Abstract
Background & Aims
Natalizumab, a humanized antibody against the α4 integrin subunit, effectively induces and maintains remission in patients with Crohn’s disease (CD) refractory to conventional treatments. Progressive multi-focal leukoencephalopathy is a rare but fatal brain infection caused by JC virus and has been associated with natalizumab use. We assessed the prevalence of and risk factors for antibodies to JC virus in serum of patients with refractory CD who were candidates for, or were already receiving, natalizumab. We also assessed the effects of natalizumab treatment of these patients.
Methods
In a retrospective study, we analyzed clinical charts from 191 patients with CD (74 male; mean age, 38.7 y; mean duration of disease, 14.9 y) tested for serum JC virus antibody from December 2012 through May 2014 at 2 medical centers in the US. We calculated JC virus antibody prevalence and compared characteristics of patients who tested negative vs those who tested positive, to identify risk factors. We also assessed the rate of subsequent natalizumab use, surgery, and seroconversion during natalizumab therapy.
Results
One hundred and twenty-nine of the patients (67.5%) tested positive for serum JC virus antibody. Multivariate analysis demonstrated that past use of thiopurine was a risk factor for testing positive for JC virus antibody (odds ratio 7.8; 95% confidence interval [CI], 2.0–30.4; P= .003). Twenty-two of the patients who tested negative for JC virus antibody (35.5%) and 16 of the 129 patients who tested positive (12.4%) had been treated with natalizumab. Cox regression analysis determined that natalizumab use was the only factor associated with avoiding subsequent surgery (hazard ratio, 0.23; 95% CI, 0.06–0.98)). Seroconversion (from testing negative to positive for JC virus antibody) occurred in 1 of the 22 patients (4.5%) who initially tested negative during natalizumab therapy.
Conclusion
The prevalence of CD patients exposed to JC virus is comparable to that of the general population. In this retrospective study, prior thiopurine use was associated with an increased risk for testing positive for JC virus antibody. Natalizumab use reduced the risk of subsequent surgery.
Keywords: inflammatory bowel disease, thiopurine, PML, biologic, IBD
INTRODUCTION
Intensive research has started to unravel the pathogenesis of Crohn’s disease (CD)1–3, however, its clinical course is still characterized by chronic and progressive inflammation of the gastrointestinal tract that often results in surgery4. Anti-tumor necrosis factor (TNF) agents are now widely used in the treatment of CD, given their efficacy for achieving and maintaining clinical remission, as well as the ability to induce mucosal healing and prevent surgery5–8. Natalizumab (Tysabri®, Elan Pharmaceuticals, South San Francisco, CA) is a humanized IgG4 anti-α4-integrin antibody that blocks the adhesion and subsequent migration of leukocytes from the blood vessels into inflamed tissues, a mechanism distinct from anti-TNF agents. Natalizumab has Food and Drug Administration (FDA) approval for the treatment of multiple sclerosis (MS) and CD, and was the first drug approved in this class to treat inflammatory bowel disease (IBD) only in the US. Although it was shown to be effective and well-tolerated in clinical trials of CD9, 10, the development of progressive multifocal leukoencephalopathy (PML), resulted in its use being limited to adult CD patients who have failed or have had intolerance to conventional treatment including anti-TNF agents11–13. Progressive multifocal leukoencephalopathy (PML) is a rare demyelinating disease of the brain, often resulting in severe disability or death, caused by John Cunningham (JC) virus in the setting of host immunosuppression14. The majority of reports of PML associated with natalizumab use have been in subjects with MS, where positive anti-JC virus antibody, prior use of immunosuppressive treatment, and longer duration of natalizumab therapy have been shown to be risk factors for PML15. The seroprevalence of anti-JC virus antibodies varies from 40 to 90% depending on the assay and population studied16, 17. In January 2012, testing for anti-JC virus antibody in the blood became commercially available to assess whether the patient has been exposed to the virus18. We and others have recently demonstrated that natalizumab was effective and safe in CD patients with a history of refractory disease19–21, however, the prevalence of JC virus antibody and their implication for CD therapy remains unknown.
