Abstract
The objective of the present study was to investigate nighttime activity of nonhuman primates during extinction and cue- and drug-primed reinstatement of methamphetamine self-administration. Adult rhesus monkeys (Macaca mulatta; n = 5) self-administered methamphetamine (0.01 mg/kg/injection, i.v.) under a fixed-ratio 20 schedule of reinforcement. Saline infusions were then substituted for methamphetamine and stimulus light (drug-conditioned stimulus presented during drug self-administration) withheld until subjects reached extinction criteria. Drug- and cue-induced reinstatement effects were evaluated after i.v. non-contingent priming injections of methamphetamine (0.03, 0.1 or 0.3 mg/kg). Activity-based sleep measures were evaluated with Actiwatch monitors a week before (baseline nighttime activity parameters) and throughout the protocol. Although methamphetamine self-administration did not significantly affect nighttime activity compared to baseline, sleep-like parameters were improved during extinction compared to self-administration maintenance. Priming injection of 0.1 mg/kg methamphetamine, but not 0.03 or 0.3 mg/kg, induced significant reinstatement effects. These behavioral responses were accompanied by nighttime outcomes, with increased sleep fragmentation and decreased sleep efficiency in the night following 0.1 mg/kg methamphetamine-induced reinstatement. In the absence of both drug and drug-paired cues (extinction conditions), nighttime activity decreased compared to self-administration maintenance. Additionally, effective reinstatement conditions impaired sleep-like measures. Our data indicate that the reintroduction of the stimulus light as a drug-paired cue increased nighttime activity.
Keywords: methamphetamine, nighttime activity, self-administration, reinstatement, rhesus monkeys
Introduction
Drug addiction is a chronic and relapsing disorder. Thus, relapse is a major problem in drug abuse, and understanding factors that might contribute or lead to relapse is mandatory in addiction research. During drug use, contextual cues where the drug is being experienced become associated with the drug effects (Kalivas, 2002). Drug-associated cues are able to induce relapse per se (Weiss, 2005), posing drug-environment conditioning as one of the biggest challenges in addiction treatment.
Sleep impairment also enhances the risk of relapse (Brower & Perron, 2010). Of note, individuals in rehabilitation from abuse of any kind of addictive substance show sleep disturbances (Brower & Perron, 2010). We have previously demonstrated that methamphetamine self-administration disrupts actigraphy-based sleep-like measures in rhesus monkeys, although nighttime activity was restored during subsequent periods of drug abstinence (Andersen et al., 2013).
No study to date has evaluated sleep patterns during the extinction and cue- and drug-induced reinstatement of methamphetamine-seeking behavior. Because the effects of sleep on substance abuse seem to be mediated by the conditioned element of addiction (Berro et al., 2014; Frussa-Filho et al., 2004) and both sleep and conditioning seem to influence relapse, this investigation is of major significance.
Accordingly, the aim of the present study was to investigate the sleep-like measures generated by nighttime activity data of nonhuman primates during extinction and cue- and drug-primed reinstatement of methamphetamine self-administration.
Methods
Subjects
Three male and 2 female adult rhesus monkeys (Macaca mulatta), weighing 9–17kg, served as subjects. Each subject was individually housed in stainless steel home cages and fed Purina monkey chow (Ralston Purina, St. Louis, MO), supplemented with fruit and vegetables. Water was continuously available in the colony. The colony was maintained at an ambient temperature of 22±2°C at 45–50% humidity, and the lights were set to a 12-h light/dark cycle (lights on at hour 07:00; lights off at hour 19:00). Environmental enrichment was provided on a regular, rotating basis. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No.85-23, revised 1985), and were approved by the Institutional Animal Care and Use Committee of Emory University. Animals were fitted with collars (Primate Products) prior to the initiation of the studies. All subjects had a previous history of methamphetamine self-administration.
Drugs
Methamphetamine HCl was provided by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, N.C., USA), and was dissolved in sterile physiological saline. The drug dose was determined as the salt.
