Abstract
The incidence of cortically induced blindness (CB) is increasing as our population ages. The major cause of CB is stroke affecting the primary visual cortex. While the impact of this form of vision loss is devastating to quality of life, the development of principled, effective rehabilitation strategies for this condition lags far behind those used to treat motor stroke victims. Here we summarize recent developments in the still emerging field of visual restitution therapy, and compare the relative effectiveness of different approaches. We also draw insights into the properties of recovered vision, its limitations and likely neural substrates. We hope that these insights will guide future research and bring us closer to the goal of providing much-needed rehabilitation solutions for this patient population.
1. Introduction
Cortically-induced blindness (CB) is a form of vision loss caused by damage to the primary visual cortex (area V1; Holmes 1918; Lawton Smith 1962; Leopold 2012b; Teuber and others 1960; Trobe and others 1973). Although extrastriate cortex is also often injured in CB, it is damage to V1 or its immediate afferents that appears to induce blindness (Holmes 1918; Lawton Smith 1962; Leopold 2012b; Teuber and others 1960; Trobe and others 1973). Stroke involving the posterior or middle cerebral arteries accounts for the great majority of cases, though traumatic brain injury, tumors or their resection, and even congenital conditions may result in similar presentation (Fujino and others 1986; Lawton Smith 1962; Reitsma and others 2013b; Trobe and others 1973; Zhang and others 2006a; Zhang and others 2006b). The incidence of CB in the general population is remarkably high (Geddes and others 1996; Gilhotra and others 2002; Pollock and others 2011b). For instance, each year in the USA, there are about 1 million new cases of stroke, with 27–57% of them exhibiting damage to V1 or its afferents (Pollock and others 2011b). Some spontaneous improvements in vision may occur within the first few months after brain damage, but significant residual visual defects usually remain (Zhang and others 2006b). These defects substantially decrease the capacity to live independently and thus, quality of life (Dombovy and others 1986; Jones and Shinton 2006; Jongbloed 1986). Many CB patients lose the ability to drive (de Jong and Warmink 2003; Papageorgiou and others 2007). However, others retain their driver’s licenses and drive routinely (Peli and Pely 2002), presenting significant danger to themselves and those around them (Bowers and others 2014; Bowers and others 2009; Bowers and others 2010). And yet, despite the prevalence of CB and its debilitating impact on everyday life, there are currently no widely-accepted, validated clinical therapies available for the restoration of these deficits (Pollock and others 2011b).
Before reviewing the latest research on rehabilitation of CB, it is worth noting that the visual defects present in CB have several features that distinguish them from other forms of blindness. First, unilateral V1 damage (occurring in only one brain hemisphere; Figure 1A) causes loss of vision in the contralateral hemifield of both eyes – i.e. the visual defect is homonymous (Figure 1). Second, depending on the extent of the V1 lesion, the loss of vision can vary greatly in size - anywhere from a small scotoma about the size of the blind spot, to a quadrant (quadrantanopia), to a full hemifield of vision (hemianopia; Figure 1C). In most cases however, central vision, including the foveal representation, remains intact (Leff 2004), though this is not always apparent on coarse automated perimetry. This is because the occipital pole, where the fovea is generally represented, receives part of its blood supply from the middle cerebral artery as well as from branches of the posterior cerebral artery (Horton and Hoyt 1991; Leff 2004; Marinkovic and others 1987). Because strokes rarely affect all branches of both arteries, stroke-induced destruction of all of V1 is extremely rare. As such, CB patients, including many of those with bilateral V1 damage, generally maintain the ability to fixate and identify small objects centrally. This, as detailed below, is of critical importance for successful rehabilitation.
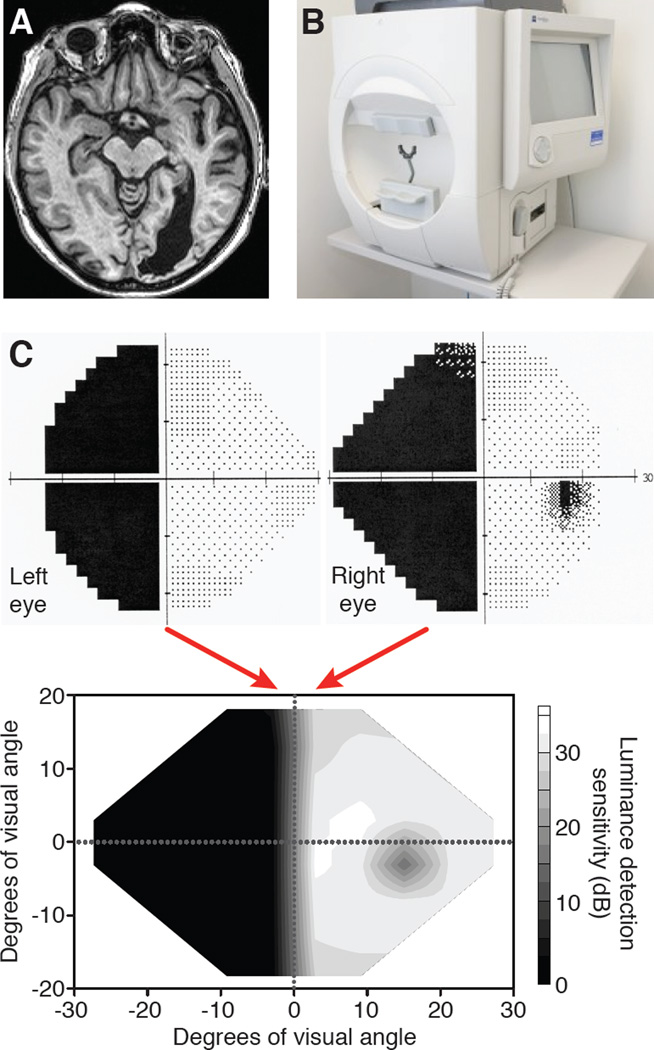
Figure 1. Assessing the impact of primary visual cortex damage in humans.
A. Horizontal magnetic resonance image of the head of a patient >6 months after a stroke affecting the occipital cortex of the right hemisphere. The eyes are clearly visible at the top of the image. Note the markedly enlarged ventricle in the posterior half of the right hemisphere, a common consequence of degenerated cortical brain matter. B. Photograph of a Humphrey visual field perimeter machine showing the “bowl” in which small spots of light are presented in a regular array. The patient’s head is fitted into a chin-forehead rest frame at the entrance of the bowl and vision is corrected monocularly using trial lenses inserted into the black holder at the center of the bowl aperture. C. A portion of the 24-2 Humphrey visual field printout generated for each eye of the patient whose brain lesion is shown in A. This patient is considered to have a large, left, homonymous hemianopia. Note also the small blind spot, visible only in the right eye of this patient. The luminance detection sensitivity measured monocularly by Humphrey perimetry can then be combined to generate a singular, interpolated map of luminance detection sensitivity in Decibels (dB) across the central visual field (bottom plot).
Third, another unique property of CB is the presence of residual, though largely unconscious, visual processing abilities in the blind field. Termed blindsight by Weiskrantz and colleagues in 1974 (Sanders and others 1974; Weiskrantz and others 1974), this phenomenon includes the ability to perform above chance when forced to detect or discriminate stimuli inside blind fields (for reviews, see Cowey 2010; Stoerig 2006; Weiskrantz 2009). Interestingly, in contrast with normal vision (Campbell and Robson 1968; Kelly 1975; Kelly 1979; Roufs 1972), blindsight can only be elicited by large, coarse stimuli moving or flickering at intermediate temporal frequencies (Barbur and others 1994; Morland and others 1999; Sahraie and others 2008; Sahraie and others 2003; Weiskrantz and others 1991). In spite of this restricted visual “range”, some CB individuals with blindsight can also discriminate and detect color (Blythe and others 1987; Pasik and Pasik 1982; Stoerig and Cowey 1995; Weiskrantz and others 1991; Zeki and ffytche 1998), luminance (Blythe and others 1987), affective/emotional stimuli (Morris and others 2001; Tamietto and others 2012), and form (Barbur and others 1993; Goebel and others 2001; Stoerig and Cowey 1997). As not all CB patients show these capacities, blindsight may not be a unitary phenomenon, but rather, one that is diverse in its properties due to heterogeneity in the underlying neurological damage across individuals.
The perceptual consequences of V1 damage have been studied extensively in both humans and non-human primates (for reviews, see Cowey 2010; Stoerig 2006; Weiskrantz 2009). This work also plays a pivotal role in the consciousness literature where it helped define neural processes that underlie visual awareness (Brogaard 2015; Foley 2015; Leopold 2012b; Overgaard and Grünbaum 2011; Overgaard and Mogensen 2015). For our purposes, blindsight research is notable because it provides a detailed documentation of spared visual processing abilities in individuals with CB. This, in turn, has helped identify the anatomical and functional substrates suitable for the development of visual rehabilitation strategies in CB.
