Abstract
Layer 5 neurons of the neocortex receive direct and relatively strong input from the thalamus. However, the intralaminar distribution of these inputs and their capacity for plasticity in adult animals are largely unknown. In slices of primary motor cortex (M1), we simultaneously recorded from pairs of corticospinal neurons associated with control of distinct motor outputs: distal forelimb versus proximal forelimb. Activation of ChR2-expressing thalamocortical afferents in M1 before motor learning produced equivalent responses in monosynaptic excitation of neurons controlling the distal and proximal forelimb, suggesting balanced thalamic input at baseline. Following skilled grasp training, however, thalamocortical input shifted to bias activation of corticospinal neurons associated with control of the distal forelimb. This increase was associated with a cell-specific increase in mEPSC amplitude, but not presynaptic release probability. These findings demonstrate distinct and highly segregated plasticity of thalamocortical projections during adult learning.
INTRODUCTION
Thalamocortical (TC) inputs to the cortex undergo significant reorganization during development (Feldman and Brecht, 2005; Huberman et al., 2008; Sur and Leamey, 2001), and aberrant developmental organization leads to compromised function (Crair et al., 1998; Fox and Wong, 2005; Li et al., 2013; Sadaka et al., 2003), illustrating the crucial role of TC synaptic patterning with regard to cortical function and behavior. While other regions of the nervous system, including the cortex and cerebellum, exhibit a capacity for plasticity throughout life, little is known regarding the ability of TC projections to undergo continued plasticity during adulthood, particularly in the context of behavioral learning.
Layer 4 is the major recipient of TC input in sensory cortex (Peters and Feldman, 1977). In motor cortex (M1), which lacks a cytoarchitecturally identifiable layer 4 (Donoghue and Wise, 1982; but see Yamawaki et al., 2014), input from motor thalamus is variably distributed across all cortical layers, including a strong input onto L5 corticospinal neurons (Hooks et al., 2013; Suter and Shepherd, 2015). Many fundamental properties of TC input to M1 are unknown, particularly with regard to the patterning of synaptic input and whether these inputs can be modified in adulthood.
The corticospinal system, wherein neighboring cells can have distinct projection patterns and participate uniquely in motor learning (Wang et al., 2011), presents an advantageous model for investigating the intralaminar distribution of TC input, and whether the allocation of thalamic inputs across functionally distinct cell populations becomes biased toward learning-relevant neurons following motor training. In the current study, we explored these questions in the context of acquisition of a skilled grasping task in adult animals. Segregation of corticospinal neuronal subtypes was made on the basis of their projection pattern to distinct segments of the spinal cord that are largely associated with the control of unique aspects of forelimb musculature (McKenna et al., 2000; Tosolini and Morris, 2012; Wang et al., 2011). This basis of segregation enabled us to probe whether potential learning-related changes in TC signaling were specific to neurons most relevant for behavioral refinements taking place during the learning. Whole-cell corticospinal recordings in slices were performed in combination with selective photostimulation of TC axon terminals expressing Channelrhodopsin-2 (ChR2). The use of paired recordings, which included at least one neuron from each functional corticospinal subtype, permitted us to assess the relative strength of synaptic drive onto neighboring L5 neurons and to determine whether learning altered the balance of synaptic drive in a functionally related manner. Our results indicate that under baseline (untrained) conditions, TC input is evenly distributed across adjacent L5 corticospinal neurons. Following motor skill training, however, TC input becomes biased toward the population of corticospinal cells most relevant to the learned behavior. Characterization of the synaptic mechanisms driving this change suggests that signaling between TC axons and learning-relevant, “trained” neurons is strengthened with learning, while input between TC axons and “untrained” L5 neurons remains static. These findings identify the existence of thalamocortical plasticity that is highly focused in learning-relevant circuits during normal adult learning.