Self-administration
All animals were surgically prepared with a chronic indwelling venous catheter under sterile conditions. The apparatus and self-administration procedure were described in detail by Howell and Wilcox (2001). Animals were previously trained to respond under a fixed-ratio (FR) 20 schedule of drug delivery. Subjects had the opportunity to self-administer methamphetamine during 60-min sessions once a day, 7 days/week in the morning (starting between 7–9 am). Animals were placed in a primate chair (Primate Products) and placed in a sound-attenuating experimental chamber for the duration of the session. The rest of the day, animals were maintained in their home-cages. Each session began with a 5 min start delay, after which the initiation of the schedule contingencies, including the availability of methamphetamine, was signaled by the illumination of a white light (discriminative stimulus). Completion of the FR 20 response requirement resulted in a change in the stimulus light from white to red (conditioned reinforcement) for 15s and a methamphetamine infusion (0.01 mg/kg in 0.5 ml). Following each methamphetamine delivery, a 1 min timeout period was initiated wherein all lights were extinguished and responding had no programmed consequences.
Extinction
During extinction sessions, the behavioral chamber was illuminated with the white light discriminative stimulus. Upon completion of the FR 20 response requirement, the red stimulus light was withheld and animals received a saline infusion.
Reinstatement
The reinstatement test day consisted of an experimenter-administered prime of methamphetamine given through the vascular access port immediately before the onset of the session. Completion of the response requirement resulted in the intravenous delivery of saline and the illumination of the conditioned reinforcer over 15s. Therefore, we describe the sessions as drug- and cue-induced reinstatement tests. Reinstatement effects were determined across a range of methamphetamine priming doses (0.03, 0.1, or 0.3 mg/kg, i.v.) in a randomized order.
Nighttime Activity
Actiwatch sensors (Mini Mitter, Bend, OR, USA) were used to assess nighttime activity in the subjects, as previously described (Andersen et al., 2010, 2013). Subjects were previously trained to cooperate with the attachment of the Actiwatch sensor in their collars. The nighttime activity data obtained from the Actiwatch devices generate the following sleep-like behavior parameters: sleep efficiency (i.e., the percentage of the dark phase spent sleeping); fragmentation index (immobile bouts during the dark phase that lasted less than 1 minute during the sleep recording period). All parameters were calculated using the Actiware software program.
Protocol Design
The Actiwatch was attached to the monkeys’ collars while in the primate chair 1 week before the beginning of methamphetamine self-administration and spontaneous baseline nighttime activity was measured for 5 days. During baseline condition, animals were kept undisturbed in their home-cages. After subjects underwent self-administration sessions for 5 days, extinction sessions began. Behavior was considered extinguished when response rates over 2 sequential sessions were <20% of the last 3 days mean rate during maintenance of methamphetamine self-administration. Starting on the day after subjects reached extinction criteria, the 3 reinstatement sessions were conducted with 1 no-drug day between sessions.
Statistical Analysis
Behavioral data and sleep parameters were analyzed using one-way repeated-measures ANOVA followed by Bonferroni’s post-hoc test. Correlational analyses were conducted using Pearson’s Correlation. Significance was accepted at an alpha of 0.05.
Results
Behavioral Data
Methamphetamine reliably maintained self-administration in all 5 subjects, with average drug intake ranging from 0.23 to 0.44 mg/kg/session. During extinction, response rates typically declined within two of sessions from ~0.6 to ~0.05 responses/s. For the reinstatement sessions, one-way repeated measures ANOVA revealed a significant main effect of the priming dose [F(3,12)=22.07, p<0.001]. Bonferroni’s post-hoc test identified that a priming injection of 0.1 mg/kg methamphetamine, but not 0.03 or 0.3 mg/kg, significantly reinstated operant behavior compared with extinction (p<0.05, Figure 1). The priming dose of 0.1 mg/kg engendered response rates which were ~50% of the rates typically observed during methamphetamine self-administration.
Figure 1.
