Abstract
Trabecular Bone Score (TBS) has been shown to predict major osteoporotic (clinical vertebral, hip, humerus, and wrist) and hip fractures in post-menopausal women and older men, but the association of TBS with these incident fractures in men independent of prevalent radiographic vertebral fracture is unknown. TBS was estimated on AP spine DXA scans obtained at the baseline visit for 5,979 men age ≥65 years enrolled in MrOS and its association with incident major osteoporotic and hip fractures estimated with proportional hazards models. Model discrimination was tested with Harrell’s C-statistic and with a categorical net reclassification improvement index, using 10-year risk cutpoints of 20% for major osteoporotic and 3% for hip fractures. For each standard deviation decrease in TBS, there were hazard ratios of 1.27 (95% C.I. 1.17 to 1.39) for major osteoporotic fracture, and 1.20 (95% C.I. 1.05 to 1.39) for hip fracture, adjusted for FRAX with BMD 10 year fracture risks and prevalent radiographic vertebral fracture. In the same model, those with prevalent radiographic vertebral fracture compared to those without prevalent radiographic vertebral fracture had hazard ratios of 1.92 (95% C.I. 1.49 to 2.48) for major osteoporotic fracture and 1.86 (95% C.I. 1.26 to 2.74) for hip fracture. There were improvements of 3.3%, 5.2%, and 6.2%, respectively, of classification of major osteoporotic fracture cases when TBS, prevalent radiographic vertebral fracture status, or both were added to FRAX with BMD and age, with minimal loss of correct classification of non-cases. Neither TBS nor prevalent radiographic vertebral fracture improved discrimination of hip fracture cases or non-cases. In conclusion, TBS and prevalent radiographic vertebral fracture are associated with incident major osteoporotic fractures in older men independent of each other and FRAX 10 year fracture risks, and these data support their use in conjunction with FRAX for fracture risk assessment in older men.
Keywords: TBS, trabecular bone score, major osteoporotic fracture, hip fracture, prediction models
Introduction
Trabecular Bone Score (TBS) is a surrogate measure of vertebral trabecular architecture derived from pixel by pixel textural analysis of the AP spine fan-beam dual energy x-ray absorptiometry (DXA) scans.(1–3) TBS correlates highly with direct measures of trabecular microarchitecture from micro-CT scans of vertebral cadaver bone,(4,5) but only weakly to modestly with lumbar spine bone mineral density (BMD).(3) The majority of individuals who have osteoporotic fractures do not have osteoporosis by bone density criteria (femoral neck T-score ≤ −2.5), and TBS has been proposed as an additional complementary method to BMD to identify individuals at high risk of fracture.(1,6) Several studies have now shown that TBS is associated with incident major osteoporotic (defined as clinical vertebral, hip, humerus, and wrist) fractures and hip fractures in Caucasian women independent of FRAX with BMD estimated fracture probabilities,(3,7,8) and in one study of Japanese women,(7) also independent of prevalent radiographic vertebral fracture. The FRAX website now allows estimated 10 year major osteoporotic and hip fracture risks to be adjusted for TBS, if it is known.
In men, the association of TBS with incident clinical fractures has been estimated in several cohorts. The recently completed meta-analysis of the FRAX cohorts (which included about 7,000 older men)(6) and analyses of smaller populations of men in the Japanese FORMEN cohort(9) and Manitoba Bone Density database(2) have indicated that TBS predicts incident major osteoporotic and hip fractures in men, after adjustment for the other clinical risk factors that are included in FRAX. To our knowledge, there has not been a prospective study in men that has estimated the ability of TBS to predict incident major osteoporotic and hip fractures in men after adjustment for both clinical risk factors included in FRAX and for prevalent radiographic vertebral fracture status. Since prevalent radiographic vertebral fracture and TBS both reflect deterioration of trabecular microarchitecture, it is important to test whether or not the associations of TBS with incident fractures in older men are attenuated with adjustment for prevalent radiographic vertebral fracture.
Our primary study objective was to use data from the Osteoporotic Fractures in Men (MrOS) study to estimate the associations of TBS with incident major osteoporotic and hip fractures in older men after adjustment for both FRAX 10 year fracture risk scores and prevalent radiographic vertebral fracture. Our second study objective was to estimate if models that include TBS, prevalent radiographic vertebral fracture, or both improve prediction of incident major osteoporotic and hip fractures compared to FRAX plus BMD alone. Improvement of prediction of major osteoporotic and hip fractures with TBS and/or lateral spine imaging to detect vertebral fractures compared to FRAX plus BMD alone would support its use in clinical practice in conjunction with FRAX to aid fracture risk assessment in older men.
