Abstract
Western diet (WD) intake induces obesity and metabolic dysfunction. The present study examined the effects of WD on hippocampal-dependent cognitive functioning and blood-brain barrier (BBB) permeability as a function of exposure duration, obesity phenotype, and peripheral markers of energy regulation. The use of hippocampal-dependent “place” or hippocampal-independent “response” strategies in a Y-maze was assessed in male rats following 10, 40, and 90 days of WD exposure in diet-induced obese (DIO) rats, in diet resistant (DR) rats that are relatively insensitive to the obesogenic properties of WD, and in chow-fed controls. Insulin, glucose, and BBB permeability throughout several loci in the hippocampus, striatum, and cerebellum were evaluated in relation to duration of WD exposure, obesity phenotype and type of strategy used.
DIO rats had increased body weight and adiposity throughout the study, and elevated 10-day glucose and 90 day insulin levels. Throughout the study, Chow-fed and DR rats reliably relied on a place strategy. DIO rats, in contrast, favored a response strategy at the 10 and 90 day time points. BBB leakage was observed in the dorsal striatum and multiple subregions of the hippocampus of DIO, but not DR or Chow-fed rats. Increased ventral hippocampal BBB permeability and blood glucose levels were associated with reduced place strategy use. These data indicate that WD-induced BBB leakage is dependent on duration of diet exposure as well as obesity phenotype, and implicates BBB leakage and impaired glucoregulation in behavioral strategy and cognitive performance.
Keywords: Obesity, Striatum, Hippocampus, Place Strategy, Response Strategy
The overconsumption of simple sugars and saturated fats has long been known to induce weight gain and fat accumulation (Fung et al., 2001; West, Boozer, Moody, & Atkinson, 1992). Throughout industrialized nations, diet-induced obesity, as well as obesity- and diet-related illnesses, are becoming ubiquitous (Flegal, Carroll, Ogden, & Curtin, 2010). Recent data suggest that extended exposure to high-fat, high-sugar “Western” diets (WD) can disturb cognitive function and increase susceptibility to neurodegenerative conditions (Hu et al., 2006; Naderali, Ratcliffe, & Dale, 2009; Wang, Beydoun, Liang, Caballero, & Kumanyika, 2008). Similarly, rats fed WD also show impaired performance on hippocampal-dependent, but not hippocampal-independent tasks (Davidson et al., 2013; Davidson, Kanoski, Schier, Clegg, & Benoit, 2007; Davidson, Kanoski, Walls, & Jarrard, 2005; Kanoski & Davidson, 2011; Kanoski, Zhang, Zheng, & Davidson, 2010; Molteni, Barnard, Ying, Roberts, & Gomez-Pinilla, 2002; Pistell et al., 2010; Pistell et al., 2009; Stranahan et al., 2008; Wu, Ying, & Gomez-Pinilla, 2004). Diet-induced obese (DIO) WD-fed rats are more likely to develop cognitive deficits than their lean (diet resistant, DR) counterparts (Davidson et al., 2013; Davidson et al., 2012). However, deficits in hippocampal-dependent task performance have also been observed prior to the onset of obesity after short-term (3–10 day) WD access (Kanoski et al., 2010). Though these impairments remitted by day 24, early deficits predicted increased weight gain over time (Davidson et al., 2013), suggesting WD need not induce obesity to alter hippocampal function, and that even transient hippocampal impairments may facilitate weight gain.
Hippocampal deficits may result from damage to the blood-brain barrier (BBB), an important structure that maintains homeostasis within the brain parenchyma, and precludes the influx of blood-borne compounds. BBB leakage can alter neuronal firing rates (Janigro, 2012) and permit neurotoxic compounds to enter the brain interstitial fluid and damage cells. In previous studies, WD-DIO rats exhibited increased sodium fluorescein (NaFl) permeability in the hippocampus (Davidson et al., 2013; Davidson et al., 2012), and decreased expression of tight-junction proteins, which comprise the BBB and choroid plexus (Kanoski et al., 2010), suggesting the BBB is damaged after maintenance on WD.
The present research further characterized the relationship between WD and BBB leakage, with three objectives. Previous investigations showed that BBB leakage following WD exposure varied as a function of obesity phenotype. However, these studies were all conducted at one time point. Our first goal was to assess the development of BBB permeability changes in DIO, DR, and Chow-fed Controls rats across three durations of WD exposure (10, 40, and 90 days). Our second objective was to expand and refine the analysis of WD-induced increases in BBB permeability on the brain. Previously, these analyses compared BBB permeability in the total hippocampus with that in other brain structures. Here, we examined the level of BBB permeability in different ventral and dorsal hippocampal subregions (subiculum, dentate gyrus) and cell fields (CA1, CA3). This was based on the preponderance of evidence showing that anatomically distinct hippocampal subregions are differentiated functionally (Abi-Saab et al., 2002; Fanselow & Dong, 2010; Gilbert, Kesner, & Lee, 2001; Goodrich-Hunsaker, Hunsaker, & Kesner, 2008; Jarrard, Davidson, & Bowring, 2004) are vulnerable to independent insults (Becker et al., 1999; Wilde, Pringle, Wright, & Iannotti, 1997), and are associated with diverse disorders (for review, see: Small, Schobel, Buxton, Witter, & Barnes, 2011). For example, loss of CA1 and subicular neurons are associated with the development of Alzheimer’s disease. The dentate gyrus appears to be vulnerable to normal aging, as BOLD signaling is altered in this region among aged adults, and alterations in cellular metabolism within the subiculum, CA1, and CA3 are associated with schizophrenia, depression, and dementia.
(for review, see: Small et al., 2011)We anticipated that BBB leakage in regions such as the dorsal CA1 and CA3, which are highly involved in the representation of space (Goodrich-Hunsaker et al., 2008), would be correlate with navigation deficits, whereas increases in obesity would be associated with BBB damage in the ventral hippocampus (Clifton, Vickers, & Somerville, 1998; Davidson et al., 2009; Kanoski, Fortin, Ricks, & Grill, 2013). Because the striatum is heavily involved in response learning (Berke, Breck, & Eichenbaum, 2009), we measured dorsal and ventral striatal NaFl accumulation. We also analyzed NaFl accumulation in the cerebellum in order to determine whether BBB permeability is altered in more extant brain areas.
Our third aim was to evaluate the relationship between BBB permeability, obesity phenotype, and learning and memory function. Expanding on previous studies, we assessed how rats solved a task in which performance could be based on either a “place” strategy, which involves learning about the location of a rewarded goal box in relation to other objects in space and has been shown in both humans and rats to depend on the hippocampus, or a hippocampal-independent “response” strategy, which involves learning what motor response leads to the rewarded goal box (Iaria, Petrides, Dagher, Pike, & Bohbot, 2003; Iglói, Doeller, Berthoz, Rondi-Reig, & Burgess, 2010; Packard & McGaugh, 1996). Thus, we examined whether (a) WD-maintained DIO and DR rats would adopt a predominantly response strategy as a function of duration of WD exposure and (b) the timing of such a shift would be linked to changes in permeability of the BBB in any of the different brain regions that we examined.
Materials and Methods
Subjects, Diets, and Study Design
All procedures were approved by the Purdue University Animal Care and Use Committee (PACUC). A total of 75 male Sprague Dawley rats (275–300g; Harlan Laboratories, Indianapolis, IN) were used (n = 63 for the primary experiment, n = 12 for the pair-feeding experiment). Rats were individually housed in a temperature and humidity controlled room with a 12/12 light/dark cycle with access to water at all times. Rats were fed chow (CHOW; Harlan Teklad 2018, Harlan Teklad, Indianapolis, IN) or a WD comprised largely of saturated fats and dextrose (WD; Harlan TD.10768). See Table 1 for composition.
Table 1.