RESULTS
Measurement of selective thalamocortical input onto subpopulations of corticospinal neurons
To label largely discrete populations of L5 corticospinal neurons associated with different motor outputs, fluorescent retrograde tracers were injected into C4 and C8 segments of the spinal cord in young adult (P35) rats (Akintunde and Buxton, 1992; Biane et al., 2015; Wang et al., 2011) (Figure 1A): spinal cord segment C8 primarily contains motor neurons controlling musculature of the distal forelimb involved in skilled forelimb grasping, while C4 projects to proximal forelimb musculature (McKenna et al., 2000; Tosolini and Morris, 2012). In support of the independent functional roles for these subpopulations of corticospinal neurons are studies demonstrating that C4- and C8-projecting cells are independently modulated following skilled grasp training, with C8-projecting cells displaying selective increases in dendritic complexity and spine density with learning (Wang et al., 2011), and partial spinal cord lesions at C8 in primates result in significant decrements in manual dexterity and grasping (Nakagawa et al., 2015).
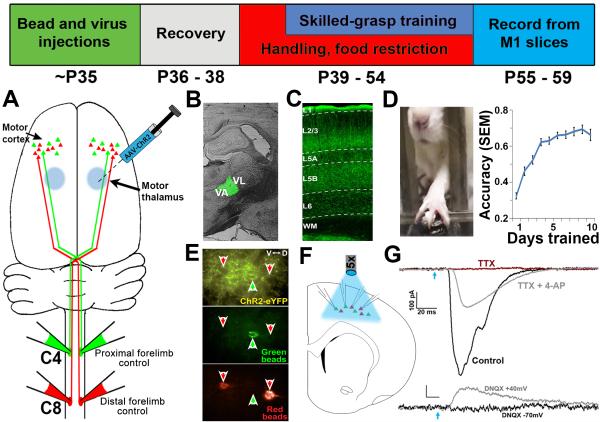
Figure 1. Experimental overview.
Top: Timeline of experiments. (A) Retrograde tracer injections at levels C4 and C8 of the spinal cord label distinct corticospinal projection populations originating in layer 5 of M1. (B) To selectively express ChR2 in motor thalamic nuclei, AAV-ChR2 was targeted to the VA/VL complex of the thalamus. (C) TC axonal expression of ChR2-EYFP was robust in layer 5 (the location of corticospinal cell bodies) of the caudal forelimb region of M1. (D) Skilled grasp training was conducted over 10 days, leading to a significant increase in pellet retrieval accuracy (repeated-measures ANOVA, p < 0.001). (E) Following the completion of training, slices of M1 were prepared and C4- and C8-projecting corticospinal neurons were targeted for simultaneous whole-cell recording. Disambiguation of eYFP signal from green bead fluorescence was achieved via a narrow band GFP filter. (F) Photostimulation at ~470nm was applied across the cortical slice via 5x objective to selectively stimulate thalamocortical axons. (G) Corticospinal neurons receive direct monosynaptic input from TC axons; upper traces: photo-induced EPSCs were abolished in the presence of 1μM TTX (red trace), but rescued following addition of 100 μM 4-AP (grey trace); lower traces: the presence of the AMPAR antagonist DNQX abolished EPSCs when neurons were held at −70mV (black trace). Releasing the Mg2+ block from NMDA channels via depolarization to +40mV unmasked monosynaptic NMDA-mediated currents (grey trace). Blue arrow = light onset.
Injection of ChR2-expressing virus was targeted to the ventroanterior / ventrolateral (VA/VL) complex of the motor thalamus (Figure 1B), resulting in robust expression of thalamocortical (TC) axons in primary motor cortex (M1; Figure 1C). Approximately 3 weeks later (P55-P59), acute slices containing the caudal forelimb region were prepared and C4- and C8-projecting corticospinal neurons near the center of the ChR2-expressing axon band in L5 were targeted for whole-cell recording (Figure 1E). Activation of TC terminals was achieved via blue light pulses delivered through a 5x objective centered over the recorded cells in M1 (Figure 1F).
Thalamocortical input is balanced across L5 corticospinal subpopulations at baseline
To assess the distribution of TC input across distinct subpopulations of L5 corticospinal neurons, we compared TC-specific EPSCs between paired recordings of neurons associated with control of either the distal (C8-projecting) or proximal (C4-projecting) forelimb (Figure 2A). To control for possible variability in ChR2 expression or intensity of light activation across animals and slices, pairs of neurons containing one cell from each functional subtype were recorded simultaneously; accordingly, we recorded 42 pairs across 26 slices from 17 rats (for non-normalized EPSC amplitudes, see Figure S1). Importantly, C4- and C8-projecting corticospinal subpopulations are intermingled within M1 (Wang et al., 2011), enabling recording of neuronal pairs in close proximity to one another (average intersomatic distance for pairs recorded was 92 ± 53 μm).
