Abstract
The Honolulu-Asia Aging Study (HAAS) is a longitudinal epidemiologic investigation of rates, risk factors, and neuropathologic abnormalities associated with cognitive decline and dementia in aged Japanese-American men. The project was established in 1991 and will be brought to closure in 2012. Age-specific rates of total dementia and the major specific types of dementia in HAAS participants are generally similar to those reported from other geographic, cultural, and ethnic populations. Risk factors for dementia in the HAAS include midlife hypertension and other factors previously shown to influence cardiovascular disease. The autopsy component of the project has yielded novel findings, the most illuminating of which is the demonstration of 5 important lesion types linked independently to cognitive impairment. While one of these – generalized atrophy – is strongly associated with both Alzheimer lesions and microinfarcts, it also occurs in the absence of these lesions and is independently correlated with dementia. Each lesion type is viewed as representing a distinct underlying pathogenic process. Their summed influences is an especially robust correlate of dementia in the months and years prior to death.
Keywords: Cognitive impairment, neuropathology, dementia, epidemiology, Alzheimer's disease, autopsy
INTRODUCTION
The Honolulu-Asia Aging Study (HAAS) was established in 1991 with the goal of determining the prevalence, incidence, and risk factors for dementia. At that time, the major causes of dementia were widely recognized as Alzheimer's disease (AD), vascular dementia (VaD), and the dementia of Parkinson's disease (PD). AD was considered to be more common in the US and Europe, whereas VaD was felt to be more prevalent in Asia. A primary aim of HAAS and its sister studies in Seattle (the KAME project) and Hiroshima (at the Radiation Effects Research Foundation), collectively referred to as the Ni-Hon-Sea project, was to clarify the relative frequencies of dementing diseases among Japanese-ancestry persons living in Japan, Hawaii, and the mainland-US.
The HAAS cohort is comprised of the surviving participants in the Honolulu Heart Program (HHP), a prospective, community-based cohort study of heart disease and stroke established in 1965. The first HHP examination (1965-1968) included two-thirds of the Japanese-American men residing on Oahu, Hawaii, who were born 1900-1919. Details of the study have been described elsewhere [1, 2]. The decision to limit the HHP to men reflected the recognition that the higher rates of heart disease and stroke in men meant power estimates were reasonable and that the cost of the study could be held to an acceptable level. It seems doubtful that the same decision would be made were the study to be done today. Three HHP examination cycles were conducted between 1965 and 1974. Information was collected through structured interviews on participant characteristics, including demographics, education, occupation, diet, physical activity, cigarette smoking, and medical history. Physical examinations included anthropometric, cardiovascular, and respiratory measures. The availability of such a rich archive of unbiased, prospectively data represents an extraordinarily rare and important resource for dementia research.
Of the original 8006 HHP participants, 3734 (age 71-93 years) participated in the first HAAS examination (1991-1993), which represented approximately 80% of surviving members of the original cohort [2]. HAAS examination cycles have occurred every 2-3 years since 1991. The 8th HAAS examination (the 11th including three prior HHP cycles) was completed in 2010.
CLINICAL DEMENTIA AND COGNITIVE IMPAIRMENT ASSESSMENT IN THE HAAS
At each HAAS cycle, participants receive standardized interviews and examinations focused on cognition and motor function, as well as other aging-related endpoints. Individuals whose examinations indicate cognitive impairment or Parkinsonism receive further evaluations to identify and classify cases using accepted criteria for AD, VaD, and PD. Additional examination items and diagnostic criteria related to cortical Lewy body (LB) dementia were added at the 1997-99 examination cycle.
Cognitive function of participants is assessed at every examination cycle with the 100-point Cognitive Abilities Screening Instrument (CASI) [3]. At the baseline HAAS examination, participants with poor CASI scores (<74) and stratified samples from subsets with higher scores were invited to return for the dementia evaluation, accompanied by a proxy informant. Cases were identified and classified through a multi-step procedure according to a previously described protocol that included screening and a standardized dementia evaluation [2]. Most men with dementia had CASI scores 0-64. When a full dementia evaluation was not completed for a participant, we considered CASI scores <65 as indicating definite cognitive impairment, 65-73 as marginal cognitive impairment, 74-82 as low-normal, and >82 as normal. CASI scores <74 represented the 16th percentile for all men examined at the first cycle. This corresponds approximately to a score of 23 on the Mini-Mental State Examination, a level often used to indicate cognitive impairment [4]. In subsequent HAAS examinations, full dementia examinations have been limited to participants with poor CASI scores.
Dementia evaluations include a neurologic examination, neuropsychological testing, review of extensive information related to health and functioning from prior examinations, and informant interview about changes in cognitive function and behavior. Computed tomography or magnetic resonance brain imaging is performed, and routine blood tests are conducted, for men provisionally classified as demented. Based on these data, a consensus diagnosis for dementia is provided by the study neurologist and two physicians with expertise in dementia, according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM III-R) [5].