The present retrospective cohort study was performed to identify the prevalence and risk factors of JC virus antibody in patients with CD in whom natalizumab was either used or contemplated being used, and to assess their impact on subsequent natalizumab use and surgery.
METHODS
This was a retrospective cohort study of patients with CD who have had an inadequate response to anti-TNF alpha agents and immunomodulators, and were tested for the presence of anti-JC virus antibody in their blood (STRATIFY JCV; Quest Diagnostics, Madison, NJ) in consideration for natalizumab use between December 2012 and May 2014 at the University of Chicago IBD Center and Oregon Health & Science University. The study was approved by both institutions’ institutional review boards. Patient charts were screened and for those eligible, details about disease phenotype, disease duration, treatment history, previous or subsequent natalizumab use and surgery were obtained.
Outcomes
Outcome measures were defined prior to performing the study. The primary analyses were 1) prevalence of anti-JC virus antibody in these CD patients, and 2) identification of risk factors for the presence of blood anti-JC virus antibody. Secondary analysis included 1) the rate of natalizumab use following the results of JC virus antibody test, 2) the incidence of subsequent CD related surgery and identification of risk factors for surgery, 3) seroconversion from a negative to positive JC virus antibody status in patients receiving natalizumab who had more than one JC virus antibody test. Intestinal resection, stricturoplasty, perianal surgery, creation of diverting ileostomy/colostomy or abscess drainage were defined as CD-related surgeries. Ileostomy revision or exam under anaesthesia without seton placement or abscess drainage were excluded.
Statistical methods
For statistical analysis, data were processed by EZR22 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0, Vienna, Austria). Standard descriptive statistics, such as mean, median, range and standard deviations were computed for continuous variables. Statistical analysis was done as noted in each section. All p-values are two-sided and p < 0.05 was considered statistically significant. Where indicated, multivariate analysis was performed with factors that showed a p-value of <0.10 in univariate analysis. Duration to surgery after the JC virus test was compared between groups by Kaplan-Meier survival analysis or Cox proportional hazards regression analysis where indicated. Log-rank trend test was performed to assess the discrepancy of linear trend between groups where applicable.
RESULTS
Patients
The characteristics of the 191 patients who had anti-JC virus antibody tested during the study period are shown in Table 1. They were all tested to assess prior to or after initiation of natalizumab therapy. Twenty-four patients (12.6%) were already on natalizumab at the time of their first JC virus antibody test (median 26.5 months, range 1–46). Seventy-four (38.7%) were male with a mean age of 38.7 years. Mean duration of disease was 14.9 years. One hundred-twenty-two patients (63.9%) had a history of CD-related surgeries. 86.2 and 96.9% of patients had a history of previous thiopurine and anti-TNF agent use, respectively, confirming that the majority of patients had refractory disease.
