errors on a radial-arm maze [66] and more recently demonstrating delayed acquisition and a bias toward a non-spatial “response” strategy on a two-arm choice task [67].
Another recent study showed that WD-fed rats that become obese, exhibit elevated hippocampal expression of the cytokine IL-1β, and show impairments on tasks of contextual fear conditioning. This behavioral effect was normalized after maintenance on standard laboratory chow or antagonism of the IL-1 receptor, indicating cytokines may influence hippocampal-dependent cognition, though the mechanism behind this effect is not yet known [42]. Recently, it has been demonstrated that performance on hippocampal-dependent relational memory was perturbed in children with high levels of abdominal adiposity [68], or high saturated fatty acid intake [69], indicating these effects translate to humans and may impact individuals even at a young age.
We have also observed impairments in hippocampal-dependent serial feature negative (sFN) discrimination performance [see 70] after WD exposure [56]. This finding is of special interest because sFN perforamnce appears to require rats to learn a set of associative relations analogous to that shown in F.2, except that external cues are substituted for satiety signals [11]. Performance of the same rats on a hippocampal-independent simple discrimination problem involving the same reinforcer and response requirements was not impaired by WD, indicating that motivational or physical deficits did not underlie impaired sFN performance. Recent evidence suggests WD intake is associated with a distinct temporal pattern of sFN deficits. Performance is temporarily impaired after short-term (10 day) access, remits, then returns again after a more extended (e.g., 90 day) period [57]. These deficits often correlate with the pernicous brain changes associated with WD intake and obesity [56, 57, 71]. Our research program has observed the greatest degree of pathology (e.g., BBB permeability) in rodents that also showed hippocampal-dependent cognitive dysfunction and excess weight gain [57, 67, 72]. This suggests that these diets promote weight gain as a result of their ability to disrupt hippocampal function, and therefore the inhibitory control of food intake.
Further, WD-fed animals are less adept at using interoceptive food cues to exert discriminative control over appetitive behavior. In a recent example [73], rats were simultaneously trained to use interoceptive (deprivation state) and exteroceptive (tone, white noise) cues to predict the presence of a sucrose reward. Rats were then either maintained on chow or switched to the WD, the latter of which weakened the ability of deprivation state to modulate food intake. WD-fed rats maintained the ability to utilize exteroceptive cues to predict reward, suggesting that a WD dimishes the discriminatory power of internal cues, relative to external stimuli.
The “outward” spiral
Here, we have described a vicious cycle in which high-energy diets harm the hippocampus (see F.1). This progressively weakens the ability of satiety signals to inhibit the capacity of environmental food-related stimuli to elicit appetitive behaviors, and results in positive energy balance, weight gain, and further harm to the hippocampus. We have described how this cycle may be initiated by WD consumption or the obesity that results from WD maintenance. However, it could commence with hippocampal injury resulting from disease, trauma, exposure to environmental toxins, or other factors. Rather than damaging the hippocampus directly, the effects of a WD, toxic substances, or illnesses could also alter hippocampal function indirectly by compromising the integrity of the BBB.
This model does not deny the possibility that, in addition to the dampening of inhibitory controls, external cues have become more powerful and prevalent elicitors of appetitive behavior. So-called obesogenic environments, which are rife with cheap, palatable, energy-dense foods, are common throughout the industrialized world. Residents of these communities are bombarded with advertising and marketing materials that serve as almost constant reminders to eat [74–77]. However, we suggest that at least part of the rise in the power of environmental stimuli to evoke intake is a reduced ability of interoceptive satiety signals to offset that power.