Probable or possible AD is diagnosed according to criteria from the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association [6]. Dementia attributed to AD, but with minor evidence of co-prevalent conditions possibly contributing to cognitive impairment, is classified as possible AD. In a subset of the HAAS cohort, 65% of men with the clinical diagnosis of pure AD demonstrated sufficient neuritic plaques to meet neuropathological criteria for definite or probable AD, while those classified as possible were less often confirmed as definite [7]. Probable or possible VaD is diagnosed using criteria of the California Alzheimer's Disease Diagnostic and Treatment Centers [8].
Unlike subjects in most other epidemiologic studies of dementia, HAAS participants are not clients of a specific clinic, hospital, or health care system. Instead, participants were initially drawn from a sampling frame that included all Japanese-American men born 1900 through 1919, who were living on Oahu when the HHP began. As a result of this community survey design, no treatments, interventions, advice, or diagnostic services are provided. This design, as a longitudinal study of a community cohort without ties to a health care providing system, results in substantial cohort differences as compared with research projects conducted in the context of a clinical referral and evaluation center. Following examinations, patients and their physicians are notified of urgent health-related findings, and summaries of clinically relevant information are provided to physicians with the participant's consent. Although consensus diagnosis committees review the accrued information to arrive at clinical diagnostic classifications, these were not utilized in patient care.
OBSERVATIONS FROM EXAMINATIONS DURING LIFE
At the baseline HAAS examination, the prevalence of overall clinical dementia in the HAAS cohort was estimated at 2.1% for men age 71-74 years, and 33.4% at age 85-93, with an overall prevalence of 7.6% at age 71-93 years. Clinically, AD as the primary or contributing cause of dementia was diagnosed in 5.4% of all men, as compared to VaD in 4.2%, with overlapping clinical diagnoses (more than one possible cause) in 26% of the dementia cases. In the HAAS cohort (Japanese-American men), AD appeared more common than in Japan, with age-specific prevalence levels similar those reported for white populations, whereas VaD appeared less common than in Japan but more frequent than in whites [2].
The three-year incidence of dementia was estimated based on 137 new cases among 2603 participants followed through the second HAAS examination cycle, 1994-96. The all-cause dementia incidence rate among these participants (age 71-93 at baseline) was 1.84/100 person-years. The ratio of AD to VaD was similar to that observed in the baseline prevalence survey, with an approximate doubling of rates with every 5-year increase in age. The apolipoprotein epsilon (APOE) e4 allele was associated with a relative risk of 2.39 after adjusting for age and education [9].
Risk factors for dementia identified in the HAAS cohort, in addition to the presence of the APOE e4 allele, [9] include untreated hypertension in midlife, [10] lower physical activity, [11] midlife cigarette smoking, [12] and higher body mass index [13]. Risk factors for clinical disease were also frequently associated with corresponding neuropathologic features. Among other risk factors, thyroid dysfunction [14] and midlife hypertension [15] have been associated with AD lesions. Diabetes mellitus has been associated with overall clinical dementia, clinical AD and VaD, as well as with AD lesions among men with the APOE e4 allele [16]. Midlife hypertension has also been associated with overall [15] and hippocampal [17] atrophy. PD lesions, including low substantia nigra density and LB lesions, have been found to be more common among men with constipation [18, 19] and olfactory dysfunction, [20] both risk factors for PD.
BRAIN AUTOPSIES
During the first decade of the HAAS (1991-2002), 1983 of the examined men died, and 443 received brain autopsies. Among autopsied decedents, 32% were demented at their last examination, a further 25.2% were definitely or marginally cognitively impaired (CASI <74), and 42.8% were cognitively normal or low-normal. Families of approximately 15% of decedents without dementia during life consented to autopsy, and 25% of decedents with dementia agreed to autopsy. Autopsied and non-autopsied decedents who had been cognitively normal or low-normal were similar with respect to demographic, social, and medical characteristics. Although the families of demented participants were substantially more likely to contact the study when death occurred, and to approve autopsy, than those of non-demented participants, demented decedents who were autopsied were similar to demented decedents who were not autopsied. Similar correspondence of features in autopsied and non-autopsied men was observed in the subset of decedents with cognitive impairment but without a formal diagnosis of dementia. Based on these observations, we have regarded the autopsied subset of participants as similar to a stratified sample of all cohort decedents, with stratification based on dementia recognized during life [21].
THE HAAS BRAIN AUTOPSY PROTOCOL
Standardized brain autopsies began in 1992. Details of the autopsy protocol and evaluation of the neuropathologic material have been described in detail elsewhere [21, 22]. Neuropathologists have been blinded to clinical information, to minimize potential bias. The principal endpoints for research analyses of autopsy findings have included a comprehensive set of standardized, objective measurements and numerically coded observations recorded on standard forms and subsequently entered into an electronic dataset. Summary classifications approximating conventional neuropathologic diagnoses have been generated by algorithms using coded observations, rather than by pathologists’ judgment.
