Abstract
Substantial evidence links greater impulsivity and stress exposure to poorer smoking cessation outcomes. Results from adolescents also indicate that stress-related change in risk taking can impede cessation attempts. We investigated the effects of stress-related change in impulsivity, risk taking, attention and nicotine withdrawal and craving in young adult smokers on time to smoking relapse in a relapse analogue paradigm. Twenty-six young adult smokers (50% female; mean age: 20.9±1.8) were exposed to a stress imagery session followed by a contingency management-based relapse analogue paradigm. Participants smoked at least 5 cigarettes daily, with a mean baseline CO level of 13.7 (±5.1) ppm. Repeated measures ANOVA and paired t-tests examined stress induction validity and Cox regressions of proportional hazards examined the effects of stress-related changes in nicotine withdrawal, nicotine craving, attention, impulsivity and risk taking on time to relapse. While stress-related change in impulsivity, nicotine craving and withdrawal did not predict time to relapse (all ps > .10), greater stress-related increases in reaction time variability (p = .02) were predictive of shorter time to relapse, with trend-level findings for inattention and risk taking. Also, changes in stress-related risk taking affected outcome in females more than in males, with a significant relationship between stress-related change in risk taking only in females (p = .026). Smoking cessation attempts in young adults may be adversely impacted by stress-related increases in risk taking and attentional disruption. Clinicians working with young adults attempting cessation may need to target these stress-related impairments by fostering more adaptive coping and resilience.
Keywords: Young adult, smoking relapse, stress, impulsivity, risk taking
Introduction
Despite progress in promoting cessation, young adult smoking remains a public health crisis (U.S. Department of Health and Human Services, 2012), with 13.8% of young adults smoking daily (Johnston, O'Malley, Bachman, & Schulenberg, 2012). Young adult smoking is associated with significant medical and psychosocial consequences (Brook, Brook, Zhang, & Cohen, 2004; Lipkus & Prokhorov, 2007), while permanent cessation by the age of 30 virtually eliminates premature mortality related to smoking (Doll, Peto, Boreham, & Sutherland, 2004). Perhaps because of the known benefits of cessation, over 60% of young adult smokers attempt to quit annually (Solberg, Boyle, McCarty, Asche, & Thoele, 2007).
Despite these efforts at cessation in young adults, unaided cessation has poor outcomes (Wechsler, Rigotti, Gledhill-Hoyt, & Lee, 1998), and there are few validated smoking cessation treatments for young adults (Villanti, McKay, Abrams, Holtgrave, & Bowie, 2010). Existing treatments have less than optimal cessation rates (for example, Tevyaw et al., 2009), highlighting the need to improve treatments. One way to boost cessation rates may be to identify psychosocial constructs or events that impede cessation.
Two such characteristics may be impulsivity and risk taking. Across the lifespan, smokers have higher levels of trait impulsivity and poorer response inhibition (Mitchell, 1999, 2004; Reynolds et al., 2007), and smoking cessation also appears to be adversely affected by higher levels of trait impulsivity (Doran, Spring, McChargue, Pergadia, & Richmond, 2004; VanderVeen, Cohen, Cukrowicz, & Trotter, 2008). Finally, Krishnan-Sarin and collaborators (2007) found that adolescents with poorer response inhibition were more likely to relapse to smoking than their less impulsive peers at the end of a contingency management-based cessation intervention. Heightened risk taking may also impede cessation attempts. When compared to non-smokers, smokers take greater risks on both laboratory (Anderson & Mellor, 2008; Businelle et al., 2009; Lejuez et al., 2003), and “real world” risk taking assessments (e.g., riding with an intoxicated driver; Ryb, Dischinger, Kufera, & Soderstrom, 2007). Functional imaging studies indicate that young adult smokers may process risk differently than non-smokers (Galvan et al., 2013), particularly as risk levels increase on a laboratory task, the Balloon Analogue Risk Task (BART). Schepis and colleagues (2011) found that greater stress-related changes in risk taking on the BART predicted cessation failure among treatment-seeking adolescent smokers.
A third potentially important variable is attention. Ample evidence from both pre-clinical models (Semenova, Stolerman, & Markou, 2007; Shoaib & Bizarro, 2005) and human smokers (Harrison, Coppola, & McKee, 2009; McClernon et al., 2008) indicates that attentional processes are impaired in nicotine abstinence. Nicotine abstinence worsens inattentive errors, reaction time (RT) and RT variability on a computerized attention task (Harrison et al., 2009) in human smokers, with evidence for worsened deficits in those with pre-existing attentional liabilities (McClernon et al., 2008). Indeed, other commentators have noted attentional deficits in smokers in early abstinence across a variety of tasks (Heishman, Kleykamp, & Singleton, 2010). In turn, smoking, or nicotine administration in a lab setting has consistently restored attention to pre-withdrawal levels of functioning in smokers (Ernst, Heishman, Spurgeon, & London, 2001; Heishman, Taylor, & Henningfield, 1994; Henningfield, Shiffman, Ferguson, & Gritz, 2009). Thus, some smokers may be motivated to continue to administer nicotine to ameliorate the attentional disruption in early abstinence.
Finally, stress also may adversely affect nicotine cessation, with ample evidence in animal models of stress-induced nicotine seeking following a period of abstinence (Mantsch, Baker, Funk, Le, & Shaham, in press). In humans, nicotine abstinence elevates self-reported levels of stress (Parrott & Kaye, 1999), leading to increases in nicotine craving (Doherty, Kinnunen, Militello, & Garvey, 1995). In turn, craving is predictive of relapse (Doherty et al., 1995; Shiffman et al., 2002). Impulsivity increases following exposure to stressors in both animals (Tonissaar et al., 2008) and humans (Swann, 2003), and smokers with higher levels of trait impulsivity experience stronger craving over the first 48 hours of abstinence (VanderVeen et al., 2008). Risk taking also appears to be affected by stress, though evidence using financial risk paradigms appears to show increased risk taking only under certain conditions (e.g., Porcelli & Delgado, 2009). Thus, impulsive or risk-prone smokers may experience greater urges to smoke over early abstinence, and this may be heightened by exposure to stressors.
While the literature suggests the influence of stress exposure on impulsivity, risk taking and attentional processes, and of those traits on smoking cessation outcome, no investigations have examined interactions between stress exposure and impulsivity, risk taking or attentional processes in young adult smokers. Young adults may be a particularly important cohort for such investigation because of the unique salience of impulsivity (Steinberg et al., 2008) and risk taking (Gardner & Steinberg, 2005; Steinberg, 2010; Van Leijenhorst et al., 2010) in this population. Young adults transitioning to college and non-college young adults also appear to have unique and significant stressors (Arnett, 2014; Compas, Wagner, Slavin, & Vannatta, 1986), and young adults have stress hormone responses that differ from both adolescents and older adults (Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004). Thus, changes in risk taking, impulsivity or attention following stress exposure may be particularly salient avenues for investigation in young adult smokers attempting cessation.