Source of diets and their macronutrient composition. The caloric composition of CHOW was 24% protein, 18% fat, and 58% complex carbohydrates, with a total caloric density of 3.4 kCal/g. WD also contained 24% kCal from protein; however, 38% of WD kCal were derived from fat, 20% from dextrose, and 18% from complex carbohydrates, resulting in a total caloric density of 4.5 kCal/g.
| CHOW Harlan 2018 |
WD Harlan TD.10768 |
|
|---|---|---|
| % kCal Protein | 24 | 24 |
| % kCal Fat | 18 | 38 |
| % kCal Carbohydrate | 58 | 38 |
| % kCal Dextrose | 0 | 20 |
|
| ||
| Caloric Density (kCal/g) | 3.4 | 4.5 |
After at least 1 week acclimation, rats (n = 63) were weighed and analyzed for body composition via nuclear magnetic resonance (NMR; Echo-MRI-900, Echo Medical Systems, LLC, Houston, TX), then matched into diet (CHOW or WD) and exposure duration (10, 40 or 90 days) groups (n = 10–11 per group) based on body weight and adiposity. These groups were then further divided into four balanced cohorts in order to accommodate apparatus scheduling and to minimize time variance on sacrifice days; diet administration was staggered by one week per cohort. Rats were weighed five days per week and analyzed for body composition approximately once every 10 days. Food intake (kCal) was measured by weighing food hoppers and spillage five times per week. Behavioral tests were performed at days 8, 38, or 88. Two days later, rats were sacrificed for BBB analyses.
Blood collection and analysis
Following a 2h fast, a drop of tail blood was analyzed for glucose in duplicate via glucometer (Novamax Plus, Nova Diabetes Care, Inc., Billerica, MA). Approximately 1 mL blood was then collected into iced K3EDTA+ tubes (Vacutainer, BD Medical, Franklin Lakes, NJ), and centrifuged 15m at 4°C at 2500 RPM. Plasma was aspirated and stored at −80°C until analysis could be completed. The tail vein was allowed to fully clot before further procedures were attempted. Plasma insulin was assessed in duplicate via radioimmunoassay (Rat Insulin RIA Kit, Millipore, St. Charles, MO). The range of sensitivity for the kit was from 0.081–10 ng/mL.
Blood-Brain Barrier Permeability Assessment
Rats were given a light IP dose (0.33 mL/kg) of Beuthanasia, and when sedated were administered a 10% sodium fluorescein (NaFl) solution (1mL/kg). NaFl circulated for 10 minutes, a duration within the window of time (5–15 minutes) previously demonstrated (Farkas et al., 1998; Martinez Jr. & Koda, 1988; Natah, Srinivasan, Pittman, Zhao, & Dunn, 2009) to deliver the best dye penetration. These doses and time frames were validated prior to experimentation in our lab using positive control animals that had been exposed to middle cerebral artery occlusion (not shown). Rats were then perfused with PBS for 5 minutes, then fixed with 4.0% iced paraformaldehyde, then post-fixed for 2 hours. No cryoprotectant was used, as these have been shown to reduce NaFl fluorescence (Natah, Mouihate, Pittman, & Sharkey, 2005).
Tissue was sectioned at 50 μM and mounted to slides under low ambient light conditions. Imaging and quantification were performed by a researcher blinded to the treatment conditions. Photomicrographs were obtained at 40x and regional densitometric analyses were performed using FIJI (Schindelin et al., 2012). NaFl was expressed as mean background-subtracted grey values, with higher numbers indicating increased BBB permeability.
Behavioral Assessment
Apparatus
Rats were tested in a three armed, Y-shaped maze in a moderately lit room containing numerous diverse extra-maze cues (see Fig. 1A for schematic). Each arm was 1m long and 20 cm wide, bordered by a 20 cm high wall. The interior of the maze was lined with lightly soiled bedding, which was tossed between trials to neutralize odor cues. One arm was designated the start arm, the goal arm was baited with a cup containing a sucrose pellet (45 mg Dustless Precision Pellets, Bio-Serv, Frenchtown, NJ), which was positioned 10 cm from the end of the arm. The third arm was unbaited (dummy), but contained a cup identical to the one placed in the goal arm. These designations remained constant throughout the experiment.
Figure 1.
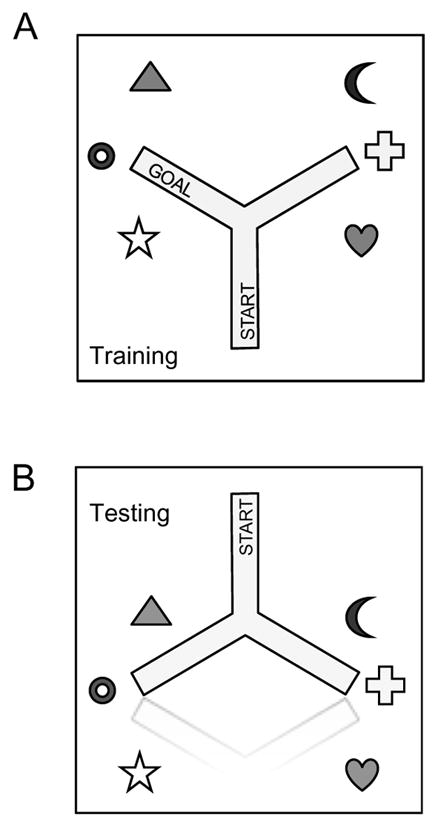
Spatial learning apparatus. (A) Acquisition setup. Extra-maze cues were placed in fixed positions throughout the room; the goal arm remained consistent throughout training, allowing rats to use either a PLACE or a RESPONSE strategy to learn the task. (B) During testing, the maze was flipped 180° in order to uncouple the arms that would predominantly be used in PLACE or RESPONSE strategy-prone rats.
Training
Rats were pre-exposed to sucrose pellets for three days prior to testing, and all rats readily consumed the sucrose pellets by the third day. After overnight food deprivation, rats were acclimated to the apparatus, then trained to reliably locate the goal arm. Training began at the onset of the dark cycle. During each trial, the rat was placed within the distal 25 cm of the start arm, and given one minute to enter another arm, then 30 seconds to explore and consume the sucrose reward. Rats consumed the pellet after nearly every correct trial, and there were no diet- or weight-based differences in the percent of pellets consumed during correct trials. Rats were then returned to their home cages for 5–10 minutes, after which this procedure was repeated. Training persisted until the following criteria were reached: the rat 1.) entered and explored both arms, 2.) consumed at least 5 sucrose pellets, 3.) made at least 20 arm entries, and 4.) entered the goal arm in 80% of the final 10, and 100% of the final 5 trials. Training ceased if rats failed to fulfill these criteria within 100 trials, as was the case in two WD-fed rats from the 10 day group. In these animals, behavioral data were not analyzed.
Intra-maze cues were made irrelevant by rotating the apparatus 120 degrees and repositioning the cups in their correct spatial location every 10 trials. Because the location of the rewarded cup remained constant relative to both the start arm location and extra-maze cues, the animal could solve the task reliably using either egocentric, directional (RESPONSE) cues, or spatial extra-maze (PLACE) cues.
Strategy preference testing
PLACE and RESPONSE cues were then uncoupled by reversing the direction of the maze while holding the extra-maze cues constant (Fig. 1B), and rats were then given 10 un-baited trials to assess which strategy was used to solve the task. If all four paws entered the arm corresponding to the rewarded extra-maze cues, it was designated PLACE; other choices were designated RESPONSE.
Body Weight and Behavioral Strategy Groups
To assess the relationship between diet, body weight, duration of diet exposure, cognitive performance, and BBB permeability, rats were divided into groups based on body weight (WD-DR and WD-DIO) via median split (WD-DR n = 6, 6, and 5; WD-DIO n = 5, 5, and 6 for 10, 40 and 90 days, respectively), and cognitive strategy (PLACE, INTERMEDIATE, RESPONSE) based on the strength and nature of their strategy preference (PLACE n = 6, 12, and 4; INTERMEDIATE n = 7, 6, and 9; RESPONSE n = 6, 3, and 8 for 10, 40 and 90 days, respectively). Rats that turned toward the extra-maze cues 70% of the time or more were designated PLACE rats, while rats that turned the opposite direction 70% of the time or more were designated RESPONSE rats. Those that did not show a reliable pattern toward one direction (40–60% of the turns towards the extra-maze cues) were designated INTERMEDIATE rats. Two rats from the 10 day WD-DIO group were unable to learn the spatial task within 100 trials and were excluded from this analysis.
