Abstract
This study aimed to evaluate the efficacy and safety of reirradiation with intensity-modulated radiation therapy (IMRT) for spinal metastases. We retrospectively analyzed 23 patients with spinal metastases who underwent IMRT reirradiation between December 2006 and July 2013. We evaluated the spinal radiation doses during the first and second radiation therapy courses, the interval between the courses, and the clinical outcomes after reirradiation, including skeletal-related events, local control rates (LCRs), overall survival (OS), and toxicities. The median time from the first irradiation to reirradiation was 13 months (range, 2–75 months). The median reirradiation dose delivered to 90% of the planning target volume was 24.5 Gy in 5 fractions (range, 14.7–50 Gy in 3–25 fractions). Nineteen patients experienced pain at reirradiation, and 15 of these attained pain relief. Two of the three patients with paresis in the upper or lower extremities upon initiation of reirradiation demonstrated improvement. Local progression was identified in four patients. The median time to local progression was 37 months. The 1- and 2-year LCRs after reirradiation were 88% and 75%, respectively. The 1- and 2-year OS rates after reirradiation were 45% and 20%, respectively, with a median OS of 12 months. No late toxicities occurred. In conclusion, spinal metastasis reirradiation using IMRT appears safe; pain relief and paresis improvement and/or prevention can be expected, along with a reduced risk of radiation-induced toxicity, especially in the spinal cord.
Keywords: spinal metastasis, reirradiation, intensity-modulated radiation therapy, dose to the spinal cord
INTRODUCTION
Spinal metastases are the most frequently observed among bone metastases as a whole. These metastases cause symptoms such as back pain and weakness due to metastatic spinal cord compression [1]. Metastatic spinal cord compression occurs in 5% to 10% of all cancer patients [2]. Various symptoms caused by bone metastases, particularly pain and neurological deficits, affect a patient's quality of life [3]. Palliative radiation therapy has been recognized as an established treatment to relieve pain and prevent morbidity due to bone metastases [4]. Recent advancements in chemotherapy and targeted therapy have been contributing to improved survival, even in patients with bone metastases. Longer survival, however, can result in the development of recurrences in previously irradiated spinal regions [5]. Rades et al. reported that the local control rates (LCRs) of metastatic spinal cord compression at 12 months were 61% after 8 Gy in 1 fraction or 20 Gy in 5 fractions and 81% after 30 Gy in 10 fractions, 37.5 Gy in 15 fractions, or 40 Gy in 20 fractions [6]. At our institution, the radiotherapy schedule for patients with bone metastases is selected by predicting prognosis on the basis of previously reported clinical results [7–10]. For most patients, the initially calculated dose of radiation to the bone metastasis is found to be adequate to relieve the pain, improve paresis, and prevent relapse; however, some patients experience in-field recurrence, and the necessity of reirradiation must be considered in these patients. In case of reirradiation, the tolerance dose of the spinal cord is an important problem that needs to be considered. Intensity-modulated radiation therapy (IMRT) is one of the techniques that have the potential to reduce the dose to the spinal cord and allow a greater dose to be delivered to the spinal metastasis at the same time. IMRT has been employed at our institution for reirradiation in the case of recurrence of spinal metastases since 2006. This study aimed to evaluate the efficacy and safety of spinal metastasis reirradiation with IMRT.
MATERIALS AND METHODS
Patients
We identified a total of 28 patients with spinal metastases who underwent reirradiation with IMRT at our institution between December 2006 and July 2013. In 5 of these patients, the initial radiation therapy had been with a definitive intent or had not targeted local relapse of spinal or paraspinal lesions. We retrospectively analyzed the 23 patients who underwent palliative radiation therapy to spinal metastases in both the first and the second treatment. The analyses were carried out with the approval of the Institutional Review Board. The treatment policy for each patient was discussed in a multidisciplinary bone metastasis board (including radiation oncologists and orthopedic surgeons) to determine the appropriate therapeutic approach. The indications for reirradiation were as follows: (i) exacerbation confirmed clinically or with computed tomography (CT) and/or magnetic resonance (MR) imaging at the previously irradiated site, and (ii) surgery and other treatments not indicated. All of the patients were informed of the risk of reirradiation, especially of the risk of radiation-induced myelopathy, and provided written informed consent.