Aims and Hypotheses
The aim of this work was to evaluate the influences of stress-related changes in impulsivity, risk taking, sustained attention, nicotine craving and withdrawal in young adult smokers on time to smoking relapse in 11-session contingency management-based relapse paradigm adapted from Juliano and colleagues (2006). A secondary aim was also to examine potential sex differences in these relationships, given the significant evidence of differences in clinic-based smoking cessation outcomes between men and women (Perkins & Scott, 2008; Scharf & Shiffman, 2004; Wetter et al., 1999). We hypothesized that young adult smokers with greater stress-related increases in risk taking and impulsivity would relapse to smoking more quickly, and we also expected that greater stress-related increases in nicotine withdrawal or craving would correspond to more rapid smoking relapse. We also conjectured that greater increases in stress-related inattention and in attentional variability would correspond to a more rapid relapse to smoking. Given the lack of previous research on sex differences in this area, we had no a priori hypotheses.
Methods
Participants
Participants were young adult university students, aged 18 to 25 years (inclusive), who were recruited through advertisements at a large, public university and postings on the area craigslist service. Smokers were eligible if they smoked ≥5 cigarettes daily and had exhaled carbon monoxide (CO) values of at least 8 ppm. Thirty-six smokers signed consent and met criteria for participation. Thirty completed the stress induction session and 26 participated in the relapse follow-up paradigm (see Procedures, below). Data from those 26 smokers is used here.
Participants were 50% female, with a mean age of 20.9 years (SD= 1.81). They smoked a mean of 9.9 (SD= 3.35) cigarettes per day in the 28 days prior to the assessment section, had a mean exhaled CO of 13.7 (SD= 5.08) and a mean Fagerström Test for Nicotine Dependence (FTND) score of 2.4 (SD= 1.60) at the assessment session; 14 were randomized to overnight abstinence at the stress induction session, and 12 were randomized to smoke normally prior to the induction session. Within the 26 eligible smokers, there were no significant differences by randomization status in age (t(24)= .02, p= .99), gender (χ2(1, N = 26)= .62, p= .43), baseline exhaled CO (t(24)= .11, p= .91), FTND score (t (24)= .52, p= .61) or total number of cigarettes smoked in the 28 days prior to the assessment session (t(24)= .09, p= .93).
Measures
Carbon Monoxide (CO) was assessed using the MicroCO meter from Micro Direct (Lewiston, ME). The MicroCO meter has a sensitivity of 1 ppm and sensor drift of less than 2% per month. To ensure correct measurement, the MicroCO meter was recalibrated biannually (Micro Direct, 2010).
Fagerström Test for Nicotine Dependence (FTND) assessed nicotine dependence with adequate internal consistency and validity (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991).
Sociodemographic information was assessed on a questionnaire capturing age, sex and race/ethnicity.
Questionnaire of Smoking Urges-Brief (QSU) assessed nicotine craving in 10 items, using a 7-point Likert scale. It is a valid measure of nicotine craving (Cox, Tiffany, & Christen, 2001; Toll, Katulak, & McKee, 2006).
Positive and Negative Affect Schedule (PANAS) assessed current affective state using 20 adjectives for both positive (PA) and negative affective (NA) states, using a 5-point Likert scale. The PANAS has good internal consistency and construct validity (Crawford & Henry, 2004; Watson, Clark, & Tellegen, 1988).
Stress Visual Analogue Scale (VAS) assessed stress levels throughout the stress induction session. Participants reported their stress level by placing a mark on a 100-millimeter line with anchors ranging from “No sensation” to “Strongest imaginable stress level of any kind”.
Minnesota Nicotine Withdrawal Scale (MNWS) assessed nicotine withdrawal, including craving and negative affect. It has adequate to good internal consistency (Hughes & Hatsukami, 1986; Toll, O'Malley, McKee, Salovey, & Krishnan-Sarin, 2007).
Conners' Continuous Performance Test – II (CPT-II) assessed inattention (failure to respond to target stimuli), reaction time (RT), RT standard error and commission errors (response inhibition or on-response to non-target stimuli). The CPT-II produces T-scores that are normed for age and gender; it is free from practice effects and is reliable and valid (Conners, 2000),
Balloon Analogue Risk Task (BART) assessed risk-taking as the mean number of pumps on balloon trials that do not burst (adjusted pumps [AP]), which is the most common outcome measure (White, Lejuez, & de Wit, 2008). The BART appears to be reliable and valid (Lejuez et al., 2003; White et al., 2008). Participants were not paid for their BART performance, but the participant with the best performance received a $25 gift card at the end of the study.
Procedures
All procedures listed below were approved by the university's Institutional Review Board.
Screening and Assessment Session
Interested young adults were phone screened and eligible individuals were scheduled for an assessment appointment. At the assessment appointment, participants completed paper-and-pencil assessments, including the FTND, and provided a breath sample for exhaled CO. Participants were asked to describe one of the most stressful situations they experienced in the past year and two recent situations they found relaxing. In order to avoid the confounding effects of tobacco use imagery, participants were asked to develop scripts without tobacco use (see Sinha, 2009; Sinha, Catapano, & O'Malley, 1999 for more on scripting procedures). Finally, smokers were randomized to return to the stress induction session either after overnight nicotine abstinence or non-abstinent. Overnight abstinence (as measured by what exhaled CO was incentivized by a $30 bonus.
Stress Induction Session
All stress induction sessions were scheduled between 9 and 11 AM to limit diurnal stress hormone variation (Sinha et al., 1999), and participants were paid $20 for completion of this session. Abstinent participants provided an immediate CO sample, which had to be at least 50% lower than their baseline CO (Leeman, O'Malley, White, & McKee, 2010). On two occasions, participants failed to reach the 50% reduction criterion, and both were rescheduled for later appointments to retry; both achieved 50% reduction at the second try and took part in the relapse analogue paradigm (below). Smokers in the non-abstinent condition smoked one cigarette in the presence of a research assistant to prevent the confounding effects of nicotine withdrawal. To ensure low baseline levels of stress, all participants then listened to one of their relaxing scripts before completing assessments. Following the first relaxing script, participants completed the VAS, PANAS, QSU, MNWS, BART and CPT-II (in that order). Then, participants listened to their stressful script and completed a second, identical, set of assessments. Finally, participants listened to a second relaxing script to reduce residual stress and completed a final VAS.
