Abstract
We used high-performance liquid chromatography to separate urine obtained from whole-body gamma-irradiated mice (4 Gy) before analyzing each fraction with matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry to identify radiation-responsive molecules. We identified two candidates: hepcidin antimicrobial peptide 2 (hepcidin-2) and peptide fragments of kidney androgen-regulated protein (KAP). We observed that peak increases of hepcidin-2 in urine were delayed in a dose-dependent manner (1 Gy and above); however, the amount of KAP peptide fragments showed no correlation with radiation dose. In addition, an increase in hepcidin-2 after exposure to relatively low radiation doses (0.25 and 0.5 Gy, respectively) was biphasic (at 8–48 h and 120–168 h, respectively, after irradiation). The increase in hepcidin-2 paralleled an increase in hepcidin-2 gene ( Hamp2 ) mRNA levels in the liver. These results suggest that radiation exposure directly or indirectly induces urinary excretion of hepcidin-2 at least in part by the upregulation of Hamp2 mRNA in the liver.
Keywords: radiation-responsive molecules, urine, hepcidin-2, mass spectrometry
INTRODUCTION
As the worldwide demand for energy is increasing rapidly, nuclear energy is expected to play a crucial role in providing sustainable and clean energy in the future. In addition, diagnostic radiology, nuclear medicine, and radiation therapy are evolving as essential technologies that are beneficial to human health. It is thus important to ensure that nuclear energy and medical radiation are used safely and that a radiation emergency medical system for unexpected nuclear accidents or disasters is established. Counting the frequency of unstable chromosome aberrations (dicentrics or centric rings) is currently the most accurate method for evaluating radiation dose (0.1 Gy and above). However, this is a time-consuming procedure and one that requires specific technical expertise. Since victims of radiation accidents may be exposed to a range of radiation doses, early biomarkers of radiation exposure would be useful when evaluating and managing victims.
In the case of a radiation emergency event, many people are likely to be exposed to ionizing radiation. Therefore, the ability to obtain an immediate dose estimation for individuals would improve treatment and ultimately survival. Assessing radiation dose by means of urine samples would be advantageous because urine collection is faster and less invasive than blood sampling. In several recent studies, researchers used high-sensitivity mass spectrometry (MS; e.g. liquid chromatography-tandem MS (LC - MS/MS)) to detect metabolites in the urine of mice exposed to ionizing radiation [ 1–6 ]. In these studies, researchers showed that several metabolites, including thymidine, 2′-deoxyuridine, 2′-deoxyxanthosine, xanthine, xanthosine, taurine and cytosine are biomarkers of radiation exposure. Compared with radiation exposure–associated metabolites, radiation exposure–associated proteins/peptides are more difficult to identify. In a recent study, Rithidech and colleagues used 2D electrophoresis coupled with matrix-assisted laser desorption/ionization–time-of-flight MS (MALDI-TOF MS) to detect radiation exposure–associated proteins in mouse plasma [ 7 ]. The advantage of using proteins/peptides as biomarkers of radiation exposure is that radiation dose can be evaluated with quantitative, high-throughput methods, such as the enzyme-linked immunosorbent assay or other antibody-based assays.
In this study, we aimed to identify candidate radiation-responsive molecules in mouse urine with MS and to develop a sensitive biodosimetry technique that could be used for patient triage in a radiation medical emergency. We identified hepcidin antimicrobial peptide 2 (hepcidin-2), which was significantly raised in the urine samples of gamma-irradiated mice. Importantly, this increase lasted for several days, depending on the radiation dose. This increase could, at least in part, be ascribed to increased expression of the hepcidin-2 gene ( Hamp2 ) in the liver. These results suggest that, in a mouse model, hepcidin-2 is a good candidate radiation-responsive molecule over a wide dose range.
MATERIALS AND METHODS
Ethics
The Animal Experimentation Committee of Hiroshima University, Hiroshima, Japan, approved all the animal experiments.
Animal experiments and gamma irradiation
We purchased 8-week-old male B6C3F1/Crlj mice from the Charles River Laboratories Japan Inc. (Yokohama, Japan). The mice were kept in autoclaved cages, maintained under temperature (23 ± 2°C) and humidity (55 ± 10%) control and a 12:12 h light/dark cycle; we fed mice a laboratory animal diet (MF; Oriental Yeast Co. Ltd, Tokyo, Japan) and gave them water ad libitum . We acclimatized mice for a week before conducting experiments. At 9 weeks of age, we either exposed them to whole-body gamma irradiation from a 137 Cs source (Gammacell; Best Theratronics, Ottawa; Canada) at a dose rate of 1.0 Gy/min ( n = 10) or sham irradiated them ( n = 15). We collected urine samples from each mouse directly into 1.5 ml tubes before and after gamma or sham irradiation. Because urine composition varies according to the stage of the circadian cycle [ 8 ], we started the experiments at the same time each day. We could not collect urine from all mice at each time point; irradiated mice appeared to have smaller urine volumes [ 2 ]. For this reason, we analyzed the urine of only a subset of mice (at least five urine samples/dose/time-point). For molecular analysis, we sacrificed mice ( n = 3 or 4) using ether anesthesia; we collected liver samples and stored them at −80°C. For mRNA analysis, we stored the harvested tissues in RNA Save (Biological Industries Israel Beit-Haemek Ltd, Kibbutz Beit-Haemek, Israel) at −80°C. Urine samples were thawed once and subsequently analyzed.