Treatment
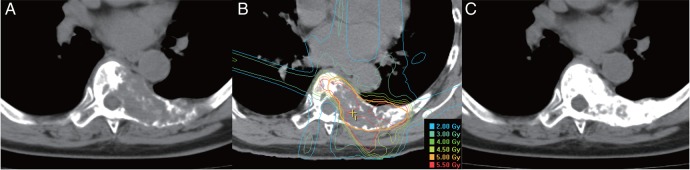
First, patients underwent treatment-planning CT. Immobilization devices were selected in accordance with the level of the spine treated. A facemask was used for the cervical and upper thoracic spine. A vacuum bag and a body shell were used for the thoracic and lumbar spine. The images from the treatment-planning CT were fused with MR images to enable us to accurately contour the metastatic lesions and spinal cord. Target volume delineation was based on the MR images. The treatment plans were designed using BrainSCAN (BrainLAB AG, Feldkirchen, Germany) or Pinnacle3 (Philips Healthcare, Andover, MA, USA). The gross tumor volume (GTV) was contoured to include clearly osteolytic lesions. The clinical target volume (CTV) was created by encompassing the domain of the lesions that suggested metastasis. The planning target volume (PTV) consisted of the CTV plus an appropriate margin, which was adjusted in areas where overlaps with organs at risk (OARs) were identified. OARs included organs such as the spinal cord and esophagus. An IMRT plan was then designed such that the dose that covered 95% of the PTV (D95) was 5 Gy per fraction, and the volume of spinal cord that was irradiated with 2 Gy per fraction was minimized to be as small as possible. The number of fractionations was 5 in principle. All patients (except 1 patient, who was treated in July 2013) were irradiated with a Primus (Siemens Healthcare, Erlangen, Germany) linear accelerator. We used an m3 (BrainLAB) micro multi-leaf collimator for most of the patients treated with the Primus. The patient position was verified with a CT scanner installed in the same room as the linear accelerator. We commenced performing IMRT using a TrueBeam (Varian Medical Systems, Palo Alto, CA, USA) linear accelerator at our institution in July 2013; only one patient was irradiated using the TrueBeam, with cone-beam CT for the verification of patient position. The images shown in Fig. 1 are the CT images and the dose distribution of IMRT from one patient, who was treated using the Primus with m3.
Fig. 1.
Computed tomography images and dose distribution of one case: a 63-year-old breast cancer patient with spinal metastases underwent reirradiation with intensity-modulated radiation therapy (IMRT). (A) Before reirradiation, (B) dose distribution of IMRT, and (C) 5 months after reirradiation.
Analysis
We evaluated the radiation doses to the spinal cord during the first and second radiotherapy courses, the interval between the courses, and the clinical outcomes after reirradiation, including skeletal-related events (SREs). SREs were defined in our study as pain and paresis due to spinal cord compression and a pathologic fracture. We also analyzed local control rates (LCRs), overall survival (OS), and toxicities.
The cumulative spinal cord dose was calculated for each case from the biological effective dose (BED) to the spinal cord, because the dose-fraction sizes varied between the first and second treatments. The BED was calculated according to the formula, BED = n · d (1 + d/(α/β)), where n is the number of fractions, d is the dose per fraction, and α and β are the linear and quadratic coefficients of the linear–quadratic model. Based on the literature, the α/β value for the spinal cord was assumed to be 2 Gy [11].
The interval between the first treatment and reirradiation with IMRT was defined as the time from the last day of the first radiation therapy to the first day of reirradiation with IMRT. We assessed two kinds of symptoms: pain and paresis. These were assessed by referring to electronic medical records: whether the patients had these symptoms before reirradiation and whether the status of these symptoms improved after reirradiation. The LCR was calculated as the time from reirradiation to a local relapse in the reirradiation field, which was confirmed by CT and/or MR images. The local relapse was defined as any tumor regrowth within the reirradiation field. OS was calculated as the time from reirradiation to death from any cause. The Kaplan–Meier method was used to analyze LCR and OS. All statistical analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA). Acute and late toxicities were assessed with the Common Terminology Criteria for Adverse Events (CTCAE) version 4 of the National Cancer Institute.