Relapse Analogue Paradigm
Participants then took part in a relapse analogue paradigm, adapted from Juliano et al. (2006) Following the induction session, participants took part in a 15-minute motivational interviewing-based skill building session to promote abstinence. Participants returned to the lab on the day of the stress induction (4 PM) and twice daily for the next 5 days (9 AM and 4 PM) for CO measurement of abstinence. CO ≥8 PPM indicated relapse (SRNT Subcommittee on Biochemical Verification, 2002).
Participants received incentives for abstinence on a decreasing schedule to encourage relapse: $8 for the day 1 visit and day 2 visits, $5 for the day 3 and 4 visits and $2 for the day 5 and 6 visits. Participants were paid $5 at each session for completion of assessments, regardless of abstinence status. Relapsed participants no longer earned abstinence-based incentives, but they were asked to attend the remaining visits to reduce the chance that follow-up requirements affected relapse outcome (Juliano et al., 2006).
Data Analysis
Participant characteristics were first examined, both across the sample and between participants by randomization status. These results are summarized above (see Participants). Second, the validity of the stress induction was examined by analyzing stress-related changes in VAS, using repeated measures ANOVA, and the PANAS PA and NA scales, using paired measures t-tests.
Primary analyses utilized Cox regression for proportional hazards, with number of abstinent follow-up visits completed as the time variable. Five sets of analyses were conducted: the first examined the effect of abstinence status on time to relapse; the last four examined the effects of change in nicotine withdrawal (MNWS), nicotine craving (QSU), risk taking (BART AP) and attentional variables and impulsive responding (CPT-II outcomes) on time to relapse. In the final four Cox regressions, the pre-stressor assessment was entered in block one, and the post-stressor assessment was entered in block two, allowing for examination of pre- to post-stressor change on time to relapse. These analyses were repeated to examine potential sex differences. In these analyses, the pre-stressor assessment was entered in block one with a term for the interaction of sex and the post-stressor assessment in block two. Finally, Post hoc analyses also examined whether abstinence status was related to change in VAS score, using repeated measures ANOVA, and whether VAS scores and abstinence status interacted to predict number of abstinent follow-up visits, using Cox regression.
Results
Follow-up Visit Outcomes
The 26 participants completed 175 total follow-up visits, for a mean of 6.73 visits per participant and a standard deviation of 4.15. At the visits where participants self-reported abstinence from smoking since the last visit, the mean CO value was 1.98 PPM and a standard deviation of 1.92. Only 5 participants appeared at the lab endorsing relapse to smoking (with the rest endorsing relapse via phone contact). In those 5 individuals, the mean CO value at the non-abstinent visit was 15.4 PPM, with a standard deviation of 8.1. All means and standard deviations for the stress induction validity and outcome measures, outlined below, are in Table 1.
Table 1. Sex, Stress Induction Validity and Outcome Measures by Abstinence Status at the Stress Induction Session.
| Sex | Abstinent | Non-Abstinent |
|---|---|---|
| 6 females, 8 males | 7 females, 5 males | |
| Stress Induction Validity (mean ± SD) | ||
| Pre-Stressor VAS | 5.29 ± 5.58 | 4.00 ± 5.95 |
| Post-Stressor VAS | 33.14 ± 20.12 | 38.08 ± 14.75 |
| Final (post-relaxation) VAS | 8.29 ± 8.53 | 5.50 ± 5.40 |
| Pre-Stressor Positive Affect | 24.00 ± 7.97 | 21.56 ± 8.26 |
| Post-Stressor Positive Affect | 18.00 ± 5.42 | 16.75 ± 7.02 |
| Pre-Stressor Negative Affect | 12.14 ± 3.9 | 12.17 ± 2.33 |
| Post-Stressor Negative Affect | 16.75 ± 7.02 | 22.83 ± 5.92 |
| Outcome Measures (mean ± SD) | ||
| Pre-Stressor Craving (QSU) | 32.43 ± 10.78 | 21.83 ± 10.16 |
| Post-Stressor Craving (QSU) | 41.29 ± 13.75 | 35.00 ± 14.30 |
| Pre-Stressor Withdrawal (MNWS) | 6.00 ± 3.78 | 3.92 ± 4.32 |
| Post-Stressor Withdrawal (MNWS) | 13.29 ± 6.46 | 12.67 ± 6.62 |
| Pre-Stressor BART Adjusted Pumps | 38.86 ± 13.62 | 29.50 ± 13.69 |
| Post-Stressor BART Adjusted Pumps | 45.43 ± 13.24 | 34.00 ± 11.81 |
| Pre-Stressor Inattention T-Score | 46.37 ± 5.93 | 48.83 ± 10.96 |
| Post-Stressor Inattention T-Score | 56.57 ± 23.02 | 51.20 ± 9.58 |
| Pre-Stressor Impulsivity T-Score | 55.91 ± 9.33 | 56.72 ± 13.36 |
| Post-Stressor Impulsivity T-Score | 56.91 ± 11.84 | 60.67 ± 13.91 |
| Pre-Stressor RT T-Score | 34.94 ± 12.56 | 38.14 ± 9.12 |
| Post-Stressor RT T-Score | 41.31 ± 12.67 | 37.69 ± 8.75 |
| Pre-Stressor RT SE T-Score | 37.69 ± 8.75 | 43.29 ± 15.01 |
| Post-Stressor RT SE T-Score | 52.25 ± 19.03 | 51.01 ± 9.57 |
Notes: SD = Standard Deviation; VAS = Visual Analogue Scale (100 cm) for current stress; QSU = Questionnaire of Smoking Urges; MNWS = Minnesota Nicotine Withdrawal Scale; BART = Balloon Analogue Risk Task; RT = Reaction Time; SE = Standard Error
Stress Induction Validity
Pre- to post-stressor comparisons in self-reported levels of stress (VAS), PA and NA (PANAS) all produced results in the expected directions. Participants endorsed a significant quadratic change in VAS scores (F(1, 25)= 105.57, p< .00001, partial η2= .81) through the three assessed timepoints. Bonferroni-corrected post hoc comparisons indicated a significant increase in stress VAS scores from pre- (4.7±5.68) to post-stressor (35.4±17.68; p< .00001), followed by a significant decrease from post-stressor to after the second relaxing script (7.0±7.26; p< .00001). Significant changes were found for both PA and NA, with significant decreases in PA (pre-stressor: 22.9±8.04; post-stressor: 17.4±6.11; t(25)= 4.55, p= .0001) and significant increases in NA (pre-stressor: 12.2±3.21; post-stressor: 21.5±6.61; t(25)= -8.21, p < .00001).