High-performance liquid chromatography and matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry
To identify radiation-responsive molecules in the urine of mice, we injected 20 μl of urine into an Inertsil ODS-80A column (GL Sciences Inc., Tokyo, Japan) using a high-performance liquid chromatography (HPLC) system (PU-980; JASCO Corporation, Tokyo, Japan) with a gradient mobile phase comprising 0.05% trifluoroacetic acid (TFA) solution (A) and 80% acetonitrile/0.05% TFA solution (B). We resolved each sample for 40 min at a flow rate of 1.0 ml/min and dispensed 500 μl into 1.5 ml tubes every 30 s. The gradient consisted of 90% (A)/10% (B) for 10 min, 90% (A)/10% (B) – 10% (A)/90% (B) for 20 min, and finally 10% (A)/90% (B) for 10 min. We mixed each fraction with 10 μl of 50 nM angiotensin II solution and dried it under reduced pressure using a centrifugal concentrator (VC-96N; TAITEC CORPORATION, Saitama, Japan) at 2500 rpm at 28°C. We extracted samples with 15 μl of 50% acetonitrile/0.1% TFA and froze them at −30°C until processing.
To measure urinary hepcidin-2, we desalted the urine samples and concentrated them with OMIX tips (C18; Agilent Technologies, Palo Alto, CA; USA), which are disposable pipette tips that contain a tiny bed of silica column chromatography media (C18). We equilibrated the OMIX tips with 60% acetonitrile/0.1% TFA and then with 10% acetonitrile/0.1% TFA. We aspirated and dispensed the pretreated samples with 0.1% TFA several times, then desalted them with 10% acetonitrile/0.1% TFA, eluted them with 2 μl of 60% acetonitrile/0.1% TFA, and froze them at −30°C until processing.
MALDI-TOF MS analysis using an ultraflex TOF spectrometer (ultraflex TOF/TOF; Bruker Daltonics, Billerica, MA, USA) has been described previously [ 9 ]. For semiquantitation of hepcidin-2, relative intensity is shown as the ratio of hepcidin-2 intensity to that of the peptide fragment of Tamm–Horsfall urinary glycoprotein ( m/z 2001), which we used to normalize the urine samples.
Tandem mass spectrometry analysis to identify candidate radiation-responsive molecules
To identify candidate radiation-responsive molecules, we conducted MS/MS analysis, primarily with ultraflex TOF/TOF. Because of their ability to detect the peptide sequence, we further employed a MALDI-quadrupole ion trap (QIT)-TOF MS/MS (AXIMA-QIT; Shimadzu Scientific Instruments, Kyoto, Japan) or electrospray ionization (ESI)-Q-TOF MS (Q-STAR; Applied Biosystems, Foster City, CA, USA). After MS analysis, peptides were fragmented using the MS/MS mode. We performed MALDI-TOF MS using post-source decay by LIFT mode on the ultraflex. The MS/MS data we obtained were submitted to the Mascot Server to search against the mouse entries of the Mascot protein database ( http://www.matrixscience.com/ ).
For the identification of m/z 1721 (sodium adduct mass) and m/z 2821, we analyzed samples with the AXIMA-QIT using 2,5-dihydroxybenzoic acid as a matrix in the positive ion QIT collision-induced dissociation mode. We analyzed the results with the Shimadzu Biotech MALDI-MS software (Shimadzu Scientific Instruments).
Initially, we identified m/z 2821 using Q-STAR. We loaded each urine sample (5 µl) into a nano-spray tip (HUMANIX, Hiroshima, Japan). We used the reflector-positive mode to obtain all results. For protein identification, we used the obtained data to search against the mouse FASTA protein sequence database ( http://www.genome.jp/tools/fasta/ ).