2. Can a V1-damaged visual system be retrained to see?
The clinical perspective
Whether visual deficits can be reversed in CB patients is one of the most controversial topics in rehabilitative medicine. The general mindset in the field is probably best summarized by findings of the Cochrane Review on Interventions for Visual Field Defects in Patients with Stroke (Pollock and others 2011a). This review was conducted by the Cochrane Collaboration, an independent, not-for-profit, non-governmental organization, whose goal is to organize and evaluate medical research information according to the principles of evidence-based medicine (see http://community.cochrane.org/about-us/our-principles). The group conducts and publishes highly respected, systematic reviews of randomized controlled trials for health-care interventions, and it has an official relationship with the World Health Organization (WHO), allowing it to provide input into WHO resolutions.
With respect to stroke-induced CB, the 2011 Cochrane Review (Pollock and others 2011b) examined three classes of interventions: (1) restitution therapies, which aim to recover visual field deficits and are the subject of this review, (2) compensation therapies, which use saccadic eye movement strategies to capture visual information that would normally fall onto blind portions of the visual field (e.g. Kerkhoff 1999; Kerkhoff 2000; Spitzyna and others 2007; Weinberg and others 1977), and (3) substitution therapies, which use prisms or other optical devices to present/overlay stimuli that would normally fall in the blind field, onto intact portions of the visual field (e.g. Peli 2000; Rossi and others 1990; Szlyk and others 2008).
Although a few of the examined studies demonstrated benefits for reading and quality of life (Spitzyna and others 2007; Weinberg and others 1977), the Cochrane Review concluded that randomized, double-masked, controlled clinical trials conducted to date had failed to demonstrate the efficacy of any of the current interventions used in the clinic at improving vision in CB (Pollock and others 2011b; Pollock and others 2012).
The basic science perspective
As mentioned above, the present review deals only with one of the therapeutic approaches examined in the Cochrane Review - restitution therapy. Restitution of vision is fundamentally appealing in CB because it targets reversal of, rather than compensation for, the underlying disability. However, the only restitution approach considered by the 2011 Cochrane Review was the commercially-available, Visual Restitution Therapy (VRT, NovaVision Inc.). None of the other [still experimental] restitution approaches described below were included because at the time of the Review, as none of them had been used in published, randomized, double-blind, placebo-controlled clinical trials in the U.S.
VRT is at its heart, a luminance detection paradigm very similar to common perimetry techniques (for detailed review of VRT work, see Turco and others 2015). Patients detect spots of light on a black screen at multiple locations across the border between the blind and intact visual hemifields (Figure 2A) - an exercise that authors claim could shift the blind field border by about 5 degrees (Kasten and Sabel 1995; Kasten and others 1998). A series of articles by Sabel’s group supported this benefit (for review, see Turco and others 2015). The claims of Sabel and collegues, however, were put into questioned with the publication of two studies conducted by Trauzettel-Klosinski, Reinhard and colleagues (Reinhard and others 2005; Schreiber and others 2006). The first study (Reinhard and others 2005) carefully controlled the impact of eye movements during pre- and post-training tests using scanning laser ophthalmoscopy. Under those conditions, perimetric improvements in the visual field could no longer be demonstrated following VRT. Similar findings emerged from a study using carefully controlled Tuebingen perimetry (Schreiber and others 2006). Both studies concluded that patients likely developed compensatory, saccadic eye movements during VRT, and that these eye movements, rather than restoration of vision in parts of the blind field, accounted for the previously-reported positive results with VRT (Horton 2005). Another problem was that VRT required patients to detect bright stimuli on a black background, which can allow stimulus detection based on light scatter reaching intact regions of the visual field (Bach-Y-Rita 1983; Pelak and others 2007). Finally, NovaVision clinical studies also tested efficacy with an evaluation task identical to the training task (High Resolution Perimetry or HRP), confounding vision restoration with improved performance on just the training task.
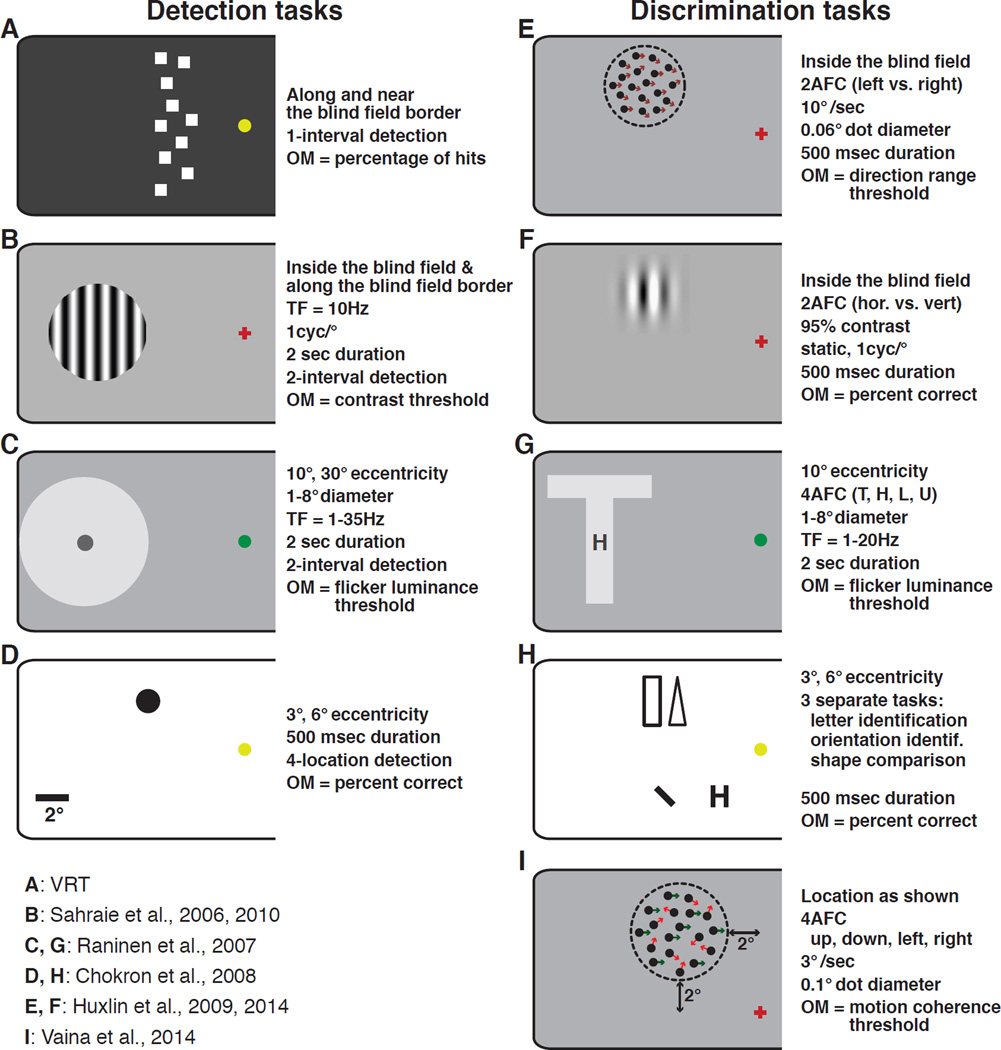
Figure 2. Details of stimuli and tasks employed for visual retraining of CB subjects.
Except for VRT (A), all stimuli are drawn to scale, with the scale bar shown in panel D. Panels C and D show the largest and smallest stimuli used in retraining by Raninen and Chokon and colleagues. Note that during training, only one stimulus was actually shown on each trial. Panel H illustrates three different tasks used by Chokron and colleagues (2008). Each of three tasks was used in separate sessions. Arrows and dashed circles in E and I illustrate dot motion directions and spatial extent of the stimuli. Neither was shown during the actual task. Where specific stimulus locations were indicated, their eccentricity is shown next to each stimulus schematic. Where stimulus locations were variable or not indicated, a descriptor of whether the stimuli were presented inside the blind field or near/along the blind field border is provided next to the appropriate stimulus schematic. OM = outcome measure, AFC = alternative forced choice.
In response to concerns raised about NovaVision’s VRT results, several different laboratories initiated experiments aimed at establishing whether visual recovery could be attained with other stimuli, tasks, and when stringent fixation control was applied during pre- and post-training tests. Success was achieved using an impressively large variety of restitution training approaches that included recognition of static (Chokron and others 2008; Das and others 2014), flickering (Raninen and others 2007; Sahraie and others 2010; Sahraie and others 2006; Trevathan and others 2012) or moving targets (Das and others 2014; Huxlin and others 2009; Vaina and others 2014). Figure 2B–I shows the wide range of stimuli and tasks used in these studies. In another important distinction from VRT, most of these groups presented targets fully in the blind field rather than straddling the border between intact and impaired vision, and a significant proportion (Bergsma and others 2012; Bergsma and van der Wildt 2010; Das and others 2014; Huxlin and others 2009; Sahraie and others 2010; Sahraie and others 2013; Sahraie and others 2006) used infrared eye trackers to enforce fixation during testing. The key principle behind this approach was that for visual restitution therapy to work, one had to force the blind field (not portions of the intact visual field) to process stimuli using spared cortical circuits that functioned abnormally post-lesion.
An interesting example among this set of restitution studies used a training paradigm called Restorative Function Training (RFT), which involved training patients to detect Goldmann perimetry-like stimuli inside the blind field. Over time, such training gradually decreased the size of the blind field, as measured by Goldmann perimetry (Bergsma and others 2012; Bergsma and van der Wildt 2008; Bergsma and van der Wildt 2010), while also improving reading speed in most of the patients, and color/shape perception in just under half the patients tested (Bergsma and others 2012).