Effect of Abstinence Status on Time to Relapse
Randomized abstinence status at the stress induction did not predict time to smoking relapse or change in self-reported stress on the VAS, and abstinence status and VAS score did not significantly interact to predict number of follow-up visits (all ps > .10). The analyses examining the effect of abstinence status on number of abstinent follow-up visits are illustrated in Figure 1. As abstinence status was not related to time to relapse, it was not controlled for in further analyses.
Figure 1. Time to Cigarette Smoking Lapse by Abstinence Status at the Stress Induction Session.
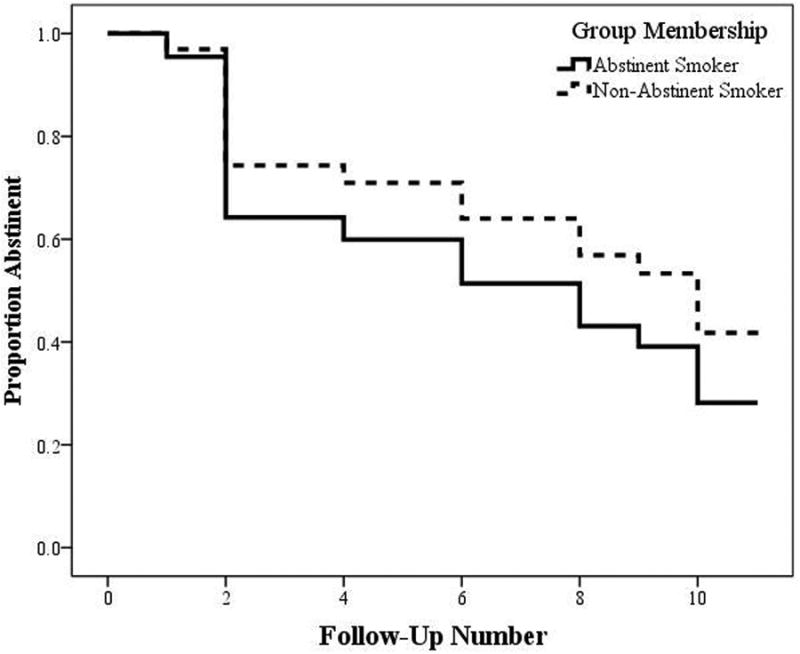
Abstinent smokers (6 females, 8 males) are captured in the solid line, while non-abstinent smokers (7 females, 5 males) are captured in the dashed line. Values on the y-axis are the proportion, from 1.0 (100%) to 0.0 (0%) of smokers who remained abstinent at the visit captured on the x-axis, from follow-up 1 to 11.
Effect of Nicotine Withdrawal and Craving on Time to Relapse
After controlling for pre-stressor levels, neither post-stressor nicotine withdrawal (B= -.03, SE= .047, p= .50, HR= 0.97, 95%CI= 0.89-1.06) nor craving significantly predicted time to smoking relapse (B= -.01, SE= .024, p= .75, HR= 0.99, 95%CI= 0.95-1.04). Similarly, no significant interactions were found between sex and nicotine withdrawal (B= .02, SE= .025, p= .40, HR= 1.02, 95%CI= 0.97-1.07) or between sex and nicotine craving (B= .01, SE= .010, p= .23, HR= 1.01, 95%CI= 0.99-1.03).
Effect of Risk Taking on Time to Relapse
After controlling for pre-stressor performance, post-stressor number of BART AP had a trend-level association with time to relapse (B= .05, SE= .024, p= .057, HR= 1.05, 95%CI= 1.00-1.10). For every extra AP on the BART, the likelihood of relapse increased by 5%. Further analysis of potential sex differences revealed a significant interaction between post-stressor BART AP score and sex (B= .02, SE= .008, p= .026, HR= 1.02, 95%CI= 1.00-1.04), after controlling for pre-stressor performance. Post hoc analyses, run separately by sex, clarified this by finding evidence of a significant difference time to relapse associated with BART AP performance in females (B= .16, SE= .072, p= .026, HR= 1.17, 95%CI= 1.02-1.35) but not males (B= .01, SE= .034, p= .83, HR= 1.01, 95%CI= 0.94-1.08). Thus, every extra point on the BART AP increased the likelihood of relapse in females by 17%. Figure 2 captures the sex by BART AP interaction on number of follow-up visits completed, using a median split in stress-related change in BART AP scores. Females below the median completed 10.63 visits (± 2.20), while females above the median completed 8.40 visits (± 3.85). Males evidenced a smaller difference by median split, with those below the median completing 5.80 visits (± 4.50), while females above the median completing 4.75 visits (± 4.93).
Figure 2. Sex Differences in Time to Cigarette Smoking Lapse by Stress-related Change in Risk Taking (BART Average Adjusted Pumps [AP]).
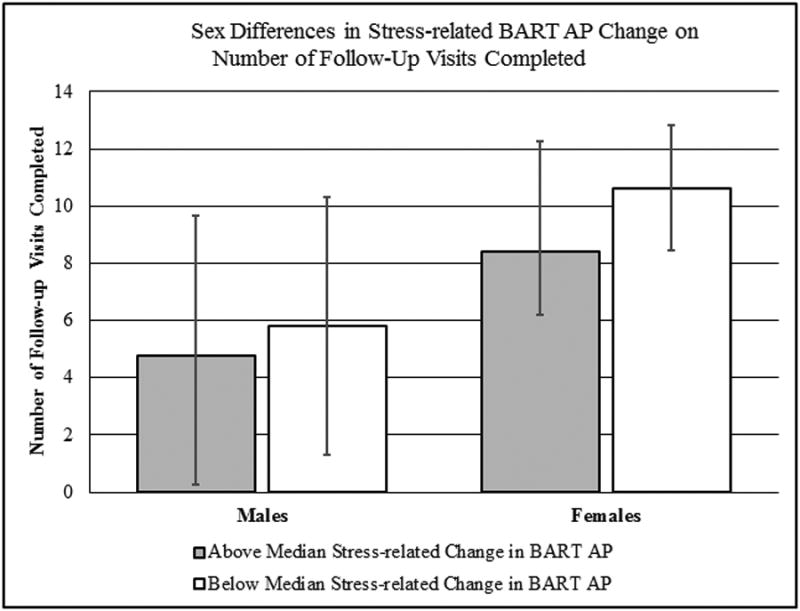
Males are on the right side of the figure, while females are on the left, with 13 participants of each sex. The grayed column captures those with a greater than median stress-related change in risk taking (8 males, 5 females) and the white column captures those with below median stress-related changes (5 males, 8 females).