RNA extraction and quantitative reverse transcription-polymerase chain reaction of mRNA
We extracted the total RNA from homogenized tissues using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions; we carried out quantitative reverse transcription-polymerase chain reaction (qRT-PCR) for mRNA expression analysis as previously described [ 10 ]. We used the primer pairs of Hamp (forward primer, 5′-CCTATCTCCATCAACAGATG-3′; reverse primer, 5′-AACAGATACCACACTGGGAA-3′), Hamp2 (forward primer, 5′-CCTATCTCCATCAACAGATG-3′; reverse primer, 5′-AACAGATACCACAGGAGGGT-3′) and glyceraldehyde-3-phosphate dehydrogenase ( Gapdh ) (forward primer, 5′-GTCAGCAATGCATCCTGCA-3′; reverse primer, 5′-GTGGTCATGAGCCCTTCCA-3′) for amplification [ 11 ]. We used Gapdh expression as an internal standard. We calculated relative gene expression levels with the 2 –ΔΔCT method [ 12 ].
Statistical analysis
The results are presented as mean ± SD. We tested any statistically significant differences between groups with Welch's t -test. We considered a P < 0.05 as statistically significant.
RESULTS
Identification of radiation-responsive molecules in mouse urine using MALDI-TOF MS
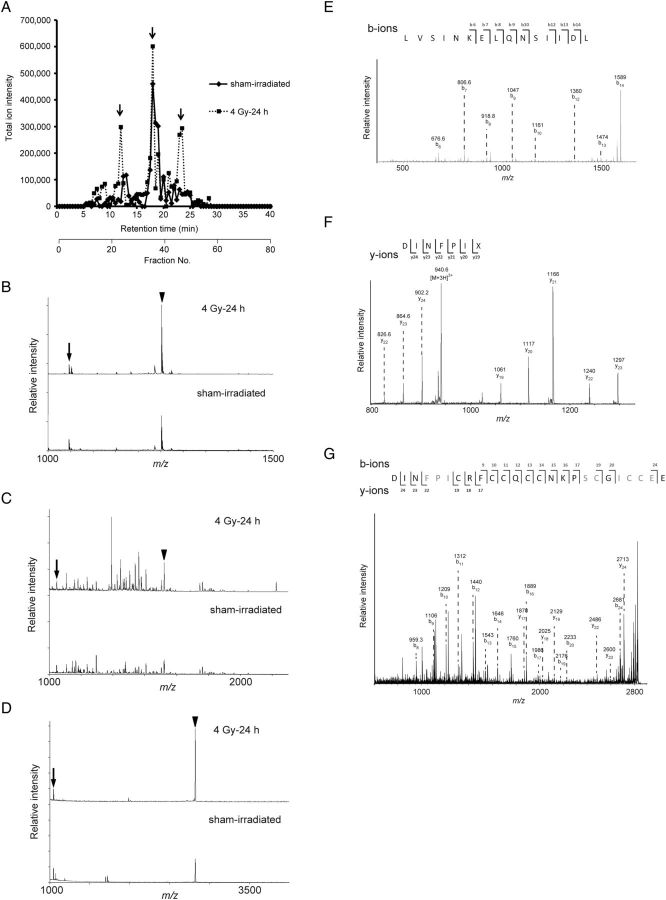
To identify radiation-responsive molecules in mouse urine, we used the MALDI-TOF MS to analyze urine samples collected from irradiated mice. We first analyzed urine from irradiated mice directly with the MALDI-TOF MS, but we did not observe clear mass spectra for the range from m/z 1000 to m/z 4000 (data not shown). We then separated the urine with HPLC; we recorded the total ion intensity obtained from each fraction with MALDI-TOF MS. We observed an increase in total ion intensity in urine collected 24 h after irradiation with 4 Gy in HPLC fractions 24, 35 and 46 compared with the urine from sham-irradiated mice (Fig. 1 A). In these fractions, the peak increased according to radiation exposure (Fig. 1 B–D); we attempted to identify the peptide sequences with MS/MS. However, we could not identify any peptide sequence with the ultraflex TOF/TOF. The peptide fragment m/z 1721 (sodium adduct mass) was identified as a KAP peptide fragment in fraction 35 with the AXIMA-QIT (Fig. 1 E). Initially, we partially identified (six amino acids; DINFPI) the peptide sequence for m/z 2821 in fraction 46 with Q-STAR (Fig. 1 F) before hepcidin-2 was finally identified with the AXIMA-QIT after urine sample reduction with dithiothreitol (Fig. 1 G). Hepcidin-1 has been shown to have eight cysteines connected by intramolecular disulfide bonds [ 13 ]. However, we could not determine the molecule corresponding to m/z 1250 in fraction 24 (Fig. 1 B) (Table 1 ).
Fig. 1.