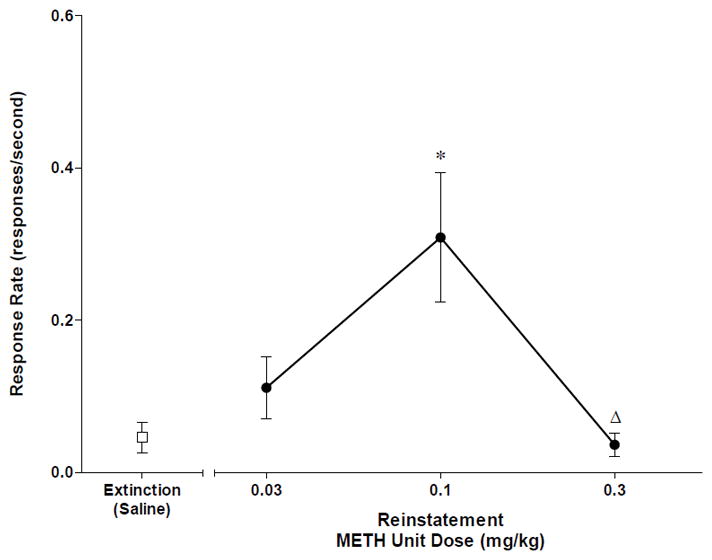
Response rates during extinction and reinstatement after priming injections of methamphetamine (METH, 0.03, 0.1 or 0.3 mg/kg, IV). Data points represent mean±SEM. *p<0.05 compared to Extinction(Saline); Δ p<0.05 compared to 0.1 METH Reinstatement. One-way repeated measures ANOVA followed by Bonferroni’s post-hoc test.
Nighttime Activity
Figure 2 shows sleep-like behavior parameters across the different experimental periods. Data for baseline sleep and self-administration maintenance are combined across a 5-day span of time. Sleep-like parameters are presented as normalized data (percentage of baseline). Individual-subject baseline data are presented on Table 1. In general, the 2 female rhesus macaques presented higher nighttime activity (lower sleep efficiency and higher sleep fragmentation) than the 3 males on baseline conditions. Although the sample size is inadequate to statistically examine these differences, it demonstrates marked individual subject differences with respect to activity-based sleep measures, what might account for the results obtained. One-way repeated measures ANOVA revealed a significant difference across experimental conditions for both sleep efficiency [F(5,20)=11.39; p<0.001] and sleep fragmentation [F(5,20)=7.26; p<0.01]. Bonferroni’s post-hoc test indicated that methamphetamine self-administration did not significantly affect nighttime activity compared to baseline. However, nighttime activity was significantly lower during extinction compared to self-administration maintenance (increased sleep efficiency in ~28%, p<0.05; decreased sleep fragmentation in ~26%, p<0.01). For reinstatement, sleep-like behavior was significantly impaired in the night following 0.1 mg/kg, but not 0.03 or 0.3 mg/kg, methamphetamine-induced reinstatement compared to extinction (decreased sleep efficiency in ~21%, p<0.05; increased sleep fragmentation in ~31%, p<0.05).
Figure 2.
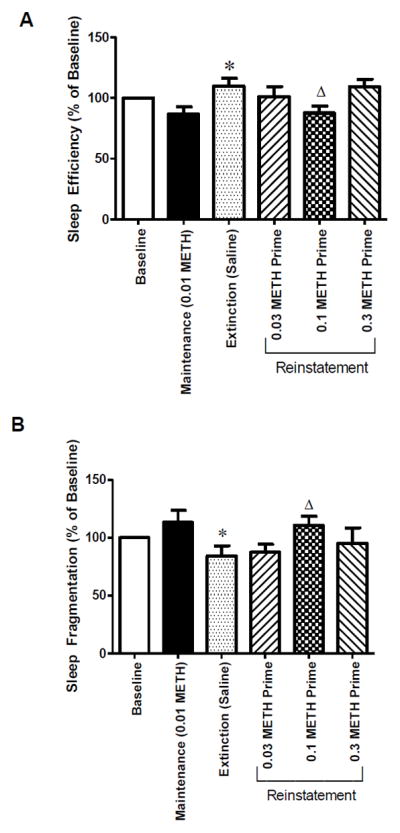
(A) Sleep efficiency and (B) sleep fragmentation at baseline, methamphetamine self-administration maintenance (0.01 mg/kg/infusion), extinction, or reinstatement test sessions conducted with three different doses of methamphetamine (METH, 0.03, 0.1 or 0.3 mg/kg, IV). Nighttime activity data for baseline and self-administration maintenance were combined across a 5-day span of time. Nighttime activity data for extinction were recorded as the night after the first effective extinction session. Data are expressed as mean±SEM. *p<0.05 compared to Maintenance (0.01 METH); Δ p<0.05 compared to Extinction(Saline). One-way repeated measures ANOVA followed by Bonferroni’s post-hoc test.