In contrast to the use of perimetry-like detection tasks, teams led by A. Sahraie (Sahraie and others 2006) and A. Raninen (Raninen and others 2007) used forced choice training paradigms and localized stimuli to retrain CB patients. In these paradigms, the subjects were forced to choose between two or more response options, indicating which of two intervals contained a target stimulus (Figures 2B,C) or which of 4 letters were presented in a single interval. Among other things, the forced-choice nature of the training tasks helped reduce response biases, which can plague simpler (i.e., perimetry-based) detection paradigms. In addition, the target stimuli flickered, a property that typically induces blindsight (Sahraie and others 2008; Weiskrantz and others 1995). The stimuli used by Sahraie and coleagues were large, vertical, flickering gratings (6 or 10° diameter, 1 cycle per degree, 10Hz flicker; Figure 2B), which decreased in contrast in order to continue to challenge subjects as they improved (Sahraie and others 2010; Sahraie and others 2006). In addition to improvements on the trained tasks, most patients also showed significant improvements on automated perimetry, although the amount of visual field recovered varied widely between individuals. In those who showed no beneficial effects of training, Sahraie and others suggested, based on examination of structural MRIs, that damage affecting both V1 and its immediate sub-cortical inputs, such as the dLGN and/or pulvinar nucleus, may eliminate the ability to regain vision – at least with the perceptual training techniques employed thus far (Sahraie and others 2010; Sahraie and others 2013).
The last major class of visual restitution approaches tried in CB patients involved training on discrimination, identification or comparison tasks. This required subjects not only to detect, but also to make judgments about the nature of stimuli presented in their blind field. Raninen and colleagues (Raninen and others 2007) trained 2 patients to discriminate flickering letters (T, L, H and U; Figure 2G), in addition to training them to detect flickering luminance targets in a separate location of the blind field (Figure 2C). Both subjects improved gradually over time, on both tasks, although no significant changes were observed in the size of their blind field, as defined by Goldmann perimetry. Chokron and others (2008) sequentially trained CB patients on four different tasks: detection of a static shape (Figure 2D), shape comparison (rectangle vs. triangle) between the intact and blind hemifields, orientation discrimination, and letter identification in their blind field (Figure 2H). Performance on all tasks improved significantly in the subjects’ blind fields, though never to levels measured in their intact hemifield of vision. In spite of this, a significant reduction in the size of the blind field was observed upon performing Humphrey automated perimetry (Chokron and others 2008). Huxlin and colleagues reported that re-learning of coarse (left/right) global motion discriminations using dark, random dot stimuli presented on a bright background (to minimize light scatter; Figure 2E), as well as static orientation discrimination of non-flickering Gabors (Figure 2F) could both be achieved in cortically blind fields. In fact, CB subjects were able to relearn to discriminate global motion and to attain normal integration thresholds at trained, blind field locations, while using fixation control with an infrared eye tracker (Cavanaugh and others 2015; Das and others 2014; Huxlin and others 2009). Both training tasks (global motion and static orientation discrimination) also decreased the size of the patients’ blind field, as defined by Humphrey automated perimetry (Huxlin and others 2009). Finally, Vaina and colleagues performed a different form of global motion discrimination training (Figure 2I) in a single hemianopic patient, and also reported significant improvement on the trained task, as well as on Humphrey automated perimetry and on discriminating motion-defined form (Vaina and others 2014).
In summary, multiple studies by different research groups involving a diversity of training techniques indicate that visual training can be used to recover some of the vision lost in CB, even when one controls for fixation and light scatter during testing. Therefore, despite extensive damage to the primary visual cortex and seriously impaired awareness and visual sensitivity, the adult visual system may in fact maintain its capacity for relearning both simple and complex visual discriminations across blind portions of the visual field. Although controlled, multi-center clinical trials with post-VRT training techniques should be performed to establish the clinical efficacy of new vision restoration therapies in CB, several observations can already be made about the properties of the vision recovered. For instance, the diversity of stimuli and tasks that can induce visual improvement challenges the notion that only blindsight “channels” mediate training-induced visual recovery in CB fields. This is most evident in retraining with both complex motion and static stimuli – classes of stimuli that fail to elicit strong blindsight performance on their own (Azzopardi and Cowey 2001; Barbur and others 1994; Sahraie and others 2008; Sahraie and others 2003; Weiskrantz and others 1995). In addition, shrinkage of the blind field was usually observed using visual perimetry, even when perimetry represented a radically different task than that on which the patients were trained. This observation has significant practical implications. For one, transfer of learning – whether to untrained tasks or blind field locations – could significantly decrease the time needed to rehabilitate the large and multi-modal visual field defects exhibited by hemi- and quadrantanopes. Second, researchers can now shift their focus beyond just proving that vision can be recovered in chronic CB, towards defining the properties of recovered vision, its neural substrates and ultimately, its limitations.
3. How normal is recovered vision?
While extensive training with a large variety of stimuli and tasks improves performance to levels that sometimes match those in the intact hemifield of vision (Das and others 2014; Huxlin and others 2009; Raninen and others 2007; Vaina and others 2014), does this mean that the vision recovered in CB fields is completely normal? Perimetric techniques can help measure global changes in the size of the visual deficit from pre- to post-training, but they reveal little about the nature and quality of recovered visual abilities. Below, we attempt to answer this question by describing experimental work that examined transfer of learning to untrained stimuli and tasks in CB fields.
Properties of recovered vision following detection training
Both VRT and RFT claimed to produce similar effects: expansion of the visible field by ∼5%, or reducing the deficit by ∼6° (Bergsma and others 2012; Kasten and others 1999; Pouget and others 2012; Turco and others 2015). Kasten and colleagues were the first to address the nature of color and form transfer following VRT (Kasten and others 2000), finding that both improved, though more modestly than luminance detection (the trained task). By measuring the blind field border separately using luminance, form, and color stimuli, Kasten and colleagues reported ∼3.8° of improvement of the blind field border using luminance detection versus a ∼1.7° shift using form and color perception (Kasten and others 2000). However, placebo-trained controls showed similar results in all conditions but color mapping, making interpretation of these findings difficult. Bergsma and colleagues also reported transfer from RFT detection training to flicker fusion, color, form and reading ability in 3 patients following training (Bergsma and van der Wildt 2008). In a follow-up study, 9/12 subjects showed an improvement in reading speed, while 3/7 showed an improvement in color and shape discrimination, though one of these 3 subjects exhibited poor fixation (Bergsma and others 2012). In short, perimetry-like detection training appears to transfer to untrained stimuli, but the reason why it transfers for some CB patients and not others remains unclear.
Meanwhile, Sahraie and colleagues found that training to detect a 1 cycle/deg, flickering grating improved the patients’ ability to detect gratings at both trained and untrained spatial frequencies, and also at an untrained, blind field location (Sahraie and others 2006). This suggested that recovery might not always be restricted to the locus of training (see also Figure 4A in Huxlin et al., 2009 and Figure 3). This is a potentially important benefit for these patients, whose blind fields can be very large. Finally, another important contribution of studies by Sahraie and colleagues was that they examined the impact of training on awareness. Subjects were asked to make a binary response indicating whether they were aware of the stimulus to which they had just responded. The majority reported some increase in awareness over the period of multiple training sessions, though in one out of the 12 patients, a complete lack of awareness accompanied marked recovery in forced choice detection performance. Subsequently, Sahraie and others (2013) further explored the relationship between detection training and awareness in a new cohort of 5 patients; 4/5 improved on stimulus detection, but subjects fell into one of two “awareness” groups: blindsight type I (lack of awareness in spite of near-normal detection) or blindsight type II (some awareness of stimuli accompanying detection). This highlights potential differences between recovered sight and intact vision. While there are many instances of unconscious perception affecting conscious perception (e.g. Dieter and others 2015; Lin and He 2009), typical perceptual experience is marked by conscious discriminations, while blindsight is marked by unconscious discriminations. Furthermore, there appear to be differences in the efficacy of training in different subjects in terms of restoring awareness. What factors control this phenomenon and whether conscious vision can be restored in all subjects remains to be determined.