Pair-Feeding
Because WD-fed rats consumed a significantly greater number of calories during the first 10 days of the study, pair-feeding was performed to assess the role of WD independent from caloric intake. Twelve Sprague-Dawley rats were weight matched into two groups, CHOW and WD-PF (n = 6/group). Each WD-PF rat was paired with the CHOW rat closest in body weight and adiposity. For CHOW rats, food and spillage was weighed daily. Caloric intake for each 24 hour period was calculated for each CHOW rat, and a portion of WD that was equal in calories was distributed to the corresponding WD-PF rat approximately 30 minutes prior to the onset of the dark cycle. Because of this feeding paradigm, experimental procedures for WD-PF rats lagged one day behind their CHOW counterparts. Other than this delay, procedures were identical to those performed during the first 10 days of the previous experiment; rats were food deprived overnight after 7 days diet exposure, and tested for spatial learning and PLACE or RESPONSE preference on day 8. Food access was then restored, and body weights were allowed to recover, and at 10 days, body composition was analyzed, and blood glucose and plasma insulin were assayed as described above.
Statistical Analyses
Body weight, caloric intake, and body composition data were analyzed via repeated measures two-way ANOVA with multiple comparison-corrected Bonferroni post-hoc tests (specific factors analyzed are indicated in their respective results sections) The distribution of strategies within dietary treatment groups was analyzed via Chi-square test. Terminal body weight, adiposity, blood glucose, plasma insulin, and blood-brain barrier measurements were analyzed via two-way ANOVA with Bonferroni post-hoc tests. Significance was set at p < 0.05.
Results
Differences Between Diet/Body Weight Groups
Food intake
Food intake was measured 5 times per week, and average weekly caloric intake was assessed via two-way ANOVA with Bonferroni post-hoc tests. A significant interaction between diet and exposure duration was noted for caloric intake (F(24,400) = 8.56, p < 0.0001), along with main effects of diet type (F(2,400) = 6.41, p = 0.0018) and exposure duration (F(12,400) = 33.33, p < 0.0001). Post-hoc analysis revealed a period of hyperphagia in WD-fed rats during the first week of diet administration (mean kcals: 87.48, 105.8, and 113.8 for chow, WD-DR, and WD-DIO rats). This increase caloric intake during the first week was significant for both WD groups, compared to chow (p < 0.001). WD-DIO rats also consumed significantly more calories than WD-DR rats during week 1 (p < 0.05). However, at no other time points did food intake levels differ between any groups.
Body weight
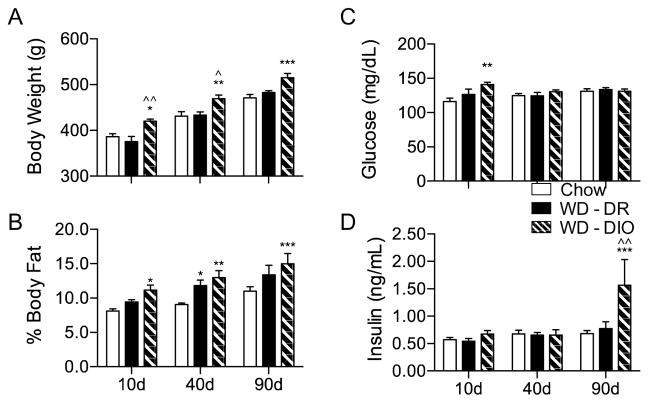
Body weight (Fig. 2A) was analyzed via two-way ANOVA with planned Bonferroni post-hoc tests at 10, 40, and 90 days. A significant interaction was observed for body weight gain (F(4,54) = 5.33, p = 0.0011), with WD-DIO rats gaining significantly more weight than both chow and WD-DR rats at 90 days (p < 0.001 for both comparisons), and weighing significantly more than chow (p < 0.05, p < 0.01, and p < 0.001 at 10, 40, and 90 days, respectively) and WD-DR (p < 0.01, p < 0.05, at 10 and 40 days, respectively). WD-DIO rats also weighed marginally more than WD-DR rats at 90 days (p = 0.0907).
Figure 2.
Markers of the metabolic syndrome in rats fed CHOW or WD. (A) Body weight of WD-DIO rats was significantly higher than CHOW rats at all time points, and WD-DR rats at 10 and 40 days. (B) Compared to CHOW, percent body fat was significantly higher in WD-DIO rats at all time points, and significantly higher in WD-DIO rats at 40 days. (C) At 10 days, blood glucose was significantly higher in WD-DIO rats, compared to CHOW. (D) At 90 days, plasma insulin was significantly higher in WD-DIO rats, compared to CHOW and WD-DR rats. * indicates results significantly different from CHOW rats; ^ indicates results significantly different from WD-DR rats (* and ^ indicate p < 0.05; ** and ^^ indicate p < 0.01; *** and ^^^ indicate p < 0.001).
Body adiposity
Body fat measurements (Fig. 2B) were expressed as a percentage of total body weight, and analyzed via two-way ANOVA with planned Bonferroni post-hoc tests. Main effects of both diet type (F(2, 54) = 19.48, p < 0.0001) and exposure duration (F(2,54) = 16.20, p < 0.0001). While WD-DR rats only had higher adiposity than chow at the 40 day time point (p < 0.05), WD-DIO rats had a greater proportion of body fat than chow rats at 10, 40, and 90 days (p < 0.05, p < 0.01, and p < 0.001, respectively), but not WD-DR rats.
Peripheral markers of energy regulation
Blood was collected after a two-hour fast on the day of sacrifice, glucose and insulin were measured in CHOW, WD-DR, and WD-DIO rats. Compared to CHOW, WD-DIO rats showed a main effect of diet on blood glucose (F(2,54) = 3.62, p = 0.0332; Fig. 2C) and an interaction between diet and time on plasma insulin (F(3,53) = 3.00, p = 0.0263 ; Fig. 2D). WD-DIO rats had elevated 10 day glucose levels compared to CHOW (p < 0.01), and increased 90 day insulin levels compared to both CHOW (p < 0.001) and WD-DR rats (p < 0.01).
Effects of diet on cognitive performance and strategy
Rats were trained to a set of criteria on an appetitive, spatial learning task which could be solved via the use of PLACE or RESPONSE strategy, then probed for PLACE or RESPONSE strategy preference. Acquisition and strategy data were analyzed via two-way ANOVA with planned Bonferroni post-hoc tests. For acquisition (Fig. 3A), an interaction between diet and time was observed (F(4,54) = 3.81, p = 0.0084). Compared to CHOW, rats fed WD had a significantly longer latency to learn the task at 10 (WD-DR: p < 0.01; WD-DIO: p < 0.05) and 90 (WD-DR: p < 0.05; WD-DIO: p < 0.01), but not 40 days. This was not dependent on body weight, as WD-DR rats weighing the same as CHOW showed the same acquisition delay as their DIO counterparts at 10 and 90 days.
Figure 3.
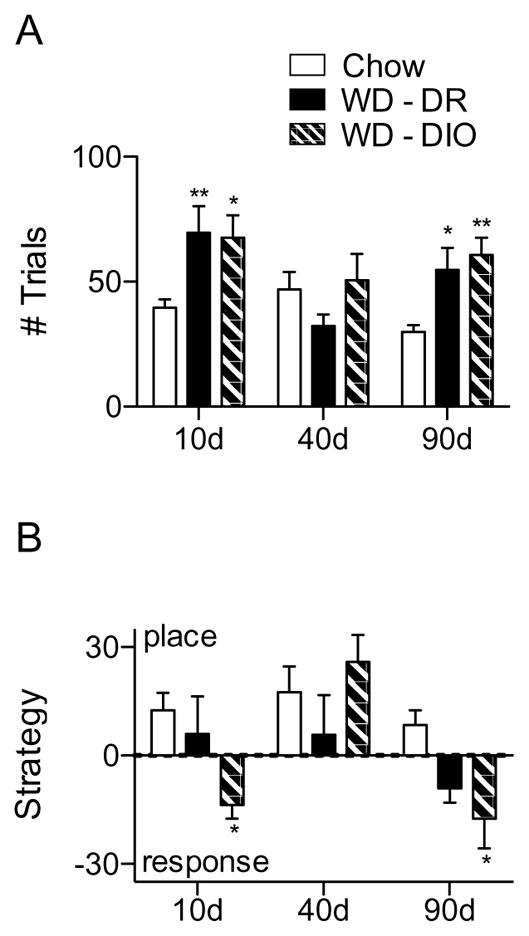
Spatial learning and strategy assessment performance. (A) Number of trials needed to reach acquisition criteria. After 10 and 90, but not 40 days, rats fed WD required a significantly greater number of trials to reach criteria. (B) Extent to which rats used a PLACE or RESPONSE strategy. At 10 and 90 days, WD-DIO rats were significantly more likely to use a RESPONSE strategy than CHOW rats. * indicates results significantly different from CHOW (* indicates p < 0.05; ** indicates p < 0.01).