Effect of Attention and Impulsive Responding on Time to Relapse
Post-stressor inattentive errors on the CPT-II were also a trend-level predictor of time to relapse after controlling for pre-stressor performance (B= .02, SE= .012, p= .074, HR= 1.02, 95%CI= 0.99-1.05), with a 2% increase in the likelihood of relapse for each T-score point increase in inattentive errors. Neither change in impulsive errors on the CPT-II (B= .05, SE= .037, p= .17, HR= 1.05, 95%CI= 0.97-1.13) nor change in RT (B= .02, SE= .029, p= .40, HR= 1.02, 95%CI= 0.97-1.08) predicted time to relapse. Finally, post-stressor change in RT standard error (i.e., RT variability) significantly predicted time to relapse (B= .04, SE= .018, p= .019, HR= 1.04, 95%CI= 1.01-1.08). Each T-score point increase in RT variability corresponded to a 4% increase in relapse likelihood. A median split by change in RT variability indicated that those with greater increases in RT variability completed fewer abstinent follow-up visits (mean: 5.8±4.02) than those with smaller increases or decreases in RT variability (mean: 8.6±4.35). This split is illustrated in Figure 3.
Figure 3. Time to Cigarette Smoking Lapse by Stress-related Change in Reaction Time Variability (CPT-II).
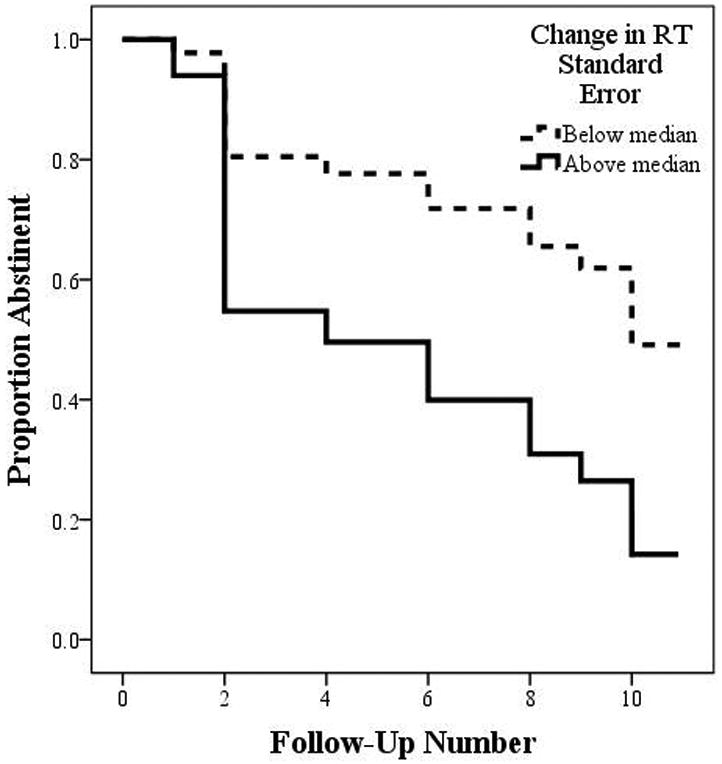
Those with a greater than median change in reaction time (RT) variability (6 females, 7 males) are captured in the solid line, while those with a smaller than median change in RT variability (7 females, 6 males) are captured in the dashed line. Values on the y-axis are the proportion, from 1.0 (100%) to 0.0 (0%) of smokers who remained abstinent at the visit captured on the x-axis, from follow-up 1 to 11.
Only one of the examined attentional variables evidenced potential sex differences in their effects on time to relapse, and this was the interaction between sex and reaction time variability (B= .02, SE= .006, p= .009, HR= 1.02, 95%CI= 1.00-1.03). Analyses separately by sex found no significant interactions in either females or males, but females evidenced trend-level relationships (B= .13, SE= .073, p=.069, HR= 1.14, 95%CI= .99-1.32), while the relationship was fully nonsignificant in males (B= .01, SE= .026, p=.69, HR= 1.01, 95%CI= .96-1.06). A median split by change in RT variability indicated that females had a larger change in visits attended from those below (mean: 10.86±2.27) to those above the mean (7.80±3.35) than did males (below median: 6.00±4.90; above median: 4.43±4.08). Nonetheless, this finding of a trend-level relationship between RT variability and outcome in females should be interpreted cautiously and examined in future research. The median split findings are captured in Figure 4.
Figure 4. Sex Differences in Time to Cigarette Smoking Lapse by Stress-related Change in Reaction Time (RT) Variability (CPT-II).
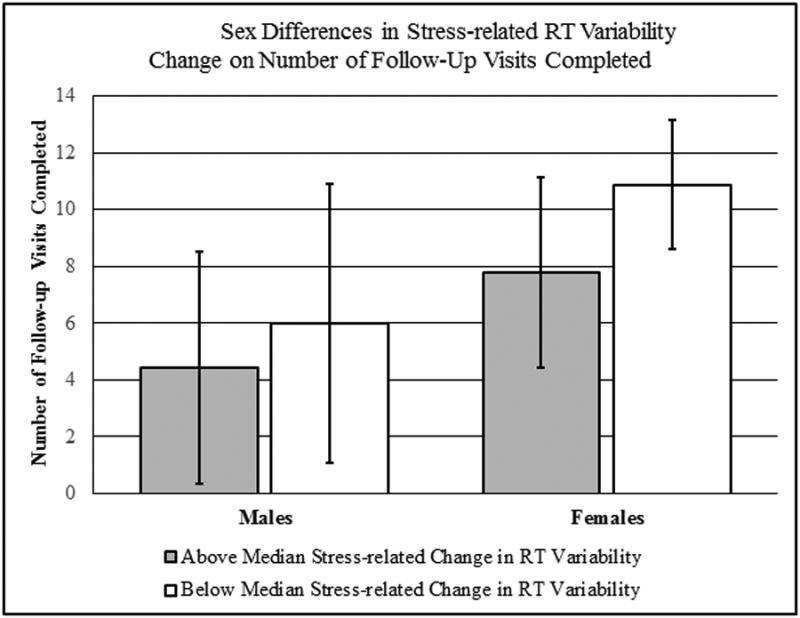
Males are on the right side of the figure, while females are on the left, with 13 participants of each sex. The grayed column captures those with a greater than median stress-related change in RT variability (7 males, 6 females) and the white column captures those with below median stress-related changes (6 males, 7 females).
Conclusions
This study suggests that stress-related increases attention-related reaction time variability predicted more rapid smoking relapse in a CM-based relapse analogue paradigm. Specifically, young adult smokers with greater variability in RT in a sustained attention task were abstinent for a shorter time (5.7 and 5.8 visits, respectively) than those with smaller increases or decreases in inattentive errors or RT variability (9.0 and 8.6 visits, respectively). Furthermore, this work found two significant sex differences, with one in the effect of stress-related risk taking on time to relapse, and the other in the effect of RT variability on time to relapse. For both, females seemed more impacted by larger stress-related changes in either risk taking or in RT variability than did males.