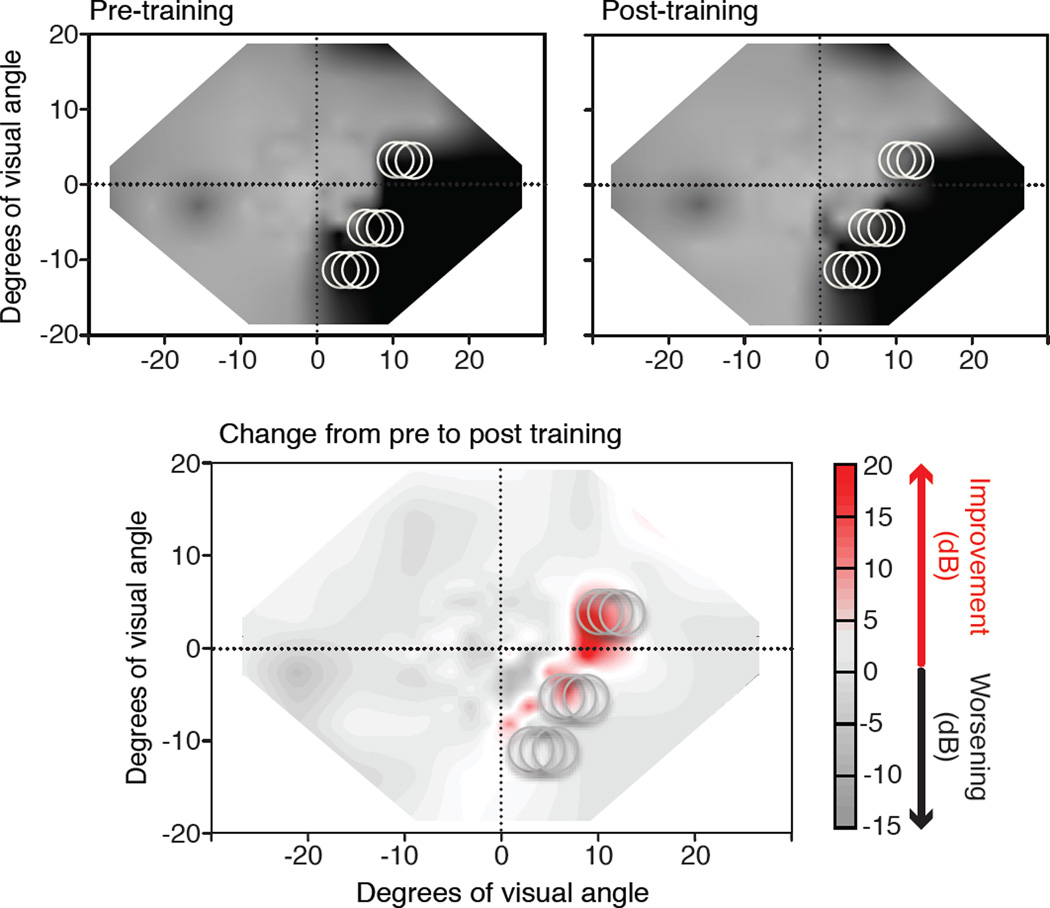
Figure 3. Impact of visual discrimination training on Humphrey visual fields.
Composite visual field maps were obtained from Humphrey perimetry as described in Figure 1 in a chronic CB patient trained using the methods of Das, Tadin and Huxlin (2014). The top graphs show composite visual fields obtained prior to, and then after left-right, global direction discrimination training at locations indicated by light grey circles (see Figure 2E). The bottom graph is a subtraction map between the two top visual field maps. Shades of red indicated regions that improved by >6 dB of sensitivity; shades of grey indicate areas that decreased in sensitivity. Note that the regions of improved sensitivity largely occur at sites of visual training, but there are regions of improvement at locations along the blind field border where training was not directly administered.
Properties of recovered vision following discrimination training
Of the retraining studies that used discrimination tasks in CB subjects, both Raninen and colleagues (2007) and Chokron and colleagues (2008) used two or more methodologies at different blind field locations. Although their approach was likely intended to elicit maximal improvements, it made it very difficult to individuate the impact of different types of training, and the transfer of learning from one task to another. To begin addressing these questions, Das and colleagues trained 3 hemianopic patients to discriminate the coarse orientation (vertical vs. horizontal) of static, slow onset/offset, non-flickering, Gabor patches (Das and others 2014). The subjects were post-tested on the trained task, as well as on their abilities to discriminate other static and dynamic stimuli to which they had never been exposed (even during pre-training tests). Post-tests showed that all subjects could now discriminate coarse and fine orientation differences at both trained and untrained orientation axes. In addition, training on static discrimination transferred to the perception of both simple and global motion. However, subjects failed to discriminate the global direction of stimuli containing a large range of dot directions (Das and others 2014). In an attempt to overcome this problem, 6 additional CB subjects were ‘double-trained’ on static orientation discrimination and global direction discrimination at two separate, blind field locations. Such double training induced complete transfer of learning across tasks and training locations (Das and others 2014). Aside from suggesting one possible method for increasing training efficacy in CB, this finding also provided insight into the mechanisms by which the training-induced improvements in CB fields may occur. In particular, it appears that when the residual visual circuitry is trained with different paradigms at different blind field locations, it may be able to generalize learning across these locations, creating significant savings in time and effort for the patients.
The body of work detailing transfer of learning to untrained stimuli and tasks in CB fields is excellent news for rehabilitation. It also begins to address the issue of whether recovered vision is completely normal. Improvement back to levels of performance measured in the intact hemifield of vision has been reported following specific training on direction range thresholds (Huxlin and others 2009), simple left-right motion discrimination (Huxlin and others 2009; Vaina and others 2014), high-contrast, coarse orientation discriminations (Huxlin and others 2009), and flicker detection of both luminance discs and letters (Raninen and others 2007). However, other visual faculties do not appear to recover completely. Color perception (Bergsma and van der Wildt 2008), form perception (Bergsma and van der Wildt 2008), acuity (Bergsma and van der Wildt 2008), contrast sensitivity (Das and others 2014), fine direction (Das and others 2014) and orientation discrimination (Chokron and others 2008; Das and others 2014), shape and letter discriminations (Chokron and others 2008), and overall awareness (Sahraie and others 2010; Sahraie and others 2013; Sahraie and others 2006) all exhibit residual, post-training deficits. In many cases, we cannot exclude the possibility that “incomplete” recovery occurred because those particular functions were not specifically retrained (i.e. they were measured in pre/post-training tests only). An alternative hypothesis is that some visual functions can never be fully recovered after V1 damage sustained in adulthood. Cavanaugh and colleagues (Cavanaugh and others 2015) considered possible causes for residual deficits in fine direction discrimination at retrained, blind field locations within the computational framework of noise processing and perceptual templates (Dosher and Lu 1999; Dosher and Lu 1998; Legge and others 1987; Ling and others 2009; Pelli 1981). Their results suggest that increased internal, equivalent noise (rather than less efficient filtering of external noise in the stimulus) was responsible for the residual deficits in retrained portions of the blind field (Cavanaugh and others 2015). It is not yet clear to what extent additional, targeted training can overcome such deficits and internal noise, or whether there is a limit to the type and quality of vision that be restored.
The emerging picture is that training can recover conscious vision in CB fields, but that what is recovered is not completely normal. Assessment of the comparative merits of different retraining paradigms is complicated by the fact that different groups have used different visual training and testing methods as well as different outcome measures. An exception is Humphrey automated perimetry with eye tracking, which has been used as an outcome measure by several groups (Chokron and others 2008; Das and others 2014; Huxlin and others 2009; Sahraie and others 2010; Sahraie and others 2013; Sahraie and others 2006; Trevathan and others 2012; Vaina and others 2014). However, beyond providing a measure of luminance detection sensitivity used to assess the size of visual field defects, it gives little information as to the quality of recovered vision. Thus, a systematic comparison of the relative efficacy of different training paradigms at restoring functional vision is needed, and that will require common ground in study design and outcome measures.
4. Limitations of visual training approaches and considerations for future research
While it is encouraging that visual retraining can partially recover vision lost after V1 damage, we do not yet understand the mechanisms of recovery and factors that may limit or speed up this process. Working with a diverse patient population presents a myriad of challenges. Unsurprisingly, this leads to methodological and practical compromises, as well as incidental findings that can provide useful insights into mechanisms and neural substrates of training-induced recovery in damaged visual systems. Furthermore, many of the questions addressed in the study of CB have bearing on other fundamental questions, such as the neural basis of awareness. Here, we consider both some interesting observations and possible limitations of retraining approaches described thus far in CB with an eye towards guiding future research in the field.
Can CB fields ever completely disappear?
CB fields are generally large. Even when unilateral, they often occupy a quarter to a half of the entire left or right hemifield of vision in both eyes. Most research to date, with some exceptions (Bergsma and van der Wildt 2010; Raninen and others 2007; Sahraie and others 2013), has involved retraining blind field regions close to the intact field (Cavanaugh and others 2015; Chokron and others 2008; Das and others 2014; Huxlin and others 2009; Sahraie and others 2010; Sahraie and others 2006). Such regions arguably present a high potential for recovery since retinotopic areas close to the blind field border are most likely to contain spared, albeit abnormal, neural tissue (i.e. representing areas of visual field that are effectively blind prior to training). However, it is of scientific and clinical importance to determine how deep into the blind field recovery can occur. For instance, if it was possible to induce recovery 20–30 degrees deep in the blind field, where no spared V1 tissue exists, it would argue against a critical role of spared V1 tissue along the lesion border in this phenomenon. Instead, it would suggest possible mediation by extrastriate visual areas, which may be intact and able to process visual information from across the entire contralateral visual field, as long as their inputs from subcortical centers (namely, dLGN, superior colliculus and/or pulvinar) remain intact (Leopold 2012a; Schmid and others 2010; Sincich and others 2004).