During the initial decade (1991-2001), a single neuropathologist, John Hardman (deceased), performed all of the gross autopsies. During this same interval, a team of neuropathologists (W. Markesbery, J. Nelson, D. Davis) completed all microscopic evaluations. This team initially trained together to ensure the inter-rater comparability of their reports and subsequently consulted whenever difficult issues arose. Results summarized in this review are from neuropathologic observations generated during this initial decade. Events in 2002 interrupted the continuity of gross and microscopic readings, requiring the training and certification of a new team of neuropathologists. Comparability of readings among three “new” neuropathologists, and with the prior team, was assured by training and mentoring provided by Dr. Hardman (for the gross examinations), Dr. Markesbery (for the microscopic examinations), and one year of rigorous training and certification. Future reports will present results from the second decade of HAAS autopsies.
NEUROPATHOLOGIC CODING AND CLASSIFICATION
Each gross brain autopsy generated observations from 1 cm coronal sections from the entire brain plus thinner sections of midline structures to the spinal cord. Standardized microscopic examinations were performed on sections from 35-50 brain regions and lesion sites, depending on the number of lesions sampled for further evaluation. A standardized, rigorously defined microscopic examination protocol generated a comprehensive set of numerically coded observations. Focal ischemic lesions, assessments of gliosis and neuron loss, identification of specific abnormalities and other features were assessed on H&E stained sections. An immunohistochemical stain for amyloid was used to assess vascular amyloid. Neurofibrillary tangles (NFT), neuritic amyloid plaques (NP), and diffuse amyloid plaques (DP) were counted on modified Bielschowski stained sections, with all counts standardized to areas of 1 square millimeter. Braak staging employed sections stained using a modified Bielschowski and Gallyas stain. An immunohistochemical stain for alpha synuclein was used to quantify Lewy bodies.
As previously reported, an extensive search of the accrued neuropathologic observations identified 5 distinct lesion types having strong and independent associations with cognitive impairment or dementia in the final years of life: (1) lesions representing the Alzheimer process, (2) microinfarcts and (to a lesser degree) lacunar infarcts, (3) cortical Lewy bodies, (4) hippocampal sclerosis, and (5) generalized brain atrophy [24]. A three-level HAAS index of severity for each of these was then defined by reference to the associated level of cognitive impairment or dementia, so that severe or moderate grades of lesion severity were strongly or moderately associated with impairment.[21] For more recent analyses, we have further divided the lowest severity category into none or negligible lesion numbers, producing a 4-level index for each of the 5 lesion types.
AD LESIONS (ISOCORTICAL NEUROFIBRILLARY TANGLES AND NEURITIC PLAQUES)
Three summary variables have been employed to assess the extent of AD lesions: (1) conventional AD Braak staging, (2) average isocortical NFT and/or NP counts (left hemisphere only), and (3) the HAAS index previously described. [9] The HAAS index summarizes AD pathology using an algorithm that closely approximates Reagan AD criteria, with severe lesions (grade=3) corresponding to a Braak stage of 6 and/or a high isocortical NP count. A HAAS AD index of 2 (moderate lesions) is assigned when low or moderate counts of both isocortical NFT and NP are recorded. An index of 1 is assigned when any isocortical NFT or NP are observed. In linear regression models with the final CASI score as the dependent variable, each of these summary AD variables contributed significantly and independently to the variance explained, despite very strong intercorrelations. Of these three AD assessment variables, the strongest association with poor CASI test scores was observed with the average isocortical NFT count.
VASCULAR LESIONS
No widely accepted neuropathologic criteria for VaD currently exist. This reflects a recognition that dementia is not sufficiently reliably and consistently associated with gross infarct type, location, number, and volume for these to serve as neuropathologic criteria for VaD. More recently, microinfarct number and location have been identified as associated with cognitive impairment and dementia [21, 23-28].
In the HAAS autopsy protocol, large and lacunar infarcts were identified at the gross examination. Lacunar infarcts were nearly all subcortical, with central cysts, mostly in the deep gray nuclei or white matter, with the longest dimension <1.0 cm as assessed on the cut surface of the coronal brain section. Large infarcts were mostly cortical with longest dimension ≥1cm on the cut surface of the same sections. Microinfarcts were defined as microscopic (not seen on gross examination) foci of neuronal loss and gliosis, or focal leukoencephalopathy when the lesion occurred in white matter. They typically had a longest dimension of 50-500 m. Microinfarcts were assessed on slides from all cortical lobes, the caudate, putamen, globus pallidus, thalamus, internal capsule, hippocampus, cerebellum, and brainstem.
While large, lacunar, and microinfarcts at autopsy were associated with poor cognition during life, they were also found among men with normal cognition at their last examination and in those with clinical diagnoses of AD [21, 22, 24]. Although numbers of large infarcts and lacunar infarcts were individually associated with cognitive impairment, the strength of those associations was weaker than the association between microinfarct count and final CASI score. Extensive multivariable analyses have identified microinfarct counts as more strongly associated with dementia and cognitive impairment than either lacunar or large infarcts. When the microinfarct count was considered in the models, the volumes and number of large infarcts were not significantly associated with cognition, while the number of lacunar infarcts fell to borderline significance. Examining the regional distribution of microinfarcts, poor cognition was most strongly associated with total isocortical microinfarcts (summed across 4 left and 4 right lobes) and, to lesser extent, with total microinfarcts in the left and right caudate nuclei, putamen, and thalami [21]. This finding violates the conventional neurologic expectation that the site of the lesion determines clinical manifestations, but is consistent with the idea that dementia – in which the functional impairments are multiple and widely dispersed – might usually require multiple and widely distributed structural lesions.