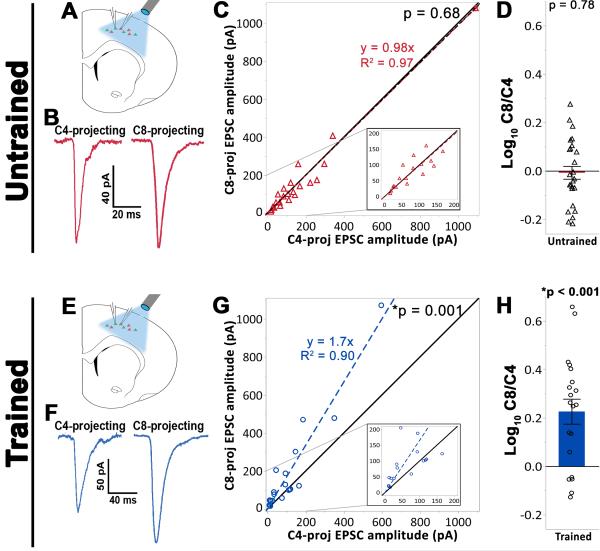
Figure 2. Plasticity of thalamocortical projections onto trained, grasp-related C8-projecting layer 5 corticospinal neuronal subpopulations.
(A) – (D) Experimental results from baseline (untrained) animals. (A) Neighboring C4- and C8-projecting cell pairs were targeted for whole-cell patch clamp in slices containing M1. (B) Sample bulk-stimulation EPSCs from a simultaneously patched C4- and C8-projecting cell pair demonstrating comparable evoked response amplitude in both neuronal subtypes. (C) Analysis across all cell pairs indicated that thalamocortical input was balanced across corticospinal subpopulations under baseline conditions (Wilcoxon signed-rank test against unity, p = 0.95). Black line = unity. Colored dashed line = linear fit of data. Inset: magnified view for smaller amplitude responses. (D) The log10 of the ratio of C8-projecting to C4-projecting EPSC peak amplitudes. Individual data points represent the average of all cell pairs recorded in a single slice (see methods). Under baseline conditions, EPSC amplitude did not differ across corticospinal subpopulations (one-sample t-test against 0, p = 0.78). (E) – (H) Experimental results from trained animals. (E) The recording setup was the same as for untrained animals. (F) Sample bulk stimulation EPSCs from a simultaneously patched cell pair indicating a larger evoked response in the C8-projecting cell relative to the C4-proceting cell. (G) Analysis across all cell pairs indicated that skilled motor learning is associated with a greater thalamocortical drive onto the C8-vs C4-projecting cell population (Wilcoxon signed-rank test against unity, p < 0.001). (H) The ratio of C8-projecting:C4-projecting EPSC peak amplitudes (C8/C4) following training deviated significantly from unity (one sample t-test against 0, p < 0.001), and was also significantly greater than that observed in untrained animals ((D) vs (H); (Welch’s unequal variances t-test, p < 0.001).
Under baseline conditions, the amplitude of evoked monosynaptic TC responses was equivalent across C4- and C8-projecting cell pairs (Wilcoxon matched-pairs signed-rank test, p = 0.68, Figure 2B,C; one-sample t-test against 0, p = 0.78, Figure 2D), indicating that the allocation of thalamic resources is typically distributed evenly across L5 corticospinal subpopulations regardless of their functional association.
Following training, thalamocortical input is biased toward learning-related neurons
To evaluate whether TC-L5 circuitry is modulated with learning in adulthood, we next investigated whether acquisition of a skilled motor behavior impacts the nature of TC input to these distinct corticospinal cell populations. Specifically, we examined whether acquisition of a behavior requiring a refinement of control of the distal forelimb musculature (Gharbawie and Whishaw, 2006; Monfils et al., 2005) would selectively bias TC input onto the grasp-related, C8-projecting subpopulation. Over 10 consecutive days, animals were trained on a single-pellet retrieval task, which requires advancing the forepaw through a slot in order to grasp and retrieve a sugar reward pellet (Figure 1D). Although pellet retrieval accuracy is poor during initial training sessions (~30%), as the grasping motor pattern is refined over subsequent sessions (Gharbawie and Whishaw, 2006), animals become considerably more proficient (~70% success rate). Indeed, animals demonstrated a significant increase in retrieval accuracy over 10 days of training (p < 0.001, Figure 1D).