Materials and Methods
The population based MrOS study recruited 5,994 community dwelling men age 65 and older between 2000 and 2002 in six U.S. geographic locations (Birmingham AL, Minneapolis MN, Palo Alto CA, Pittsburgh PA, Portland OR, and San Diego, CA). The methods of study recruitment have been previously described.(10,11) The cohort for this analysis was comprised of 5,863 men who had TBS and FRAX measurements and BMI<37 kg/m2 at baseline.
Measurement of TBS
TBS was scored on baseline visit AP spine DXA scans for 5,979 men using Med-Imaps Software (version 2.1) for the lumbar vertebrae L1 to L4 that had not been excluded in the AP spine BMD measurement. The remainder of men enrolled in MrOS either did not have a spine DXA or had one of insufficient quality for TBS to be accurately scored.
Measurement of bone mineral density
Bone mineral density was measured at the femoral neck and total hip with QDR-4500 fan-beam densitometers (Hologic, Bedford, MA, USA), at the baseline MrOS visit. Central training of densitometry technologists and cross-calibration of densitometers across study centers with a phantom was done to ensure consistency and quality of bone mass measurement.(12)
Measurement of other covariates
At the baseline visit, height loss was calculated as the difference between recalled young height at age 25 and height measured at the baseline visit with a Harpenden stadiometer. Current weight was measured at the baseline visit with a balance beam or electronic scale, and body mass index (BMI) calculated as weight (kg) divided by height (meters) squared. Participants were asked if they experienced any fractures since age 50, and if so, the skeletal location of the fracture(s). They were asked whether or not they were currently smoking cigarettes, whether or not they were taking glucocorticoids, whether or not either their mother or their father had a hip fracture, and whether or not they had been diagnosed as having rheumatoid arthritis. Data for MrOS men was sent to the World Health Organization Collaborating Centre for Metabolic Bone Disease at the University of Sheffield, U.K. for 10 year major osteoporotic and hip fracture risk scores to be estimated using the FRAX with femoral neck BMD scoring algorithm.(13) For each of these men, if data was missing for any dichotomous clinical risk factors in the FRAX algorithm, this risk factor was assumed to be absent; baseline visit FRAX with BMD fracture risk scores ultimately were calculated for 5,991 men.
Assessment of lateral spine radiographs for prevalent vertebral fracture
Lateral lumbar and thoracic spine radiographs were obtained for all men at the baseline study visit with an x-ray tube to film distance of 40 inches using a breathing technique, with thoracic x-rays centered at T7 and lumbar x-rays centered at L3. X-rays for which more than 6 of the vertebrae from T4 through L4 were not evaluable (34 men) were considered technically inadequate. In most of these instances, either the thoracic or lateral view from the baseline study visit could be located. All technically adequate x-rays (for 5,962 men) were scanned to digital format; among these, only 174 (2.9%) had any vertebrae that were not evaluable. A triage process that has been validated in other populations was used to identify those (2,749 men) with unequivocally normal x-rays.(14) The lateral spine radiographs of the remaining 3,213 men were evaluated for vertebral fracture using the Genant semi-quantitative (SQ) criteria,(15) detailed in a previous publication.(16) Prevalent radiographic vertebral fracture was defined as the presence of one or more SQ grade 2 or grade 3 fractures.
Assessment of incident fractures
Participants were contacted by mailed postcard every 4 months and queried whether or not they had a fracture. The radiographic reports in the participant’s medical record pertaining to the fracture event were obtained and reviewed at the MrOS Coordinating Center to confirm that an incident fracture had taken place. For incident clinical vertebral fractures, spine images taken at the time of the clinical encounter were obtained and reviewed by the study radiologist, who used the Genant semi-quantitative method(15) to confirm that the community imaging study showed in increase of ≥ 1 SQ grade in one or more vertebrae compared to the study baseline lateral spine radiographs. Over the 10 year period following the baseline visit, 99% of these contacts were completed. Mean follow-up was 8.5 years (sd 2.6 years) for incident major osteoporotic fractures and 8.6 years (sd 2.5 years) for incident hip fracture.
Statistical analysis
The distributions of FRAX with BMD fracture risk scores, particularly for hip fracture, were right skewed such that regression models (described below) were poorly calibrated. Therefore, FRAX fracture risk estimates were modeled as the log of the FRAX with BMD 10 year major osteoporotic and hip fracture risk scores. Four sets of proportional hazards models through ten years of follow-up time were run with the following predictors; a) log of FRAX with BMD fracture risk as the sole predictor (base model); b) log of FRAX with BMD fracture risk plus TBS (model 1); c) log of FRAX with BMD fracture risk plus radiographic prevalent vertebral fracture (model 2), and d) log of FRAX with BMD fracture risk plus TBS and prevalent radiographic vertebral fracture (model 3). The proportional hazards assumption was violated for both log of FRAX with BMD major osteoporotic and hip fracture risk scores; therefore, models with one of these predictors included an interaction term between log of FRAX fracture risk scores and follow-up time.