HIPPOCAMPAL SCLEROSIS
Hippocampal sclerosis (HS) is a neuropathological entity which does not yet have a widely accepted clinical identity. It is characterized by a dramatic loss of neurons and a vigorous gliosis in CA1 and CA3, typically ending abruptly at or within the subiculum. These changes can be focal within the rostral-caudal extent of the hippocampus, and can occur in one or both hemispheres [9, 14].
NEOCORTICAL LEWY BODY LESIONS
When the HAAS began in 1991, it was recognized that persons with PD may experience a gradual but definite cognitive decline ultimately justifying a diagnosis of dementia. It was not clear, however, if these declines were entirely due to PD or other pathologic processes. Cortical (or diffuse) LB dementia is now recognized as a common cause of dementia in late life, also affecting persons not previously diagnosed with PD. Standard neuropathologic criteria for LB dementia are used in the HAAS autopsy protocol, and the contribution to cognitive impairment is assessed in analytic models using the cortical Lewy body score, as described by McKeith [29, 30].
BRAIN ATROPHY
Generalized brain atrophy has rarely been systematically examined in autopsy studies, in part because neuropathologists ordinarily examine the brain after removal from the cranium, precluding a comparison of brain volume with intracranial volume. Atrophy is ordinarily inferred pathologically from enlarged ventricles, shrunken gyri and sulcal widening.
In the HAAS protocol, brain atrophy is assessed based on brain weight, mantle thickness, and microscopic observations of neuronal loss in 8 neocortical regions. Brain weight and mantle thicknesses are referenced against the estimated intracranial volume. Size of the cranium is determined by fusion of cranial plates when brain growth nears completion, which usually occurs around age 5 years, even though myelination and some brain growth continue into adolescence. This provides the rationale for estimating peak brain size based on the volume of the cranial vault.
At the second HAAS examination cycle, intracrainial volumes were estimated for more than 500 standardized magnetic resonance images. We found head circumference to provide a reasonably reliable basis for estimating intracranial volume, with a minor additional contribution provided by midlife height. The further use of intracranial measurements from a subset of the scanned subjects who came to autopsy did not significantly improve our estimates. We now use the following algorithm to calculate imputed intracranial volumes (iicv) in ml:
Among 58 autopsied HAAS participants selected to minimize the severity of atrophy (who died before age 90, had normal CASI scores within 3 years of death, negligible neocortical tangle counts, and negligible microinfarct counts), the mean (weight/iicv) percent value was 86.3 (sd, 8.1). We view this value as representing mild atrophy, consistent with advanced age (though not necessarily normal aging).
In addition to the ratio of brain weight (in g) to iicv (in ml), we identified two additional measures of atrophy which were significantly correlated with brain weight and with one another: (1) the ratio of the sum of the left and right mantle thicknesses to the bitemporal intracranial diameter, and (2) the subjective microscopic assessment of isocortical neuronal loss by the neuropathologist. All three measures contributed independently to poor cognitive test scores. Mantle thickness was defined as the distance between the lateral margins of the left and right lateral ventricles (at the level of the head of the caudate nucleus) and the outer cortical margin (in cm). When a measured bi-temporal intracranial diameter was unavailable, it was imputed based on the head circumference (treated as a perfect circle) and an estimated 1.8 cm thickness of the skull, including skin, bone, and dura – i.e., intracranial diameter (in cm) = head circumference / 3.14 – 3.6. The average ratio of the sum of the two mantle thicknesses to the bitemporal intracranial diameter for the 58 selected brains with the least evidence of cognitive impairment was 0.699 (sd, 0.059).
Isocortical neuronal loss (nearly always associated with gliosis) was based on the sum of subjective 0/1 (absent/present) judgments made by the neuropathologist on review of 8 H&E stained sections of left and right frontal, parietal, temporal, and occipital cortex, with a possible total score range of 0-8. The three indices were standardized by transformation to z-scores using algorithms derived from a subset of 58 autopsied HAAS decedents who were less than 90 years at death, cognitively normal within 3 years of death, and who had negligible AD lesions or microvascular infarcts at autopsy. Z-scores were calculated as:
Neuronal loss, isocx z= (isocx Nloss – 0.66) / 1.15 Where iicv = imputed intracranial volume (ml), brwt= brain weight (g), icdiam = intracranial diameter (cm), mantleL = left mantle thickness (cm), mantleR = right mantle thickness (cm), isocx Nloss = isocortical neuronal loss (range 0-8). It is important to note that the heights and head circumferences of HAAS cohort members are substantially less than in most other American and European male cohorts, reflecting suboptimal childhood nutrition and growth of many of the participants.
As a summary index of generalized atrophy, we use a weighted average of z-scores for the individual atrophy indices above (weights: brain weight = 0.5, mantle thickness = 0.4, microscopic neuronal loss = 0.1).