The trend-level risk taking findings are broadly in line with a previous result in adolescents attempting smoking cessation (Schepis et al., 2011); that said, the significant sex difference finding was unexpected. It is generally accepted that males are greater risk takers than females (e.g., Dwyer, Gilkeson, & List, 2002; Powell & Ansic, 1997; Turner & McClure, 2003), though meta-analytic evidence indicates that the relationship is complex and depends on the age cohort and type of risk examined (Byrnes, Miller, & Schafer, 1999). Perhaps, given their already elevated levels of risk engagement, changes in risk taking are a less salient indicator of the effects of stress in males than in females. Put somewhat differently, perhaps male risk taking is closer to a ceiling level than is female risk taking, with any changes producing less effects than in females. Regardless, and given that smoking is an inherently risky behavior, with small (and debatable) short-term benefits that are outweighed by the longer-term health consequences, it is logical that smoking abstinence would be most difficult in those younger smokers who notably increase risk taking following stress exposure.
The attentional results for RT variability was in line with our hypotheses, as individuals with greater stress-related increases in RT variability on the CPT-II had shorter times to relapse. Greater stress-related attentional impairment could simply be a proxy for greater impact of stress exposure, requiring more urgent and immediate coping. Conversely, attentional impairment may affect relapse processes more directly. One hypothesis is that attentional impairment makes post-stressor relapse more likely by affecting the ability of the individual to hold the cessation goal, in his or her attention. Instead, the more automatic response of cigarette use may become more salient and require less attentional resources than cessation efforts, prompting smoking (Acheson & de Wit, 2008). Disruptions in attention by stress may also increase attentional bias towards nicotine cues that smokers experience during cessation attempts (Waters et al., 2013), or such attentional disruptions may be sufficiently aversive to promote relapse to ameliorate their effects (Acheson & de Wit, 2008).
As with the sex differences in RT, the sex difference in the effects of stress-related change in RT variability on time to relapse was unexpected. Research on sex differences in attentional processes, including those of RT variability, have found conflicting and complex results (Greenberg & Waldmant, 1993; Hultsch, MacDonald, & Dixon, 2002; Lin, Hsiao, & Chen, 1999; Naglieri & Rojahn, 2001). While speculative, perhaps stress-related attentional changes disrupt cognitive processes related to smoking cessation (e.g., ability to focus away from smoking cues, ability to retain abstinence goals in attention) in females more than in males. Alternatively, perhaps the sex difference findings for RT variability and risk taking simply signal a greater effect of stress in females, with no relationships between relapse and RT variability or risk taking. As noted in the results, though, the result for potential sex differences in RT variability only found a trend-level relationship in females, with no relationship in males. Given the small sample sizes involved and the pilot nature of this research, these results should be replicated in future research with larger sample sizes. Such work is needed to either confirm or disconfirm this particular sex difference and clarify the potential mechanisms underlying it.
It is notable that stress-related changes in impulsive responding, nicotine withdrawal and nicotine craving did not affect time to relapse in this trial. The lack of influence for impulsive responding is in line with our previous work in adolescents attempting cessation (Schepis et al., 2011); nonetheless, it is somewhat surprising that stress-related changes in impulsive responding do not affect relapse risk, given the importance of impulsivity in many other smoking processes. Given the theorized importance of nicotine craving (Berlin, Singleton, & Heishman, 2013) and withdrawal (Paolini & De Biasi, 2011) for relapse to smoking, it was also somewhat surprising that stress-related change in each was unrelated to time to smoking relapse. It is crucial to note, however, that the lack of findings for impulsivity, nicotine withdrawal and nicotine craving involve their interaction with stress. All three constructs could operate in other, stress-independent ways, to increase relapse risk in young adult smokers.
Limitations and Strengths
Five limitations of this work should be acknowledged. First, this was a pilot study with a small sample size, and these results should be confirmed and extended in future research. Second, the results are only generalizable to similar samples in terms of sociodemographic and tobacco use characteristics. Despite efforts to increase sample diversity, the majority of participants were Caucasian undergraduate students who were mildly dependent on nicotine (per mean FTND score), despite smoking nearly 10 cigarettes per day on average. Third, other authors (Perkins, Stitzer, & Lerman, 2006) have called for investigations of smoking cessation mechanisms to employ treatment-seeking smokers to improve clinical validity. While treatment-seeking status was not formally assessed, it seemed anecdotally that most participants were not strongly motivated to cease smoking permanently. Despite concerns about motivation to cease smoking in this population, the work of Perkins et al. (2006) and others (Gilbert, Crauthers, Mooney, McClernon, & Jensen, 1999; Juliano et al., 2006) indicates that monetary reinforcement can temporarily raise abstinence motivation. Fourth, as participants took up to 20 minutes to complete all post-stressor assessments, it is likely that the effects of the stressor had waned somewhat by the end of the final task (CPT-II). Sinha and colleagues (2003), though, found stress-induced elevations over baseline in heart rate, anxiety, systolic and diastolic blood pressure and cocaine craving continued past 30 minutes post-stressor in cocaine using adults exposed to the same stress induction paradigm.
Finally, the CO cutoff for follow-up abstinence of below 8 ppm may have been too liberal, as more recent commentators (e.g., Cropsey et al., 2014) have called for a cutoff of 4 PPM or less. As noted in the results, though, the CO value at one standard deviation above the mean (3.8 PPM) in those self-reporting abstinence was below this cutoff, and no individuals had two consecutive visits endorsing abstinence with levels above 4 PPM at both visits. Our classification may have missed individuals who lapsed to one cigarette, though, making our CO cutoff a potential limitation. This work was strengthened, however, by the use of a validated stress imagery paradigm, the adapted use of a validated relapse paradigm, and the use of validated assessments of tobacco use, risk taking, attentional variables and impulsivity.