Discrimination of models with TBS, prevalent radiographic vertebral fracture, or both were compared to the base model by Harrell’s C-statistic. Because of multiple comparisons (four each for major osteoporotic and hip fractures), we used a Bonferroni correction to set the p-value significance threshold at 0.012 when testing if Harrell’s C of two models were different.
We also tested how well TBS and/or prevalent radiographic vertebral fracture improved discrimination of fracture cases and non-cases compared to the base model using a categorical net reclassification improvement (NRI) index.(17) Based on recommended 10 fracture risk treatment thresholds,(18,19) we considered a model to have correctly classified major osteoporotic fracture and hip fracture cases if the model-predicted probabilities of major osteoporotic and hip fracture were, respectively, ≥ 20% and ≥ 3%. Conversely, we considered a model to have correctly classified fracture non-cases if the model-predicted probabilities were below these thresholds. The NRI statistic estimates the net improvement of classification of fracture cases as being at high risk for that fracture, and the net improvement of classification of fracture non-cases as being at low risk, when adding a predictor to a referent model, for example, the addition of TBS to a model with FRAX. In this example, the net improvement of classification of fracture cases was calculated as the proportion who were shifted from being low risk to high risk after adding TBS to FRAX with BMD (representing reclassification in the desired direction), minus the proportion who were shifted from being at high risk to low risk. Similar calculations were done to estimate the net proportion of fracture non-cases reclassified as being at low risk after adding TBS to FRAX with BMD.
We used logistic regression models to estimate the predicted probabilities of incident major osteoporotic and hip fractures over 10 years. Age was included as a covariate in the hip fracture models to assure good model calibration according to the Hosmer-Lemeshow statistic and plots of observed vs. expected number of fractures within each decile of predicted risk. For each fracture type, a total of six comparisons were done (three models comparisons each for fracture cases and non-cases); therefore we used a Bonferroni correction to set the p-value for significance at 0.0083.
Two sets of secondary analyses were done. First, because the calibration of FRAX in MrOS is less than optimal,(13,20) we were concerned that FRAX might be incompletely adjusting for the clinical risk factors that are part of FRAX. Therefore, to address the question as to whether or not TBS truly is associated with incident major osteoporotic and hip fractures independent of these clinical risk factors, proportional hazards models were done with femoral neck BMD, body mass index, parental history of hip fracture, personal history of a clinical fracture since age 50, current glucocorticoid use, and rheumatoid arthritis as separate predictor variables, instead of FRAX with BMD risk score. Predictors with a multivariable adjusted p-value of association >0.1 were removed in a stepwise manner.
Second, because such a large proportion of men in MrOS are at low risk of fracture, a categorical NRI index may underestimate the improvement in fracture prediction using TBS and/or lateral spine imaging to detect vertebral fracture for the subset of men at intermediate to high risk of fracture. Therefore, model predictions of incident major osteoporotic and hip fractures with the additions of TBS and/or prevalent radiographic vertebral fracture were compared in the subset of men with a femoral neck T-score < −1.0. We also compared model predictions of incident hip fracture in the subset of men with a FRAX with BMD 10 year hip fracture risk score of 1.5% to 4.5%. We considered performing a secondary analyses of model discrimination in the subset of men with FRAX predicted 10 year major osteoporotic fracture risk between 15% and 25%, but there were too few men (316) to carry this out.
Results
Of the original 5,994 enrollees, 5,863 both had a BMI < 37 kg/m2 and had AP DXA scans on which valid TBS scores could be calculated, and of these, 5,829 had lateral thoracic and lumbar spine x-rays interpretable for prevalent radiographic vertebral fracture.
The mean (sd) age and total lumbar spine trabecular bone score were, respectively, 73.7 (5.9) years, and 1.27 (0.12). Modeled in categories corresponding to degraded (≤ 1.200), partially degraded (>1.200 and < 1.350), and normal (≥ 1.350) TBS values(1) (table 1), higher TBS was strongly associated (p-values< 0.001) with greater femoral neck BMD, lower BMI, and lower 10 year hip and major osteoporotic fracture risks. Similarly, increased TBS was strongly associated (p-values<0.001) with lower proportions of older men with self-reported prior fracture or prevalent radiographic vertebral fracture.
Table 1.