CO-PREVALENCE OF THE BRAIN LESIONS AT AUTOPSY
All became more prevalent with advancing age. By age 90, the majority of HAAS brain autopsies showed lesions of at least one type of dementia-causing process. Most importantly, the sum of the severity indices for the 5 lesion types was highly and consistently correlated with dementia or impairment at the end of life. The occurrence of two lesion types at moderate levels was frequent and at least as strongly linked to impairment as a single lesion type at high levels [21]. Most cases of dementia developing after 85 years appear to be caused by a combination of at least two distinct abnormalities [21].
We observed three patterns of lesion co-prevalence at levels greater than expected by chance: (1) AD lesions and HS, (2) generalized brain atrophy and moderate/severe AD lesions, and (3) generalized brain atrophy and moderate/severe levels of microvascular infarcts. Approximately half of men with HS also have high or moderate isocortical NFT and/or NP counts, whereas HS is only marginally associated with the presence of vascular lesions. By contrast, associations of generalized brain atrophy with AD lesions and microvascular infarcts are strong. The association of HS with atrophy after adjusting for AD lesions was not statistically significant [21].
RELATIONSHIPS BETWEEN AUTOPSY FINDINGS AND COGNITIVE FUNCTION IN THE FINAL YEARS OF LIFE
Microvascular infarcts were identified as the sole or dominant lesion in 33.8% of demented or definitely cognitively impaired decedents, as compared with AD lesions in 18.6%, LB lesions in 10.9%, HS in 3.3%, atrophy alone in 7.1%, and co-dominant lesions (most often microvascular or AD lesions) in 14.2%. Moderate or high levels of at least one lesion type were found in 87.9% of demented or definitely impaired decedents, leaving 12.1% unexplained by any of the lesion types examined [21].
One of the most important observations from the HAAS autopsy study is that all of the brain lesions linked to clinical dementia occur with substantial frequencies among persons who were not recognized as demented during life. Among decedents who were cognitively normal at their last examination, 54% had moderate or high levels of one lesion type, while very few had more than one lesion type at moderate or high levels [21].
Approximately 15% of decedents with normal or borderline cognitive function at their final examination before death had moderate or high levels of AD lesions sufficient for pathological diagnosis of AD, as compared to 77% of decedents diagnosed with probable AD and 35.5% of those diagnosed with possible AD [7]. Among decedents in the HAAS with clinical probable pure AD, 77% had sufficient neuritic plaques to meet neuropathologic criteria for definite or probable AD [7]. Furthermore, the presence of a single AD lesion type (neocortical NP or NFT) did not appear to represent early AD, as decedents with a single AD lesion type did not have a higher prevalence of dementia as compared with men without any AD lesions [31]. AD lesions were also not more prevalent among decedents with VaD lesions, suggesting that AD and vascular lesions occur independently but have additive influences on cognition [32].
While large, lacunar, and microinfarcts at autopsy were associated with clinical VaD, they were also found among men with normal cognition at their last examination and those with clinical AD. Of the focal ischemic lesions, microinfarcts were most strongly and consistently associated with overall clinical dementia and cognitive test scores. High levels of microvascular infarcts were found in 10.5% of cognitively normal men and in 31% of demented or definitely impaired decedents [21].
We identified HS as a pathologic correlate of dementia, independent of its coexistence with other lesions. Moderate or high levels of HS were found in 4% of cognitively normal decedents and 15.8% of those who were demented or definitely impaired [21].
Moderate or high levels of cortical LB lesions were found in 9.5% of men who were cognitively normal at their last examination, as compared to 20.2% of those with dementia or definite impairment [21]. Nearly all men with both PD and dementia had cortical LB, however most men with cortical LB had not been diagnosed with PD.
Brain atrophy was strongly associated with both AD lesions and microvascular infarcts, but was also found in decedents with negligible levels of other lesions. With or without AD lesions and microvascular lesions, moderate or severe brain atrophy was independently associated with poor cognition, even after accounting for the influence of education and other lesions. Moderate or severe brain atrophy was found among 60.7% of demented or definitely impaired decedents and among 19.5% of cognitively normal decedents. Moderate/severe atrophy was the sole or dominant lesion in 7.1% of those who were demented or definitely impaired [21].
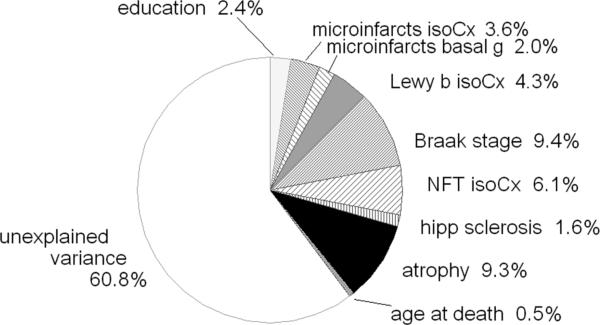
Fig. (1) shows the percent of variance in the final CASI score explained by variables representing lesion types entered in sequence in a linear regression model, beginning (at the twelve o'clock position) with education, progressing clockwise and ending with age at death. In these models we have used the two measures of microvascular infarct that together captured nearly all of the discernable influence of infarcts on cognition, and two measures of the Alzheimer process that captured nearly all of the discernable influence of this process. In such a cumulative model, the association of each independent variable with the endpoint is adjusted for the previously entered variables, but not for those entered thereafter. The total variance in last cognitive test score explained in this analysis is 39%, the sum of the R2 associated with each independent variable. All of the independent variables were significantly associated with cognitive test scores.