Table 1.
| Patient characteristics | |||||
|---|---|---|---|---|---|
| JC virus antibody status | |||||
| All patients | Negative | Positive | p-value (negative vs positive) | ||
| n = 191 | n = 62 | n= 129 | Univariate | Multivariate | |
| Gender, male : female (%male) | 74 : 117 (38.7) | 20 : 42 (32.3) | 54 : 75 (41.9) | 0.21 | |
| Age (years), mean (SD) | 38.7 (13.6) | 35.9 (12.7) | 40.0 (13.9) | 0.05 | 0.88 |
| Disease duration (years), mean (SD) | 14.9 (9.5) | 13.9 (9.7) | 15.4 (9.4) | 0.34 | |
| Disease location (ileum: colon: ileocolonic) | 20 : 63 : 107 | 4 : 27: 31 | 16: 36: 77 | 0.07 | 0.64 |
| Perianal disease, n (%) | 70 (36.6) | 27 (43.5) | 43 (33.3) | 0.20 | |
| Disease behavior (fistulizing : non-fistulizing) | 98:93 | 35 (56.5) | 58 (45.0) | 0.17 | |
| Smoker, n (%) | 15 (7.9) | 2 (3.4) | 13 (10.7) | 0.15 | |
| History of surgery (%) | 122 (63.9) | 39 (62.9) | 83 (64.3) | 0.87 | |
| CRP (mg/L), mean (SD) | 14.0 (19.1) | 18.6 (23.6) | 11.7 (16.1) | 0.07 | 0.10 |
| Ethnicity (Caucasian : others : unknown) | 143: 17:31 | 41 : 4: 17 | 102: 13: 14 | 0.99 | |
| Past medications | |||||
| Corticosteroid†, n (%) | 104 (58.8) | 28 (48.3) | 76 (63.9) | 0.05 | 0.90 |
| Natalizumab#, n (%) | 24 (12.6) | 8 (12.9) | 16 (12.4) | 1.0 | |
| Thiopurine, n (%) | 162 (86.2) | 46 (74.2) | 116 (92.1) | 0.001* | 0.003* |
| Methotrexate, n (%) | 80 (42.6) | 20 (32.3) | 60 (47.6) | 0.06 | 0.10 |
| Calcineurin inhibitor, n (%) | 10 (5.3) | 1 (1.6) | 9 (7.1) | 0.17 | |
| Anti-TNF agent, n (%) | 185 (96.9) | 61 (98.4) | 124 (96.1) | 0.67 | |
| Number of anti-TNF agents, median (range) | 2 (0–3) | 2 (0–3) | 2 (0–3) | 0.52 | |
| Outcome | |||||
| Use of natalizumab, n (%) | 38 (19.9) | 22 (35.5) | 16 (12.4) | <0.001* | |
| Surgery after JCV Ab test, n (%) | 28 (14.7) | 11 (17.7) | 17 (13.2) | 0.39 | |
p <0.05;
use of the corticosteroid > 3 months at the time of JC virus antibody check;
use of natalizumab at the time of JC virus antibody check
Risk factors for JC virus exposure
The prevalence of blood anti-JC virus antibody in our patients was 67.5% (129/191) (Table 1). In univariate analysis, prior use of thiopurines was significantly more frequent in patients who tested positive as compared to those with a negative result (92.1 vs 74.2%, p = 0.001). Older age (p = 0.05), corticosteroid use (p = 0.05), and methotrexate use (p = 0.06) showed a strong trend towards a positive serology. Multivariate analysis with all factors that had a p-value of <0.10 on univariate analysis demonstrated that only prior use of thiopurines was associated with a positive JC virus serology (odds ratio 7.8 (95%CI 2.0–30.4), p = 0.003). Methotrexate use and lower CRP level also demonstrated a non-significant trend by multivariate analysis (Table 1).