Clinical Implications
Clinically speaking, these results indicate that it is not solely stress exposure (Sinha, 2001) but aspects of the individual's reaction to stress that increase smoking relapse risk. Clinicians working with young adults attempting cessation may need to tailor their interventions in those individuals who endorse increased stress-related risk taking and/or attentional disruption to increase adaptive coping and lessen stress exposure. Replacement of risk taking behavior with cognitive and/or behavioral strategies (Villanti et al., 2010), or with mindfulness approaches, may be fruitful in those who are most likely to take risks following stress exposure. Mindfulness-based approaches or acceptance and commitment therapy-based approaches may be particularly fruitful. Both approaches combat avoidance of unpleasant cognitions or negative affect, which for smokers attempting cessation can include relapse, by promoting acceptance or detached awareness, and both are effective behavior therapies for smoking cessation (Bowen & Marlatt, 2009; Brewer et al., 2011; Gifford et al., 2004; Gifford et al., 2011). These techniques may also allow individuals who tend to relapse under stress to avoid an increased propensity towards risk taking behaviors and/or maladaptive focus on nicotine withdrawal and craving symptoms by promoting purposeful and detached awareness of urges and better self-control (Bowlin & Baer, 2012; Friese, Messner, & Schaffner, 2012; Hayes, Strosahl, & Wilson, 2011). In addition, techniques to reduce stress, including use of relaxation techniques (Wynd, 1992) and efforts to increase social support (Westmaas, Bontemps-Jones, & Bauer, 2010), may be warranted, given both the findings of this study and the significant literature on the negative effects of stress on prolonged cessation attempts (Sinha, 2007).
Conclusion
In all, this work suggests that young adult smokers who experience attentional impairment have shorter times to smoking relapse. Furthermore, sex differences were found in the relationships between time to relapse and reaction time variability or risk taking, with females more significantly affected at higher levels of pre- to post-stressor change. Combined with the trend-level finding of effects of risk taking on time to relapse across sexes, the risk taking findings largely concur with previous research in adolescents attempting smoking cessation (Schepis et al., 2011). While further research is needed to replicate and expand upon these results, this work provides initial evidence for stress-related increases in risk taking and disruptions in sustained attention in young adult smokers who are more prone to early smoking relapse.
References
- Acheson A, de Wit H. Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Experimental and Clinical Psychopharmacology. 2008;16(2):113–123. doi: 10.1037/1064-1297.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LR, Mellor JM. Predicting health behaviors with an experimental measure of risk preference. Journal of Health Economics. 2008;27(5):1260–1274. doi: 10.1016/j.jhealeco.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: The winding road from the late teens through the twenties. Oxford University Press; 2014. [Google Scholar]
- Berlin I, Singleton EG, Heishman SJ. Predicting smoking relapse with a multidimensional versus a single-item tobacco craving measure. Drug and Alcohol Dependence. 2013;132(3):513–520. doi: 10.1016/j.drugalcdep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Bowen S, Marlatt A. Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychology of Addictive Behaviors. 2009;23(4):666–671. doi: 10.1037/a0017127. [DOI] [PubMed] [Google Scholar]
- Bowlin SL, Baer RA. Relationships between mindfulness, self-control, and psychological functioning. Personality and Individual Differences. 2012;52(3):411–415. [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al. Rounsaville BJ. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug and Alcohol Dependence. 2011;119(1-2):72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Brook DW, Zhang C, Cohen P. Tobacco use and health in young adulthood. Journal of Genetic Psychology. 2004;165(3):310–323. doi: 10.3200/GNTP.165.3.310-323. [DOI] [PubMed] [Google Scholar]
- Businelle MS, Kendzor DE, Rash CJ, Patterson SM, Coffey SF, Copeland AL. Heavy smokers perform more poorly than nonsmokers on a simulated task of gambling. Substance Use & Misuse. 2009;44(7):905–914. doi: 10.1080/10826080802484173. [DOI] [PubMed] [Google Scholar]
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: A meta-analysis. Psychological Bulletin. 1999;125(3):367. doi: 10.1037/0033-2909.125.3.367. [DOI] [Google Scholar]
- Compas BE, Wagner BM, Slavin LA, Vannatta K. A prospective study of life events, social support, and psychological symptomatology during the transition from high school to college. American Journal of Community Psychology. 1986;14(3):241–257. doi: 10.1007/BF00911173. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners' Continuous PerformanceTest (CPT-II) computer program for Windows: Technical guide and software manual. North Tonawanda, NY: Multi-Health Systems, Inc; 2000. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43(Pt 3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine and Tobacco Research. 2014;16(10):1348–1355. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119(2):171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D, Pergadia M, Richmond M. Impulsivity and smoking relapse. Nicotine and Tobacco Research. 2004;6(4):641–647. doi: 10.1080/14622200410001727939. [DOI] [PubMed] [Google Scholar]
- Dwyer PD, Gilkeson JH, List JA. Gender differences in revealed risk taking: evidence from mutual fund investors. Economics Letters. 2002;76(2):151–158. doi: 10.1016/S0165-1765(02)00045-9. [DOI] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25(3):313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Friese M, Messner C, Schaffner Y. Mindfulness meditation counteracts self-control depletion. Consciousness and Cognition. 2012;21(2):1016–1022. doi: 10.1016/j.concog.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Galvan A, Schonberg T, Mumford J, Kohno M, Poldrack RA, London ED. Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41(4):625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MP, Rasmussen-Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behavior Therapy. 2004;35(4):689–705. doi: 10.1016/S0005-7894(04)80015-7. [DOI] [PubMed] [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, Palm KM. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy. 2011;42(4):700–715. doi: 10.1016/j.beth.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA. Effects of monetary contingencies on smoking relapse: influences of trait depression, personality, and habitual nicotine intake. Experimental and Clinical Psychopharmacology. 1999;7(2):174–181. doi: 10.1037/1064-1297.7.2.174. [DOI] [PubMed] [Google Scholar]
- Greenberg LM, Waldmant ID. Developmental normative data on The test of variables of attention (TOVA™) Journal of Child Psychology and Psychiatry. 1993;34(6):1019–1030. doi: 10.1111/j.1469-7610.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Harrison EL, Coppola S, McKee SA. Nicotine deprivation and trait impulsivity affect smokers' performance on cognitive tasks of inhibition and attention. Experimental and Clinical Psychopharmacology. 2009;17(2):91–98. doi: 10.1037/a0015657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. Guilford Press; 2011. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: a review of effects on human performance. Experimental and Clinical Psychopharmacology. 1994;2(4):345. [Google Scholar]
- Henningfield JE, Shiffman S, Ferguson SG, Gritz ER. Tobacco dependence and withdrawal: science base, challenges and opportunities for pharmacotherapy. Pharmacology & Therapeutics. 2009;123(1):1–16. doi: 10.1016/j.pharmthera.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57(2):101–115. doi: 10.1093/geronb/57.2.P101. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2011: Volume II, College students and adults ages 19-50. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology. 2006;115(1):166–173. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83–98. doi: 10.1016/S0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Leeman RF, O'Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology (Berl) 2010;212(1):25–32. doi: 10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2003;11(1):26–33. doi: 10.1037/1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lin CC, Hsiao CK, Chen WJ. Development of sustained attention assessed using the continuous performance test among children 6–15 years of age. Journal of Abnormal Child Psychology. 1999;27(5):403–412. doi: 10.1023/A:1021932119311. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Prokhorov AV. The effects of providing lung age and respiratory symptoms feedback on community college smokers' perceived smoking-related health risks, worries and desire to quit. Addictive Behaviors. 2007;32(3):516–532. doi: 10.1016/j.addbeh.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Neuropsychopharmacology. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DW, Redman C, Rose JE. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology (Berl) 2008;197(1):95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- Micro Direct. Breath CO The MicroCO. 2010 Retrieved from http://www.breathcotest.com/microCO.asp.