Transneuronal retrograde degeneration
Retinal ganglion cells and dLGN neurons deprived of their targets appear to exhibit transneuronal retrograde degeneration (for review, see Van Buren 1963). In monkeys with V1 lesions, cell death occurs first in the dLGN, then in the retina, peaking 1 to 3 years post-lesion (Cowey and others 2011; Cowey and others 1989; Cowey and others 1999), and appearing to vary proportionally with the size of the lesion (Cowey and others 1999). Retinal abnormalities suggestive of retrograde degeneration have also been reported in humans following occipital lobe lesions (Cowey and others 2011; Jindahra and others 2009; Jindahra and others 2012; Porrello and Falsini 1999). Such patients can exhibit decreased optic tract size as measured by high resolution MRI (Bridge and others 2011; Millington and others 2014) and decreased thickness of the retinal optic nerve fiber layer, as measured by optical coherence tomography (Jindahra and others 2009; Jindahra and others 2012). In monkeys, retrograde degeneration after V1 damage appears to be largely specific to Pβ retinal ganglion cells, which project to parvocellular layers of the dLGN (Cowey and others 1989). Degeneration of this class of ganglion cells and dLGN neurons is consistent with the notion that koniocellular dLGN neurons may mediate blindsight (Cowey and Stoerig 1989; Sincich and others 2004).
Retrograde degeneration of visual structures is an important challenge in CB, because death of retinal ganglion cells and dLGN neurons will effectively preclude true and complete recovery of normal vision in visual field locations represented by lost cells. To date, most re-training interventions have been applied long after the acute phase of brain damage (generally greater than 6 months post-insult). This was done in order to distinguish training effects from spontaneous recovery, which can occur during the first few months post-lesion (Zhang and others 2006b). However, as the effectiveness of retraining interventions becomes better defined for chronic patients, it may be advantageous to begin studies that involve training of acute patients. We speculate here that early training, by stimulating weakened visual circuits sufficiently, could slow or halt some of the neuronal degeneration that would normally occur.
Cohort size
Much of the interesting research on CB involves case studies of only 1–3 patients (for review of those involving rehabilitation, see Pouget and others 2012). This makes it difficult to generalize findings and draw conclusions, especially when considering the diverse nature of the damage suffered by CB patients. A classic example of this are studies of the well-known patient GY, who not only represents a single case, but one whose insult to the visual system occurred when he was seven years old —considerably earlier in life than the majority of CB patients. Patients such as GY are incredibly valuable, as they offer an opportunity to study in detail the effects of a single lesion without the variability of multiple patients. However, such patients should be considered carefully, as GY exhibits changes typically not seen in older patients, particularly regarding reorganization of subcortical [and likely cortical] connections (Bridge and others 2008).
Lesion type
Another common problem in the field of CB research is that studies often group together patients whose visual field defects result not just from stroke, but also from traumatic brain injury, tumor or tumor resection, epilepsy or congenital defects. For example, Reitsma and colleagues reported that 3 out of the 27 patients they examined possessed an interesting representation of the ipsilateral visual field in cortex that would normally represent the contralateral visual field (Reitsma and others 2013a). However, these 3 patients had very different damage to their visual system (collateral damage from epilepsy surgery, congenital cerebral malformation, removed tumor), sustained at different ages, which complicates the interpretation of results. Similarly, Elliot and colleagues used a narrow-band, high-frequency flicker stimulation paradigm to try and restore vision in a cohort of 3 heterogeneous subjects (stroke, TBI, and a surgical optic nerve lesion - Elliott and others 2015). While people with different lesions can present with similar vision loss, differences in the type of damage sustained can significantly impact their potential for plasticity and compensation (Teo and others 2012). Given that data from individual cases are highly valuable, one possible solution may be to compile detailed information about lesion types, response to training and other information into a shareable database available to the neuroscience and neuro-medicine communities (for examples, see Press and others 2001; Van Essen 2002). Having the ability to examine comparable subjects across multiple testing sites, especially if consistent outcome measures from retraining are also provided, would greatly benefit our endeavors to understand both the functional visual deficits that result from different lesions, and the potential for recovery.
Importance of eye movement control
Proper control of eye movements is critical both for retraining vision and assessing success of such interventions. Small and often subconscious eye movements toward stimuli shown in CB fields can bring them at least partially into the intact field of view. Especially in the case of simple detection paradigms, this can erroneously appear to indicate visual recovery. As detailed above, the lack of adequate eye movement control is a significant confound in VRT-based studies (for review, see Pouget et al., 2012; see also discussion by Turco et al., 2015). Whereas many of the more recent experimental studies have adopted stringent monitoring of eye position, some groups continue to utilize more indirect approaches, such as central fixation tasks (Jobke and others 2009), false positive detection or eye-monitoring by an experimenter (Chokron and others 2008; Gall and others 2011; Paramei and Sabel 2008; Poggel and others 2009; Raninen and others 2007) as proxies for eye tracker enforced fixation control. The fact that VRT’s High Resolution Perimetry (HRP) is still carried out without online eye tracking complicates interpretation of new results emerging from the VRT paradigm. Ideally, eye-tracker fixation control should be utilized during training as well as during pre- and post-training vision assessments. However, training is lengthy and often done in patients’ homes, where eye tracking is not currently feasible. One practical solution is to utilize proper fixation control via a calibrated eye tracker during both pre- and post-training tests in the laboratory or clinic (e.g. Bergsma and others 2012; Bergsma and van der Wildt 2010; Cavanaugh and others 2015; Das and others 2014; Huxlin and others 2009; Sahraie and others 2010; Sahraie and others 2006).
Ways to enhance and speed up vision re-learning
A key disadvantage of current training interventions for CB is that they require lengthy, difficult and repetitious (i.e., boring) visual training. Researchers are beginning to explore ways of overcoming these limitations. As alluded to earlier, training using different stimuli and tasks at multiple blind field locations in the same patient is one such approach (Das and others 2014). Other promising directions involve use of brain stimulation and pharmacology during perceptual training.
Brain stimulation has been used together with VRT (Plow and others 2012; Plow and others 2011). Specifically, this involved anodal trans-cranial direct current stimulation (tDCS) over occipital cortex. tDCS is a noninvasive, electric brain stimulation method capable of increasing excitatory or inhibitory neural responses depending on the direction of current applied (Nitsche and others 2003). Though interpretation of these results should take into account the limitations of VRT as a training tool, patients treated with anodal tDCS appeared to show larger improvements than those treated in the sham condition. This early work with tDCS in CB suggests that electrical brain stimulation could be a promising tool for enhancing training-induced visual restitution in chronic CB, and perhaps spontaneous recovery in acute CB. At the very least, it would be beneficial for future work to evaluate and contrast the efficacy of different forms of non-invasive brain stimulation, include anodal and cathodal tDCS over different brain regions, transcranial random noise stimulation (tRNS) and related magnetic stimulation paradigms (Parkin and others 2015).
At this time, the role of pharmacology in enhancing vision relearning in CB has also not been systematically explored. The important limiting factor for the development of pharmacological interventions is uncertainty as to what neural changes are necessary for vision recovery in CB. However, even without clarity about underlying mechanisms, accumulating evidence suggests that selective serotonin reuptake inhibitors (SSRIs) can significantly improve motor function in motor stroke survivors (Chollet and others 2011). SSRIs, including Fluoxetine, are commonly prescribed as anti-depressants; as such, their influence on neuronal excitability (among other factors) could indeed enhance neural plasticity post-stroke. Alternatively, patients taking SSRIs may be more motivated to do rehabilitation therapy. In the Fluoxetine for Motor Recovery after Acute Ischemic Stroke (FLAME) trial, significant effort was made to disambiguate the anti-depressant and motor effects of Fluoxetine (Chollet and others 2011). Yet, the benefits of Fluoxetine in terms of motor recovery were still significant when the authors adjusted for this potential confound (Chollet and others 2011). Another SSRI, Escitalopram, has also shown promise for improving post-stroke cognitive outcomes (Jorge and others 2010). Whether these medications can benefit patients with visual cortex strokes remains to be determined, but the question certainly opens up a potentially exciting avenue for future research. Encouragingly, Fluoxetine appears to restore critical periods levels of neural plasticity in the visual system of adult rodents (Maya Ventecourt and others 2008). If adult visual circuits respond positively to SSRIs, these medications could significantly enhance the beneficial impact of retraining in CB fields - whether in terms of speed, amount or quality of vision recovered.
5. Conclusion
This is a promising time for research into CB. Early behavioral investigations focused on blindsight, which, while intriguing, offered limited functional benefits to patients with lesions of primary visual cortex and its afferent pathways. For several decades now, there has been an increase in work aimed at developing interventions to recover lost vision in CB. The first studies in this area of research offered encouraging results but suffered from methodological limitations, especially inadequate control for eye movements. The last 10 years have seen an increase in well-controlled studies, which appear to show that vision lost in CB can indeed be partially recovered with appropriate training.
In our opinion, there are three key areas in which further progress would be particularly beneficial. First, we need a better understanding of neural mechanisms that underlie visual retraining. For example, comprehensive functional imaging and tractography in a large number of patients with standardized lesions and visual defects, both before and after retraining, could provide invaluable data as to the changes that underlie recovery. In turn, this should lead to better prediction of retraining outcomes. Second, it will be important to establish the limits of recovery attainable with current retraining methods in order to then determine whether these limits can be overcome via complementary or alternative means. Third, systematic investigations are needed using novel behavioral techniques, pharmacological interventions and/or brain stimulation, to determine if we can enhance and/or accelerate recovery in CB patients. Finally, the long-term goal of research efforts should be to develop effective, evidence-based interventions for CB. Only then can we begin translating these treatments into standard clinical practice, similar to the now well-established and validated interventions that are prescribed for motor cortex strokes.