Subsequent natalizumab use and effect on surgery
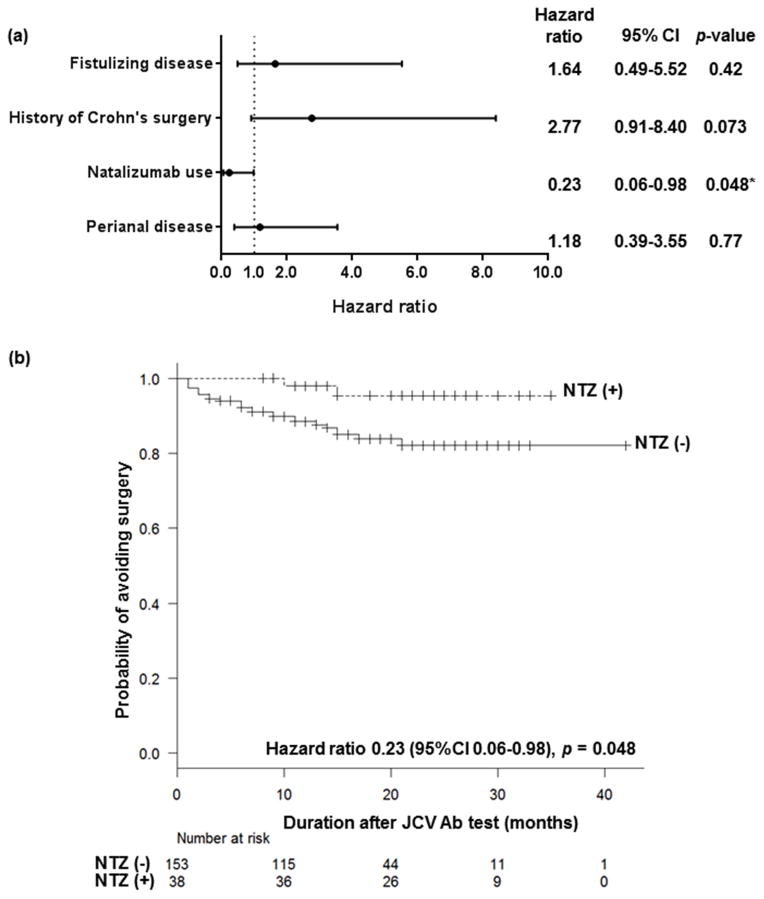
More patients were treated with natalizumab following a negative JC virus antibody test than those with a positive result (Table 1; 35.5% and 12.4%, respectively, p<0.001). Natalizumab was given as monotherapy, and no patients received thiopurines, methotrexate, or anti-TNF agents while on therapy. Corticosteroids were allowed, but were tapered according to the patients’ symptoms. 14.7% of patients (28/191) who had JC virus status checked underwent a bowel surgery during the study period (median 6.5 months, range 1–21). Based on log-rank tests (Kaplan-Meier analyses), fistulizing disease (p = 0.028), history of Crohn’s surgery (p = 0.015), and perianal disease (p = 0.048) significantly increased the risk of subsequent surgery, whereas subsequent natalizumab use significantly lowered the risk (p = 0.043). Cox regression analysis with these four factors showed that the use of natalizumab was the only factor associated with modifying the risk of subsequent surgery (Figure 1a; hazard ratio 0.23 (95%CI 0.06–0.98), p = 0.048). Figure 1b shows the Cox regression analysis of the probability of avoiding surgery in the study population according to the subsequent use (or no use) of natalizumab after JC virus testing adjusted with fistulizing disease, history of Crohn’s surgery, and perianal disease as the covariates.
Figure 1.
(a) Cox proportional hazards regression analysis was performed to identify risk factors for surgery in the present population. Fistulizing disease, history of Crohn’s surgery, natalizumab use after JCV testing, and perianal disease were included in the Cox regression analysis as they demonstrated relevance with increasing/reducing the risk of surgery based on a p value of <0.10 on a log-rank test (Kaplan-Meier analysis). Natalizumab use after JCV testing was associated with significantly reducing the risk of surgery (Hazard ratio 0.23, 95%CI 0.06–0.98, p = 0.048). (b) Probability of avoiding surgery in the study population according to the use of natalizumab or not after JC virus testing adjusted with fistulizing disease, history of Crohn’s surgery, and perianal disease as the covariates. Time to surgery after JCV testing was analyzed using Kaplan-Meier estimator. Patients without follow-up at a certain time point or those who discontinued due to adverse effects were regarded as censored and shown as ticks on the graph.
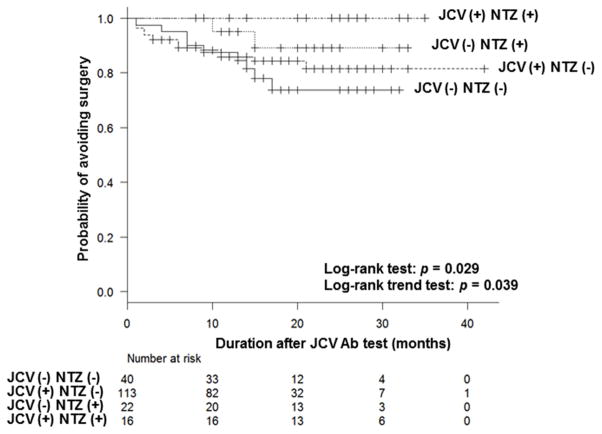
We subcategorized the patients based on the results of the JC virus antibody status and the subsequent natalizumab use, and assessed the risk of surgery. Interestingly, as shown in Figure 2, patients who tested positive for JC virus and were treated with natalizumab had an excellent outcome, followed by those who tested negative and were treated with natalizumab. Regardless of the JC virus status, patients who were not treated with natalizumab were more likely to undergo surgery. None of the patients who were treated with natalizumab developed PML.