In addition to obesogenic environmental stimuli, western and westernized societies contain numerous external cues designed to inhibit intake, such as nutritional information labels, public service announcements describing the health risks associated with obesity, frequent advertisements for dietary interventions, and widespread cultural penchants for slimness. Given the continuing obesity pandemic, the effectiveness of these “obesolytic” cues can also be questioned [but see 75, 78]. It may be that, like external cues in sFN discrimination problems, WD intake also reduces the ability of obesolytic environmental stimuli to antagonize the eating evoked by obesogenic environmental cues. This speculation suggests that interference with hippocampal-dependent mechanisms may diminish the potential for both interoceptive and exteroceptive stimuli to inhibit appetitive and consummatory behaviors.
Regardless of its etiology, new findings summarized here indicate that the “outward spiral” may arise based, at least in part, on WD-induced pathologies that interfere with hippocampal function. In rats, this interference can be observed after relatively brief exposure (90 days or less) to a WD and may be manifested in the form of reduced ability to use interoceptive satiety signals to counter the power of food-related environmental stimuli to evoke appetitive and eating behaviors.
Highlights.
The hippocampus is a substrate for the contextual stimulus control of behavior.
Satiety signals are contextual cues that underlie the inhibitory control of eating.
The Western diet (WD) is associated with hippocampal pathology and dysfunction.
Both hippocampal damage and WD impair discriminative control by satiety cues.
WD may induce a vicious cycle of overeating and hippocampal-based memory decline.
Acknowledgments
This manuscript was supported by a grant from National Institute of Child Health and Human Development, HD29792 (TLD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sara L. Hargrave, Email: Hargrave@american.edu.
Sabrina Jones, Email: sj8677a@student.american.edu.
Terry L. Davidson, Email: terryd@american.edu.
References
- 1.Kanoski SE, Grill HJ. Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parent MB, Darling JN, Henderson YO. Remembering to eat: hippocampal regulation of meal onset. Am J Physiol Regul Integr Comp Physiol. 2014;306:R701–R713. doi: 10.1152/ajpregu.00496.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blessing EM, Beissner F, Schumann A, et al. A data-driven approach to mapping cortical and subcortical intrinsic functional connectivity along the longitudinal hippocampal axis. Hum Brain Mapp. 2015 doi: 10.1002/hbm.23042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrovich GD. Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav. 2013;121:10–18. doi: 10.1016/j.physbeh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluth S, Sommer T, Rieskamp J, Buchel C. Effective Connectivity between Hippocampus and Ventromedial Prefrontal Cortex Controls Preferential Choices from Memory. Neuron. 2015;86:1078–1090. doi: 10.1016/j.neuron.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Palombo DJ, Keane MM, Verfaellie M. How does the hippocampus shape decisions? Neurobiol Learn Mem. 2015;125:93–97. doi: 10.1016/j.nlm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013;63:119–128. doi: 10.1016/j.appet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Front Aging Neurosci. 2014;6:88. doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacka FN, Cherbuin N, Anstey KJ, et al. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015;13:215. doi: 10.1186/s12916-015-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Davidson TL, Tracy AL, Schier LA, Swithers SE. A View of Obesity as a Learning and Memory Disorder. Journal of Experimental Psychology-Animal Behavior Processes. 2014;40:261–279. doi: 10.1037/xan0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shefer G, Marcus Y, Stern N. Is obesity a brain disease? Neurosci Biobehav Rev. 2013;37:2489–2503. doi: 10.1016/j.neubiorev.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Squire LR, Dede AJ. Conscious and unconscious memory systems. Cold Spring Harb Perspect Biol. 2015;7:a021667. doi: 10.1101/cshperspect.a021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor AM, Bus T, Sprengel R, et al. Hippocampal NMDA receptors are important for behavioural inhibition but not for encoding associative spatial memories. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130149. doi: 10.1098/rstb.2013.0149. Mice without dentate gyrus or CA1 NMDA receptors could learn hippocampal-dependent spatial tasks, but were impaired at reversal learning, indicating a role for these receptors, and the hippocampus in general, in conflict resolution and behavioral inhibition.