Fig. (1).
Cumulative analysis of variance, dependent variable: final CASI score before death. Percentages refer to percent of variance in the final CASI score explained by the model with entry of explanatory variables beginning at 12 o'clock with education and progressing sequentially clockwise.
In this model, the first variables entered in the model after adjusting for education were the number of microinfarcts counted in the isocortex, and three deep, gray nuclei (caudate, putamen, thalamus). Together these explained 8.0% of the variance in the last CASI before death. Cortical LB (measured as the McKeith LB score) explained a further 4.3%. Braak stage, isocortical NFT counts (20 fields, 4 major lobes, left side only), and HS collectively added another 17.1%. Even after considering the explanatory sums for the aforementioned lesions, generalized brain atrophy added another 9.3%. The negligible contribution of age at death (0.5%), considered after adjusting for the 8 previously entered variables, implies that the well known, strong association of older age with cognitive decline and dementia appears almost fully attributable to the combined influences of the other variables included in the model.
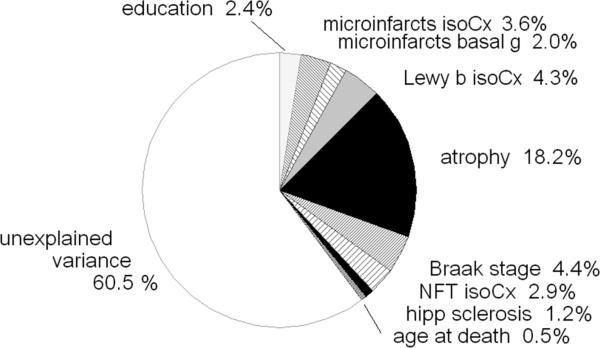
Fig. (2) presents data from a similar regression model, but with a change in the order in which the independent variables were entered – with generalized atrophy moved to a prior position in the model, after the cortical LB, and before AD lesions. The change in entry order increased the variance attributed to atrophy, with a corresponding reduction attributed to AD lesions, leaving the total R2 unchanged. This phenomenon occurred because approximately 8% of the variance could be attributed (statistically) to either atrophy or AD lesions, since they were co-prevalent in the same brains. This emphasizes that a substantial proportion of persons with poor CASI test scores possess both AD lesions and generalized atrophy.
Fig. (2).
Cumulative analysis of variance identical to Fig. (1), except with altered order of entry of the predictor variables with ‘atrophy’ entered after isocortical Lewy bodies, and before Alzheimer lesions.
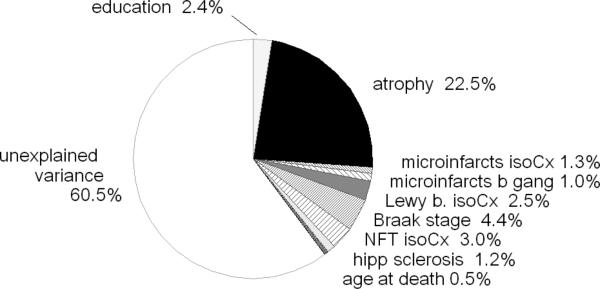
Fig. (3) provides another modification of the model, with generalized atrophy entered immediately after education. Again, the variance explained by atrophy in this model increases by an amount balanced by the further reduced variance attributed to the isocortical and subcortical microinfarct counts. These observations indicate that a rather large proportion of the HAAS decedents who had shown declines in the final CASI score before death had brain atrophy in combination with elevated microinfarct counts.
Fig. (3).
Cumulative analysis of variance identical to Figs. (1) and (2), except for entry of ‘atrophy’ after education and prior to all other predictor variables.
Figs. (1, 2 and 3) indicate a strong correlation of generalized brain atrophy with AD lesions and with microvascular infarcts. In addition, atrophy also occurs frequently without an apparent strong association with any specific lesion type, suggesting three separate mechanisms linking generalized brain atrophy with cognitive impairment: an AD-associated atrophy, an ischemia-associated atrophy, and atrophy not apparently linked to either AD or ischemic lesions.
(Table 1) shows the frequencies of AD lesions (moderate or high isocortical NFT and NP counts), microvascular infarcts (moderate or high numbers), and generalized atrophy (moderate or severe) -- individually and in all possible combinations – among all 443 decedents autopsied. Among 156 decedents in whom none of the three lesion types occurred at moderate or high levels, 32 were nonetheless demented or definitely impaired at their last examination before death. In about half of these decedents, the brains showed HS or cortical LB, leaving approximately 8% of the demented or impaired decedents without histopathology features sufficient for attribution. In the 200 brains from decedents who had been demented or definitely impaired, 14 (7%) had only AD lesions, 23 (11.5%) had only microvascular infarcts, 34 (17%) had only atrophy, 11 (5.5%) had AD lesions plus microvascular infarcts, 29 (14.5%) had AD lesions plus atrophy, 36 (18%) had microvascular infarcts plus atrophy, and 21 (10.5%) had AD lesions, microvascular infarcts, and atrophy.