Figure 2.
Survival analysis based on the JCV Antibody serology and the subsequent use of natalizumab. Patients were categorized into 4 groups (JCV (−) NTZ (−), JCV (−) NTZ (+), JCV (+) NTZ (−), JCV (+) NTZ (+)). The probability of avoiding surgery was significantly different among groups as assessed by log-rank test (p = 0.029). Log-rank trend test demonstrated a trend in outcome across the four groups (p = 0.039). Analysis was done as in Figure 1b.
Seroconversion rate
Among the 62 patients who were negative for JC virus antibody, 22 patients (35.5%) went on to receive natalizumab. All patients except for those with a short follow-up period had a follow-up JC virus antibody test at 6–12 months intervals while receiving natalizumab therapy (mean follow-up period 17.6 months, range 3–37). One patient (4.5%), a 25 year old female with a previous history of thiopurine and anti-TNF use, seroconverted at 22 months, and, at the time of this analysis, the patient still remained on therapy with stable disease control.
DISCUSSION
The present study is the first to describe the seroprevalence of JC virus in refractory CD patients, who were candidates for natalizumab treatment. The majority of our patients were exposed to immunosuppressive and anti-TNF therapies, and we described a seroprevalence rate of 67.5%. This result is in range of those of previously reported rates in MS and the general population. We further identified that prior use of thiopurines was a risk factor for a positive serology and that subsequent use of natalizumab decreased the likelihood of surgery.
Natalizumab is an efficacious treatment for CD, however, its use has been limited due to the risk of PML10. PML, which is caused by reactivation of JC virus, exclusively occurs in severely immunocompromised patients such as acquired immune deficiency syndrome, and more recently is associated with the use of certain immunosuppressives or biological therapies (natalizumab, rituximab, etc.)23, 24.The rate of seroprevalence of JC virus antibody ranges from 30 to 70% in the general population and increases with age25, 26. In MS, the rate is reportedly between 40–90%27, 28. Bozic et al., in a study of over 7000 MS patients, found that seroprevalence was significantly associated with increasing age, gender and geography16. However, they found no significant association with MS disease characteristics, including duration and number of therapies16, 27. Studies assessing the rate of JC virus infection in CD are limited, but Verbeeck et al. showed a JC virus seroprevalence of 76% in Belgian CD patients, where the use of natalizumab is not yet approved29. No risk factors were described, but they demonstrated that the median viral load in urine was higher among CD patients than in patients with other digestive diseases or controls.
The majority of reports of PML associated with natalizumab use come from subjects with MS16, 17, 27. Among 99,571 MS patients treated with natalizumab, 212 cases of PML were reported (2.1/1000)15. The incidence of PML was 0.09/1000 in subjects negative for anti-JC virus antibody, whereas in those with positive anti-JC virus antibody, prior use of immunosuppressives, and duration of natalizumab therapy of more than 2 years, the risk was as high as 11.1/1000. Combined evidence demonstrates that JC virus infection is required for the development of PML, thus it is important to identify risk factors for JC virus infection. In contrast to MS, we found in our CD patients that previous use of thiopurine was associated with an increased risk of JC virus serology. Also distinct from the findings in MS, age or gender did not affect the result. While it is known that previous use of immunosuppressives increases risk of PML in JC virus positive MS patients, it has not been shown to increase seroprevalence of JC virus in MS. This discrepancy may be due to the difference in the type of immunosuppressive used to treat each condition. It remains unclear if this is due to increased immunosuppression, but methotrexate use also showed a non-significant trend towards a positive serology. Anti-TNF agents were not associated with a risk, however, it should be noted that 97% of our patient population had been exposed to them, so it was not possible to assess this properly.