Strategy was then assessed (Fig. 3B); a marginally significant interaction between diet and time was present (F(4,52) = 2.36, p = 0.0661, and main effects of both diet and exposure time were present (F(2,52) = 2.35, p = 0.249 and F(2,52) = 7.42, p = 0.0015, respectively), and post-hoc tests revealed that WD-DIO rats were significantly more likely than CHOW to use a RESPONSE strategy at 10 and 90 days (p < 0.05). There were no significant differences in strategy between WD-DIO and DR and the DR and CHOW diet groups.
Blood-brain barrier permeability
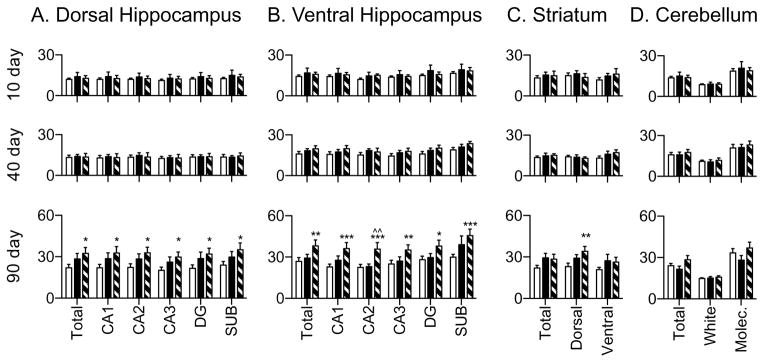
Hippocampal, striatal, and cerebellar BBB permeability was assessed via NaFl injection after 10d, 40d and 90d diet exposure in CHOW, WD-DR and WD-DIO rats, and analyzed via two-way ANOVA. There were no significant interactions between diet and treatment group. Significant main effects of time were observed for all regions (p < 0.0001). The smallest F(2,54) values were: 27.88 (dorsal hippocampus), 23.52 (ventral hippocampus), 15.95 (striatum), and 14.71 (cerebellum). Main effects of diet were also noted in several regions of the ventral hippocampus (F(2,54) = 3.68, p = 0.0318; F(2,54) = 5.07, p = 0.0097; F(2,54) = 5.07, p = 0.0099; and F(2,54) = 5.20, p = 0.0086 for total ventral hippocampus, CA1, CA2, and ventral subiculum, respectively)
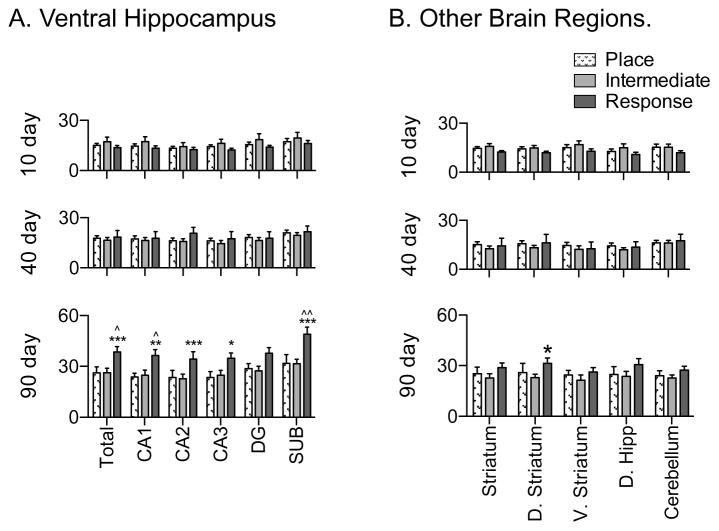
Compared to CHOW, WD-DIO rats had significantly higher 90 day BBB permeability in all regions of the hippocampus that were measured (p < 0.05 throughout the dorsal hippocampus [Fig. 4A, 5A] and the ventral dentate gyrus; p < 0.01 in the entire ventral hippocampus and ventral CA3 region; p < 0.001 in the ventral CA1, CA2, and subiculum, [Fig. 4B, 5B]). WD-DIO rats also had increased 90 day ventral CA2 permeability compared to WD-DR rats (p < 0.01). This is consistent with previous observations showing WD-induced hippocampal BBB leakage (Davidson et al., 2013; Davidson et al., 2012; Kanoski et al., 2010).
Figure 4.
Regional, background subtracted NaFl permeability after 10, 40 and 90 days diet maintenance. (A) At 90 days, WD-DIO rats had significantly increased NaFl levels in all measured fields in dorsal hippocampus, compared to CHOW rats. (B) At 90 days, WD-DIO rats had significantly elevated NaFl levels in all ventral hippocampal cell fields, compared to CHOW, and elevated ventral CA2 NaFl compared to WD-DR rats. (C) At 90 days, there were no significant differences in total or ventral striatal NaFl expression, but WD-DIO rats showed significantly elevated dorsal striatal NaFl compared to CHOW. (D) There were no differences in NaFl permeability in the cerebellum at any time point. * indicates results significantly different from CHOW rats; ^ indicates results significantly different from WD-DR rats (* and ^ indicate p < 0.05; ** and ^^ indicate p < 0.01; *** and ^^^ indicate p < 0.001).
Figure 5.
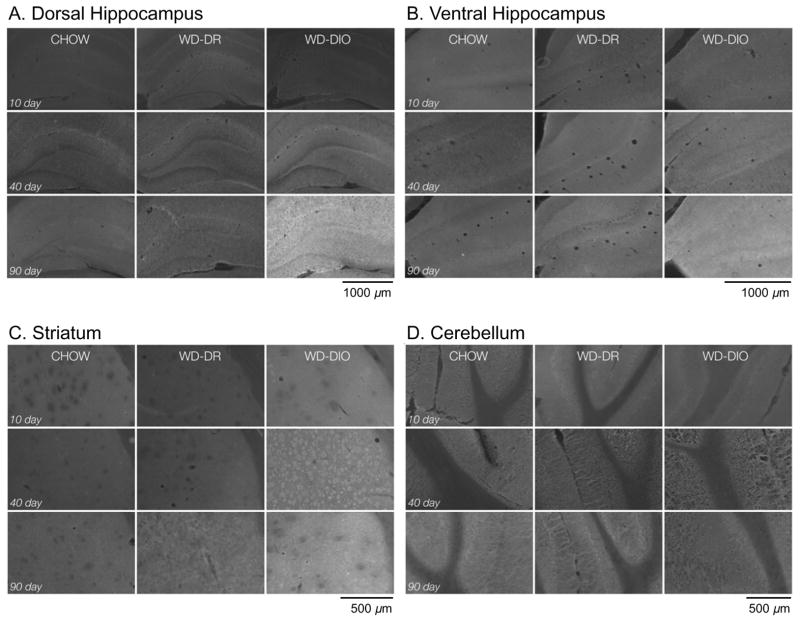
Representative images of NaFl permeability in (A) dorsal hippocampus, (B) ventral hippocampus, (C) striatum, and (D) cerebellum in Chow (left columns), DR (middle columns) and DIO (right columns) rats after 10 (top rows), 40 (middle rows), and 90 (bottom rows) days WD access.