After completion of training, slices containing M1 were prepared and C4- and C8-projecting corticospinal neurons were targeted for paired recordings (Figure 2E,F). We recorded a total of 35 cell pairs over 20 slices in 15 skilled grasp-trained rats. Following training, the amplitude of light evoked TC responses was ~70% greater in the grasp-related C8-projecting population compared to the C4-projecting population associated with more proximal forelimb musculature (Wilcoxon matched-pairs signed-rank test, p = 0.001, Figure 2G; one-sample t-test against 0, p <0.001, Figure 2H). Significance was also found when directly comparing C8/C4 EPSC ratios in trained vs baseline conditions (Figure 2D vs. 2H; Welch’s unequal variances t-test, p < 0.001). Hence, TC projections to L5 motor cortex are not static in adulthood, but exhibit behaviorally-associated plasticity and become biased toward a learning-relevant subpopulation of L5 corticospinal neurons following motor skill training.
Importantly, the training-associated shift in the C8/C4 EPSC amplitude ratio could be a product of increased input onto the C8-projecting population, decreased input onto the C4-projecting population, or some combination thereof. In addition, several synaptic properties could contribute to observed change in EPSC amplitude, including alterations in presynaptic release probability (pr), postsynaptic quantal amplitude (q), and/or the number of synaptic contacts (n). Thus, we subsequently attempted to identify the directionality of TC signaling changes associated with training, and the site(s) of corresponding synaptic modifications.
Thalamocortical release probability is unchanged following training
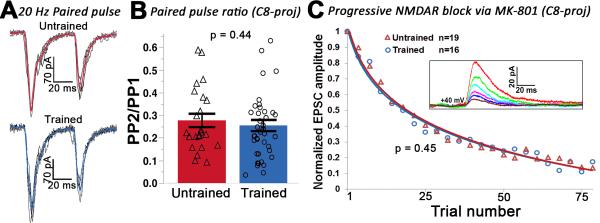
Presynaptic release probability (pr), or the probability that an action potential invading the presynaptic terminal results in release of neurotransmitter, varies considerably across central synapses (Dobrunz and Stevens, 1997) and is modified under a variety of circumstances, including learning (Li et al., 2011). We first examined whether pr was altered during adult motor learning by measuring the paired-pulse ratio (PPR) of light-evoked, 20 Hz stimulation of TC-L5 input (Figure 3A). Our results show that PPR was not altered in either the C8- or C4-projecting subpopulation as a function of training (C8-projecting: p = 0.44. Figure 3B. C4-projecting: untrained = 0.28 ± 0.13, trained = 0.22 ± 0.18, p = 0.36, data not shown), indicating that pris unaffected by learning.
Figure 3. Presynaptic release probability is unaffected by skilled motor training.
(A) Postsynaptic responses to a paired light pulse in C8-projecting neurons from an untrained (upper) or trained (lower) animal. Black traces = individual responses. Colored traces = averaged response. (B) The average paired-pulse ratio did not differ across training conditions for either C8-projecting or C4-projecting cells (C8: t-test on log transformed data (see methods), p = 0.44; C4: t-test on log transformed data, p = 0.36; C4-projecting data not shown). (C) The NMDAR-mediated EPSC declined at a similar rate in the C8-projecting subpopulation in trained and untrained animals (t-test, p = 0.45). Inset: progressive decline of the NMDAR-mediated response from a sample neuron. All cells were held at +40mV in the presence of 20 μM MK-801, 20 μM DNQX, and 20 μM picrotoxin.
