Abstract
Densely ionizing charged particle irradiation offers physical as well as biological advantages compared with photon irradiation. Radiobiological data for the combination of such particle irradiation (i.e. therapeutic carbon ions) with commonly used chemotherapeutics are still limited. Recent in vitro results indicate a general prevalence of additive cytotoxic effects in combined treatments, but an extension of established multimodal treatment regimens with photons to the inclusion of particle therapy needs to evaluate possible peculiarities of using high linear energy transfer (LET) radiation. The present study investigates the effect of combined radiochemotherapy using gemcitabine and high-LET irradiation with therapeutic carbon ions. In particular, the earlier observation of S-phase specific radiosensitization with photon irradiation should be evaluated with carbon ions. In the absence of the drug gemcitabine, carbon ion irradiation produced the typical survival behavior seen with X-rays—increased relative biological efficiency, and depletion of the survival curve's shoulder. By means of serum deprivation and subsequent replenishment, ∼70% S-phase content of the cell population was achieved, and such preparations showed radioresistance in both treatment arms—,photon and carbon ion irradiation. Combined modality treatment with gemcitabine caused significant reduction of clonogenic survival especially for the S-phase cells. WIDR cells exhibited S-phase–specific radioresistance with high-LET irradiation, although this was less pronounced than for X-ray exposure. The combined treatment with therapeutic carbon ions and gemcitabine caused the resistance phenomenon to disappear phenotypically.
Keywords: gemcitabine, radiosensitization, carbon ion irradiation, S-phase
INTRODUCTION
The antimetabolite gemcitabine (2′2′-difluoro-2′-deoxycytidine, dFdCyd) has structural similarity to cytarabine; however, it differs considerably in its biophysical properties, such as mechanism of action, metabolism and antitumor effect. It is well known for its broad spectrum of antineoplastic effectiveness in solid malignancies [1–3]. Applied as a prodrug it is transformed intracellularly into dFdCMP and its active di- and triphosphate metabolites (dFdCDP/dFdCTP) [4], which inhibit DNA polymerase and ribonucleotide reductase. After insertion of only one more deoxynucleotide, chain termination occurs [5], which makes damage detection and subsequent repair by exonucleases more difficult [6]. A self-potentiating effect develops through increasing depletion of available deoxynucleotide triphosphates and inhibition of dCMP deaminase and CTP synthetase, resulting in accumulating intracellular levels of the active metabolite, thereby increasing the probability of incorporation [7]. The predominant mechanism of inactivation is mediated by cytidine deaminase which transforms the active metabolite into 103 less active dFdUridine; alternatively, dFdCMP is converted into dFdUMP by cytidilate deaminase [8]. Furthermore, gemcitabine is well known for its radiosensitizing properties. Radiosensitizing potential increases with longer exposure and higher concentration. This effect was also observed in combination experiments with low dose application of gemcitabine, in which no cytotoxicity was developed if it was administered as a single agent [7]. Several factors are considered to be responsible for this phenomenon, because Lawrence et al. showed that depletion of dATP by ribonucleotide reductase inhibition is a key step in producing radiosensitization. Cell lines with overexpression of ribonucleotide reductase are resistant to effects mediated by gemcitabine [9]. Furthermore, radiosensitization in vitro apparently requires cellular activities linked to S-phase progression [10].
High-LET radiation in general offers several biological as well as physical advantages compared with photon radiation—such as inverse depth–dose profile and increased relative biological effectiveness (RBE). However, there is only limited radiobiological data for the effects of combined treatment with dFdCyd and heavy ion irradiation. Recently, a respective combination effect was described for tumor cells from log-phase culture, and only additivity was observed [11]. In light of our earlier results with photon irradiation, we attempted to test whether cell-cycle stage-specific radiosensitization was also observed with carbon ion exposure.
MATERIALS AND METHODS
Cell culture
In the present study, the human colorectal tumor cell line WIDR was used—cells expressing mutant p53 (p53mut). The cell line was obtained from the American Type Culture Collection (ATCC; Manassas, USA) and cultured in Dulbecco's modified Eagle medium (DMEM) (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (FCS) (Biochrom, Berlin, Germany). Cultures were maintained in an exponential monolayer at 37°C in 5% CO2 and 95% humidity. Cell population doubling times were 14–18 h for WIDR. Cultures were passaged once a week.