We also identified that the use of natalizumab after checking the JC virus status was associated with reduced risks of surgery regardless of antibody status. As previously mentioned, our patient population had failed various other CD treatments, which normally would result in surgery as the only remaining standard treatment option. Indeed, in a previous study, we showed that more than half of the patients who failed natalizumab required surgery19. The present study demonstrates that the use of natalizumab reduced the risk of surgery not only in those who were negative for JC virus antibody, but also among those who were positive. Although natalizumab has not been widely used mainly due to the risk of PML, we again demonstrate its benefit in refractory CD. Given that PML only occurs after >9–12 months of natalizumab exposure suggests that those who are JC virus antibody positive and willing to accept this risk may do so initially to assess if the therapy or this mechanism even works, before having surgery. However, it should also be noted that the risk of PML in CD patients treated with natalizumab remains largely unknown and whether risk stratification similar to that of MS can be applied needs further investigation. For those who test negative for anti-JC virus antibody, natalizumab would be an effective long term treatment as the seroconversion rate appears to be low. We still recommend that they have repeat JC virus tests done every 6–12 months to assure that they remain negative. The recent regulatory approval of vedolizumab, a gut specific α4β7-integrin inhibitor, which potentially may carry no risk of PML30, 31, suggests another consideration. If vedolizumab is used as first choice in this class and the patient initially responds but then develops anti-vedolizumab antibodies or other secondary loss of response, similarly as seen in anti-TNF agents, consideration of a second anti-integrin inhibitor, i.e. natalizumab (and the patient’s associated JCV serology), can take into consideration the findings from this study as well. Furthermore, based on the mechanism of action, natalizumab may carry a broader immunosuppressive spectrum when compared to vedolizumab, and it remains to be determined whether the two have a similar efficacy in CD.Only one patient that received natalizumab seroconverted from a negative to a positive JCV serology during the study period. This is not surprising considering the limited follow-up period and the reported low annual seroconversion rate of 1–2%32.
One limitation of our study is the retrospective nature of our analysis, and our findings need to be confirmed in prospectively designed studies with larger numbers of samples. In our study, none of the predefined outcomes underwent power analysis, however, our results can be applied for such analysis in future prospective studies. Furthermore, our patient population can be considered as one of the most refractory patients and whether the rates of JC virus serology and risk factors can be applied to the general CD population remains unclear. However, at present, the indication of natalizumab is limited to refractory CD, and our data is meaningful to be applied to such patients.
In summary, we have identified the prevalence of positive JC virus serologyn refractory CD patients, and have shown that the use of thiopurines was associated with a higher risk. Furthermore, the subsequent use of natalizumab appeared to reduce the risk of surgery regardless of the antibody status and seroconversion was infrequent, suggesting its utility in this selected population. This information contributes significantly to discussions of risk and benefit of natalizumab therapy.
Acknowledgments
AS was supported by the Foreign Clinical Pharmacology Training Program of the Japanese Society of Clinical Pharmacology and Therapeutics.
Footnotes
Author contribution: EB, study design and analysis of data; KK, JP, RDC and DTR, patient recruitment and critical review; AS, concept, study design, analysis of data and writing of manuscript.