Acknowledgments
The authors thank Tatiana Pasternak, Marisa Carrasco, Lorella Battelli, Antoine Barbot and Matthew Cavanaugh for insightful comments on the manuscript. We also thank Matthew Cavanaugh for generating the interpolated Humphrey visual field maps in Figures 1 and 3. This work was supported by grants from the NIH (EY021209 to KRH, EY019295 to DT, Core Center Grant P30 EY001319 to the Center for Visual Science (CVS) and by training grant T32 EY007125 to CVS and MDM), by a Collaborative Grant from the Schmitt Program on Integrative Brain Research (to KRH) and by an unrestricted grant from the Research to Prevent Blindness (RPB) Foundation to the Flaum Eye Institute.
References
- Azzopardi P, Cowey A. Motion discrimination in cortically blind patients. Brain. 2001;124:30–46. doi: 10.1093/brain/124.1.30. [DOI] [PubMed] [Google Scholar]
- Bach-Y-Rita P. Controlling variables eliminates hemianopia rehabilitation results. Behavioural and Brain Sciences. 1983;6:448. [Google Scholar]
- Barbur JL, Harlow AJ, Weiskrantz L. Spatial and temporal response properties of residual vision in a case of hemianopia. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1994;343:157–166. doi: 10.1098/rstb.1994.0018. [DOI] [PubMed] [Google Scholar]
- Barbur JL, Watson JDG, Frackowiak RSJ, Zeki S. Conscious visual perception without V1. Brain. 1993;116(Part 6):1293–1302. doi: 10.1093/brain/116.6.1293. [DOI] [PubMed] [Google Scholar]
- Bergsma DP, Elshout JA, van der Wildt GJ, van den Berg AV. Transfer effects of training-induced visual field recovery in patients with chronic stroke. Topics in Stroke Rehabilitation. 2012;19(3):212–225. doi: 10.1310/tsr1903-212. [DOI] [PubMed] [Google Scholar]
- Bergsma DP, van der Wildt GJ. Properties of the regained visual field after visual detection training of hemianopsia patients. Restorative Neurology and Neuroscience. 2008;26(4–5):365–375. [PubMed] [Google Scholar]
- Bergsma DP, van der Wildt GJ. Visual training of cerebral blindness patients gradually enlarges the visual field. British Journal of Ophthalmology. 2010;94(1):88–96. doi: 10.1136/bjo.2008.154336. [DOI] [PubMed] [Google Scholar]
- Blythe IM, Kennard C, Ruddock KH. Residual vision in patients with retro-geniculate lesions of the visual pathways. Brain. 1987;110:887–905. doi: 10.1093/brain/110.4.887. [DOI] [PubMed] [Google Scholar]
- Bowers AR, Ananyev E, Mandel AJ, Goldstein RB, Peli E. Driving with hemianopia, IV: head scanning and detection at intersections in a simulator. Investigative Ophthalmolgy and Visual Science. 2014;55(3):1540–1548. doi: 10.1167/iovs.13-12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers AR, Mandel AJ, Goldstein RB, Peli E. Driving with hemianopia, I: Detection performance in a driving simulator. Investigative Ophthalmolgy and Visual Science. 2009;50(11):5137–47. doi: 10.1167/iovs.09-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers AR, Mandel AJ, Goldstein RB, Peli E. Driving with hemianopia, II: lane position and steering in a driving simulator. Investigative Ophthalmolgy and Visual Science. 2010;51(12):6605–6613. doi: 10.1167/iovs.10-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, Jindahra P, Barbur J, Plant GT. Imaging reveals optic tract degeneration in hemianopia. Investigative Ophthalmolgy and Visual Science. 2011;52(1):382–388. doi: 10.1167/iovs.10-5708. [DOI] [PubMed] [Google Scholar]
- Bridge H, Thomas O, Jbabdi S, Cowey A. Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain. 2008;131(Pt 6):1433–44. doi: 10.1093/brain/awn063. [DOI] [PubMed] [Google Scholar]
- Brogaard B. Type 2 blindsight and the nature of visual experience. Consciousness and Cognition. 2015;32:92–103. doi: 10.1016/j.concog.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier Analysis to the visibility of gratings. Journal of Physiology. 1968;197:551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh MR, Zhang R, Melnick MD, Das A, Roberts M, Tadin D, et al. Visual recovery in cortical blindness is limited by high internal noise. Journal of Vision in press. 2015 doi: 10.1167/15.10.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokron S, Perez C, Obadia M, Gaudry I, Laloum L, Gout O. From blindsight to sight: Cognitive rehabilitation of visual field defects. Restorative Neurology and Neuroscience. 2008;26:305–320. [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischemic stroke (FLAME): a randomized placebo-controlled trial. Lancet Neurology. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- Cowey A. The blindsight saga. Experimental Brain Research. 2010;200:3–24. doi: 10.1007/s00221-009-1914-2. [DOI] [PubMed] [Google Scholar]
- Cowey A, Alexander I, Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in monkeys and humans. Brain. 2011;134:2149–2157. doi: 10.1093/brain/awr125. [DOI] [PubMed] [Google Scholar]
- Cowey A, Stoerig P. Projection patterns of surviving neurons in the dorsal lateral geniculate nucleus following discrete lesions of striate cortex: implications for residual vision. Experimental Brain Research. 1989;75(3):631–638. doi: 10.1007/BF00249914. [DOI] [PubMed] [Google Scholar]
- Cowey A, Stoerig P, Perry VH. Transneuronal retrograde degeneration of retinal ganglion cells after damage to striate cortex in macaque monkeys: selective loss of Pb cells. Neuroscience. 1989;29(1):65–80. doi: 10.1016/0306-4522(89)90333-3. [DOI] [PubMed] [Google Scholar]
- Cowey A, Stoerig P, Williams C. Variance in transneuronal retrograde ganglion cell degeneration in monkeys after removal of striate cortex: effects of size of the cortical lesion. Vision Research. 1999;39(21):3642–3652. doi: 10.1016/s0042-6989(99)00097-8. [DOI] [PubMed] [Google Scholar]
- Das A, Tadin D, Huxlin KR. Beyond blindsight: properties of visual relearning in cortically blind fields. Journal of Neuroscience. 2014;34(35):11652–11664. doi: 10.1523/JNEUROSCI.1076-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong P, Warmink HH. Homonymous hemianopia and driving. Eye. 2003;17(4):545–545. doi: 10.1038/sj.eye.6700395. [DOI] [PubMed] [Google Scholar]
- Dieter KC, Tadin D, Pearson J. Dissociating perceptual bistability and consciousness: Motion-induced blindness outside awareness. Scientific Reports. 2015;5:11841. doi: 10.1038/srep11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombovy ML, Sandok BA, Basford JR. Rehabilitation for stroke: a review. Stroke; a journal of cerebral circulation. 1986;17(3):363–369. doi: 10.1161/01.str.17.3.363. http://stroke.ahajournals.org/content/17/3/363. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu Z. Mechanisms of perceptual learning. Vision Research. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZI. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proceedings of the National Academy of Science USA. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MA, Seifert D, Poggel DA, Strasburger H. Transient increase of intact visual field size by high-frequency narrow-band stimulation. Consciousness and Cognition. 2015;32:45–55. doi: 10.1016/j.concog.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Foley R. The case for characterising type-2 blindsight as a genuinely visual phenomenon. Consciousness and Cognition. 2015;32:56–67. doi: 10.1016/j.concog.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Fujino T, Kigizawa K, Yamada R. Homonymous hemianopia: a retrospective study of 140 cases. Neuro-Ophthalmology. 1986;6:17–21. [Google Scholar]
- Gall C, Sgorzaly S, Schmidt S, Brandt S, Fedorov A, Sabel BA. Noninvasive transorbital alternating current stimulation improves subjective visual functioning and vision-related quality of life in optic neuropathy. Brain Stimulation. 2011;4(4):175–188. doi: 10.1016/j.brs.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Geddes JM, Fear J, Tennant A, Pickering A, Hillman M, Chamberlain MA. Prevalence of self-reported stroke in a population in northern England. Journal of Epidemiology and Community Health. 1996;50:140–143. doi: 10.1136/jech.50.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhotra JS, Mitchell P, Healey PR, Cumming RC, Currie J. Homonymous Visual Field Defects and Stroke in an Older Population. Journal of the American Heart Association. 2002;33:2417–20. doi: 10.1161/01.str.0000037647.10414.d2. [DOI] [PubMed] [Google Scholar]
- Goebel R, Muckli L, Zanella FE, Singer W, Stoerig P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies in hemianopic patients. Vision Research. 2001;41:1459–1474. doi: 10.1016/s0042-6989(01)00069-4. [DOI] [PubMed] [Google Scholar]
- Holmes G. Disturbances of vision by cerebral lesions. British Journal of Ophthalmology. 1918;2:353–384. doi: 10.1136/bjo.2.7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC. Disappointing results from Nova Vision’s visual restoration therapy. Br J Ophthalmol. 2005;89(1):1–2. doi: 10.1136/bjo.2004.058214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Archives of Ophthalmology. 1991;109:816–824. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- Huxlin KR, Riley ME, Martin T, Kelly KN, Friedman DI, Burgin WS, et al. Perceptual re-learning of complex visual motion after V1 damage in humans. Journal of Neuroscience. 2009;29(13):3981–3991. doi: 10.1523/JNEUROSCI.4882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132(3):628–634. doi: 10.1093/brain/awp001. [DOI] [PubMed] [Google Scholar]
- Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135(2):534–541. doi: 10.1093/brain/awr324. [DOI] [PubMed] [Google Scholar]
- Jobke S, Kasten E, Sabel BA. Vision restoration through extrastriate stimulation in patients with visual field defects: a double-blind and randomized experimental study. Neurorehabilitation and Neural Repair. 2009;23(3):246–255. doi: 10.1177/1545968308324221. [DOI] [PubMed] [Google Scholar]
- Jones SA, Shinton RA. Improving outcome in stroke patients with visual problems. Age and Ageing. 2006;35:560–565. doi: 10.1093/ageing/afl074. [DOI] [PubMed] [Google Scholar]
- Jongbloed L. Prediction of function after stroke: a critical review. Stroke. 1986;17(4):765–776. doi: 10.1161/01.str.17.4.765. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Acion L, Moser D, Adams HP, Robinson RG. Escitalopram and enhancement of cognitive recovery following stroke. Archives of General Psychiatry. 2010;67:187–196. doi: 10.1001/archgenpsychiatry.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten E, Poggel DA, Muller-Oehring E, Gothe J, Schulte T, Sabel BA. Restoration of vision II: residual functions and training-induced visual field enlargement in brain-damaged patients. Restorative Neurology and Neuroscience. 1999;15(2–3):273–287. [PubMed] [Google Scholar]
- Kasten E, Poggel DA, Sabel BA. Computer-based training of stimulus detection improves color and simple pattern recognition in the defective field of hemianopic subjects. Journal of Cognitive Neuroscience. 2000;12(6):1001–1012. doi: 10.1162/08989290051137530. [DOI] [PubMed] [Google Scholar]
- Kasten E, Sabel BA. Visual field enlargment after computer-training in brain-damaged patients with homonymous deficits - an open pilot trial. Restorative Neurology and Neuroscience. 1995;8:113–127. doi: 10.3233/RNN-1995-8302. [DOI] [PubMed] [Google Scholar]
- Kasten E, Wüst S, Behrens-Baumann W, Sabel BA. Computer-based training for the treatment of partial blindness. Nature Medicine. 1998;4(9):1083–1087. doi: 10.1038/2079. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Spatial frequency selectivity in the retina. Vision Research. 1975;15:665–672. doi: 10.1016/0042-6989(75)90282-5. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Motion and vision. II. Stabilization spatio-temporal threshold surface. J. Opt. Soc. Am. 1979;69:1340–1345. doi: 10.1364/josa.69.001340. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G. Restorative and compensatory therapy approaches in cerebral blindness - a review. Restorative Neurology and Neuroscience. 1999;15:255–271. [PubMed] [Google Scholar]
- Kerkhoff G. Neurovisual rehabiliation: recent developments and future directions. Journal of Neurology, Neurosurgery and Psychiatry. 2000;68:691–706. doi: 10.1136/jnnp.68.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton Smith J. Homonymous hemianopia. American Journal of Ophthalmology. 1962;54:616–623. [PubMed] [Google Scholar]
- Leff AP. A historical review of the representation of the visual field in primary visual cortex with special reference to the neural mechanisms underlying macular sparing. Brain and Language. 2004;88:268–278. doi: 10.1016/S0093-934X(03)00161-5. [DOI] [PubMed] [Google Scholar]
- Legge GE, Kersten D, Burgess AE. Contrast discrimination in noise. Journal of the Optical Society of America A, Optics and Image Science. 1987;4:391–404. doi: 10.1364/josaa.4.000391. [DOI] [PubMed] [Google Scholar]
- Leopold DA. Primary visual cortex, awareness and blindsight. Annual Reviews of Neuroscience. 2012a;35:91–109. doi: 10.1146/annurev-neuro-062111-150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA. Primary visual cortex: awareness and blindsight. Annual Review of Neuroscience. 2012b;35:91–109. doi: 10.1146/annurev-neuro-062111-150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, He S. Seeing the invisible: the scope and limits of unconscious processing in binocular rivalry. Progress in Neurobiology. 2009;87(4):195–211. doi: 10.1016/j.pneurobio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Liu T, Carrasco M. How spatial and feature-based attention affect the gain and tuning of population responses. Vision Research. 2009;49:1194–1204. doi: 10.1016/j.visres.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic SV, Milisavljevic MM, Lolic-Draganic V, Kovacevic MS. Distribution of the occipital branches of the posterior cerebral artery. Correlation with occipital lobe infarcts. Stroke. 1987;18:728–732. doi: 10.1161/01.str.18.4.728. [DOI] [PubMed] [Google Scholar]
- Maya Ventecourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Millington RS, Yasuda CL, Jindahra P, Jenkinson M, Barbur JL, Kennard C, et al. Quantifying the pattern of optic tract degeneration in human hemianopia. Journal of Neurology, Neurosurgery & Psychiatry. 2014;85:379–386. doi: 10.1136/jnnp-2013-306577. [DOI] [PubMed] [Google Scholar]
- Morland AB, Jones SR, Finlay AL, Deyzac E, Le S, Kemp S. Visual perception of motion, luminance and color in a human hemianope. Brain. 1999;122:1183–1198. doi: 10.1093/brain/122.6.1183. [DOI] [PubMed] [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124(6):1241–1252. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation-technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56(3):255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Grünbaum T. Consciousness and modality: On the possible preserved visual consciousness in blindsight subjects. Consciousness and Cognition. 2011;20(4):1855–1859. doi: 10.1016/j.concog.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Mogensen J. Reconciling current approaches to blindsight. Consciousness and Cognition. 2015;32:33–40. doi: 10.1016/j.concog.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Papageorgiou E, Hardiess G, Schaeffel F, Wiethoelter H, Karnath H-O, Mallot H, et al. Assessment of vision-related quality of life in patients with homonymous visual field defects. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2007;245(12):1749–1758. doi: 10.1007/s00417-007-0644-z. [DOI] [PubMed] [Google Scholar]
- Paramei GV, Sabel BA. Contour-integration deficits on the intact side of the visual field in hemianopia patients. Behavioural Brain Research. 2008;188(1):109–124. doi: 10.1016/j.bbr.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Parkin BL, Ekhtiari H, Walsh VF. Non-invasive Human Brain Stimulation in Cognitive Neuroscience: A Primer. Neuron. 2015;87(5):932–945. doi: 10.1016/j.neuron.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Pasik P, Pasik T. Visual functions in monkeys after total removal of visual cerebral cortex. Contributions to Sensory Physiology. 1982;7:147–200. [Google Scholar]
- Pelak VS, Dubin MW, Whitney E. Homonymous Hemianopia: A Critical Analysis of Optical Devices, Compensatory Training, and NovaVision. Current Treatment Options in Neurology. 2007;9(1):41–47. doi: 10.1007/s11940-007-0029-y. [DOI] [PubMed] [Google Scholar]
- Peli E. Field expansion for homonymous hemianopia by optically induced peripheral exotropia. Optometry and Vision Science. 2000;77(9):453–64. doi: 10.1097/00006324-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Peli E, Pely D. Driving with confidence: A practical guide to driving with low vision: World Scientific. 2002 [Google Scholar]
- Pelli DG. Effects of Visual Noise. Cambridge: Cambridge University; 1981. [Google Scholar]
- Plow EB, Obretenova SN, Fregni F, Pascual-Leone A, Merabet LB. Comparison of visual field training for hemianopia with active versus sham transcranial direct cortical stimulation. Neurorehabilitation and Neural Repair. 2012;26(6):616–626. doi: 10.1177/1545968311431963. [DOI] [PubMed] [Google Scholar]
- Plow EB, Obretenova SN, Halko MA, Kenkel S, Jackson M-L, Pascual-Leone A, et al. Combining visual rehabilitative training and noninvasive brain stimulation to enhance visual function in patients with hemianopia: a comparative case study. PM&R. 2011;3(9):825–835. doi: 10.1016/j.pmrj.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Poggel DA, Mueller I, Kasten E, Bunzenthal U, Sabel BA. Subjective and objective outcome measures of computer-based vision restoration training. Neurorehabilitation. 2009;27(2):173–187. doi: 10.3233/NRE-2010-0594. [DOI] [PubMed] [Google Scholar]
- Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, Langhorne P, et al. Interventions for visual field defects in patients with stroke. Cochrane Database Syst Rev 10. 2011a doi: 10.1002/14651858.CD008388.pub2. [DOI] [PubMed] [Google Scholar]
- Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, Langhorne P. In: Interventions for visual field defects in patients with stroke (Review) Group CS, et al., editors. The Cochrane Library: John Wiley & Sons, Ltd; 2011b. pp. 1–83. [DOI] [PubMed] [Google Scholar]