Synchronized cell populations
Cell cultures were incubated for 60 h with DMEM and only 0.5% FCS. After serum deprivation, they were stimulated with medium of usual composition. Progression through the cell cycle was monitored every 2–4 h by FACS analysis for 38 h, resulting in an up to 70% synchronized cell population. The maximum G1 percentage was achieved immediately after serum stimulation, whereas 22 h later, most of the cells had progressed into S-phase (Fig. 1A).
Fig. 1.
Baseline experiments. A: Proportions of cell cycle distribution after stimulation of serum-deprived (60-h) WIDR cells. Added is data of cell cycle distribution for the untreated controls (right portion). Means of three independent experiments, each performed in triplicate, are presented. B: Clonogenic survival curve for human colorectal cell line WIDR treated with increasing doses of gemcitabine for 2 h. Means and standard deviations from three independent experiments are presented. C: Clonogenic survival curve for human colorectal cell line WIDR after treatment with photon or carbon ion irradiation alone. Means and standard deviations from three independent experiments are presented.
Cell cycle analysis
The distribution of the various cell cycle phases was measured prior to irradiation by routine flow-cytometry (FACScan, Becton-Dickinson, Heidelberg, Germany). Cells were stained with propidium iodide (Sigma–Aldrich, Deisenhofen, Germany), and histograms were created and analyzed using ModFit software (Verity Software House, Topsham, USA).
Radiation and drug treatment
Adherent cells were irradiated with 6-MV X-rays from a clinical linear accelerator (100-cm reference distance) at a dose rate of 2.2 Gy/min and at room temperature in T25 culture flasks with 5 ml medium. Carbon ion irradiation was performed at HIT (Heidelberg Ion Therapy Center) with a horizontal beam line with a rasterscanning technique using an extended 8-mm Bragg peak, energies varying from 1.47 to 1.64 GeV/ion. Cell monolayers were positioned in the middle of the extended Bragg peak (dose averaged LET of 103 keV/µm). Due to the experimental settings, T25 flasks were completely filled with medium during irradiation. The dFdCyd stock solution (1 mM) was stored at –20°C and diluted in double-distilled water for the required concentrations. Immediately before performing radiotherapy and after incubation with dFdCyd for 2 h, the cell layer was rinsed twice with phosphate-buffered saline and fresh medium was added.
Clonogenic assay and statistical analysis
After performing a cell viability test with 0.4% trypan blue, vital log phase tumor cells were plated in T25 culture flasks in appropriate numbers to obtain 50–150 colonies for each different treatment. A colony was defined as 50 or more cells and was identified microscopically. Data for survival curves were obtained from three independent experiments, each performed in triplicate. Sigma Plot's (Systat Software GmbH, Erkrath, Germany) non-linear least-squares regression was used to fit the linear–quadratic expression S = exp (–αD–βD²) to the resulting averaged survival fractions after the plating efficiency (of treated cells) was normalized to the untreated controls.
RESULTS AND DISCUSSION