( A ) Representative total ion intensity in each fraction of mouse urine individually separated by high-performance liquid chromatography (HPLC): bold line, sham-irradiated; broken line, 24 h after 4 Gy irradiation. The sum intensity of each fraction is shown by retention time. Arrows show the changes in response to radiation. ( B , C and D ) Representative matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry spectra of HPLC fractions no. 24, 35 and 46, respectively. The arrows represent the peak of angiotensin II ( m/z 1046) as the internal control, and the arrowheads represent m/z 1250 (B), 1721 (C) and 2821 (D), respectively. ( E ) Representative tandem mass spectrometry (MS/MS) spectra of m/z 1721 and (F) 2821. ( G ) Full MS/MS spectra of m/z 2821 were obtained from samples treated with dithiothreitol.
Table 1.
Putative urinary biomarkers of radiation exposure identified by MS/MS analysis
| Fraction | m/z | Predicted protein | Peptide sequence |
|---|---|---|---|
| 24 | 1250 | not determined | not determined |
| 35 | 1721 (Na adduct mass) | kidney androgen–regulated protein | LVSINKELQNSIIDL |
| 46 | 2821 | hepcidin-2 | DINFPICRFCCQCCNKPSCGICCEE |
Semiquantitation of candidate radiation-responsive molecules with MALDI-TOF MS
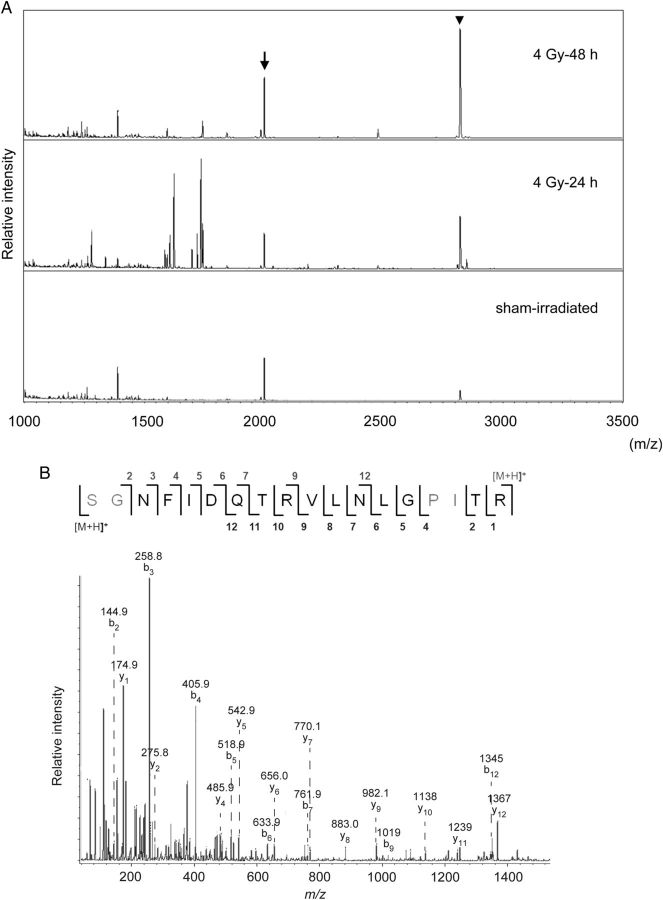
To evaluate the possibility of using the KAP and hepcidin-2 peptide fragments in mouse urine as radiation-responsive molecules, we analyzed time-dependent changes in these molecules with MALDI-TOF MS. Figure 2 A shows the mass spectrum from unfractionated urine, which we concentrated and deionized with OMIX tips. In this analysis, we used m/z 2001 as an internal standard, which we identified as the peptide fragment of Tamm–Horsfall glycoprotein (uromodulin; amino acid sequence: SGNFIDQTRVLNLGPITR), the most abundant urinary protein in mammalian urine, using MALDI-TOF MS (Fig. 2 B) [ 14 ]. After radiation exposure, the intensity of hepcidin-2 increased in a time-dependent manner compared with the corresponding intensity of uromodulin (Fig. 2 A).
Fig. 2.
( A ) Representative matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry spectra from unfractionated urine, which we concentrated and deionized with OMIX tips. We collected urine samples from mice before irradiation (bottom) or 24 h (middle) or 48 h (top) after 4 Gy irradiation. Arrow: peptide fragment of uromodulin ( m/z 2001); arrowhead: hepcidin-2 ( m/z 2821). ( B ) Representative tandem spectrometry spectrum of m/z 2001.