Baseline characteristics of Study Population, According to TBS Level
| Characteristics | TBS Level [Range] (number) |
||
|---|---|---|---|
| Degraded [≤ 1.200] (n=1348) |
Partially degraded [>1.209 to <1.350] (n=2945) |
Normal [≥ 1.350] (n=1570) |
|
| *Age, years (sd) | 73.4 (5.7) |
74.0 (5.9) |
73.3 (5.8) |
| ^FRAX 10 year Hip Risk w/ BMD, median (IQR) | 0.017 (0.008–0.034) |
0.016 (0.007–0.030) |
0.011 (0.005–0.021) |
| ^FRAX 10 year MOF Risk w/ BMD, median (IQR) | 0.075 (0.053–0.105) |
0.068 (0.050–0.094) |
0.057 (0.045–0.077) |
| *Femoral Neck BMD, g/cm2 | 0.753 (0.121) |
0.773 (0.122) |
0.825 (0.131) |
| *BMI kg/m2, sd | 29.6 (3.5) |
26.8 (3.1) |
25.6 (2.9) |
| ‡‽Prior Clinical Fracture, % | 300 (22.2%) |
513 (17.4%) |
194 (12.4%) |
| †‽Parental Hip Fx, % | 195 (14.5%) |
377 (12.8%) |
175 (11.1%) |
| ‡‽Current Smoking, (%) | 69 (5.1%) |
92 (3.1%) |
41 (2.6%) |
| ‽≥ 3 Alcohol Drinks per day, n (%) | 61 (4.5%) |
115 (3.9%) |
58 (3.7%) |
| †‽Rheumatoid Arthritis, n (%) | 90 (6.7%) |
144 (4.9%) |
71 (4.5%) |
| ‡‽Glucocorticoid Medication Use, n (%) | 44 (3.3%) |
62 (2.1%) |
18 (1.1%) |
| ‡Prevalent Vertebral Fracture, % | 175 (13.0 %) |
199 (6.8%) |
62 (3.9%) |
p-value of association < 0.001 by ANOVA
p-value of association <0.001 by Kruskal Wallis rank test
p-value of association <0.001 by chi-square test
p-value of association >0.001 &<0.05 by chi-square test
Assumed to negative if datum was missing in calculating FRAX scores
Association of TBS and prevalent radiographic vertebral fracture with incident fractures
Over a 10 year follow-up period, 448 men (7.6%) and 181 men (3.1%) had incident major osteoporotic and hip fractures, respectively. Each standard deviation decrease in TBS was associated with a hazard ratio of 1.31 (95% C.I. 1.20 to 1.43) of incident major osteoporotic fracture, adjusted only for the log of FRAX 10 year major osteoporotic fracture risk score (model 1, table 2). Further adjustment for prevalent radiographic vertebral fracture did not significantly change this association (model 3, table 2). Men with one or more prevalent radiographic vertebral fracture had a hazard ratio of 2.10 (95% C.I. 1.63 to 2.70) of incident major osteoporotic fracture compared to those with no prevalent vertebral fracture, adjusted only for log of FRAX risk score (model 2, table 2); this association was not substantially altered with further adjustment for TBS (model 3, table 2).
Table 2.
Associations of TBS and prevalent radiographic vertebral fracture with incident major osteoporotic fractures, and model discrimination compared to base model*†
| Predictor | Model 1 (n=5862) |
Model 2 (n=5829) |
Model 3 (n=5829) |
|---|---|---|---|
| TBS (per sd decrease) | 1.31 (1.20 to 1.43) |
1.27 (1.17 to 1.39) |
|
| Prevalent Radiographic Vertebral Fracture | 2.10 (1.63 to 2.70) |
1.92 (1.49 to 2.48) |
|
| Harrell’s C (95% C.I.) | 0.69^ (0.67 to 0.72) |
0.69** (0.67 to 0.72) |
0.70‡ (0.68 to 0.72) |
Base model has log of FRAX with BMD 10 year major osteoporotic fracture risk as sole covariate; Harrell’s C for base model 0.69 (95% C,I, 0.66 to 0.71) .
Model 1 covariates: log of FRAX with BMD plus TBS; Model 2 covariates; log of FRAX with BMD plus prevalent radiographic vertebral fracture; Model 3 covariates; log of FRAX with BMD plus TBS and prevalent radiographic vertebral fracture
p-value=0.067 compared to base model
p-value=0.054 compared to base model
p-value=0.010 compared to base model
Each standard deviation decrease in TBS was associated with a hazard ratio of 1.24 (95% C.I. 1.08 to 1.43; model 1, table 3) of incident hip fracture adjusted only for log of FRAX with BMD 10 year hip fracture risk score, and this did not change with further adjustment for prevalent radiographic vertebral fracture (model 3, table 3). Those with one or more prevalent radiographic vertebral fractures had a hazard ratio of 2.02 (95% C.I. 1.38 to 2.90) of incident hip fracture compared to those with no prevalent vertebral fracture, adjusted for FRAX risk score alone (model 2, table 3). This association remained significant after additional adjustment for TBS (model 3, table 3).
Table 3.