In the baseline experiment, increasing concentrations of gemcitabine (2-h exposure) allowed us to determine a concentration (EC50 = 70 nM) with appropriate cytotoxicity for use as a single agent in all further combination experiments (Fig. 1B). Survival analysis for treatment with photons alone compared with therapeutic carbon ion irradiation produced characteristic differences, as shown in Fig. 1C. Gemcitabine monotherapy for 2 h at 70 nM resulted in reduction of survival to 65% (G1-preparation) and 51% (S-phase), respectively. Combined treatment of G1-phase preparations with X-rays showed additive cytotoxic effects (Fig. 2A, after normalization for the monotoxicity of dFdCyd). S-phase cells, however, display radioresistance, which is at least qualitatively similar to our earlier observation [10]. After normalization for the monotoxicity of dFdCyd, the radioresistant phenotype is reversed, which is equivalent to an expression of supra-additive toxicity in the S-phase preparations (Fig. 2B). For combination experiments with carbon ions, radiation doses of up to 3 Gy were used, due to higher radiobiological effectiveness. RBE values ranged between 2.6 and 3.1, depending on dose level and surviving fraction. Combination of gemcitabine with carbon ions instead of photons again revealed cell-cycle dependence for killing efficiency (Fig. 3A and B). This is summarized in Table 1, in which the initial slopes of the survival curves are listed. The main finding of the present investigation is the S-phase–specific radiosensitizing effect of dFdCyd in combination with therapeutic carbon ion irradiation, which is in agreement with earlier findings, when chemoradiation employing X-rays had been tested for the same in vitro cell system [12] and which was confirmed for clonogenic cell survival, here. While monotoxicity of gemcitabine was similar for the G1-phase and S-phase –enriched cell populations, the combination with radiation elicited radiosensitization for cells in S-phase by overcoming the well-known S-phase–associated radioresistance phenotype. The latter is thought to result—at least in part—from the increased contribution in S-phase cells of homology-directed DNA double-strand breaks [13], which could selectively be targeted by gemcitabine [14]. A deficiency in the mismatch repair system (MMR), however, has also been implicated in radiosensitization by gemcitabine [15], but the WIDR cells used in the present investigation are MMR-proficient [16]. Remarkably, even with high-LET irradiation, S-phase–specific radioresistance was observed, even though not as pronounced as with photons. This is particularly interesting in that one of the characteristics of densely ionizing radiation is to generally overcome radiation resistance phenomena by generating more complex damage to DNA [17–19], including contributions to apoptosis induction [17, 20]. Wang et al. could show that the role of Ku-dependent non-homologous end joining in particular is reduced by densely ionizing radiation, but not other repair mechanisms [21]. Therefore, homology-directed repair via removal of heavy ion–induced DNA damage and its inhibition by gemcitabine needs to be taken into consideration.
Fig. 2.

Clonogenic survival curve of WIDR G1-phase cell preparation (A) or S-phase cell preparation (B) after combined treatment with X-rays and with or without (mock-treated) 2-h exposure to 70 nM dFdCyd. Survival curves are normalized for dFdCyd monotoxicity. Means and standard deviation from three independent experiments are presented.
Fig. 3.
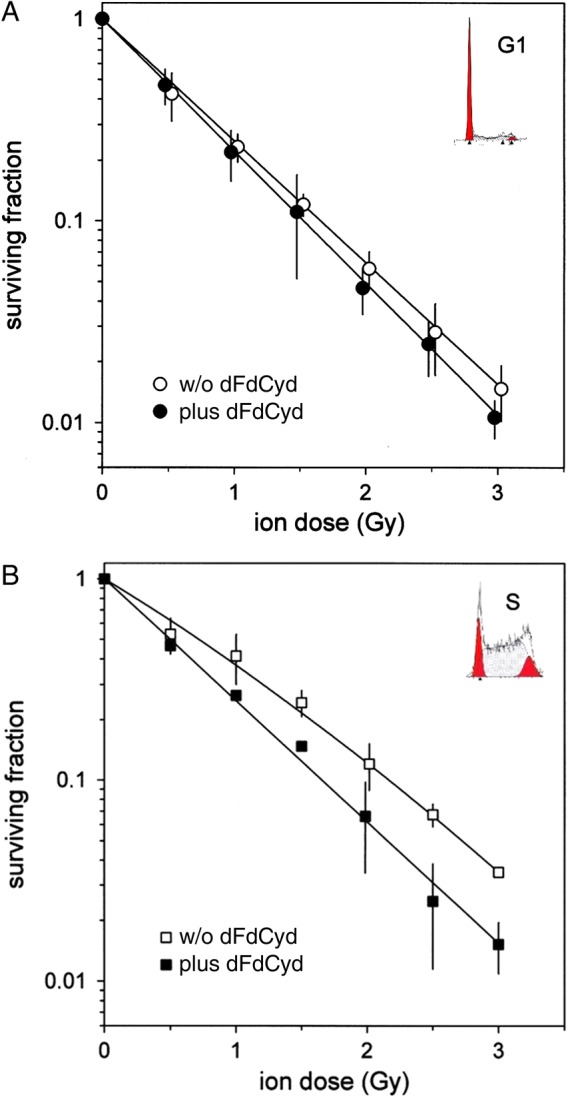
As in Fig. 2, but after combined treatment with therapeutic carbon ions.
Table 1.
Comparison of α-values from monoexponentially fitted survival curves of WIDR cells after carbon ion irradiation, treated either with or without dFdCyd
| Cell phase | W/o dFdCyd | Plus dFdCyd | P |
|---|---|---|---|
| G1 | 1.39 (0.036) | 1.51 (0.017) | Not significant |
| S | 1.02 (0.074) | 1.39 (0.051) | <0.001 |
| P | <0.001 | Not significant |
dFdCyd = 2′2′-difluoro-2′-deoxycytidine, dFdCyd (‘gemcitabine’).