RESULTS
The patient characteristics are shown in Table 1. All patients but 3 (who were lost to follow-up) were dead at the time of the analysis. The median follow-up time from reirradiation was 10 months (range, 1–54 months). The treatment characteristics are shown in Table 2. The median initial radiation dose to the spinal cord was 37.5 Gy (range, 30–40 Gy). The median interval from the first treatment to reirradiation was 13 months (range, 2–75 months). The median prescribed dose of reirradiation to 90% of the PTV was 24.5 Gy in 5 fractions (range, 14.7–50 Gy in 3–25 fractions). The median maximum reirradiation dose to the spinal cord was 14.5 Gy in 5 fractions (range, 7.8–32.5 Gy in 3–25 fractions). The BED delivered to the spinal cord for each patient is shown in Table 3. The median BED to the spinal cord for the first treatment and upon reirradiation was 80 Gy2 and 35.5 Gy2, respectively. We also evaluated the dose to the irradiated volume of 0.5 cm3 of spinal cord (D0.5cm3). The median D0.5cm3 of the spinal cord at reirradiation was 10 Gy in 5 fractions (range, 4.8–25 Gy in 3–25 fractions). Accordingly, the median cumulative D0.5cm3 of the spinal cord was 91 Gy2 (range, 81.5–106.5 Gy2). As for the esophagus, the median D1cm3 was 14.2 Gy in 5 fractions (range, 5.5–35 Gy in 3–25 fractions).
Table 1.
Patient characteristics (n = 23)
| Characteristic | No. (%) |
|---|---|
| Age, years | |
| Median 68 (range, 42–85) | |
| Gender | |
| Male | 14 (61) |
| Female | 9 (39) |
| Performance Status | |
| 0 | 1 (4) |
| 1 | 11 (48) |
| 2 | 6 (26) |
| 3 | 5 (22) |
| Primary cancer site | |
| Breast | 4 (17) |
| Thyroid | 4 (17) |
| Liver | 4 (17) |
| Unknown | 3 (13) |
| Lung | 2 (9) |
| Esophagus/Stomach | 2 (9) |
| Others | 4 (17) |
Table 2.
Treatment characteristics (n = 23)
| Characteristics | No. (%) |
|---|---|
| Spinal region treated | |
| Cervical | 2 (9) |
| Thoracic | 18 (78) |
| Lumbar | 3 (13) |
| Initial dose to spinal cord | |
| 30 Gy/10 fr | 9 (39) |
| 40 Gy/20 fr | 6 (26) |
| 37.5 Gy/15 fr | 2 (9) |
| 40 Gy/16 fr | 2 (9) |
| Others | 4 (17) |
| Interval, month | |
| Median 13 (range, 2–75) | |
| <6 | 4 (17) |
| ≥6 | 19 (83) |
| GTV volume (cm3) | |
| Median 47.4 (range, 11.2–292.4) | |
| PTV volume (cm3) | |
| Median 69.8 (range, 11.2–316.7) | |
| Reirradiation dose (dose to 90% of the PTV) | |
| Median 24.5 Gy/5 fr (range, 14.7–50 Gy/3–25 fr) | |
Table 3.