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146(4):455–464. doi: 10.1007/PL00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behavioral and Cognitive Neuroscience Reviews. 2004;3(4):261–275. doi: 10.1177/1534582305276838. [DOI] [PubMed] [Google Scholar]
- Naglieri JA, Rojahn J. Gender differences in planning, attention, simultaneous, and successive (PASS) cognitive processes and achievement. Journal of Educational Psychology. 2001;93(2):430. [Google Scholar]
- Paolini M, De Biasi M. Mechanistic insights into nicotine withdrawal. Biochemical Pharmacology. 2011;82(8):996–1007. doi: 10.1016/j.bcp.2011.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Kaye FJ. Daily uplifts, hassles, stresses and cognitive failures: in cigarette smokers, abstaining smokers, and non-smokers. Behavioral Pharmacology. 1999;10(6-7):639–646. doi: 10.1097/00008877-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine and Tobacco Research. 2008;10(7):1245–1251. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl) 2006;184(3-4):628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Delgado MR. Acute stress modulates risk taking in financial decision making. Psychological Science. 2009;20(3):278–283. doi: 10.1111/j.1467-9280.2009.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell M, Ansic D. Gender differences in risk behaviour in financial decision-making: An experimental analysis. Journal of Economic Psychology. 1997;18(6):605–628. doi: 10.1016/S0167-4870(97)00026-3. [DOI] [Google Scholar]
- Reynolds B, Patak M, Shroff P, Penfold RB, Melanko S, Duhig AM. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2007;15(3):264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- Ryb GE, Dischinger P, Kufera J, Soderstrom C. Smoking is a marker of risky behaviors independent of substance abuse in injured drivers. Traffic Injury Prevention. 2007;8(3):248–252. doi: 10.1080/15389580701272353. [DOI] [PubMed] [Google Scholar]
- Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled-and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99(11):1462–1469. doi: 10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- Schepis TS, McFetridge A, Chaplin TM, Sinha R, Krishnan-Sarin S. A pilot examination of stress-related changes in impulsivity and risk taking as related to smoking status and cessation outcome in adolescents. Nicotine and Tobacco Research. 2011;13(7):611–615. doi: 10.1093/ntr/ntr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacology, Biochemistry and Behavior. 2007;87(3):360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, et al. Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111(4):531–545. doi: 10.1037/0021-843X.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) 2005;178(2-3):211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berlin) 2001;158(4):343–359. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current Psychiatry Reports. 2007;9(5):388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142(4):343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170(1):62–72. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Solberg LI, Boyle RG, McCarty M, Asche SE, Thoele MJ. Young adult smokers: are they different? American Journal of Managed Care. 2007;13(11):626–632. [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psycholology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Swann AC. Neuroreceptor mechanisms of aggression and its treatment. Journal of Clinical Psychiatry. 2003;64 Suppl 4:26–35. [PubMed] [Google Scholar]
- Tevyaw TO, Colby SM, Tidey JW, Kahler CW, Rohsenow DJ, Barnett NP, et al. Monti PM. Contingency management and motivational enhancement: a randomized clinical trial for college student smokers. Nicotine and Tobacco Research. 2009;11(6):739–749. doi: 10.1093/ntr/ntp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, Katulak NA, McKee SA. Investigating the factor structure of the Questionnaire on Smoking Urges-Brief (QSU-Brief) Addictive Behaviors. 2006;31(7):1231–1239. doi: 10.1016/j.addbeh.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, O'Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychology of Addictive Behaviors. 2007;21(2):216–225. doi: 10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonissaar M, Herm L, Eller M, Koiv K, Rinken A, Harro J. Rats with high or low sociability are differently affected by chronic variable stress. Neuroscience. 2008;152(4):867–876. doi: 10.1016/j.neuroscience.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Turner C, McClure R. Age and gender differences in risk-taking behaviour as an explanation for high incidence of motor vehicle crashes as a driver in young males. Injury Control and Safety Promotion. 2003;10(3):123–130. doi: 10.1076/icsp.10.3.123.14560. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- VanderVeen JW, Cohen LM, Cukrowicz KC, Trotter DR. The role of impulsivity on smoking maintenance. Nicotine and Tobacco Research. 2008;10(8):1397–1404. doi: 10.1080/14622200802239330. [DOI] [PubMed] [Google Scholar]
- Villanti AC, McKay HS, Abrams DB, Holtgrave DR, Bowie JV. Smoking-cessation interventions for U.S. young adults: a systematic review. American Journal of Preventive Medicine. 2010;39(6):564–574. doi: 10.1016/j.amepre.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Waters AJ, E HS, Wetter DW, Cinciripini PM, Robinson JD, Li Y. Cognition and Craving During Smoking Cessation: An Ecological Momentary Assessment Study. Nicotine and Tobacco Research. 2013;16(Suppl. 2):S111–S118. doi: 10.1093/ntr/ntt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Rigotti NA, Gledhill-Hoyt J, Lee H. Increased levels of cigarette use among college students: a cause for national concern. JAMA. 1998;280(19):1673–1678. doi: 10.1001/jama.280.19.1673. [DOI] [PubMed] [Google Scholar]
- Westmaas JL, Bontemps-Jones J, Bauer JE. Social support in smoking cessation: reconciling theory and evidence. Nicotine and Tobacco Research. 2010;12(7):695–707. doi: 10.1093/ntr/ntq077. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67(4):555. doi: 10.1037/0022-006X.67.4.555. [DOI] [PubMed] [Google Scholar]
- White TL, Lejuez CW, de Wit H. Test-retest characteristics of the Balloon Analogue Risk Task (BART) Experimental and Clinical Psychopharmacology. 2008;16(6):565–570. doi: 10.1037/a0014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynd CA. Relaxation imagery used for stress reduction in the prevention of smoking relapse. Journal of Advanced Nursing. 1992;17(3):294–302. doi: 10.1111/j.1365-2648.1992.tb01907.x. [DOI] [PubMed] [Google Scholar]