Dorsal striatal permeability was increased in WD-DIO rats compared to CHOW (p < 0.01) after 90 days diet access. No differences were observed for the total striatum or the ventral (nucleus accumbens and olfactory tubule) subregions (Fig. 4C, 5C). These data are consistent with our previous observations which revealed no diet-induced permeability differences within the aggregate striatum, but expands upon them by revealing the increased permeability of the dorsal striatum and highlights the importance of this more refined BBB analysis. No differences were observed in the cerebellum at any time (see Fig. 4D, 5D). Collectively, these findings indicate that diet-induced changes in BBB permeability, while not global, extend beyond the hippocampus. However, a comparison of all brain regions revealed that NaFl accumulation in WD-fed rats was higher in the ventral hippocampus, compared to both the striatum (p < 0.05) and cerebellum (p < 0.001) at 90 days. Permeability was likewise higher in the dorsal hippocampus of WD-fed rats, compared to the cerebellum (p < 0.05). This suggests that the BBB of the hippocampal formation may be more vulnerable to insult than the other regions measured in the present experiments.
Effects Related To Behavioral Phenotype
Rats were then grouped by strategy preference (PLACE, RESPONSE, or INTERMEDIATE) and analyzed via Chi-squared test. The proportion of rats to use PLACE, INTERMEDIATE, and RESPONSE strategies (respectively) differed significantly as a result of the nature and duration of the dietary treatment used, χ2 (10 df) = 23.70; p < 0.01. Data was reanalyzed by strategy group at 10, 40 and 90 days via two-way ANOVA in order to assess the relationship between the behavioral phenotype and other factors influenced by WD maintenance.
Cognitive performance and learning strategy
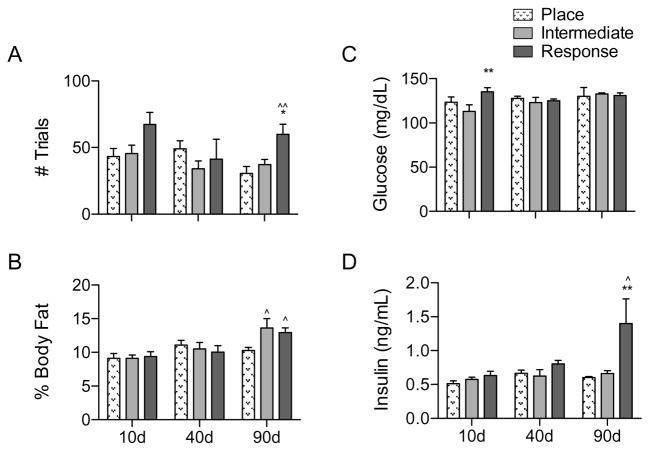
Latency to learn the task was quantified for PLACE, RESPONSE, and INTERMEDIATE strategists, collapsed across diet and obesity phenotype. A main effect was observed for strategy (F(2,52) = 4.30, p = 0.0186), and a RESPONSE strategy was associated with slower acquisition than PLACE (p < 0.01) or INTERMEDIATE (p < 0.05) at 90 days (Fig. 6A), and marginally so at 10 days (p = 0.052 and 0.062; PLACE and INTERMEDIATE, respectively).
Figure 6.
Behavioral data and markers of the metabolic syndrome by strategy group. (A) At 90 days, rats using a RESPONSE strategy required a significantly greater number of trials to learn to locate the sucrose reward than PLACE or INTERMEDIATE rats. (B) At 90 days, RESPONSE and INTERMEDIATE rats had significantly higher adiposity levels than PLACE rats. (C) Blood glucose was significantly higher in RESPONSE rats, compared to INTERMEDIATE rats. (D) Plasma insulin levels were significantly increased at 90 days in RESPONSE rats, compared to PLACE or INTERMEDIATE rats. * indicates results significantly different from INTERMEDIATE rats; ^ indicates results significantly different from PLACE rats (* and ^ indicate p < 0.05; ** and ^^ indicate p < 0.01; *** and ^^^ indicate p < 0.001).
Relationships between strategy and body weight, adiposity, and blood composition
Though a main effect of time was detected for body weight (F(2,54) = 56.30, p < 0.0001) due to growth throughout the experimental period, there were no significant differences in weight between strategy groups at any point (Fig. 6B). A main effect of time was observed for adiposity (F(2,54) = 10.49, p < 0.0001), which was significantly higher in both RESPONSE and INTERMEDIATE rats at 90 days (p < 0.05).
Although no significant effects of body weight and adiposity on learning strategy were observed, glucose levels were higher in RESPONSE (Fig. 6C) compared to INTERMEDIATE rats at 10 days (p < 0.01). A main effect of strategy was detected for insulin (F(2,54) = 4.43, p = 0.0166; Fig. 6D); RESPONSE animals had significantly higher 90 day plasma insulin levels than INTERMEDIATE (p < 0.01) or PLACE (p < 0.05) rats.
Relationship between strategy and BBB permeability
NaFl permeability in the hippocampus, striatum and cerebellum were analyzed for PLACE, INTERMEDIATE, and RESPONSE rats at 10, 40, and 90 days. Interactions were observed between strategy and time in the ventral hippocampus (for total ventral hippocampus, CA1, and ventral subiculum, F(2,54) = 3.04, p = 0.05; F(2,52) = 3.04, p < 0.05; and F(2,52) = 6.09, p < 0.001; respectively. See Fig. 7A). Main effects of time were observed for all brain regions analyzed (p < 0.0001). The smallest F(2,52) values were: 19.68 (dorsal hippocampus), 20.89 (ventral hippocampus), 24.84 (striatum), and 16.19 (cerebellum). A main effect of strategy was noted for the ventral subiculum (F(2,52) = 4.06, p < 0.05). These data suggest that rats with more pronounced BBB permeability in the ventral hippocampus were more likely to use a RESPONSE strategy at 90, but not 10 or 40 days, suggesting that this region may be particularly tied to the behavioral consequences of extended WD maintenance.
Figure 7.
BBB permeability data by strategy group. (A) In the ventral hippocampus (total and all regions except the dentate gyrus), 90 day BBB permeability was significantly higher in rats using a RESPONSE strategy, compared to an INTERMEDIATE strategy. In the entire ventral hippocampus, CA1, and subiculum, RESPONSE-prone rats had higher NaFl levels than PLACE rats. (B) NaFl levels in other brain areas measured, by strategy. There were no significant strategy-based differences in the total or ventral striatum, dorsal hippocampus or cerebellum. Rats using a RESPONSE strategy had increased NaFl in the dorsal hippocampus compared to those using an INTERMEDIATE strategy. * indicates results significantly different from INTERMEDIATE rats; ^ indicates results significantly different from PLACE rats (* and ^ indicate p < 0.05; ** and ^^ indicate p < 0.01; *** and ^^^ indicate p < 0.001).
RESPONSE rats had significantly higher 90 day BBB permeability in most regions of the ventral hippocampus, compared to both INTERMEDIATE (p < 0.05 in the ventral CA3, p < 0.01 in the total ventral hippocampus, and CA1, and p < 0.001 in the ventral CA2 and subiculum) and PLACE (p < 0.05 in the total ventral hippocampus, CA1, and CA3 region; p < 0.01 in the ventral subiculum) strategists. No differences were present in the dorsal hippocampus, cerebellum, or ventral striatum (Fig. 7B), however the permeability of the entire hippocampus was significantly increased in RESPONSE strategy-prone rats, compared to both PLACE and INTERMEDIATE animals (p < 0.05). Though they did not differ from PLACE strategists, RESPONSE-prone rats had increased 90 day dorsal striatal permeability compared to INTERMEDIATE rats (p < 0.05); total and ventral striatum did not differ between strategy groups.
Pair-feeding Experiment
Because pronounced hyperphagia was observed in WD-fed rats during the first week of the experiment, it was challenging to determine whether the behavioral and physiological results obtained at the 10-day time point were the result of the diet per se, or excess energy intake in general. In order to determine whether these early changes could be attributed WD intake, an additional group of rats (WD-PF) were pair-fed WD for 10 days, in direct proportion to the caloric intake of a CHOW cohort, after which cognitive performance was assessed. Food was measured daily, though there were no differences in intake between groups prior to the period of food deprivation.
Body weight gain was analyzed via two-way ANOVA for CHOW and WD-PF rats for the seven days prior to food deprivation and behavioral testing, and separately thereafter. During the first week, a significant interaction and a main effect of time were observed (F(7,80) = 2.14, p < 0.05; and F(7,80) = 42.56, p < 0.0001, respectively) Although WD-PF rats weighed the same as the CHOW rats to which they were pair-fed, they had significantly elevated adiposity (p < 0.01; Fig. 8A). Blood was sampled from CHOW and WD-PF rats; there were no significant differences between these groups on blood glucose (Fig. 8B) or plasma insulin (Fig. 8C).