Cumulative BED to the spinal cord of individual patients
| Patient | First irradiation BED (Gy2) | Interval (m) | Second irradiation BED (Gy2) | Cumulative BED (Gy2) | Follow-up from second irradiation (m) |
|---|---|---|---|---|---|
| 1 | 80 | 40 | 33.9 | 113.9 | 11 |
| 2 | 75 | 6 | 22.2 | 97.2 | 1 |
| 3 | 75 | 19 | 26.4 | 101.4 | 8 |
| 4 | 75 | 13 | 23.1 | 98.1 | 14 |
| 5 | 97.5 | 75 | 28.1 | 125.6 | 35 |
| 6 | 82.5 | 2 | 24.7 | 107.2 | 42 |
| 7 | 79 | 5 | 26.4 | 105.4 | 10 |
| 8 | 75 | 11 | 29.9 | 104.9 | 13 |
| 9 | 80 | 9 | 72.0 | 152.0 | 9 |
| 10 | 75 | 3 | 57.5 | 132.5 | 12 |
| 11 | 75 | 9 | 20.0 | 95.0 | 1 |
| 12 | 80 | 39 | 38.1 | 118.1 | 2 |
| 13 | 80 | 21 | 43.7 | 123.7 | 54 |
| 14 | 75 | 7 | 35.5 | 110.5 | 5 |
| 15 | 80 | 7 | 84.5 | 164.5 | 5 |
| 16 | 75 | 4 | 28.1 | 103.1 | 4 |
| 17 | 75 | 19 | 23.1 | 98.1 | 27 |
| 18 | 90 | 52 | 52.7 | 142.7 | 9 |
| 19 | 90 | 31 | 60.0 | 150.0 | 23 |
| 20 | 78.8 | 34 | 53.6 | 132.4 | 21 |
| 21 | 80 | 15 | 67.7 | 147.7 | 3 |
| 22 | 84.4 | 30 | 67.9 | 152.3 | 18 |
| 23 | 84.4 | 9 | 62.5 | 146.9 | 9 |
BED = biological effective dose.
Of the 23 patients, 19 patients experienced pain and 15 (65%) of these patients were relieved after reirradiation. Three patients had paresis in the upper or lower extremities at the beginning of reirradiation. The degree of paresis was Frankel C for all of them [12]. Two of the three patients showed improvement to Frankel D or E thereafter, while the other patient showed no change.
Of the 23 patients, 20 underwent CT or MR examination after reirradiation; local progression was identified in 4 of these patients. The details of these patients are presented in Table 4. The primary tumor sites were all different. The intervals between the first irradiation and reirradiation for two patients were <6 months. Among the four patients with local progression, SREs occurred in three patients; paraparesis in the lower extremities was seen in all of them. The location of the bone metastasis in which SREs occurred for the three patients was T10, T2–4, and T7 of the thoracic spine, respectively. The median time to local progression was 37 months. The 1- and 2-year LCRs after reirradiation were 88% and 75%, respectively (Fig. 2). With regard to OS, the 1- and 2-year OS rates after reirradiation were 50% and 20%, respectively, with a median OS of 12 months (Fig. 3).
Table 4.
The details of the four patients who presented with local failure
| Patient | Primary site | First irradiation (dose/fractions) | Interval (m) | Second irradiation (dose/fractions) | Size of GTV (cm3) | Time to failure (monts) | SRE |
|---|---|---|---|---|---|---|---|
| 4 | Liver | 39 Gy/13 fr | 13 | 26.5 Gy/5 fr | 74.5 | 6 | – |
| 6 | Thyroid | 39 Gy/18 fra | 2 | 25.5 Gy/5 fr | 11.2 | 37 | + |
| 7 | Lung | 58 Gy/28 frb | 5 | 24.5 Gy/5 fr | 69.8 | 1 | + |
| 13 | Kidney | 40 Gy/20 fr | 21 | 23.5 Gy/5 fr | 25.0 | 13 | + |
SRE = skeletal-related event. aPatient 6 received 9 Gy in 3-Gy fractions followed by 30 Gy in 2-Gy fractions. bPatient 7 received 6 Gy in 3-Gy fractions followed by 52 Gy in 2-Gy fractions. The spinal cord was irradiated to a cumulative dose of 38 Gy.
Fig. 2.

Kaplan–Meier estimate of local control after reirradiation.
Fig. 3.
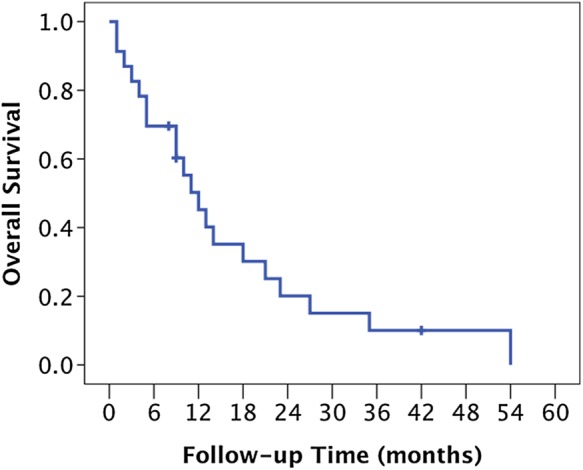
Kaplan–Meier estimate of overall survival.