- 15. Oehrn CR, Baumann C, Fell J, et al. Human Hippocampal Dynamics during Response Conflict. Curr Biol. 2015;25:2307–2313. doi: 10.1016/j.cub.2015.07.032. Human response conflict was tested using the Stroop test in epileptic patients using intracranial electroencephalography. Activation of the hippocampus was increased during response conflict, and higher theta oscillations were associated with successful conflict resolution.
- 16. Schumacher A, Vlassov E, Ito R. The ventral hippocampus, but not the dorsal hippocampus is critical for learned approach-avoidance decision making. Hippocampus. 2015 doi: 10.1002/hipo.22542. Excitotoxic lesions of the ventral, but not the dorsal hippocampus led to increased preference for a cue that was associated with both appetitive and aversive stimuli. This indicates the ventral hippocampus is necessary to exert inhibitory control over previously rewarded, but presently aversive cues.
- 17.Jurado-Parras MT, Sanchez-Campusano R, Castellanos NP, et al. Differential contribution of hippocampal circuits to appetitive and consummatory behaviors during operant conditioning of behaving mice. J Neurosci. 2013;33:2293–2304. doi: 10.1523/JNEUROSCI.1013-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee I, Byeon JS. Learning-dependent Changes in the Neuronal Correlates of Response Inhibition in the Prefrontal Cortex and Hippocampus. Exp Neurobiol. 2014;23:178–189. doi: 10.5607/en.2014.23.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramirez S, Liu X, Lin PA, et al. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. Mice were exposed to a novel context, after which fos-expressing cells in the dentate gyrus and CA1 regions of the hippocampus were tagged with an optogenetic probe. In a second context, concurrent foot shock and optogenetic activation of these neurons occurred. Mice demonstrated increased freezing behaviors in the first context, despite no fear conditioning taking place there; this was context-specific, as little freezing occurred in a third novel context.
- 20. Smith DM, Bulkin DA. The form and function of hippocampal context representations. Neurosci Biobehav Rev. 2014;40:52–61. doi: 10.1016/j.neubiorev.2014.01.005. This excellent review discusses the role of the hippocampus in contextual representations, particularly with regard to priming relevant memories, and preventing interference from irrelevant memories.
- 21.Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiology of Learning and Memory. 2014;108:172–184. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection -Case HM. Behav Neurosci. 1985;99:1031–1039. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- 23.Clifton PG, Vickers SP, Somerville EM. Little and often: Ingestive behavior patterns following hippocampal lesions in rats. Behavioral Neuroscience. 1998;112:502–511. doi: 10.1037//0735-7044.112.3.502. [DOI] [PubMed] [Google Scholar]
- 24.Schmelzeis MC, Mittleman G. The hippocampus and reward: Effects of hippocampal lesions on progressive-ratio responding. Behavioral Neuroscience. 1996;110:1049–1066. doi: 10.1037//0735-7044.110.5.1049. [DOI] [PubMed] [Google Scholar]
- 25.Davidson TL, Kanoski SE, Chan K, et al. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124:97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23:100–107. doi: 10.1002/hipo.22062. [DOI] [PubMed] [Google Scholar]
- 28.Magnan C, Levin BE, Luquet S. Brain lipid sensing and the neural control of energy balance. Mol Cell Endocrinol. 2015;418(Pt 1):3–8. doi: 10.1016/j.mce.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Picard A, Rouch C, Kassis N, et al. Hippocampal lipoprotein lipase regulates energy balance in rodents. Mol Metab. 2014;3:167–176. doi: 10.1016/j.molmet.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu TM, Hahn JD, Konanur VR, et al. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40:327–337. doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanoski SE, Hayes MR, Greenwald HS, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36:1859–1870. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2013;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Veldhuizen MG, Wray AE, et al. The neural signature of satiation is associated with ghrelin response and triglyceride metabolism. Physiol Behav. 2014;136:63–73. doi: 10.1016/j.physbeh.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy J, Dimitropoulos A. Influence of feeding state on neurofunctional differences between individuals who are obese and normal weight: a meta-analysis of neuroimaging studies. Appetite. 2014;75:103–109. doi: 10.1016/j.appet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Wallner-Liebmann S, Koschutnig K, Reishofer G, et al. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity (Silver Spring) 2010;18:1552–1557. doi: 10.1038/oby.2010.26. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes D, Clemens J, Goldman J, et al. 2011–2012 What We Eat in America, NHANES Tables 1–40. Worldwide Web Site: Food Surveys Research Group. 2014 [Google Scholar]