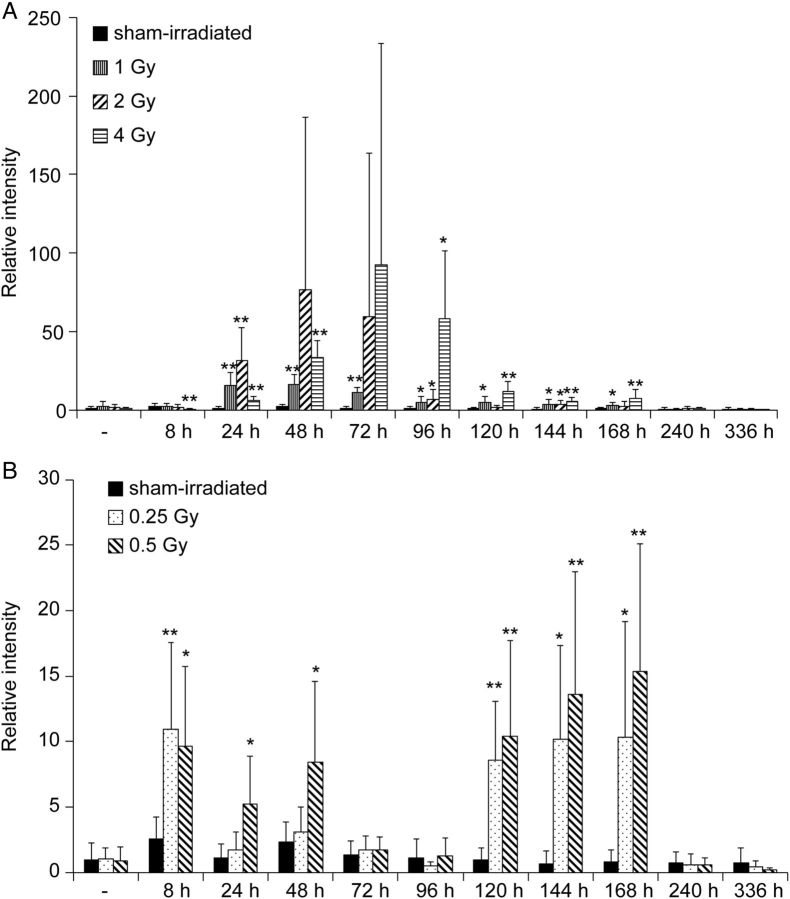
Figure 3 shows the changes in the level of urinary hepcidin-2 in mice exposed to various doses of gamma irradiation. Hepcidin-2 levels in urine from sham-irradiated mice were almost identical throughout the experiments. In irradiated mice, however, the increase in urinary hepcidin-2 started 24 h after irradiation (1 Gy and above) and lasted for ∼168 h (Fig. 3 A). Peak increases in hepcidin-2 in mouse urine were delayed in a dose-dependent manner. Interestingly, after low-dose irradiation (0.25 or 0.5 Gy), hepcidin-2 levels increased significantly at 8 h, decreased at 72 h, and increased again from 120 to 168 h (Fig. 3 B).
Fig. 3.
( A ) Urinary excretion of hepcidin-2 by high-dose irradiation. ( B ) Urinary excretion of hepcidin-2 by low-dose irradiation. We used the uromodulin peptide fragment as normalization, and we calculated relative intensity compared with the levels found in mice prior to sham irradiation. * P < 0.05, ** P < 0.01, compared with sham-irradiated mice at the same time-point.
We next measured the changes in the level of KAP peptide fragment in the urine of mice exposed to various doses of gamma irradiation. In the urine from sham-irradiated mice, however, the level of this peptide fragment increased 8 h after sham irradiation, suggesting that the experimental procedure itself induced a stress response. This peptide fragment was upregulated by radiation exposure, but this phenomenon was not significant and did not occur in a time- or dose-dependent manner ( Supplementary Fig. 1 ).