Associations of TBS and prevalent radiographic vertebral fracture with incident hip fractures, and model discrimination compared to base model*†
| Predictor | Model 1 (n=5862) |
Model 2 (n=5829) |
Model 3 (n=5829) |
|---|---|---|---|
| TBS (per sd decrease) | 1.24 (1.08 to 1.49) |
1.20 (1.05 to 1.39) |
|
| Prevalent Radiographic Vertebral Fracture | 2.02 (1.38 to 2.96) |
1.86 (1.26 to 2.74) |
|
| Harrell’s C (95% C.I.) | 0.79 (0.76 to 0.82) |
0.79 (0.76 to 0.82) |
0.79‡ (0.76 to 0.82) |
Base model (with log of FRAX with BMD 10 year hip fracture risk estimate as sole covariate; Harrell’s C for base model is 0.79 (95% C.I. 0.76 to 0.92).
Model 1 covariates: log of FRAX with BMD plus TBS; Model 2 covariates; log of FRAX with BMD plus prevalent radiographic vertebral fracture; Model 3 covariates; log of FRAX with BMD plus TBS and prevalent radiographic vertebral fracture
p-value >0.5 compared to base model
When clinical risk factors were entered into the regression model as separate covariates instead of overall FRAX with BMD risk score, the association of prevalent radiographic vertebral fracture with incident major osteoporotic fracture remained significant (HR 1.71, 95% C.I. 1.16 to 2.53, table 4). However, TBS was no longer significantly associated with incident hip fracture after adjustment for individual clinical risk factors instead of FRAX with BMD score (table 4).
Table 4.
Associations of TBS and prevalent radiographic vertebral fracture with incident major osteoporotic and hip fractures, adjusted for other clinical risk factors individually instead of FRAX with BMD score
| Predictor | Hazard Ratio (95% C.I.) |
|
|---|---|---|
| Incident Major Osteporotic Fracture (n=5827) |
Incident Hip Fracture (n=5828) |
|
| TBS (per sd decrease)† | 1.23 (1.14 to 1.37) |
1.06 (0.88 to 1.28) |
| Prevalent Radiographic Vertebral Fracture | 1.72 (1.33 to 2.23) |
1.71 (1.16 to 2.53) |
| Age (per 5 year increase) | 1.45 (1.34 to 1.57) |
1.83 (1.61 to 2.09) |
| Femoral Neck BMD (per sd decrease) | 1.81 (1.61 to 2.03) |
2.98 (2.42 to 3.67) |
| BMI (per sd increase) | * | 1.21 (1.00 to 1.47) |
| Prior Clinical Fracture | 1.62 (1.31 to 2.00) |
* |
| Rheumatoid Arthritis | 1.57 (1.11 to 2.22) |
* |
| Current Smoking | 1.59 (0.99 to 2.57) |
3.31 (1.81 to 6.07) |
| Harrell’s C (95% C.I.) | 0.74^ (0.72 to 0.76) |
0.82^ (0.79 to 0.85) |
Predictor variable dropped from final model because p-value of association >0.1
P-value < 0.001 compared to base model with log of FRAX with BMD score as the sole predictor
Discrimination of those with from those without incident fractures
By Harrell’s C statistic, model discrimination of those who had from those who did not have an incident major osteoporotic fracture or an incident hip fracture did not significantly change when TBS and/or prevalent radiographic vertebral fracture were added as predictors to log of FRAX risk score (tables 2 and 3). Models with clinical risk factors entered as separate covariates discriminated those who had an incident hip or major osteoporotic fracture from those who did not moderately better than models that adjusted for overall FRAX fracture risk score (table 4).
The net reclassification improvement index showed small to modest increases in the proportions of major osteoporotic fracture cases correctly classified as being at high risk with the addition of TBS to FRAX (3.3%, p-value 0.005), prevalent radiographic vertebral fracture to FRAX (5.2%, p-value<0.001), and the addition of both TBS and prevalent vertebral fracture status to FRAX (6.2%, p-value<0.001, Figure 1). For the subset of men with a femoral neck T-score of less than −1.0 (using young female norms), slightly higher proportions of major osteoporotic fracture cases were classified as being at high risk (5.2%, 7.7%, and 8.7%, [p-values all <0.001] respectively), with the addition of TBS, prevalent radiographic vertebral fracture, or both to FRAX with BMD. There was a minimal decrease in the percentage of those who did not have a major osteoporotic fracture classified as being at low risk (−0.3% to −1.5%) in all of these model comparisons.
Figure 1.