In the C8-projecting population, we further substantiated that training does not affect pr by recording NMDAR-mediated responses in the presence of MK-801, an NMDAR antagonist that selectively and irreversibly blocks open NMDAR channels (Hessler et al., 1993). The collective NMDA response thus shows a progressive decrease in amplitude with repeated activation of presynaptic terminals (Figure 3C, inset). The rate of this decay is attributable to properties such as NMDAR open time, affinity of MK-801 for the channel, and, notably, pr. Our MK-801 findings corroborated those obtained with the paired-pulse paradigm: there was no difference in the NMDAR-mediated EPSC decay rate in trained versus untrained animals, as the mean number of stimuli required to reach 50% of baseline did not differ between untrained (21.8 ± 7.8) and trained (19.3 ± 8.2) animals (p = 0.45. Figure 3C). Taken together, our results suggest that pr at the TC-L5 synapse is not altered by skilled motor training.
Thalamocortical mEPSC amplitude is selectively augmented in learning-relevant cells following training
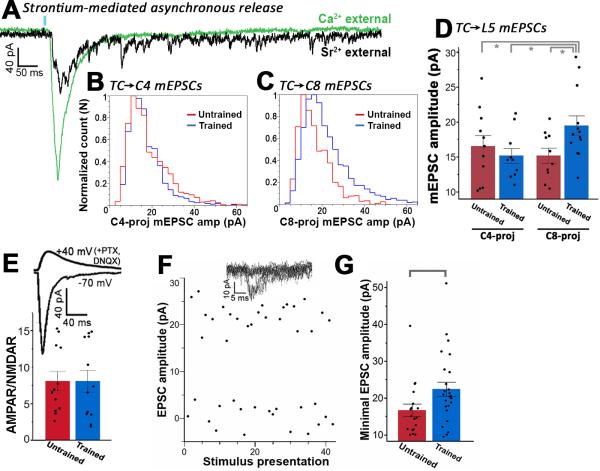
An additional mechanism that could drive changes in synaptic strength is the postsynaptic response to the release of a single presynaptic vesical, or the quantal amplitude (q). To investigate the contribution of quantal amplitude changes to the learning-mediated change in EPSC, we isolated putative single release events at TC terminals by replacing calcium (Ca2+) with strontium (Sr2+) in the external solution (Dodge et al., 1969). As seen in Figure 4A, substituting Ca2+ with Sr2+ desynchronized vesical release across presynaptic terminals following bulk stimulation, inducing prolonged, asynchronous release that enabled measurement of individual evoked mEPSCs due to putative single release events at ~60 - 500 ms post activation of TC terminals. Consistent with our previous finding that TC input is balanced across corticospinal subpopulations at baseline, the quantal amplitude at TC-L5 synapses did not differ among C4- and C8-projecting neurons under baseline, untrained conditions (t-test, p = 0.5; Figure 4B,D). Skilled forelimb reach training markedly altered the balance in quantal amplitude resulting in a greater mEPSC amplitude in the C8-projecting subpopulation (t-test, p = 0.02; Figure 4C,D). This increase was not attributable to a disproportionate increase in AMPAR or NMDAR signaling at the time of recording, as the AMPAR:NMDAR ratio did not differ between training conditions (t-test, p = 0.99; Figure 4E). These results demonstrate that TC-L5 quantal amplitude is plastic in adulthood, and provides a synaptic mechanism for increasing TC signaling onto learning-relevant neurons following training.
Figure 4. Postsynaptic quantal amplitude increases selectively onto the C8-projecting subpopulation following skilled motor training.
(A) Sample postsynaptic responses to photostimulation (blue bar) of TC axons. Green trace = external solution containing 2.5 mM calcium. Black trace = same cell after calcium was replaced with 3 mM strontium. Note the diminished synchronous release in the presence of strontium, and the presence of individual release events that persist for 500+ ms following photostimulation. (B) The mEPSC amplitude distribution did not differ across training conditions in the C4-projecting population (K-S test, p = 0.6), (C) but the frequency of larger responses increased significantly with training in the C8-projecting population (K-S test, p < 0.01). (D) The mean mEPSC amplitude for trained C8-projecting cells was also significantly greater compared to all other conditions (Wilcoxon test, p< 0.05). (E) Upper trace: NMDAR-mediated EPSC with cell held at +40mV in the presence of DNQX and PTX. Lower trace: AMPAR-mediated response with cell held at −70mV. The AMPAR/NMDAR ratio for the TC✧C8 pathway was unaffected by training (t-test, p = 0.99). (F) Top traces: superimposed postsynaptic responses to light presentation at the minimal stimulation amplitude. Main: minimal optical stimulation parameters resulted in a ~50% failure rate and successes that were of consistent amplitude, suggesting the same TC axon was being stimulated across trials. (G) For C8-projecting cells, mean EPSC amplitude for putative single TC axon stimulation was significantly higher in trained vs untrained animals (Wilcoxon test, p < 0.05).