- 37.Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. The American journal of clinical nutrition. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 38.Hargrave SL, Davidson TL, Lee TJ, Kinzig KP. Brain and behavioral perturbations in rats following Western diet access. Appetite. 2015 doi: 10.1016/j.appet.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gan L, England E, Yang JY, et al. A 72-hour high fat diet increases transcript levels of the neuropeptide galanin in the dorsal hippocampus of the rat. BMC Neurosci. 2015;16:51. doi: 10.1186/s12868-015-0188-9. Acute consumption of high-fat diets led to increased histone deacetylase expression in the ventral hippocampus.
- 40.Boitard C, Cavaroc A, Sauvant J, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Miller AA, Spencer SJ. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behavior and Immunity. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 42. Sobesky JL, Barrientos RM, De May HS, et al. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1 beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behavior and Immunity. 2014;42:22–32. doi: 10.1016/j.bbi.2014.06.017. Hippocampal memory function was impaired in rats fed high-fat diets, which also showed elevated hippocampal cytokine expression. After being switched back to standard chow, memory and inflammation were normalized, but body weight remained elevated compared to rats that had not been maintained on high-fat diet.
- 43.Bazar KA, Yun AJ, Lee PY, et al. Obesity and ADHD may represent different manifestations of a common environmental oversampling syndrome: a model for revealing mechanistic overlap among cognitive, metabolic, and inflammatory disorders. Medical Hypotheses. 2006;66:263–269. doi: 10.1016/j.mehy.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 44.White CL, Pistell PJ, Purpera MN, et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiology of disease. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pistell PJ, Morrison CD, Gupta S, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. Journal of neuroimmunology. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of neurochemistry. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 47.Molteni R, Barnard RJ, Ying Z, et al. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 48.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behavioural brain research. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korte M, Carroll P, Wolf E, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proceedings of the National Academy of Sciences. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizuno M, Yamada K, Olariu A, et al. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. The Journal of Neuroscience. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karimi SA, Salehi I, Komaki A, et al. Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: an in vivo study. Brain Res. 2013;1539:1–6. doi: 10.1016/j.brainres.2013.09.029. Consumption of high-fat diets led to reductions in long-term potentiation in the dentate gyrus. Supplementation of antioxidants reversed these effects.
- 53. Krishna S, Keralapurath MM, Lin Z, et al. Neurochemical and electrophysiological deficits in the ventral hippocampus and selective behavioral alterations caused by highfat diet in female C57BL/6 mice. Neuroscience. 2015;297:170–181. doi: 10.1016/j.neuroscience.2015.03.068. Consumption of high-fat diets for 11–12 weeks led to reduced long-term potentiation and paired-pulse ratio in the ventral hippocampus.
- 54. Grayson BE, Fitzgerald MF, Hakala-Finch AP, et al. Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.100. Though both bariatric procedures elicited metabolic improvements and general improvements in the cognitive performance of formerly obese rats, Roux en Y gastric bypass, but not vertical sleeve gastrectomy, rescued spatial learning deficits on the Morris water maze, and reduced hippocampal inflammation.