FUNDING
This work was supported by a grant from Deutsche Forschungsgemeinschaft, clinical research group KFO 214, to KJW.
ACKNOWLEDGEMENTS
The authors express their gratitude to Mrs Sylvia Trinh and Mrs Ludmilla Frick for excellent technical assistance. Data obtained in this study were presented at the 13th Annual Meeting of the German Society for Biological Radiation Research in Hamburg, Germany.
REFERENCES
- 1.Burris HA., III Recent updates on the role of chemotherapy in pancreatic cancer. Semin Oncol 2005;32 Suppl 6:S1–3. [DOI] [PubMed] [Google Scholar]
- 2.Jones J, Takeda A, Tan S, et al. Gemcitabine for the treatment of metastatic breast cancer. Health Technol Assess 2009;13 Suppl 2:1–7. [DOI] [PubMed] [Google Scholar]
- 3.Sheth S. Current and emerging therapies for patients with advanced non-small-cell lung cancer. Am J Health Syst Pharm 2010;67 Suppl 1:S9–14. [DOI] [PubMed] [Google Scholar]
- 4.Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine). Drug Resist Updat 2002;5:19–33. [DOI] [PubMed] [Google Scholar]
- 5.Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol 2007;25:4043–50. [DOI] [PubMed] [Google Scholar]
- 6.Mini E, Nobili S, Caciagli B, et al. Cellular pharmacology of gemcitabine. Ann Oncol 2006;17 Suppl 5:v7–12. [DOI] [PubMed] [Google Scholar]
- 7.Pauwels B, Korst AEC, Lardon F, et al. Combined modality therapy of gemcitabine and radiation. Oncologist 2005;10:34–51. [DOI] [PubMed] [Google Scholar]
- 8.Wong A, Soo RA, Yong W-P, et al. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev 2009;41:77–88. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol 2003;13:13–21. [DOI] [PubMed] [Google Scholar]
- 10.Latz D, Fleckenstein K, Eble M, et al. Radiosensitizing potential of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) within the cell cycle in vitro. Int J Radiat Oncol Biol Phys 1998;41:875–82. [DOI] [PubMed] [Google Scholar]
- 11.Schlaich F, Brons S, Haberer T, et al. Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat Oncol 2013;8:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen A, Debus J, Weber K-J. S-phase cell-specific modification by gemcitabine of PFGE-analyzed radiation-induced DNA fragmentation and rejoining. Int J Radiat Biol 2008;84:770–7. [DOI] [PubMed] [Google Scholar]
- 13.Tamulevicius P, Wang M, Iliakis G. Homology-directed repair is required for the development of radioresistance during S-phase: interplay between double-strand break repair and checkpoint response. Radiat Res 2007;167:1–11. [DOI] [PubMed] [Google Scholar]
- 14.Wachters F, van Putten J, Maring J. Selective targeting of homologous DNA recombination repair by gemcitabine. Int J Radiat Oncol Biol Phys 2003;57:553–62. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan S, Robinson B, Krokosky C, et al. Mismatched nucleotides as the lesions responsible for radiosensitization with gemcitabine: a new paradigm for antimetabolite radiosensitizers. Mol Cancer Ther 2008;6:1858–68. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler J, Beck N, Kim H, et al. Mechanisms of inactivation of mismatch repair genes in human colorectal cancer cell lines: the predominant role of hMLH1. Proc Natl Acad Sci U S A 1999;96:10296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamada N, Hara T, Omura-Minamisawa M, et al. Energetic heavy ions overcome tumor radioresistance caused by overexpression of Bcl-2. Radiother Oncol 2008;89:231–6. [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Suzuki Y, Ohno T, et al. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin Cancer Res 2006;12:2185–90. [DOI] [PubMed] [Google Scholar]
- 19.Hirakawa H, Fujisawa H, Masaoka A, et al. The combination of Hsp90 inhibitor 17AAG and heavy-ion irradiation provides effective tumor control in human lung cancer cells. Cancer Med 2015;4:426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori E, Takahashi A, Yamakawa N, et al. High LET heavy ion radiation induces p53-independent apoptosis. J Radiat Res 2009;50:37–42. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Wang X, Zhang P, et al. The Ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair (Amst) 2008;7:725–33. [DOI] [PubMed] [Google Scholar]