No Grade 2 or higher toxicity was identified in either the acute or the late period. Only two patients developed mild acute toxicities, including dermatitis (one patient) and nausea (one patient). Most importantly, no late toxicities (including radiation-induced myelopathy and compression fracture) occurred.
DISCUSSION
The present study indicated that reirradiation of spinal metastases using IMRT was performed safely; pain relief and paresis improvement and/or prevention could be expected. Reirradiation to spinal metastases poses a challenge: namely, administering a larger dose to the metastatic lesion while reducing the dose to the spinal cord as much as possible. For a patient with a poor prognosis, reirradiation can be performed with a tolerable dose to the spinal cord because the incidence of radiation myelopathy seems to be relatively low. However, it is difficult to accurately predict the prognosis of an individual patient given that the recent advancements in systemic therapy have been contributing to improvements in survival time. There have been a few reports on reirradiation to spinal metastases with various irradiation methods, such as IMRT, volumetric-modulated arc therapy (VMAT), and stereotactic body radiation therapy (SBRT) [13–17].
Regarding reirradiation of spinal metastases using IMRT, Milker-Zabel et al. reported clinical results of retreatment of vertebral bone metastases by 3D conformal radiotherapy (3D-CRT) and IMRT [13]. Their study had significance as one of the initial reports on reirradiation for spinal metastases with IMRT.
Table 5 shows a summary of the previous reports on reirradiation to spinal metastases. With regard to the clinical outcomes, our results presented here, such as pain relief, paresis improvement, survival, local control, and toxicities, seem to be comparable with those of the previous reports. Thus, it can be said that our multidisciplinary bone metastasis board was helpful for discussing the indications for reirradiation.
Table 5.
Comparison of previous reports with our present study
| Author/year [Ref.] | Total no. metastases | Reirradiation modality | Interval, months, median (range) | Spinal cord dose (BED, Gy2), median (range) |
||
|---|---|---|---|---|---|---|
| First irradiation | Second irradiation | Cumulative | ||||
| Wright et al./2006 [13] | 23 | IMRT | 19 (2–125) | 75 (11–98) | 20 (6–53) | 95 (22–113) |
| Navarria et al./2012 [14] | 31 | VMAT | 17 (6–105) | 75 (40–100.3) | 23.6 (7.3–68.9) | 102.5 (55.7–115.1) |
| Sahgal et al./2009 [15] | 22 | SBRT | 11 (3–85) | 47 (10–64) | 36 (20–98) | NA |
| Grosu et al./2002 [18] | 8 | Conventional | 30 (6–63) | 77.5 (49.4–100) | 60 (55.1–105) | 136.6 (120.1–205) |
| Present study | 23 | IMRT | 13 (2–75) | 80 (75–97.5) | 35.5 (20–84.5) | 118.1 (95–164.5) |
BED = biological effective dose, IMRT = intensity-modulated radiation therapy, VMAT = volumetric-modulated arc therapy, SBRT = stereotactic body radiation therapy.
Sahgal et al. reported on the clinical outcomes for SBRT in patients with previously irradiated spinal and paraspinal metastases [16]. As for pain improvement, they could not determine a true benefit because of the retrospective nature of the data collection and the lack of use of a standardized pain outcome tool. The same kind of difficulty in evaluating pain relief also occurred in our study. We also examined whether pain was improved or not by referring to the electronic medical records. We could not quantitatively determine pain improvement accurately because we did not objectively evaluate pain before and after reirradiation for most of the patients. It was difficult to evaluate pain by taking analgesic use into consideration, as well. These factors are thought to be limitations of our present study. In order to standardize the evaluation of pain, we currently use patient self-report on the Numerical Rating Scale to evaluate pain from bone metastases before and after the radiotherapy [18].