An increase of urinary hepcidin-2 is caused by the upregulation of its mRNA in the liver
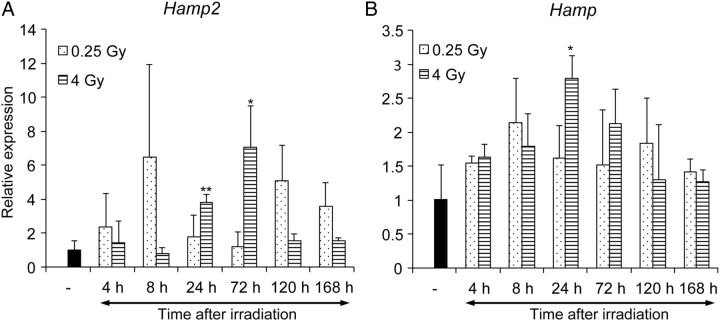
We next examined the source of hepcidin-2 found in mouse urine. Mouse hepcidin mRNA encodes an 83-residue precursor that includes a signal peptide; this is subsequently cleaved, and then prohepcidin is proteolytically cleaved by a conserved proprotein convertase to the 25-residue hepcidin-2 [ 15 ]. Liver and pancreas have abundant Hamp2 mRNA [ 11 ]. Thus, we examined Hamp2 in the liver and pancreas with qRT-PCR. Hamp2 expression in the liver was significantly upregulated from 24 to 72 h after 4 Gy irradiation, and increased biphasically at 8 h and 120–168 h after 0.25 Gy irradiation (Fig. 4 A). Hamp2 expression in the liver was not identical but was similar to the pattern of hepcidin-2 in mouse urine. On the other hand, Hamp2 expression in the pancreas was almost unchanged after irradiation ( Supplementary Fig. 2 A). We also assessed Hamp expression, which encodes another isoform of hepcidin-2 and correlates with radiation exposure [ 16 , 17 ]. Indeed, we found that Hamp was also upregulated after radiation exposure (Fig. 4 B), but the increase was transient and relatively low compared with that of Hamp2 (Fig. 4 A). These results suggested that the increase of hepcidin-2 in mouse urine after radiation exposure was caused at least in part by the upregulation of hepcidin-2 mRNA in the liver.
Fig. 4.
Expression of Hamp and Hamp2 genes in the liver after whole-body irradiation. We analyzed Hamp2 ( A ) and Hamp ( B ) expression levels with quantitative reverse transcription-polymerase chain reaction. We calculated the relative expression levels compared with the levels found in sham-irradiated mice. * P < 0.05, ** P < 0.01.
DISCUSSION
In this study, we identified hepcidin-2 as a candidate radiation-responsive molecule in mouse urine. Hepcidin-2 began to increase significantly in a dose-dependent manner 24 h after irradiation and lasted for ∼168 h; peak urinary excretion of hepcidin-2 was delayed with increasing doses above 1 Gy (Fig. 3 ). Interestingly, hepcidin-2 increased in a biphasic manner after relatively low doses of irradiation (0.5 Gy and below); we observed peaks after 8 h and 120–168 h post irradiation. The radiation response of hepcidin-2 was also observed in C57BL/6 mice ( Supplementary Fig. 3 ). We ascribe the increase in hepcidin-2 in mouse urine, at least in part, to the increase in Hamp2 expression in the liver. In contrast to biodosimetric methods that use gamma-H2AX staining, and which are applied within 1 day after radiation exposure, hepcidin-2 may be used to evaluate the irradiation dose up to 1 week after exposure.
Hepcidin was first identified in human blood ultrafiltrate with antimicrobial activity and termed liver-expressed antimicrobial peptide 1 [ 18 ]. Humans have only one hepcidin gene ( hepcidin antimicrobial peptide , HAMP ); however, mice have two, Hamp and Hamp2 . Both Hamp and Hamp2 mRNA in the liver are upregulated by iron overload [ 19 ]. Exposure to lipopolysaccharide increases Hamp expression both in the liver and pancreas, suggesting that Hamp is an acute-phase reactant; however, Hamp2 does not respond to lipopolysaccharide treatment [ 19 ]. Hamp knockout mice show severe tissue iron overload [ 20 ], and Hamp transgenic mice show severe anemia. On the other hand, Hamp2 transgenic mice show normal hematological parameters, suggesting that hepcidin-2 has no function in iron metabolism [ 21 ]. Hamp2 was also reported to be downregulated in nephrotoxic serum nephritis [ 22 ] and ethanol-loaded mouse models [ 23 , 24 ], whereas it was significantly increased in the liver of diet-induced obese mice compared with normal mice [ 25 ]. These previous observations suggest that disorder (iron overload, nephritis, obesity and alcoholic liver disease) might interfere with the evaluation of radiation exposure by means of hepcidin-2 measurements. Instead, because hepcidin-2 shares some common features with fish peptides, it has been suggested that it functions in innate immunity similarly to fish hepcidin-like peptides [ 21 ]. Although the biological functions of hepcidin-2 have not been clearly demonstrated, finding that Hamp2 was induced in the liver after whole-body irradiation may provide a clue to its function. Moreover, Hamp2 in the liver was induced biphasically by low-dose irradiation (<0.5 Gy). This biphasic response is consistent with previous studies that showed interleukin 6 has a biphasic response in the spleen following irradiation [ 26 ]. Further investigation of the role of hepcidin-2 and related molecules in response to radiation is required in order to identify a reliable candidate for biodosimetry. The biological mechanisms behind this phenomenon are unknown and should be investigated further.