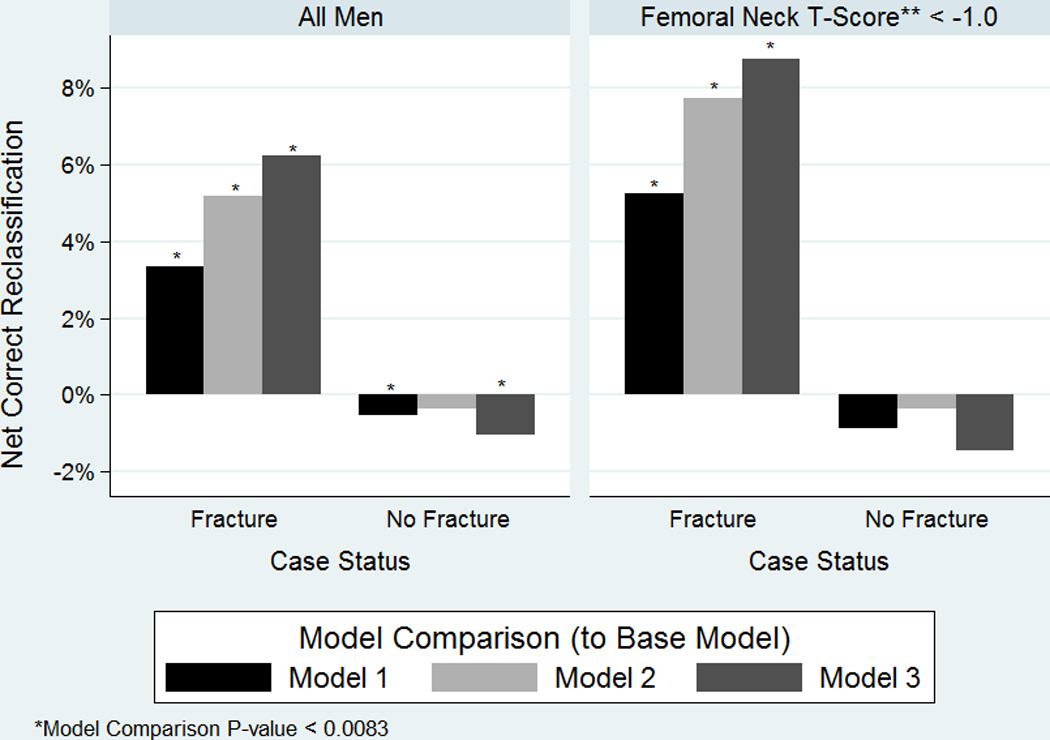
Net Correct Reclassification Improvement of Major Osteoporotic Fracture Cases and Non-Cases with addition of TBS, Prevalent Radiographic Vertebral Fracture, or Both to FRAX with BMD
On the other hand, there was minimal net reclassification of hip fracture cases or non-cases with the addition of TBS, prevalent radiographic vertebral fracture, or both to FRAX with BMD (Figure 2), when all men were included. Additional analyses of NRI subset of men with FRAX with BMD hip fracture risk scores between 1.5% and 4.5% yielded similar results (data not shown).
Figure 2.
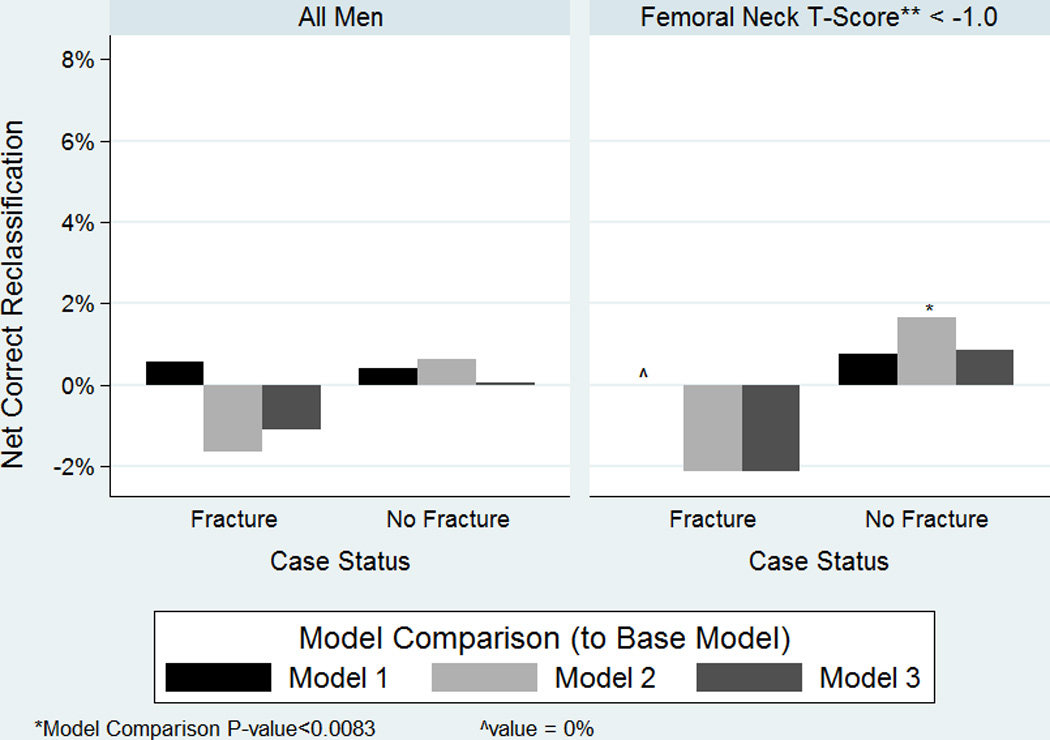
Net Correct Reclassification Improvement of Hip Fracture Cases and Non-Cases with addition of TBS, Prevalent Radiographic Vertebral Fracture, or Both to FRAX with BMD
Discussion
In this community dwelling cohort of older U.S. men, TBS was modestly associated with incident major osteoporotic and hip fractures after adjustment for FRAX with BMD fracture risk score and prevalent radiographic vertebral fracture. There was also a small improvement in correct classification of major osteoporotic fracture cases (but not hip fracture cases) when TBS was added to FRAX with BMD risk score as a predictor, especially in the subset of men with low bone mass at the femoral neck, with minimal loss of correct classification of non-cases. Our data are the first to show that TBS remains significantly associated with incident major osteoporotic and hip fractures after adjustment for prevalent radiographic vertebral fracture status in older men
Additionally, our data show that prevalent radiographic vertebral fracture are associated with incident major osteoporotic and hip fractures, even after adjustment for TBS and other clinical fracture risk factors. These data suggest that prevalent radiographic vertebral fracture status modestly discriminates those who will from those who will not have an incident major osteoporotic fracture. However, TBS has the large advantage that it can be seamlessly obtained from AP spine DXA scans done as part of routine densitometry. Even though TBS values may alter management decisions for only a minority of older men with estimated fracture risks closer to treatment thresholds (before TBS is known), beyond an initial software purchase there is no additional cost to estimate TBS on everyone who has bone densitometry, and additional physician and/or bone densitometry technician time to discern a priori who would benefit from TBS is unnecessary. In contrast, although bone densitometry technologists can use an algorithm to select the minority of those having a bone density test who would benefit from VFA,(21) this does require additional densitometry technologist time to obtain and physician time to interpret a lateral spine image. Moreover, additional resources to train physicians to expertly interpret lateral spine images for vertebral fracture is required for more widespread use of VFA in clinical practice, whereas that is unnecessary for widespread clinical application of TBS.