Table 1.
Individual-subject baseline sleep parameters
| Subject | Sleep Efficiency (%) | Fragmentation Index |
|---|---|---|
| ROf8 (M) | 83.1 ± 0.74 | 24.86 ± 0.87 |
| RVm8 (F) | 64.28 ± 4.46 | 71.13 ± 2.76 |
| RLk4 (M) | 68.83 ± 1.74 | 50.75 ± 3.04 |
| RZs9 (F) | 34.42 ± 2.38 | 88.22 ± 4.26 |
| RJl8 (M) | 86.21 ± 0.56 | 30.95 ± 2.45 |
M = male rhesus monkey; F = female rhesus monkey. Data are expressed as mean±SEM.
Correlation: Behavioral data vs Nighttime Activity
Pearson’s Correlation analysis indicated a significant correlation between methamphetamine intake during active self-administration and sleep fragmentation [r=0.93, p<0.01], but not sleep efficiency [r=0.42, p>0.05] (Figure 3). Individual variability with respect to activity-based sleep measures, as observed in Table 1, might contribute to the absence of difference in nighttime activity between baseline and active self-administration conditions, and the presence of a positive correlation between sleep fragmentation and methamphetamine intake during active self-administration.
Figure 3.
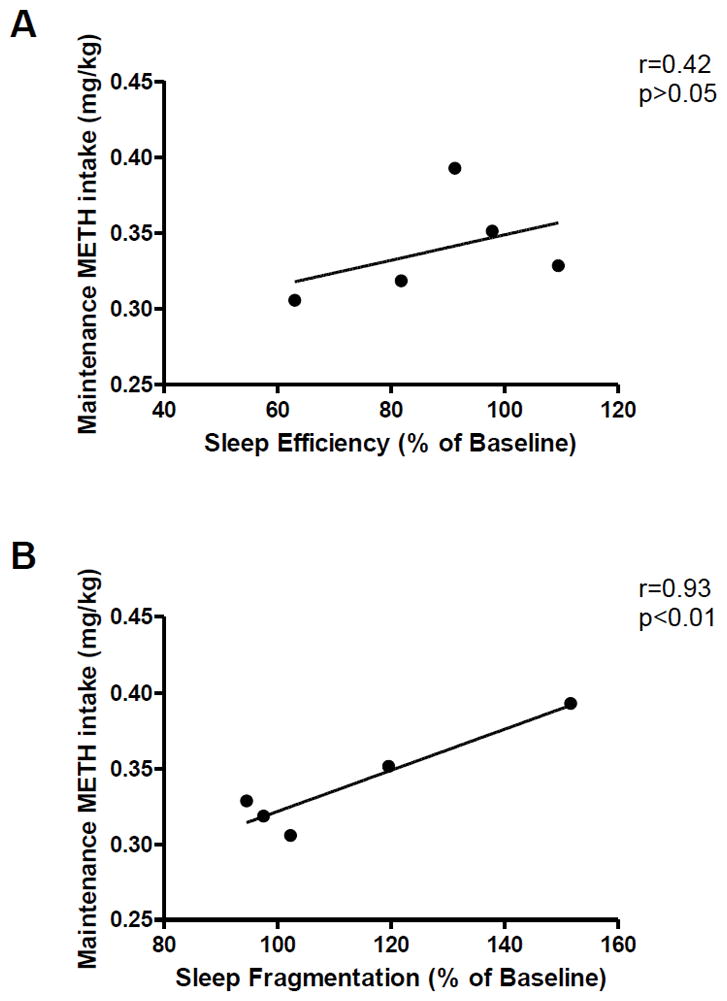
Correlation between methamphetamine intake (0.01 mg/kg/injection self-administration) and sleep (A) efficiency or (B) fragmentation following self-administration days. Pearson‘s Correlation.