Table 1.
Associations Between Dementia (Or Definite Cognitive Impairment) At last Examination and the Presence of Neuropathologic Lesions at Autopsy Among 443 Decedents
| Group | NFT/NP | Microvascular Infarcts | Atrophy | Total no. of Men in Lesion Group | No. of Men (%) with Dementia or Definite Impairment | Mean CASI | Odds Ratio (95%CI) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 156 | 32 (20%) | 76 | 1.0 (ref) |
| 1 | + | 0 | 0 | 32 | 14 (44%) | 64 | 2.5 (1.1- 5.9) |
| 2 | 0 | + | 0 | 66 | 23 (38%) | 67 | 2.1 (1.1- 4.1) |
| 3 | 0 | 0 | + | 55 | 34 (62%) | 55 | 5.7 (2.8- 11.3) |
| 4 | + | + | 0 | 19 | 11 (58%) | 50 | 5.2 (1.9- 14.5) |
| 5 | + | 0 | + | 37 | 29 (78%) | 32 | 12.1 (4.9- 30.1) |
| 6 | 0 | + | + | 53 | 36 (68%) | 48 | 8.9 (4.3- 18.2) |
| 7 | + | + | + | 25 | 21 (84%) | 33 | 20.6 (6.4- 66.5) |
| All groups | 443 | 200 (45%) |
(Table 1) also shows associations between neuropathologic lesions at autopsy and the presence of dementia (or definite cognitive impairment, CASI <65) at last clinical examination. As indicated by the odds ratios, the three lesion types show an approximately geometrically additive influence on dementia. By this, we mean that the combined influences of lesion types can be estimated by summing their coefficients of association defined in a logistic model. In logistic models, the coefficients are exponents. Adding exponents corresponds to multiplying their arithmetic base values – i.e., multiplying the odds ratios. The result is that a logistically additive influence of risk factors appears to be a synergistic effect, since the combined influences of the independent risk factors is approximated by their product. The data suggest that even in individuals who have the misfortune to have Alzheimer brain lesions, dementia may be substantially less likely if other lesions (as microvascular infarcts or atrophy) are not present. This concept – the role of co-prevalent brain lesions – is of major relevance to public health efforts to reduce the prevalence of clinical dementia in late life.
While the importance of atrophy in Alzheimer's disease is well recognized, the data shown indicate that moderate or marked generalized brain atrophy was present in 26% of the decedents without significant levels of either Alzheimer or microvascular lesions. While it is accepted that brain atrophy often develops as part of the Alzheimer process, it was common in HAAS autopsied decedents even in the absence of these primary lesions. Despite the absence of these other lesions, 62% of such subjects (with isolated brain atrophy) had been demented or cognitively impaired.
It is clear that having one type of brain lesion, or one type of dementia-causing pathogenic process, does not protect against the development of others. It seems reasonable to assume that in some persons with Alzheimer brain lesions, the associated atrophy is entirely secondary to the Alzheimer process, while in others it is attributable to the combined influences of AD and at least one other pathogenic process. The same argument can be made for the development of atrophy in persons with microvascular infarcts. This idea emphasizes the importance of viewing generalized brain atrophy not just as a consequence of the Alzheimer process or microvascular ischemia, but also as developing independently in some persons, as a result of some other (unrecognized) pathogenic process.
The findings summarized here represent two decades of data collection and analysis. While progress in understanding age-related cognitive decline has been impressive during this time, many questions remain unanswered. Some of the findings from the HAAS autopsy study challenge commonly accepted views. These include the expectation that clinically diagnosed Alzheimer's disease can be wholly attributed to high levels of neuritic amyloid plaques and/or neurofibrillary tangles in the isocortex, and that vascular dementia can be wholly attributed to large and lacunar infarcts. The design of the HAAS autopsy study departed from approaches conventionally used for most such investigations in that it imposed epidemiologic methods for rigorous, standardized assessment of a large, defined set of neuropathologic measures for all autopsies without regard to clinical features or diagnoses. To avoid bias, HAAS pathologists were denied knowledge of clinical information for the decedents. The neuropathologic assessments were comprised of a large set of standardized measurements rather than diagnoses or interpretations. This approach, which appears to devalue judgements, training, respect for convention, and professional acumen on the part of the participating neuropathologists represents a major operational hurtle. We were able to carry out this study design largely because of the extraordinary wisdom, dedication, and humility of Drs. Bill Markesbery and John Hardman, both now deceased.
The strengths of the HAAS include its longitudinal design, the representativeness of the cohort for a clearly defined community population, the availability of prospectively collected, unbiased measurements representing candidate risk factors collected during three HHP examination cycles before the HAAS was established, and during the early examination cycles of the HAAS. The design of the autopsy study, as described above, was a second major strength. Weaknesses of the HAAS include the limitation of participants to Japanese-American men, and a delay in initiating the HAAS until the youngest men were already 71 years of age. A further unfortunate limitation is a failure to incorporate standardized neuroimaging in the examinations except for CTs for participants provisionally diagnosed as demented.