Our data support the practical clinical use of TBS to adjust FRAX with BMD fracture risk estimates for TBS values (as can currently be done using the FRAX website) when using FRAX fracture risk thresholds for treatment decisions. However, our results suggest that its overall impact on identification of men at high risk of major osteoporotic fracture in clinical practice will be correspondingly small, and at least in US men, will not improve identification of men at high risk of hip fracture. The impact of TBS on fracture risk is modest enough that, considered by itself, we cannot establish any TBS cutpoints in these men above or below which treatment should be given or withheld. We believe that current fracture risk assessment standards of care, such as those recommended by the National Osteoporosis Foundation,(18) should indicate that TBS is an optional addition to FRAX for fracture risk assessment for men, but is not necessary or mandatory for good clinical practice.
The hazard ratio estimate of incident major osteoporotic fracture with each standard deviation decrease in TBS in our study (1.31) is nearly identical to what thus far has been reported out of the FRAX TBS meta-analysis for men and women together (1.32).(6) However, our results are different from that of the Manitoba study, which found TBS in men to be associated with incident hip but not incident major osteoporotic fractures.(2) The Manitoba cohort consists of all individuals referred for bone densitometry testing in the province of Manitoba, Canada,(22) whereas the MrOS study recruited US men age 65 and older from general population listings (such as state driver’s license registrations). Whether or not the difference in enrollment strategy between these two studies explains these different associations of TBS with major osteoporotic fracture in older men is not known.
Our results are also in contrast with those of the FORMEN study, which suggested a large improvement in net correct reclassification of osteoporotic fractures in men with use of TBS in addition to FRAX.(9) However, there were only 22 fracture cases in FORMEN (in contrast to 441 major osteoporotic fractures in our study), and a category free net reclassification index was used in FORMEN. Category free NRI indices are highly susceptible to bias if they are applied to poorly calibrated regression models,(23,24) and it is unclear that the models in FORMEN were in fact well calibrated.