In a study on reirradiation before the widespread use of IMRT and SBRT, Grosu et al. reported on retreatment of spinal metastases with conventional radiotherapy in the 1990s [19]. A single posterior field was used for most of the patients, with a total dose ranging from 29 to 38 Gy (1.8–4 Gy per fraction) for reirradiation. The median interval to reirradiation was 30 months (range, 6–63 months). No patient showed any neurologic abnormalities affecting motor or sensory function. All of the patients were reirradiated after an interval of 6 months or longer. There is a possibility that a longer interval resulted in the absence of the occurrence of myelopathy after reirradiation. From a radiobiological point of view, the spinal cord has a capacity for recovery from occult radiation injury [20]. Such recovery increases with longer time intervals [21]. Thus, reirradiation to spinal metastases can be considered after a certain time interval from the first irradiation.
In case of reirradiation, the most important adverse effect to be avoided is radiation-induced myelopathy. Nieder et al. suggested that the risk of myelopathy appears small, with a cumulative BED ≤ 135.5 Gy2, when there is an interval of ≥6 months between treatments and the dose of each course is ≤98 Gy2 [11]. In light of this, our maximum reirradiation dose to the spinal cord was thought to be relatively tolerable. The criteria of Nieder et al. are useful indices for reference in evaluating the safety of reirradiation. Although the cumulative BED of seven patients was >135.5 Gy2 (Table 3), the calculation of the volume of the spinal cord irradiated with a cumulative dose exceeding 135.5 Gy2 was 1.4 cm3 or smaller. As a result, no radiation-induced myelopathy occurred to the patients, including these seven patients.
The numbers (1–23) of patients in Table 3 are sorted in the time series from old to new. Comparing the BEDs of the spinal cord of the second irradiation for the early (1–12) and latter (13–23) patients, the value of the latter patients (52.7 Gy2) was higher than that of the early patients (33.5 Gy2). The reason for this difference is thought to be that we made an alteration to the prescription strategy in the middle because we experienced two relapses (patients 4 and 7) in the early patients within 6 months after reirradiation. In order to improve the coverage of irradiation for the PTV, we permitted the maximum dose to the spinal cord to be slightly raised. As a result, the relapse after reirradiation in the latter patients was seen in only one case. Eventually, as no radiation-induced myelopathy occurred for any of the patients, it was inferred that the accumulated doses to the spinal cord were within the permissible range in the study.
The toxicity of our reirradiation seemed to be tolerable in the current study, while four patients presented local failure (Table 4). In two of the four patients, the time to failure was within 6 months and the size of the GTVs was larger than that of the other two patients. There was a possibility that the larger size of GTV affected the shorter time to local failure. As we have accumulated experience with reirradiation with IMRT and confirmed the safety of our technique. We currently prescribe 30 Gy in 5 fractions to the PTV for the purpose of improving local control. We are expecting to obtain better clinical outcomes with dose escalation and to retain a minimal level of toxicity as well.
IMRT can be relatively flexible in regard to designing a dose distribution fitting a lesion with a concave shape and a large volume compared with other radiation therapy methods such as SBRT. This distinction can be useful in the irradiation of spinal metastases, especially in the retreatment situation. As far as we know, only a few reports of reirradiation of spinal metastases with IMRT have been published in the literature. Because even patients with bone metastases can experience long-term survival, reirradiation of spinal metastases with IMRT, which can perform greater dose prescription than conventional radiotherapy, may provide the possibility of lasting pain relief and prevention of paresis, as well as tumor control. In an analysis of latent periods from published data of radiation-induced myelopathy, Wong et al. showed that the median latent time was as long as 11.4 months (range, 3.8–25 months) as measured from reirradiation [22]. Thus the spinal metastasis reirradiation with IMRT can be indicated for patients with projected good prognoses.
With regard to the prognoses of patients with bone metastases, Katagiri et al. proposed prognostic factors and a scoring system to determine the optimal treatment [9]. Calculating the score for each patient in the present study, prognostic scores of 0–2, 3–5 and 6–8 were obtained for 7, 13 and 3 patients, respectively; the median survival of each prognostic score group after reirradiation was 35, 10 and 4 months, respectively. It can be said that the patients with comparatively good prognoses were selected as candidates for reirradiation at our institution.
When considering reirradiation to spinal metastases, it is important to determine whether IMRT is suitable or not for each patient by evaluating symptoms and prognosis, and to design a treatment plan and perform it without delay. In conclusion, reirradiation of spinal metastases using IMRT appears safe; pain relief and paresis improvement and/or prevention can be expected, along with a reduced risk of radiation-induced toxicity, especially in the spinal cord. It is therefore worth considering reirradiation via IMRT for spinal metastases in well-selected patients with projected good prognoses.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by Shizuoka Cancer Center Hospital for which the corresponding author works.