CONCLUSIONS
Clinical diagnoses of dementia type usually fail to reflect the pathologic complexity found at autopsy. Findings from the HAAS have demonstrated that the development of dementia appears more closely correlated with the combined number of lesions and their severity than with specific lesion types.
Based on the HAAS autopsy study, it appears that most brain lesions associated with dementia develop independently of one another. Since most also tend to become more prevalent with advancing age, the co-prevalence of two or more lesion types increases dramatically with age. The combined adverse influences of co-prevalent lesion types on cognitive function are logistically additive. With the exception of hippocampal sclerosis, attribution of dementia or cognitive impairment to a specific lesion type requires consideration of the distribution and numbers of lesions and co-prevalent lesions.
Preventing or slowing the development of any single disease process, including the Alzheimer process, will likely lead to modest delays and reductions in late life dementia. Strategies leading to major reductions in the risk and severity of dementia in late life will probably require interventions that simultaneously target multiple different disease processes. To understand the causes of cognitive decline and dementia, and eventually to prevent or delay their onset, we can no longer afford to regard them as singular, discrete, present or absent conditions. Rather, we must focus on the nature of the several specific pathogenic processes that are common with advanced age, and then on the determinants of their onsets, rates of development, and factors that modulate their influences on function.
ACKNOWLEDGEMENT
We are indebted to the participants in the Honolulu-Asia Aging Study for their outstanding commitment and cooperation and to the entire Honolulu-Asia Aging Study staff for their expert and unfailing assistance. This work was supported by grants 5U01 AG19349, and 5UO1 AG017155, and contract N01-AG-4-2149 from the NIA, National Institutes of Health, and by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. The information contained in this article does not necessarily reflect the position or the policy of the United States Government, and no official endorsement should be inferred.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts to disclose.
REFERENCES
- 1.Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: introduction. Am J Epidemiol. 1975;102:477–480. doi: 10.1093/oxfordjournals.aje.a112185. [DOI] [PubMed] [Google Scholar]
- 2.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- 3.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 4.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 5.Diagnostic and statistical manual of mental disorders. third edition, revised American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 6.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 7.Petrovitch H, White LR, Ross GW, Steinhorn SC, Li CY, Masaki KH, et al. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57:226–234. doi: 10.1212/wnl.57.2.226. [DOI] [PubMed] [Google Scholar]
- 8.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 9.Havlik RJ, Izmirlian G, Petrovitch H, Ross GW, Masaki K, Curb JD, et al. APOE-epsilon4 predicts incident AD in Japanese-American men: the honolulu-asia aging study. Neurology. 2000;54:1526–1529. doi: 10.1212/wnl.54.7.1526. [DOI] [PubMed] [Google Scholar]
- 10.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 11.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 12.Tyas SL, White LR, Petrovitch H, Webster Ross G, Foley DJ, Heimovitz HK, et al. Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2003;24:589–596. doi: 10.1016/s0197-4580(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 13.Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20:2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 14.de Jong FJ, Masaki K, Chen H, Remaley AT, Breteler MM, Petrovitch H, et al. Thyroid function, the risk of dementia and neuropathologic changes: the Honolulu-Asia aging study. Neurobiol Aging. 2009;30:600–606. doi: 10.1016/j.neurobiolaging.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 16.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 17.Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 18.Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord. 2007;22:1581–1586. doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- 19.Petrovitch H, Abbott RD, Ross GW, Nelson J, Masaki KH, Tanner CM, et al. Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov Disord. 2009;24:371–376. doi: 10.1002/mds.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross GW, Abbott RD, Petrovitch H, Tanner CM, Davis DG, Nelson J, et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord. 2006;21:2062–2067. doi: 10.1002/mds.21076. [DOI] [PubMed] [Google Scholar]
- 21.White L. Brain Lesions at Autopsy in Older Japanese-American Men as Related to Cognitive Impairment and Dementia in the Final Years of Life: A Summary Report from the Honolulu-Asia Aging Study. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 22.Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 23.Sonnen JA, Larson EB, Haneuse S, Woltjer R, Li G, Crane PK, et al. Neuropathology in the adult changes in thought study: a review. J Alzheimers Dis. 2009;18:703–711. doi: 10.3233/JAD-2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann NY Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 25.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- 26.Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain. 2007;130:2830–2836. doi: 10.1093/brain/awm228. [DOI] [PubMed] [Google Scholar]
- 27.Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, et al. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis. 2009;18:645–658. doi: 10.3233/JAD-2009-1182. [DOI] [PubMed] [Google Scholar]
- 28.Wang LY, Larson EB, Sonnen JA, Shofer JB, McCormick W, Bowen JD, et al. Blood pressure and brain injury in older adults: findings from a community-based autopsy study. J Am Geriatr Soc. 2009;57:1975–1981. doi: 10.1111/j.1532-5415.2009.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 30.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- 31.Petrovitch H, Ross GW, He Q, Uyehara-Lock J, Markesbery W, Davis D, et al. Characterization of Japanese-American men with a single neocortical AD lesion type. Neurobiol Aging. 2008;29:1448–1455. doi: 10.1016/j.neurobiolaging.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29:1587–1590. doi: 10.1016/j.neurobiolaging.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]