Figure 8.
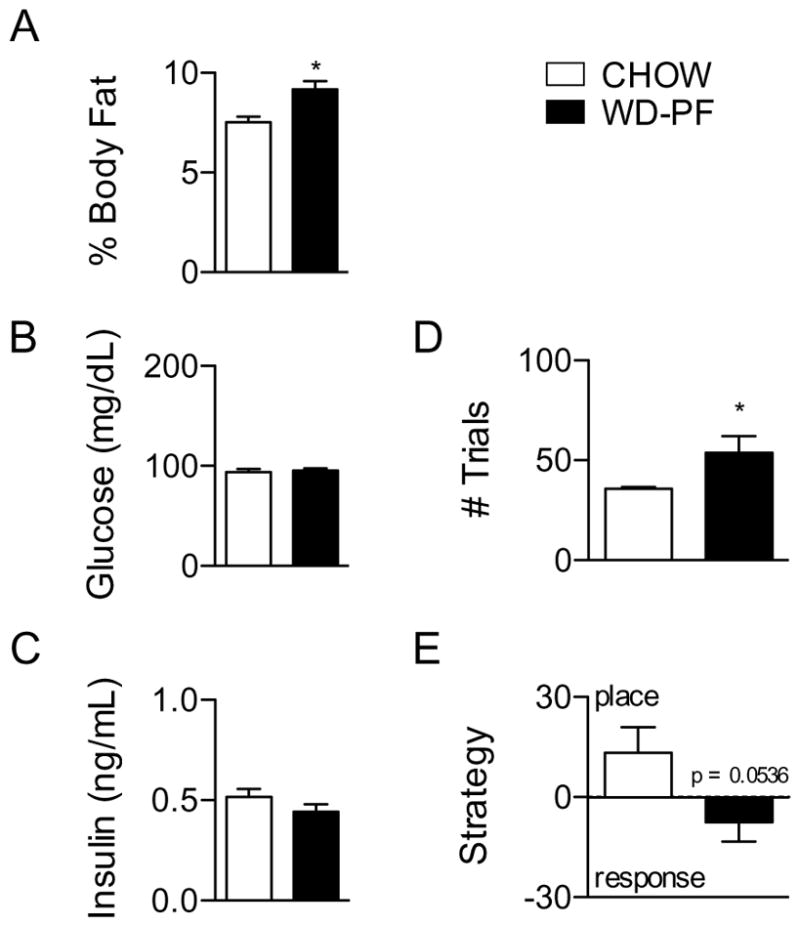
Data for rats fed CHOW (n = 6), and those pair-fed WD to the number of calories consumed by CHOW (WD-PF; n = 6). (A) Percent body fat was significantly increased in WD-PF rats compared to CHOW rats. There were no differences in (B) blood glucose or (C) plasma insulin. (D) WD-PF rats required significantly more trials to reach acquisition criteria than CHOW rats. (E) WD-PF rats were marginally more likely to use a RESPONSE strategy, compared to CHOW rats (p = 0.0536). * indicates results significantly different, compared to CHOW (p < 0.05).
At the 10 day time point, CHOW and WD-PF rats were assessed for acquisition performance (Fig. 8D) and strategy preference (Fig. 8E) on the spatial learning task, and analyzed via Student’s t-test. Relative to CHOW, WD-PF rats were significantly impaired at acquisition (p < 0.05). Along with the 90 day data from the previous experiment, these data suggest that acquisition deficits are not driven primarily by increased caloric intake or body weight, but instead may be due to features of the diet itself. However, the preferred strategy used to solve the task seems to depend heavily on body weight and adiposity, as WD-DIO but not WD-DR or CHOW rats (which were lighter and leaner than WD-DIO rats) showed a robust preference for a RESPONSE strategy at 10 and 90 days, and WD-PF rats, which had higher levels of body fat than CHOW rats, were marginally more RESPONSE-prone (p = 0.0536).
Discussion
The three primary objectives of the present study were to (1) characterize the development of WD-induced BBB permeability changes, (2) refine our analysis of BBB permeability changes to better understand where they manifest within the brain, and (3) to assess the relationship between BBB permeability, obesity phenotype, and behavioral strategy and performance. First, we showed that the integrity of the BBB varied as a function of duration of WD exposure. Increased BBB permeability was not present until WD-DIO rats had been exposed to WD for 90 days. This suggests that BBB breakdown is (a) gradual, or (b) coincides with other factors related to WD maintenance and/or obesity.
Second, we found that after 90 days on WD, BBB permeability increased in hippocampus in toto, and that these permeability increases were widespread, affecting the CA1 and CA3 cell fields, as well as the subiculum and dentate gyrus in both the dorsal and ventral hippocampus. Thus, the hippocampal BBB was broadly affected by WD. Increased BBB permeability, while not observed within the structure overall, was observed in the dorsal (but not ventral) striatum. While increased striatal BBB permeability was not observed in previous studies, these other analyses examined the entire mass of the striatum, rather than discrete subregions. The present results remain consistent with previous observations showing no global striatal BBB leakage, but the improved anatomical precision employed herein suggests further investigation into the effects of WD on dorsal striatum-dependent behaviors is warranted. Consistent with previous results, we found no differences in cerebellar BBB permeability, indicating that this region may be relatively resilient to the neurovascular damage induced by WD.
Third, we showed that the WD-induced BBB permeability disruptions were linked to hippocampal-dependent learning and memory function. Rats with BBB dysfunction exhibited a shift from a hippocampal-dependent PLACE strategy to a hippocampal-independent RESPONSE strategy to solve the Y-maze problem. The use of a response strategy is associated with increased behavioral rigidity (Ragozzino, Detrick, & Kesner, 1999; Ragozzino, Ragozzino, Mizumori, & Kesner, 2002); it may therefore be more difficult for these animals to implement lifestyle changes (e.g., diet and exercise) to regulate body weight.
Within our present design, a switch from a PLACE to a RESPONSE strategy is difficult to attribute to a nonspecific WD-induced deficit in performance (e.g., reduction in motivation, reward value, behavioral competence). Though a greater number of trials is often associated with a RESPONSE bias (Dickinson, 1985; Gibson & Shettleworth, 2005; Packard & McGaugh, 1996), hippocampal activation during spatial tasks remains constant even as rats switch from a PLACE to a RESPONSE strategy, and RESPONSE-prone rats retain the ability to solve tasks using a PLACE strategy (Chang & Gold, 2003a). We do not believe the number of trials underlies the phenotype observed here, as WD-DR and WD-DIO rats were equivalent in their number of acquisition trials, and the two factors did not significantly correlate (r = −0.1036; p = 0.419). Further, when others have examined the switch in memory systems as a result of extended training, a response bias emerged only after rats received substantial (20–40 trials) training beyond that needed to reliably solve the problem (Chang & Gold, 2003b; Packard, 1999). Since acquisition trials in the present experiment were terminated immediately upon a rat reaching criteria, animals from all groups were approximately equivalent in the number of potential “post-learning” trials. While it still remains possible that an increased number of trials influenced strategy preference, controlling for this would have resulted in different degrees of learning, which could also impact arm choice during a probe test.
BBB permeability and strategy preference were also associated with the obesity-promoting effects of WD. The BBB deficit and a shift from the place to the response strategy were observed only by the diet-induced obese (DIO) rats that exhibited marked weight gain when WD was introduced. Rats that exhibited the diet resistant (DR) phenotype failed to show significantly increased BBB permeability or decreased reliance on a place strategy relative to chow-fed controls. Interestingly, maintenance on WD led to a significant impairment in acquisition at 10 and 90 days that was seemingly independent of body weight phenotype or behavioral strategy. One possible explanation for the overall increase in number of trials among both DIO and DR rats fed WD, compared to chow, is a performance deficit due to diet-based differences in satiety or arousal. However, as we did not observe a difference in pair-fed rats, or between groups at day 40, it is unlikely that this effect is totally due to such factors. Another possibility is that WD affects other brain substrates underlying spatial learning, in a manner that is independent of body weight or BBB permeability. It is known that maintenance on WD can lead to reductions in both hippocampal and hypothalamic BDNF expression (Yu, Wang, & Huang, 2009), along with inflammation and activation of microglia (Pistell et al., 2010; Pistell et al., 2009), both of which could affect learning. Finally, considering we observed a trend toward increased BBB permeability in the hippocampus and striatum of WD-DR rats, it is possible that a milder neurological phenotype was sufficient to slow the rate of acquisition, but not impede it so much as to alter an animal’s strategy. Investigation into the effects of diet-induced obesity phenotype on place- or response-specific spatial tasks would help elucidate this. Regardless, it is clear that diet and body weight exert independent effects on brain health and its behavioral manifestations.