The majority of proteins with significantly altered expression in plasma after whole-body irradiation are acute-phase proteins, which are associated with the inflammatory response [ 7 ]. Gene expression profiles in the liver after whole-body irradiation have also revealed that the tissue is in an inflammatory state, as characterized by upregulation of positive acute-phase genes [ 27 ]. We also observed irradiation-induced expression of metallothionein 1 , one of the acute-phase genes, in the liver ( Supplementary Fig. 2 B). Taken together, these data suggest that induction of Hamp2 is associated with the inflammatory state of irradiated liver.
We do not know which organs caused the increase of hepcidin-2 in mouse urine. The results showed that Hamp2 expression in the liver was upregulated in parallel with the hepcidin-2 levels in mouse urine, suggesting upregulation of hepcidin-2 in serum. We have investigated the existence of the hepcidin-2 peptide in plasma; however, we could not find it (data not shown). This is in agreement with a previous study reporting that hepcidin-2 was detected in urine, but not in serum, by MS [ 28 ].
In this study, we detected three candidate radiation-responsive molecules by means of HPLC and MALDI-TOF MS (Fig. 1 A). We could not unequivocally identify one of the three radiation-responsive candidates, m/z 1250, by MS/MS. We speculate that m/z 1250 is not a polypeptide, given that MALDI-TOF MS can also detect lipids, glycolipids and cholesterols. Indeed, the levels of certain fatty acids in rat sebum are significantly changed after radiation exposure [ 29 ]. Further investigation is needed to identify this candidate radiation-responsive molecule.
In summary, hepcidin-2 in mouse urine is a candidate radiation-responsive molecule. Establishment of a sensitive technique for identifying radiation-responsive molecules in blood or urine will be useful in the area of radiation emergency medicine.
FUNDING
This work was supported in part by Grants-In-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 23710071). Funding to pay the Open Access publication charges for this article was also provided by this grant. A part of this study is the result of the ‘Study on the establishment of biodosimetry methods for radiation exposure’ carried out under the Fundamental Nuclear Research and Development Initiative of the Japan Science and Technology Agency.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr T. Imaoka (National Institute of Radiological Sciences) and Dr M. Sasatani for their comments, Ms M. Yano for technical assistance, and the staff at the Radiation Research Center for Frontier Science Facilities, Hiroshima University, for managing the radiation sources. This study was presented at the 2nd Asian Congress of Radiation Research (12 May 2013), the 56th Annual Meeting of the Japan Radiation Research Society (18 October 2013) and the 59th Annual Meeting of the Radiation Research Society (22 September 2014).
REFERENCES
- 1. Chen C, Brenner DJ, Brown TR . Identification of urinary biomarkers from X-irradiated mice using NMR spectroscopy . Radiat Res 2011. ; 175 : 622 – 30 . [DOI] [PubMed] [Google Scholar]
- 2. Tyburski JB, Patterson AD, Krausz KW, et al. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice . Radiat Res 2008. ; 170 : 1 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tyburski JB, Patterson AD, Krausz KW, et al. Radiation metabolomics. 2. Dose- and time-dependent urinary excretion of deaminated purines and pyrimidines after sublethal gamma-radiation exposure in mice . Radiat Res 2009. ; 172 : 42 – 57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson CH, Patterson AD, Krausz KW, et al. Radiation metabolomics. 4. UPLC-ESI-QTOFMS-based metabolomics for urinary biomarker discovery in gamma-irradiated rats . Radiat Res 2011. ; 175 : 473 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanz C, Patterson AD, Slavík J, et al. Radiation metabolomics. 3. Biomarker discovery in the urine of gamma-irradiated rats using a simplified metabolomics protocol of gas chromatography–mass spectrometry combined with random forests machine learning algorithm . Radiat Res 2009. ; 172 : 198 – 212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laiakis EC, Hyduke DR, Fornace AJ . Comparison of mouse urinary metabolic profiles after exposure to the inflammatory stressors γ radiation and lipopolysaccharide . Radiat Res 2012. ; 177 : 187 – 99 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rithidech KN, Honikel L, Rieger R, et al. Protein-expression profiles in mouse blood-plasma following acute whole-body exposure to 137 Cs γ rays . Int J Radiat Biol 2009. ; 85 : 432 – 47 . [DOI] [PubMed] [Google Scholar]