Unitary responses are greater in learning-relevant cells following training
Our strontium experiments indicate that q increases by ~35% in the C8-projecting population following training, accounting for roughly half of the 70% increase seen under the bulk stimulation conditions presented in Figure 2H. Because the bulk EPSC is directly related to the product q*n, we hypothesized that the remaining ~35% increase unaccounted for by q could reflect a doubling in the number of synaptic contacts (n) between TC axons and C8-projecting cells. To test this, we examined the unitary postsynaptic response (uEPSC) in C8-projecting cells by stimulating a single TC axon via minimal optical stimulation (Boyd et al., 2012). Briefly, photostimulation intensity was increased from zero until the threshold activation level for a single ChR2 axon was reached, with roughly half of light presentations resulting in failures (Figure 4F). In C8-projecting cells, uEPSC amplitude was ~35% greater for trained vs. untrained animals (Wilcoxon test, p = 0.02; Fig 4f). This increase in uEPSC amplitude mimicked the 35% increase obtained for q, suggesting that the number of contacts between synaptically connected TC and C8-projecting cells does not increase during training (but see discussion).
DISCUSSION
Recent findings indicate that layer 5 (L5) receives direct, relatively strong input from the primary thalamus (Constantinople and Bruno, 2013; Hooks et al., 2013). However, little is known regarding the fundamental characteristics of this thalamocortical-to-layer 5 (TC-L5) system. In the current study, we identify several core functional features of the adult TC-L5 pathway, finding that TC synaptic transmission is homogenously allocated across L5 corticospinal subpopulations under baseline conditions, yet possesses the flexibility to adapt to imposed behavioral demands following motor skill training.
Thalamocortical input is balanced across layer 5 subpopulations in naïve adult animals
The magnitude of TC input onto a specific cortical region shows substantial variation between cortical lamina and postsynaptic neuronal subtype (Gibson et al., 1999; Hooks et al., 2013; Kloc and Maffei, 2014). However, how TC input is distributed among neighboring neurons of the same subtype that mediate distinct functions has yet to be established. We addressed this question by targeting intermingled, functionally distinct L5 corticospinal subpopulations associated with control of different motor outputs. Our results demonstrate that thalamic signaling is evenly distributed across corticospinal subpopulations at a pre-training, baseline condition.
Adult plasticity of thalamocortical-layer 5 signaling following motor skill learning
Traditionally, plasticity of the thalamocortical system was thought to play a minor role in experience-dependent adaptations following the developmental critical period (De Paola et al., 2006; Fox et al., 2002; Keller and Carlson, 1999). Recent studies, however, have established that TC projections can exhibit considerable reorganization following nerve damage and sensory deprivation in adulthood (Oberlaender et al., 2012; Wimmer et al., 2010; Yu et al., 2012). Whether adult TC plasticity accompanies periodic behavioral training in the intact adult animal is unknown. Here, we have shown that not only does plasticity of the TC-L5 system exist as a component of normal adult learning, but is remarkably specific for learning-relevant neurons, with TC input becoming biased toward grasp-related corticospinal neurons following skilled grasp training.
In accordance with the quantal theory of synaptic transmission, the observed learning-related change in TC-L5 signaling could stem from alterations in multiple synaptic properties. We directly assessed the potential contributions of presynaptic release probability (pr) and postsynaptic quantal amplitude (q) to the observed change in TC-L5 input. Our results indicate that pr is unaffected by learning, whereas q is selectively increased in the learning-relevant, C8-projecting population following training. These findings are in agreement with a recent report demonstrating that TC input to layer 4 cortex following nerve resection is associated with modifications in q, but not pr (Yu et al., 2012), and suggest adult plasticity may be prominently implemented at the postsynaptic level.