To better understand the relationship between BBB permeability and behavior, we assessed NaFl expression according to strategy preference. This analysis revealed increased 90 day BBB permeability in the ventral hippocampus of RESPONSE rats, compared to both PLACE and INTERMEDIATE, and increased 90 day permeability in the dorsal striatum of RESPONSE rats compared to INTERMEDIATE. Though we did not observe strategy group differences in NaFl accumulation in the dorsal hippocampus, there was a trend toward increased permeability in RESPONSE rats compared to INTERMEDIATE and PLACE. While dorsal hippocampus is canonically associated with spatial learning, damage to the ventral hippocampus has also been shown to upset performance in spatial tasks (Broadbent, Squire, & Clark, 2004; Moser & Moser, 1998). Damage to both structures induces the most severe phenotype; the additive effects of BBB leakage in the ventral hippocampus, plus the marginal leakage in the dorsal hippocampus may induce the greatest spatial deficits. Given its role in energy homeostasis (Davidson et al., 2009), BBB leakage in the ventral hippocampus may also influence feeding behavior and promote further weight gain.
The increase in BBB permeability in both the hippocampus and dorsal striatum of rats using a RESPONSE strategy is interesting, though a bit counterintuitive. While deficits in PLACE learning have long been associated with impairments in hippocampal function, RESPONSE learning is believed to depend on an intact striatum (Yin & Knowlton, 2004). If a RESPONSE bias is presumed to indicate reduced hippocampal involvement, the present data suggest that the functional impairment due to BBB leakage was greater in the hippocampus, compared to the striatum. This may due to overall higher BBB permeability in the hippocampus relative to the striatum, which was consistent across sub-regions and animals, but except for comparisons between the striatum and ventral subiculum (p < 0.001 for all striatum regions) failed to reach statistical significance (for ventral hippocampus vs. dorsal striatum, p = 0.0584). Alternately, differences in strategy preference may be due to a higher sensitivity to the effects of BBB damage within the hippocampus. Importantly, neither explanation requires impeccable RESPONSE learning or striatum function so long as the hippocampal deficits are sufficiently profound; deficits in both PLACE and RESPONSE may occur simultaneously and may account for delays in acquisition.
The present study also showed that spatial deficits and reduced PLACE strategy use could also be obtained after 10 days WD maintenance, in the apparent absence of a leaky BBB. At this time, WD-DIO rats exhibited a mild hyperglycemia compared to Chow. Since rats were hyperphagic during the early phase of the study, we included a pair-fed cohort to investigate the effects of diet quality, rather than quantity, on behavior. While WD-PF rats had normal glucose levels compared to chow, they still required significantly more acquisition trials and showed a trend toward a RESPONSE bias, suggesting neither hyperglycemia nor caloric excess can sufficiently explain the 10 day behavioral changes. WD-PF rats, like their WD-DIO counterparts, did have increased adiposity compared to Chow animals, suggesting the initiation of lipid accumulation may be associated with these early deficits. Alternately, the performance deficits observed may be the result of altered nutrient transport into the brain. We have previously observed reductions in hippocampal glucose and monocarboxylate transporter (GLUT1 and MCT1, respectively) mRNA following 10 days maintenance on WD (Hargrave, Davidson, Lee, & Kinzig, 2015). These changes were accompanied by altered vicarious trial and error and spontaneous alternation behaviors. In humans, GLUT1 deficiency is associated with cognitive (including spatial) impairments, suggesting the hippocampus may be particularly affected by reduced GLUT1 levels (De Vivo et al., 1991; De Vivo & Wang, 2008).
We observed high levels of both adiposity and insulin in WD-DIO rats at 90 days, which were associated with NaFl accumulation as well as RESPONSE strategy preference. Insulin has been known to regulate brain endothelial cells; during states of central insulin resistance, the BBB is damaged (Banks, 2012). The functional consequences of this are severe; for instance, Alzheimer’s disease is so strongly associated with central insulin resistance that it has been ascribed the moniker “type 3 diabetes” (de la Monte, 2009). Though peripheral and central insulin levels are not directly correlated, changes in glycemic control may be one mechanism by which diet-induced impairments develop. In vitro exposure to components of WD, including glucose (Trudeau, Molina, & Roy, 2011), cholesterol (Fu et al., 2012) and lipids (Cacicedo, Benjachareowong, Chou, Ruderman, & Ido, 2005), and in vivo streptozotocin treatment (Chung, Choi, Kang, Koh, & Yoon, 2014) can lead to the deterioration of retinal pericytes. Since pericytes are essential to the maintenance of a healthy BBB, damage as a result of alterations in blood glucose or other factors as a result of diet or diabetes could have severe neurological consequences. High adiposity is also associated with increases in inflammatory cytokines, which have been shown to induce BBB remodeling (Bolton & Perry, 1998; Fiala et al., 2002; Huber, Egleton, & Davis, 2001; Minagar & Alexander, 2003), and may explain the increases in NaFl expression observed in all groups at 90 days, as well as the reason WD-DR rats were afforded some protection.
Higher-order brain functions rely on accurate, responsive signaling processes whereby neurons interpret the often subtle chemical and electrical changes to the brain microenvironment, which is strictly regulated by the BBB. Even small disruptions in interstitial fluid homeostasis may induce “noise” and alter neuronal transmission and transduction. In the case of the hippocampus, which displays pronounced cellular plasticity, fluctuations in the presence of nutrients, cytokines, and chemokines can have catastrophic consequences on brain morphology and function (Williamson & Bilbo, 2013). In addition, increased BBB permeability has been shown to induce neurodegeneration by permitting the influx of neurotoxic substances (Brace, Latimer, & Winn, 1997; Juhler et al., 1984). WD-induced BBB permeability increases may follow a similar pattern, allowing blood-borne toxins access to CNS neurons.
Understanding the mechanisms that link obesity and diet to BBB damage and increased risk for neurological impairments will be an important step toward isolating factors that could be modified to delay or prevent the onset of these pernicious health consequences.
Acknowledgments
This research was supported in part by the National Institute of Health, grants DK078654 (KPK) and HD29792 (TLD), and by the Bilsland Dissertation Fellowship, Purdue University, West Lafayette, IN.
We are grateful to Xue Fu, Tien-Jui Lee, Melissa McCurley, Terry L. Powley, Joshua Stephenson, Melissa Swisher, and Arielle Zawadski-Weist for advice and assistance with experimental procedures. These studies were supported by the US National Institute of Health grants DK078654 (KPK) and HD29792 (TLD), and the Bilsland Dissertation Fellowship (SLH).
Footnotes
Sara L. Hargrave, Department of Psychology, Purdue University, West Lafayette, IN.
Terry L. Davidson, Center for Behavioral Neuroscience, American University, Washington DC.
Wei Zheng, School of Health Sciences, Purdue University, West Lafayette, IN.
Kimberly P. Kinzig, Department of Psychology, Purdue University, West Lafayette, IN.
Sara Hargrave is now at the Center for Behavioral Neuroscience, American University, Washington, DC.
Author Contributions
SLH, KPK, and TLD conceptualized the experiment. SLH conceived the experimental design performed all experiments, analyzed data, and wrote the document. WZ advised and assisted with BBB permeability assay design. KPK and TLD supervised the project. All authors discussed the results and their implications, and commented on the manuscript.
Contributor Information
Sara L. Hargrave, Purdue University
Terry L. Davidson, American University
Wei Zheng, Purdue University.