Conflict of interest: EB, JP, KK, no conflict of interest exists. RDC is on the speakers’ bureau for Abbvie, Entera Health, Salix Pharmaceuticals, and Shire PLC; he has served as a consultant for Abbive, Cellgene, Entera Health, Hospira, Janssen, Prometheus Laboratories, Salix Pharmaceuticals, Sandoz Biopharmaceuticals, Shire PLC, Takeda, and UCB Pharma. DTR has received consulting fees and research support (Safety Registry) from Elan Pharmaceuticals and Takeda Pharmaceuticals. AS is on the speakers’ bureau for Abbvie, Eisai, JIMRO and Mitsubishi-Tanabe. This study was not supported by any pharmaceutical industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–6. doi: 10.1007/s00535-005-1744-3. [DOI] [PubMed] [Google Scholar]
- 3.Sakuraba A, Sato T, Kamada N, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–45. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 4.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 5.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–13. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–33. doi: 10.1053/j.gastro.2005.11.030. quiz 591. [DOI] [PubMed] [Google Scholar]
- 8.Sakuraba A, Sato T, Matsukawa H, et al. The use of infliximab in the prevention of postsurgical recurrence in polysurgery Crohn's disease patients: a pilot open-labeled prospective study. Int J Colorectal Dis. 2012;27:947–52. doi: 10.1007/s00384-011-1398-y. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912–25. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 11.Tyler KL, Khalili K. Natalizumab and progressive multifocal leukoencephalopathy: highlights of the International Workshop on JC Virus/PML and Multiple Sclerosis, June 3–4, 2005, Philadelphia PA. Rev Neurol Dis. 2005;2:144–9. [PubMed] [Google Scholar]
- 12.Linda H, von Heijne A, Major EO, et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med. 2009;361:1081–7. doi: 10.1056/NEJMoa0810316. [DOI] [PubMed] [Google Scholar]
- 13.Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 14.Brew BJ, Davies NW, Cinque P, et al. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6:667–79. doi: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- 15.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–80. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 16.Bozic C, Subramanyam M, Richman S, et al. Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol. 2014;21:299–304. doi: 10.1111/ene.12304. [DOI] [PubMed] [Google Scholar]
- 17.da Silva AM, Santos ME, Portuguese JSI. JCV epidemiology in MS (JEMS)--epidemiology of anti-JCV antibody prevalence in multiple sclerosis patients--Portuguese data. J Neurol Sci. 2014;337:119–22. doi: 10.1016/j.jns.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Plavina T, Castro A, et al. A second-generation ELISA (STRATIFY JCV DxSelect) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol. 2013;57:141–6. doi: 10.1016/j.jcv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Sakuraba A, Keyashian K, Correia C, et al. Natalizumab in Crohn's Disease: Results From a US Tertiary Inflammatory Bowel Disease Center. Inflamm Bowel Dis. 2013;19:621–6. doi: 10.1097/MIB.0b013e31827eea78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakuraba A, Annunziata ML, Cohen RD, et al. Mucosal healing is associated with improved long-term outcome of maintenance therapy with natalizumab in Crohn's disease. Inflamm Bowel Dis. 2013;19:2577–83. doi: 10.1097/MIB.0b013e3182a8df32. [DOI] [PubMed] [Google Scholar]
- 21.Juillerat P, Wasan SK, Fowler SA, et al. Efficacy and safety of natalizumab in Crohn's disease patients treated at 6 Boston academic hospitals. Inflamm Bowel Dis. 2013;19:2457–63. doi: 10.1097/MIB.0b013e3182a32a0d. [DOI] [PubMed] [Google Scholar]
- 22.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalkias S, Dang X, Bord E, et al. JC virus reactivation during prolonged natalizumab monotherapy for multiple sclerosis. Ann Neurol. 2014;75:925–34. doi: 10.1002/ana.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifford DB, De Luca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–46. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi F, Kajioka J, Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26:1057–64. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 26.Gu ZY, Li Q, Si YL, et al. Prevalence of BK virus and JC virus in peripheral blood leukocytes and normal arterial walls in healthy individuals in China. J Med Virol. 2003;70:600–5. doi: 10.1002/jmv.10436. [DOI] [PubMed] [Google Scholar]
- 27.Bhan V, Lapierre Y, Freedman MS, et al. Anti-JC Virus Antibody Prevalence in Canadian MS Patients. Can J Neurol Sci. 2014;41:748–52. doi: 10.1017/cjn.2014.32. [DOI] [PubMed] [Google Scholar]
- 28.Olsson T, Achiron A, Alfredsson L, et al. Anti-JC virus antibody prevalence in a multinational multiple sclerosis cohort. Mult Scler. 2013;19:1533–8. doi: 10.1177/1352458513477925. [DOI] [PubMed] [Google Scholar]
- 29.Verbeeck J, Van Assche G, Ryding J, et al. JC viral loads in patients with Crohn's disease treated with immunosuppression: can we screen for elevated risk of progressive multifocal leukoencephalopathy? Gut. 2008;57:1393–7. doi: 10.1136/gut.2007.145698. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–21. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 31.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 32.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68:295–303. doi: 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]