An additional site of plasticity potentially affecting TC signaling is an alteration in the number of synaptic contacts (n) between TC axons and L5 neurons. Previously, spine density has been demonstrated to selectively increase in C8-projecting corticospinal neurons following skilled grasp training (Wang et al., 2011). Because the learning-associated increase in q accounted for only half of the learning-associated increase seen under bulk stimulation conditions (and because the bulk EPSC is proportional to n*q), we hypothesized that the number of synaptic contacts between TC axons and C8-projecting cells would increase following training. Although stimulation of a single TC axon induced a larger uEPSC for trained vs. untrained animals, the magnitude of this increase did not differ from that observed for q, suggesting that, for synaptically coupled neurons, the number of synaptic contacts per TC axon did not increase with learning. Importantly, however, this should not be taken as evidence that the overall number of synapses between TC axons and C8-projecting cells does not increase with learning. Indeed, our results are consistent with a scenario where synaptogenesis between previously uncoupled cells accompanies learning. This possibility should be explored in future experiments.
Target-specific modulation of thalamocortical signaling
As previously mentioned, the observed biasing of TC input toward the L5, C8-projecting population could theoretically stem from numerous causes, including an increase in synaptic drive onto C8-projecting cells and/or a decrease in synaptic input onto the C4-projecting population. Our results support the hypothesis that learning results in a selective increase in synaptic drive on to the learning-relevant C8-projecting cell population, while synaptic drive onto the C4-projecting population remains unchanged. Together, these results suggest that there is not a set equilibrium of TC-L5 input that must be persistently balanced across postsynaptic populations, but that task-specific learning can drive synaptic modifications in TC transmission with distinct downstream targets.
The contribution of increased TC input to learning
Motor learning is likely distributed across multiple loci in the brain; findings of the present study for the first time identify the TC projection as one of these loci. Plasticity of TC projections onto C8-projecting neurons could impact target cells in several ways, including priming corticospinal neurons for firing of a coordinated, learned motor program (Graziano, 2015). Future experiments will explore this and other possibilities. It is also possible that the augmentation of TC input we observed is simply a function of increased activity in this projection elicited by the skilled reaching task; however, this explanation is unlikely, for several reasons. First, training was limited to only 60 reaching attempts per day, comprising a very small fraction of daily forelimb activity that includes grooming, play, moving about the environment, rearing, and consuming food. Second, both distal (C8-projecting) and proximal (C4-projecting) forelimb musculature is necessary for the reach-to-grasp task, yet plasticity only occurred in TC inputs onto C8-projecting neurons. Third, recordings were conducted 1-5 days following cessation of training, a delay over which simple use-dependent potentiation might be expected to wane.
In total, the results of the current study establish the presence of adult plasticity within the thalamocortical-layer 5 pathway in the context of adult motor learning, and demonstrate the remarkable precision with which these changes occur.
EXPERIMENTAL PRODECURES
All procedures adhered to American Association for the Accreditation of Laboratory Animal Care and institutional guidelines. Detailed methods are in Supplemental Experimental Procedures.
Supplementary Material
ACKNOWLEDGEMENTS
This research was funded by the NIH (AG10435), the Veterans Administration, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.S.B., Y.T., and J.M.C. collected/analyzed data. All authors designed experiments, discussed results, and commented on the manuscript.