- Pollock A, Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, et al. Interventions for visual field defects in patients with stroke. Stroke. 2012;43(4):e37–e38. doi: 10.1161/strokeaha.111.639815. [DOI] [PubMed] [Google Scholar]
- Porrello G, Falsini B. Retinal ganglion cell dysfunction in humans following post-geniculate lesions: specific spatio-temporal losses revealed by pattern ERG. Vision Research. 1999;39(9):1739–1745. doi: 10.1016/s0042-6989(98)00272-7. [DOI] [PubMed] [Google Scholar]
- Pouget M-C, Levy-Bencheton D, Prost M, Tiliket C, Husain M, Jacquin-Courtois S. Acquired visual field defects rehabilitation: critical review and perspectives. Annals of Physical and Rehabilitation Medicine. 2012;55:53–74. doi: 10.1016/j.rehab.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Press WA, Olshausen BA, Van Essen DC. A graphical anatomical database of neural connectivity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356(1412):1147–1157. doi: 10.1098/rstb.2001.0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raninen A, Vanni S, Hyvärinen L, Näsänen R. Temporal sensitivity in a hemianopic visual field can be improved by long-term training using flicker stimulation. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:66–73. doi: 10.1136/jnnp.2006.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard JAS, Schiefer U, Sabel BA, Kenkel S, Vontheim R, et al. Does visual restitution training change absolute homonymous visual field defects? A fundus controlled study. British Journal of Ophthalmology. 2005;89:30–35. doi: 10.1136/bjo.2003.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma DC, Mathis J, Ulmer JL, Mueller W, Maciejewski MJ, De Yoe EA. Atypical Retinotopic Organization of Visual Cortex in Patients with Central Brain Damage: Congenital and Adult Onset. Journal of Neuroscience. 2013a;33(32):13010–13024. doi: 10.1523/JNEUROSCI.0240-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma DC, Mathis J, Ulmer JL, Mueller W, Maciejewski MJ, DeYoe EA. Atypical retinotopic organization of visual cortex in patients with central brain damage: congenital and adult onset. The Journal of Neuroscience. 2013b;33(32):13010–13024. doi: 10.1523/JNEUROSCI.0240-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, Khefyets S, Reding MJ. Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect. Neurology. 1990;40(10):1597–1599. doi: 10.1212/wnl.40.10.1597. [DOI] [PubMed] [Google Scholar]
- Roufs JA. Dynamic properties of vision - 1. Experimental relationship between flicker and flash thresholds. Vision Research. 1972;12:261–278. doi: 10.1016/0042-6989(72)90117-4. [DOI] [PubMed] [Google Scholar]
- Sahraie A, MacLeod MJ, Trevathan CT, Robson S, Olson JA, Callaghan P, et al. Improved detection following Neuro-Eye Therapy in patients with post-geniculate damage. Experimental Brain Research. 2010;206:25–34. doi: 10.1007/s00221-010-2395-z. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevathan CT, MacLeod MJ, Weiskrantz L, Hunt AR. The Continuum of Detection and Awareness of Visual Stimuli Within the Blindfield: From Blindsight to the Sighted-Sight. Investigative Ophthalmolgy and Visual Science. 2013;54:3579–3585. doi: 10.1167/iovs.12-11231. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod M-J. Temporal properties of spatial channels of processing in hemianopia. Neuropsychologia. 2008;46:879–885. doi: 10.1016/j.neuropsychologia.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod MJ, Murray AD, Olson JA, Weiskrantz L. Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(40):14971–14976. doi: 10.1073/pnas.0607073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, Weiskrantz L, Olson JA, MacLeod MJ, Murray AD, et al. Spatial channels of visual processing in cortical blindness. European Journal of Neuroscience. 2003;18:1189–1194. doi: 10.1046/j.1460-9568.2003.02853.x. [DOI] [PubMed] [Google Scholar]
- Sanders MD, Warrington EK, Marshall J, Weiskrantz L. ‘Blindsight’: vision in a field defect. Lancet. 1974;1:707–708. doi: 10.1016/s0140-6736(74)92907-9. [DOI] [PubMed] [Google Scholar]
- Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M, Peters AJ, et al. Blindsight depends on the lateral geniculate nucleus. Nature. 2010;466(7304):373–377. doi: 10.1038/nature09179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A, Vonthein R, Reinhard J, Trauzettel-Klosinski S, Connert C, Scheifer U. Effect of visual restitution training on absolute homonymous scotomas. Neurology. 2006;67:143–145. doi: 10.1212/01.wnl.0000223338.26040.fb. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct genicular input fo area MT. Nature Neuroscience. 2004;7(10):1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Spitzyna GA, Wise RJS, McDonald SA, Plant GT, Kidd D, Crewes H, et al. Optokinetic therapy improves test reading in patients with hemianopic alexia. Neurology. 2007;68:1922–1930. doi: 10.1212/01.wnl.0000264002.30134.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoerig P. Blindsight, conscious vision, and the role of primary visual cortex. Progress in Brain Research. 2006;155:217–234. doi: 10.1016/S0079-6123(06)55012-5. [DOI] [PubMed] [Google Scholar]
- Stoerig P, Cowey A. Visual Perception and Phenomenal Consciousness. Behavioural Brain Research. 1995;71(1–2):147–156. doi: 10.1016/0166-4328(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Stoerig P, Cowey A. Blindsight in man and monkey. Brain. 1997;120(Pt 3):535–559. doi: 10.1093/brain/120.3.535. [DOI] [PubMed] [Google Scholar]
- Szlyk JP, Seiple WH, Stelmack J, McMahon T. Use of prisms for navigation and driving in hemianopic patients. Ophthalmic and Physiological Optics. 2008;25:128–135. doi: 10.1111/j.1475-1313.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Tamietto M, Pullens P, De Gelder B, Weiskrantz L, Goebel R. Subcortical connections to human amygdala and changes following destruction of the visual cortex. Current Biology. 2012;22(15):1449–1455. doi: 10.1016/j.cub.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Teo L, Rosenfeld J, Bourne JA. Models of CNS injury in the nonhuman primate: A new era for treatment strategies. Translational Neuroscience. 2012;3(2):181–195. [Google Scholar]
- Teuber H-L, Battersby WS, Bender MB. Visual Field Defects After Penetrating Missile Wounds of the Brain. Cambridge, Massachusetts: Harvard University Press; 1960. [Google Scholar]
- Trevathan CT, Urquhart J, Ward R, Gentleman D, Sahraie A. Evidence for perceptual learning with repeated stimulation after partial and total cortical blindness. Advances in Cognitive Psychology. 2012;8(1):29–37. doi: 10.2478/v10053-008-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobe JD, Lorber ML, Schlezinger NS. Isolated homonymous hemianopia: a review of 104 cases. Archives of Ophthalmology. 1973;89:377–381. doi: 10.1001/archopht.1973.01000040379005. [DOI] [PubMed] [Google Scholar]
- Turco S, Albamonte E, Ricci D, Fortini S, Amore FM. Bernhard Sabel and “residual vision activation theory”: a history spanning three decades. Multisensory Research. 2015;28:309–330. doi: 10.1163/22134808-00002499. [DOI] [PubMed] [Google Scholar]
- Vaina L, Soloviev S, Calabro FJ, Buonanno F, Passingham RE, Cowey A. Reorganization of retinotopic maps after occipital lobe infarction. Journal of Cognitive Neuroscience. 2014;26(6):1266–1282. doi: 10.1162/jocn_a_00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren JM. Trans-synaptic retrograde degeneration in the visual system of primates. Journal of Neurology, Neurosurgery & Psychiatry. 1963;26(5) doi: 10.1136/jnnp.26.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. Windows on the brain: the emerging role of atlases and databases in neuroscience. Current Opinion in Neurobiology. 2002;12(5):574–579. doi: 10.1016/s0959-4388(02)00361-6. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Diller L, Gordo WA, Gerstman LJ, Lieberman AR, Lakin P, et al. Visual scanning training effect on reading-related tasks in acquired right brain damage. Archives of Physical Medicine and Rehabilitation. 1977;58:479–486. [PubMed] [Google Scholar]
- Weiskrantz L. Blindsight. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- Weiskrantz L, Barbur JL, Sahraie A. Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (v1) Proceedings of the National Academy of Science USA. 1995;92:6122–6126. doi: 10.1073/pnas.92.13.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L, Harlow A, Barbur JL. Factors affecting visual sensitivity in a hemianopic subject. Brain. 1991;114:2269–2282. doi: 10.1093/brain/114.5.2269. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- Zeki S, ffytche DH. The Riddoch Syndrome: insights into the neurobiology of conscious vision. Brain. 1998;121:25–45. doi: 10.1093/brain/121.1.25. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Homonymous hemianopias: Clinical-anatomic correlations in 904 cases. Neurology. 2006a;66(906–910) doi: 10.1212/01.wnl.0000203913.12088.93. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Natural history of homonymous hemianopia. Neurology. 2006b;66:901–905. doi: 10.1212/01.wnl.0000203338.54323.22. [DOI] [PubMed] [Google Scholar]