Additional analysis also indicated that nighttime activity measures after effective reinstatement conditions (night following 0.1 mg/kg methamphetamine-induced reinstatement) did not significantly correlate with response rates during the respective reinstatement session (sleep efficiency [r=−0.12, p>0.05]; sleep fragmentation [r=0.15, p>0.05])(Figure 4).
Figure 4.
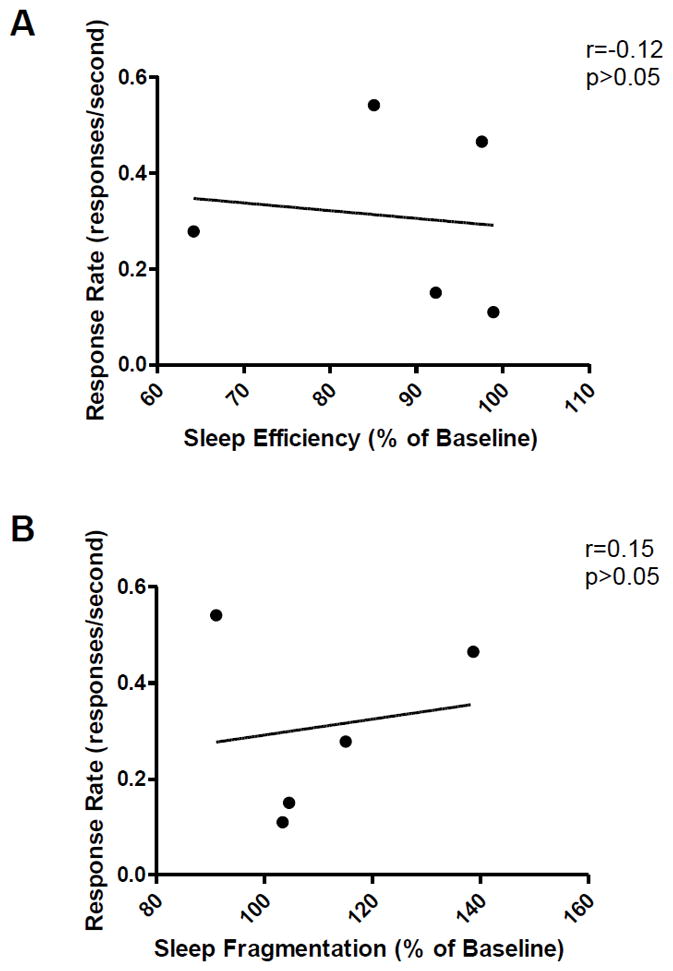
Correlation between response rates and sleep (A) efficiency or (B) fragmentation following effective reinstatement conditions (reinstatement effects induced by priming injection of 0.1 mg/kg methamphetamine). Pearson‘s Correlation.
Discussion
We evaluated nighttime activity of rhesus monkeys on baseline (no-drug) condition and on the night after methamphetamine self-administration, extinction and cue- plus methamphetamine-induced reinstatement sessions. At the dose used in the present study, methamphetamine self-administration did not significantly affect nighttime activity compared to baseline. However, methamphetamine intake was positively correlated with sleep fragmentation. Sleep-like parameters were improved during extinction compared to self-administration maintenance. Also, priming injection of 0.1 mg/kg methamphetamine, but not 0.03 or 0.3 mg/kg, induced significant reinstatement effects. These behavioral responses were accompanied by nighttime outcomes, with increased sleep fragmentation and decreased sleep efficiency in the night following 0.1 mg/kg methamphetamine-induced reinstatement.
Because an increase in nighttime activity during reinstatement was not a monotonic function of drug dose, the direct pharmacological effects of the priming injection on sleep parameters are unlikely to account for the results obtained. Rather, reinstatement effects observed in the present study were bi-phasic (i.e., highest dose of methamphetamine did not induce effective reinstatement). Average drug intake ranged from 0.23 to 0.44 mg/kg/session during active methamphetamine self-administration sessions, without inducing changes in nighttime activity. In a previous study from our group, average drug intake ranged from 0.09 to 0.33 mg/kg/session when methamphetamine self-administration sessions were conducted with the unit dose of 0.01 mg/kg/infusion (Andersen et al., 2013). Under these conditions, there was no significant effect on nighttime activity. Of importance, when the unit dose of methamphetamine during self-administration sessions was increased to 0.03 mg/kg/infusion, with drug intake increasing to 0.22–0.996 mg/kg/session, a marked increase in nighttime activity was observed. Thus, methamphetamine effects on nighttime activity are dose-dependent without evidence of a bi-phasic function.