REFERENCES
- Akintunde A, Buxton DF. Differential sites of origin and collateralization of corticospinal neurons in the rat: a multiple fluorescent retrograde tracer study. Brain Res. 1992;575:86–92. doi: 10.1016/0006-8993(92)90427-b. [DOI] [PubMed] [Google Scholar]
- Biane JS, Scanziani M, Tuszynski MH, Conner JM. Motor cortex maturation is associated with reductions in recurrent connectivity among functional subpopulations and increases in intrinsic excitability. J Neurosci. 2015;35:4719–4728. doi: 10.1523/JNEUROSCI.2792-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical feedback control of olfactory bulb circuits. Neuron. 2012;76(6):1161–74. doi: 10.1016/j.neuron.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Deep Cortical Layers are Activated Directly by Thalamus. Science (New York, NY) 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The Role of Visual Experience in the Development of Columns in Cat Visual Cortex. Science (New York, NY) 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Jr., Miledi R, Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969;200:267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: Cytoarchitecture and microstimulation mapping. The Journal of Comparative Neurology. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Fox K, Wallace H, Glazewski S. Is there a thalamic component to experience-dependent cortical plasticity? Philos Trans R Soc Lond B Biol Sci. 2002;357:1709–1715. doi: 10.1098/rstb.2002.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Wong RO. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron. 2005;48:465–477. doi: 10.1016/j.neuron.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Whishaw IQ. Parallel stages of learning and recovery of skilled reaching after motor cortex stroke: "oppositions" organize normal and compensatory movements. Behav Brain Res. 2006;175:249–262. doi: 10.1016/j.bbr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Graziano MS. Ethological Action Maps: A Paradigm Shift for the Motor Cortex. Trends Cogn Sci. 2015 doi: 10.1016/j.tics.2015.10.008. doi:10.1016/j.tics.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Mao T, Gutnisky DA, Yamawaki N, Svoboda K, Shepherd GM. Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J Neurosci. 2013;33:748–760. doi: 10.1523/JNEUROSCI.4338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms Underlying Development of Visual Maps and Receptive Fields. Annual review of neuroscience. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Carlson GC. Neonatal whisker clipping alters intracortical, but not thalamocortical projections, in rat barrel cortex. J Comp Neurol. 1999;412:83–94. doi: 10.1002/(sici)1096-9861(19990913)412:1<83::aid-cne6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kloc M, Maffei A. Target-specific properties of thalamocortical synapses onto layer 4 of mouse primary visual cortex. J Neurosci. 2014;34:15455–15465. doi: 10.1523/JNEUROSCI.2595-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R, Sestan N, Crair MC. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron. 2013;79:970–986. doi: 10.1016/j.neuron.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11:471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Ninomiya T, Yamashita T, Takada M. Reorganization of corticospinal tract fibers after spinal cord injury in adult macaques. Sci Rep. 2015;5:11986. doi: 10.1038/srep11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlaender M, Ramirez A, Bruno RM. Sensory experience restructures thalamocortical axons during adulthood. Neuron. 2012;74:648–655. doi: 10.1016/j.neuron.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Feldman ML. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. IV. Terminations upon spiny dendrites. J Neurocytol. 1977;6:669–689. doi: 10.1007/BF01176379. [DOI] [PubMed] [Google Scholar]
- Sadaka Y, Weinfeld E, Lev DL, White EL. Changes in mouse barrel synapses consequent to sensory deprivation from birth. The Journal of Comparative Neurology. 2003;457:75–86. doi: 10.1002/cne.10518. [DOI] [PubMed] [Google Scholar]
- Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- Suter BA, Shepherd GM. Reciprocal interareal connections to corticospinal neurons in mouse M1 and S2. J Neurosci. 2015;35:2959–2974. doi: 10.1523/JNEUROSCI.4287-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini AP, Morris R. Spatial characterization of the motor neuron columns supplying the rat forelimb. Neuroscience. 2012;200:19–30. doi: 10.1016/j.neuroscience.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Wang L, Conner JM, Rickert J, Tuszynski MH. Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci U S A. 2011;108:2545–2550. doi: 10.1073/pnas.1014335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Broser PJ, Kuner T, Bruno RM. Experience-induced plasticity of thalamocortical axons in both juveniles and adults. J Comp Neurol. 2010;518:4629–4648. doi: 10.1002/cne.22483. [DOI] [PubMed] [Google Scholar]
- Yamawaki N, Borges K, Suter BA, Harris KD, Shepherd GM. A genuine layer 4 in motor cortex with prototypical synaptic circuit connectivity. Elife. 2014;3:e05422. doi: 10.7554/eLife.05422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Chung S, Chen DY, Wang S, Dodd SJ, Walters JR, Isaac JT, Koretsky AP. Thalamocortical inputs show post-critical-period plasticity. Neuron. 2012;74:731–742. doi: 10.1016/j.neuron.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.