- 8. Noh JY, Han DH, Yoon JA, et al. Circadian rhythms in urinary functions: possible roles of circadian clocks? Int Neurourol J 2011. ; 15 : 64 – 73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maniwa J, Izumi S, Isobe N, et al. Studies on substantially increased proteins in follicular fluid of bovine ovarian follicular cysts using 2-D PAGE and MALDI-TOF MS . Reprod Biol Endocrinol 2005. ; 3 : 23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iizuka D, Imaoka T, Nishimura M, et al. Aberrant microRNA expression in radiation-induced rat mammary cancer: the potential role of miR-194 overexpression in cancer cell proliferation . Radiat Res 2013. ; 179 : 151 – 9 . [DOI] [PubMed] [Google Scholar]
- 11. Ilyin G, Courselaud B, Troadec MB, et al. Comparative analysis of mouse hepcidin 1 and 2 genes: evidence for different patterns of expression and co-inducibility during iron overload . FEBS Lett 2003. ; 542 : 22 – 6 . [DOI] [PubMed] [Google Scholar]
- 12. Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2 –ΔΔC T Method . Methods 2001. ; 25 : 402 – 8 . [DOI] [PubMed] [Google Scholar]
- 13. Park CH, Valore EV, Waring AJ, et al. Hepcidin, a urinary antimicrobial peptide synthesized in the liver . J Biol Chem 2001. ; 276 : 7806 – 10 . [DOI] [PubMed] [Google Scholar]
- 14. Lhotta K . Uromodulin and chronic kidney disease . Kidney Blood Press Res 2010. ; 33 : 393 – 8 . [DOI] [PubMed] [Google Scholar]
- 15. Schranz M, Bakry R, Creus M, et al. Activation and inactivation of the iron hormone hepcidin: biochemical characterization of prohepcidin cleavage and sequential degradation to N-terminally truncated hepcidin isoforms . Blood Cells Mol Dis 2009. ; 43 : 169 – 79 . [DOI] [PubMed] [Google Scholar]
- 16. Christiansen H, Saile B, Hermann RM, et al. Increase of hepcidin plasma and urine levels is associated with acute proctitis and changes in hemoglobin levels in primary radiotherapy for prostate cancer . J Cancer Res Clin Oncol 2007. ; 133 : 297 – 304 . [DOI] [PubMed] [Google Scholar]
- 17. Christiansen H, Sheikh N, Saile B, et al. x-Irradiation in rat liver: consequent upregulation of hepcidin and downregulation of hemojuvelin and ferroportin-1 gene expression . Radiology 2007. ; 242 : 189 – 97 . [DOI] [PubMed] [Google Scholar]
- 18. Krause A, Neitz S, Mägert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity . FEBS Lett 2000. ; 480 : 147 – 50 . [DOI] [PubMed] [Google Scholar]
- 19. Krijt J, Cmejla R, Sýkora V, et al. Different expression pattern of hepcidin genes in the liver and pancreas of C57BL/6N and DBA/2N mice . J Hepatol 2004. ; 40 : 891 – 6 . [DOI] [PubMed] [Google Scholar]
- 20. Lesbordes-Brion JC, Viatte L, Bennoun M, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis . Blood 2006. ; 108 : 1402 – 5 . [DOI] [PubMed] [Google Scholar]
- 21. Lou DQ, Nicolas G, Lesbordes JC, et al. Functional differences between hepcidin 1 and 2 in transgenic mice . Blood 2004. ; 103 : 2816 – 21 . [DOI] [PubMed] [Google Scholar]
- 22. Wenderfer SE, Dubinsky WP, Hernandez-Sanabria M, et al. Urine proteome analysis in murine nephrotoxic serum nephritis . Am J Nephrol 2009. ; 30 : 450 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrison-Findik DD, Lu S, Zmijewski EM, et al. Effect of alcohol exposure on hepatic superoxide generation and hepcidin expression . World J Biol Chem 2013. ; 4 : 119 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohtake T, Saito H, Hosoki Y, et al. Hepcidin is down-regulated in alcohol loading . Alcohol Clin Exp Res 2007. ; 31Suppl. 1 : S2 – 8 . [DOI] [PubMed] [Google Scholar]
- 25. Pini M, Rhodes DH, Fantuzzi G . Hematological and acute-phase responses to diet-induced obesity in IL-6 KO mice . Cytokine 2011. ; 56 : 708 – 16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang CM, Elliott TB, Dobson ME, et al. Ionizing radiation and bacterial challenge alter splenic cytokine gene expression . J Radiat Res 2000. ; 41 : 259 – 77 . [DOI] [PubMed] [Google Scholar]
- 27. Roudkenar MH, Li L, Baba T, et al. Gene expression profiles in mouse liver cells after exposure to different types of radiation . J Radiat Res 2008. ; 49 : 29 – 40 . [DOI] [PubMed] [Google Scholar]
- 28. Tjalsma H, Laarakkers CM, van Swelm RP, et al. Mass spectrometry analysis of hepcidin peptides in experimental mouse models . PLoS One 2011. ; 6 : e16762 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lanz C, Ledermann M, Slavík J, et al. The production and composition of rat sebum is unaffected by 3 Gy gamma radiation . Int J Radiat Biol 2011. ; 87 : 360 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.