Kimberly P. Kinzig, Purdue University
References
- Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Sherwin RS. Striking Differences in Glucose and Lactate Levels Between Brain Extracellular Fluid and Plasma in Conscious Human Subjects: Effects of Hyperglycemia and Hypoglycemia. Journal of Cerebral Blood Flow & Metabolism. 2002;22:271–279. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology. 2012;153(9):4111–4119. doi: 10.1210/en.2012-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AJ, Gillardon F, Blümcke I, Langendörfer D, Beck H, Wiestler OD. Differential regulation of apoptosis-related genes in resistant and vulnerable subfields of the rat epileptic hippocampus. Molecular Brain Research. 1999;67(1):172–176. doi: 10.1016/s0169-328x(99)00060-1. http://dx.doi.org/10.1016/S0169-328X(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Berke JD, Breck JT, Eichenbaum H. Striatal Versus Hippocampal Representations During Win-Stay Maze Performance. Journal of Neurophysiology. 2009;101:1575–1587. doi: 10.1152/jn.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton SJ, Perry VH. Differential Blood–Brain Barrier Breakdown and Leucocyte Recruitment Following Excitotoxic Lesions in Juvenile and Adult Rats. Experimental Neurology. 1998;154:231–240. doi: 10.1006/exnr.1998.6927. [DOI] [PubMed] [Google Scholar]
- Brace H, Latimer M, Winn P. Neurotoxicity, Blood-Brain Barrier Breakdown, Demyelination and Remyelination Associated With NMDA-Induced Lesions of the Rat Lateral Hypothalamus. Brain Research Bulletin. 1997;43:447–455. doi: 10.1016/S0361-9230(97)00064-6. [DOI] [PubMed] [Google Scholar]
- Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes roles of NAD (P) H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54(6):1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behavioural Brain Research. 2003a;144:19–24. doi: 10.1016/S0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. The Journal of Neuroscience. 2003b;23(7):3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YR, Choi JA, Kang MJ, Koh JY, Yoon YH. Endoplasmic reticulum stress is associated with pericyte loss at early retinopathy of streptozotocin-induced diabetic mice. Investigative Ophthalmology & Visual Science. 2014;55(13):5839–5839. [Google Scholar]
- Clifton PG, Vickers SP, Somerville EM. Little and often: Ingestive behavior patterns following hippocampal lesions in rats. Behavioral Neuroscience. 1998;112:502–511. doi: 10.1037/0735-7044.112.3.502. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, Zheng W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–122. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Current opinion in pharmacology. 2007;7:613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiology & Behavior. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood–brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiology & Behavior. 2012;107:26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB reports. 2009;42:475. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. The New England journal of medicine. 1991;325:703–709. doi: 10.1056/NEJM199109053251006. [DOI] [PubMed] [Google Scholar]
- De Vivo DC, Wang D. Glut1 deficiency: CSF glucose. How low is too low? Revue neurologique. 2008;164:877–880. doi: 10.1016/j.neurol.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Actions and Habits: The Development of Behavioural Autonomy. Philosophical Transactions of the Royal Society of London B, Biological Sciences. 1985;308:67–78. doi: 10.1098/rstb.1985.0010. [DOI] [Google Scholar]
- Fanselow MS, Dong HW. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas G, Márton J, Nagy Z, Mándi Y, Takács T, Deli MA, Ábrahám CS. Experimental acute pancreatitis results in increased blood–brain barrier permeability in the rat: a potential role for tumor necrosis factor and interleukin 6. Neuroscience Letters. 1998;242:147–150. doi: 10.1016/S0304-3940(98)00060-3. [DOI] [PubMed] [Google Scholar]
- Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. European Journal of Clinical Investigation. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA: the journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Fu D, Wu M, Zhang J, Du M, Yang S, Hammad SM, Lyons TJ. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012;55(11):3128–3140. doi: 10.1007/s00125-012-2692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. The American Journal of Clinical Nutrition. 2001;73(1):61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- Gibson BM, Shettleworth SJ. Place Versus Response Learning Revisited: Tests of Blocking on the Radial Maze. Behavioral Neuroscience. 2005;119:567–586. doi: 10.1037/0735-7044.119.2.567. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: A double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behavioral Neuroscience. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Hargrave SL, Davidson TL, Lee T-J, Kinzig KP. Brain and behavioral perturbations in rats following Western diet access. Appetite. 2015 doi: 10.1016/j.appet.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67:1955–1959. doi: 10.1212/01.wnl.0000247052.18422.e5. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends in Neurosciences. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. The journal of neuroscience. 2003;23(13):5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglói K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proceedings of the National Academy of Sciences. 2010;107(32):14466–14471. doi: 10.1073/pnas.1004243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigro D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood–brain barrier. Epilepsia. 2012;53:26–34. doi: 10.1111/j.1528-1167.2012.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE, Davidson TL, Bowring B. Functional differentiation within the medial temporal lobe in the rat. Hippocampus. 2004;14:434–449. doi: 10.1002/hipo.10194. [DOI] [PubMed] [Google Scholar]
- Juhler M, Barry DI, Offner H, Konat G, Klinken L, Paulson OB. Blood-brain and blood-spinal cord barrier permeability during the course of experimental allergic encephalomyelitis in the rat. Brain Research. 1984;302:347–355. doi: 10.1016/0006-8993(84)90249-X. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiology & Behavior. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin Signaling in the Ventral Hippocampus Stimulates Learned and Motivational Aspects of Feeding via PI3K-Akt Signaling. Biological Psychiatry. 2013;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. Journal of Alzheimer’s Disease. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL, Jr, Koda L. Penetration of fluorescein into the brain: a sex difference. Brain Research. 1988;450:81–85. doi: 10.1016/0006-8993(88)91546-6. [DOI] [PubMed] [Google Scholar]
- Minagar A, Alexander JS. Blood-brain barrier disruption in. Multiple Sclerosis. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Naderali EK, Ratcliffe SH, Dale MC. Review: Obesity and Alzheimer’s disease: a link between body weight and cognitive function in old age. American Journal of Alzheimer’s Disease and Other Dementias. 2009;24:445–449. doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natah SS, Mouihate A, Pittman QJ, Sharkey KA. Disruption of the blood–brain barrier during TNBS colitis. Neurogastroenterology & Motility. 2005;17:433–446. doi: 10.1111/j.1365-2982.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- Natah SS, Srinivasan S, Pittman Q, Zhao Z, Dunn JF. Effects of acute hypoxia and hyperthermia on the permeability of the blood-brain barrier in adult rats. Journal of Applied Physiology. 2009;107:1348–1356. doi: 10.1152/japplphysiol.91484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proceedings of the National Academy of Sciences. 1999;96(22):12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of Hippocampus or Caudate Nucleus with Lidocaine Differentially Affects Expression of Place and Response Learning. Neurobiology of Learning and Memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. Journal of neuroimmunology. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Nelson CM, Miller MG, Spangler EL, Ingram DK, Devan BD. Striatal lesions interfere with acquisition of a complex maze task in rats. Behavioural brain research. 2009;197:138–143. doi: 10.1016/j.bbr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. The journal of neuroscience. 1999;19(11):4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral neuroscience. 2002;116(1):105. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau K, Molina AJA, Roy S. High glucose induces mitochondrial morphology and metabolic changes in retinal pericytes. Investigative Ophthalmology & Visual Science. 2011;52(12):8657. doi: 10.1167/iovs.11-7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity. 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1992;262(6):R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- Wilde GJC, Pringle AK, Wright P, Iannotti F. Differential Vulnerability of the CA1 and CA3 Subfields of the Hippocampus to Superoxide and Hydroxyl Radicals In Vitro. Journal of Neurochemistry. 1997;69(2):883–886. doi: 10.1046/j.1471-4159.1997.69020883.x. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Bilbo SD. Chemokines and the hippocampus: A new perspective on hippocampal plasticity and vulnerability. Brain, Behavior, and Immunity. 2013;30(0):186–194. doi: 10.1016/j.bbi.2013.01.077. http://dx.doi.org/10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience. 2009;160(2):295–306. doi: 10.1016/j.neuroscience.2009.01.078. [DOI] [PubMed] [Google Scholar]