- 55. Calvo-Ochoa E, Hernandez-Ortega K, Ferrera P, et al. Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. Journal of Cerebral Blood Flow and Metabolism. 2014;34:1001–1008. doi: 10.1038/jcbfm.2014.48. Seven days of access to a high-fat chow and high-fructose corn syrup-laden water led to obesity, insulin resistance, reduced hippocampal weight, reductions in cA1 dendritic arborizations and spine density, elevated tau phosphorylation and reactive gliosis.
- 56.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davidson TL, Hargrave SL, Swithers SE, et al. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–122. doi: 10.1016/j.neuroscience.2013.08.044. Rats were fed Western diet, ketogenic diet, or chow, probed for memory deficits, then tested for blood-brain barrier permeability. Obese rats fed Western diet showed feature negative discrimination impairments and elevated hippocampal blood-brain barrier permeability; this was not the case with obese rats fed ketogenic diet, or animals that did not gain excess weight on Western diet.
- 58. Williamson LL, Bilbo SD. Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain, behavior, and immunity. 2013;30:186–194. doi: 10.1016/j.bbi.2013.01.077. The authors provide compelling evidence that hippocampal plasticity may be a double-edged sword that contributes to both increased vulnerability to external insult and remarkable capacity for learning and memory.
- 59.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Claycombe KJ, Brissette CA, Ghribi O. Epigenetics of inflammation, maternal infection, and nutrition. The Journal of nutrition. 2015;145:1109S–1115S. doi: 10.3945/jn.114.194639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood–brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 62.Fiala M, Liu QN, Sayre J, et al. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. European journal of clinical investigation. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 63.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends in neurosciences. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 64.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature Reviews Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stranahan AM, Norman ED, Lee K, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:313–319. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- 67. Hargrave SL, Davidson TL, Zheng W, Kinzig KP. Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behavioral Neuroscience. 2015 doi: 10.1037/bne0000110. in press. Western diets, but not high-fat ketogenic diets, lead to reductions in hippocampal mRNA expression of GLUT1 and MCT1 transporters, and impair spontaneous alternation and vicarious trial and error behaviors.
- 68. Khan NA, Baym CL, Monti JM, et al. Central adiposity is negatively associated with hippocampal-dependent relational memory among overweight and obese children. The Journal of pediatrics. 2015;166:302–308. e301. doi: 10.1016/j.jpeds.2014.10.008. In overweight and obese children, abdominal adipose tissue significantly predicted lower performance on hippocampal-dependent relational memory accuracy.
- 69. Baym CL, Khan NA, Monti JM, et al. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am J Clin Nutr. 2014;99:1026–1032. doi: 10.3945/ajcn.113.079624. Saturated fatty acid intake was negatively associated with both hippocampal-dependent relational and hippocampal-independent item memory in children. Among this same cohort, omega-3 fatty acid intake was positively associated with relational memory performance.
- 70.Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 71.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davidson TL, Monnot A, Neal AU, et al. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol Behav. 2012;107:26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sample CH, Martin AA, Jones S, et al. Western-style diet impairs stimulus control by food deprivation state cues: Implications for obesogenic environments. Appetite. 2015;93:13–23. doi: 10.1016/j.appet.2015.05.018. In rats fed standard laboratory chow, deprivation cues elicit comparable levels of discriminative control over conditioned responding to that of discrete auditory cues. After maintenance on Western diet, rats were unable to use deprivation state to predict reinforcement in the appetitive task above, but retained the ability to use discrete auditory cues. This indicates that Western diet and obesity diminish the potency of interoceptive, relative to external, cues to elicit behavior.
- 74.Swinburn BA, Sacks G, Hall KD, et al. Obesity 1 The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 75.Corsica JA, Hood MM. Eating disorders in an obesogenic environment. J Am Diet Assoc. 2011;111:996–1000. doi: 10.1016/j.jada.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 76.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. International Journal of Obesity. 2009;33:S8–S13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nature Reviews Endocrinology. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 78.Kaye WH, Wierenga CE, Bailer UF, et al. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends in neurosciences. 2013;36:110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]