ACKNOWLEDGEMENTS
The authors thank Masahiro Konno for collecting the data for the dose–volume histogram. The results of this study were presented at the 56th annual meeting of the American Society for Radiation Oncology (ASTRO) in San Francisco (Int J Radiat Oncol Biol Phys 2014;90:Supplement S691).
REFERENCES
- 1.Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol 2008;7:459–66. [DOI] [PubMed] [Google Scholar]
- 2.Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol 2005;23:3366–75. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s–9s. [DOI] [PubMed] [Google Scholar]
- 4.Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965–76. [DOI] [PubMed] [Google Scholar]
- 5.Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol 2006;24:3388–93. [DOI] [PubMed] [Google Scholar]
- 6.Rades D, Lange M, Veninga T, et al. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2011;79:524–30. [DOI] [PubMed] [Google Scholar]
- 7.Mizumoto M, Harada H, Asakura H, et al. Prognostic factors and a scoring system for survival after radiotherapy for metastases to the spinal column: a review of 544 patients at Shizuoka Cancer Center Hospital. Cancer 2008;113:2816–22. [DOI] [PubMed] [Google Scholar]
- 8.Mizumoto M, Harada H, Asakura H, et al. Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. Int J Radiat Oncol Biol Phys 2011;79:208–13. [DOI] [PubMed] [Google Scholar]
- 9.Katagiri H, Takahashi M, Wakai K, et al. Prognostic factors and a scoring system for patients with skeletal metastasis. J Bone Joint Surg Br 2005;87:698–703. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieder C, Grosu AL, Andratschke NH, et al. Proposal of human spinal cord reirradiation dose based on collection of data from 40 patients. Int J Radiat Oncol Biol Phys 2005;61:851–5. [DOI] [PubMed] [Google Scholar]
- 12.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 1969;7:179–92. [DOI] [PubMed] [Google Scholar]
- 13.Milker-Zabel S, Zabel A, Thilmann C, et al. Clinical results of retreatment of vertebral bone metastases by stereotactic conformal radiotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2003;55:162–7. [DOI] [PubMed] [Google Scholar]
- 14.Wright JL, Lovelock DM, Bilsky MH, et al. Clinical outcomes after reirradiation of paraspinal tumors. Am J Clin Oncol 2006;29:495–502. [DOI] [PubMed] [Google Scholar]
- 15.Navarria P, Mancosu P, Alongi F, et al. Vertebral metastases reirradiation with volumetric-modulated arc radiotherapy. Radiother Oncol 2012;102:416–20. [DOI] [PubMed] [Google Scholar]
- 16.Sahgal A, Ames C, Chou D, et al. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys 2009;74:723–31. [DOI] [PubMed] [Google Scholar]
- 17.Yamada Y, Lovelock DM, Yenice KM, et al. Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: a preliminary report. Int J Radiat Oncol Biol Phys 2005;62:53–61. [DOI] [PubMed] [Google Scholar]
- 18.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs 2005;14:798–804. [DOI] [PubMed] [Google Scholar]
- 19.Grosu AL, Andratschke N, Nieder C, et al. Retreatment of the spinal cord with palliative radiotherapy. Int J Radiat Oncol Biol Phys 2002;52:1288–92. [DOI] [PubMed] [Google Scholar]
- 20.Ang KK, Jiang GL, Feng Y, et al. Extent of kinetics of occult spinal cord injury. Int J Radiat Oncol Biol Phys 2001;50:1013–20. [DOI] [PubMed] [Google Scholar]
- 21.Nieder C, Milas L, Ang KK. Tissue tolerance of reirradiation. Semin Radiat Oncol 2000;10:200–9. [DOI] [PubMed] [Google Scholar]
- 22.Wong CS, Van Dyk J, Milosevic M, et al. Radiation myelopathy following single courses of radiotherapy and retreatment. Int J Radiat Oncol Biol Phys 1994;30:575–81. [DOI] [PubMed] [Google Scholar]