Except for the drug priming, the main difference between extinction and effective reinstatement sessions was the presentation or not of the drug-conditioned stimulus (red light). Thus, it appears that the presence or absence of conditioned stimuli mediated sleep-like behavior outcomes observed following extinction and reinstatement sessions. In the absence of both drug and drug-paired cues (extinction conditions), there was a decrease in nighttime activity compared to self-administration maintenance. Additionally, effective reinstatement conditions impaired sleep-like measures compared to the night after effective extinction conditions. We hypothesize that the reintroduction of the stimulus light as a drug-paired cue increased nighttime activity. Corroborating our hypothesis, nighttime activity was also lower after extinction compared to the night following methamphetamine self-administration sessions, during which drug-paired stimulus also occurred.
Response rates during effective reinstatement sessions did not correlate with nighttime activity parameters in the following night. Because response rates under a FR schedule of reinforcement directly reflect the presentation of the stimulus light during the session, this analysis suggests that the presentation of the conditioned stimulus light might be important but not sufficient to explain an improved nighttime activity after effective reinstatement conditions. Therefore, although our data support the hypothesis proposed herein, additional tests could be informative in order to validate this hypothesis, such as the investigation of drug- or cue-induced reinstatement on nighttime activity separately.
Our data are consistent with previous studies suggesting that sleep loss exerts its effects on psychostimulant addiction through manipulations in the conditioned component of substance abuse. In rodents, sleep deprivation specifically potentiates context-dependent amphetamine-induced behavioral sensitization (Frussa-Filho et al., 2004) and impairs the extinction of cocaine-induced environmental conditioning (Berro et al., 2014). Our results further indicate that the repeated introduction of drug-paired stimuli is sufficient to induce sleep disruption, inducing an alarming cycle that might ultimately contribute to the continuation of drug abuse.
In summary, we provide evidence that drug-seeking behavior culminating in the presentation of drug-paired stimuli has a negative impact on sleep. Because drug-environment conditioning plays a key role in the addictive cycle (Kalivas, 2002) and sleep impairment is considered a risk factor for drug relapse (Brower & Perron, 2010), sleep patterns must be considered in treatment strategies. A preserved sleep architecture during abstinence periods and after relapse could facilitate the rehabilitation process.
References
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology. 2010;210:439–48. doi: 10.1007/s00213-010-1839-2. http://dx.doi.org/10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ML, Diaz MP, Murnane KS, Howell LL. Effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Psychopharmacology. 2013;227:101–7. doi: 10.1007/s00213-012-2943-2. http://dx.doi.org/10.1007/s00213-012-2943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro LF, Hollais AW, Patti CL, Fukushiro DF, Mári-Kawamoto E, Talhati F, …Frussa-Filho R. Sleep deprivation impairs the extinction of cocaine-induced environmental conditioning in mice. Pharmacology Biochemistry and Behavior. 2014;124:13–8. doi: 10.1016/j.pbb.2014.05.001. http://dx.doi.org/10.1016/j.pbb.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Medical Hypotheses. 2010;74:928–33. doi: 10.1016/j.mehy.2009.10.020. http://dx.doi.org/10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frussa-Filho R, Gonçalves MT, Andersen ML, de Araujo NP, Chinen CC, Tufik S. Paradoxical sleep deprivation potentiates amphetamine-induced behavioural sensitization by increasing its conditioned component. Brain Research. 2004;1003:188–93. doi: 10.1016/j.brainres.2003.11.050. http://dx.doi.org/10.1016/j.brainres.2003.11.050. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurocircuitry of Addiction. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 1357–1366. [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. http://dx.doi.org/10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. http://dx.doi.org/10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]