In the case of hip fracture, we were unable to show any meaningful improvement in discrimination of hip fracture cases and non-cases. Moreover, we were unable to confirm that TBS is independently associated with incident hip fracture in older men, after full adjustment for other clinical risk factors. In these respects, TBS in these men was a more robust predictor of major osteoporotic fracture than of hip fracture. We suspect that the aggregate FRAX with BMD fracture risk scores incompletely capture the effects of the individual clinical risk factors that are used in FRAX; this may be particularly true in the MrOS population since the calibration of FRAX in the MrOS population is less than optimal.(13,20)
There are many strengths to our study. The MrOS study population is broadly representative of the population of U.S. men age 65 and older, with characteristics similar to those of the National Health and Nutrition (NHANES) Survey study. To our knowledge, ours is the largest single study of the association of TBS with incident fractures in older men. Ascertainment of incident fractures in MrOS was excellent, with 99% completion of follow-up contacts to ascertain self-reported fractures and confirmation of positive self-reports by review of clinical radiographic reports.
There most important limitation to our study is that robust fracture prediction using TBS combined with FRAX may well require different coefficients of the other risk factors than those of the FRAX algorithm version (3.3) used to generate the FRAX fracture risk scores for MrOS men. Moreover, it is possible that prediction within FRAX may be improved with use of interaction terms between TBS and other risk factors within the FRAX model.(25) We could not explore these issues, since we only have the aggregate FRAX risk scores and no additional details of the FRAX algorithm. Second, it is possible that discrimination of those with major osteoporotic fracture with TBS would be even better in subsets of men with pre-TBS FRAX major osteoporotic fracture risk scores closer to the threshold value of 20%, e,g. between 15% and 25%. However, we could not obtain reliable estimates of NRI for this subset of men because of its very small size (316 men, 5.4% of the cohort). Although 10% of the MrOS study population is non-white, our results cannot be generalized beyond non-Hispanic white populations. Finally, as is true of any prospective observational study, there is the possibility that there is some residual confounding of our estimated associations of TBS with incident fractures by unmeasured or unknown characteristics.
In conclusion, TBS is independently associated with incident major osteoporotic and hip fractures in older men after adjustment for FRAX fracture risk score and prevalent radiographic vertebral fracture status. TBS and lateral spine imaging to detect prevalent vertebral fracture are complementary methods that may modestly aid fracture risk assessment and treatment decisions in older men.
Acknowledgments
This study was conducted with support by the National Institutes of Health primarily under grant number 1R21 AG046571-01.
The Osteoporotic Fractures in Men (MrOS) Study is also supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128
Footnotes
Disclosures
Dr. Ensrud serves as a consultant on a Data Monitoring Committee for Merck Sharpe & Dohme. All other authors have nothing to disclose.
References
- 1.Silva BC, Leslie WD, Resch H, et al. Trabecular Bone Score: A Noninvasive Analytical Method Based Upon the DXA Image. Journal of Bone and Mineral Research. 2014;29(3):518–530. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 2.Leslie WD, Aubry-Rozier B, Lix LM, Morin SN, Majumdar SR, Hans D. Spine bone texture assessed by trabecular bone score (TBS) predicts osteoporotic fractures in men: The Manitoba Bone Density Program. Bone. 2014;67:10–14. doi: 10.1016/j.bone.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Hans D, Goertzen AL, Krieg M-A, Leslie WD. Bone Microarchitecture Assessed by TBS Predicts Osteoporotic Fractures Independent of Bone Density: The Manitoba Study. Journal of Bone and Mineral Research. 2011;26(11):2762–2769. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 4.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg M-A. Correlations Between Trabecular Bone Score, Measured Using Anteroposterior Dual-Energy X-Ray Absorptiometry Acquisition, and 3-Dimensional Parameters of Bone Microarchitecture: An Experimental Study on Human Cadaver Vertebrae. Journal of Clinical Densitometry. 2011;14(3):302–312. doi: 10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Roux JP, Wegrzyn J, Boutroy S, Bouxsein ML, Hans D, Chapurlat R. The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: an ex vivo study. Osteoporosis International. 2013;24(9):2455–2460. doi: 10.1007/s00198-013-2316-7. [DOI] [PubMed] [Google Scholar]
- 6.Harvey NC, Gluer CC, Binkley N, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–224. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iki M, Tamaki J, Kadowaki E, et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J Bone Miner Res. 2014;29(2):399–407. doi: 10.1002/jbmr.2048. [DOI] [PubMed] [Google Scholar]
- 8.Briot K, Paternotte S, Kolta S, et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone. 2013;57(1):232–236. doi: 10.1016/j.bone.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Iki M, Fujita Y, Tamaki J, et al. Trabecular bone score may improve FRAX(R) prediction accuracy for major osteoporotic fractures in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Cohort Study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(6):1841–1848. doi: 10.1007/s00198-015-3092-3. [DOI] [PubMed] [Google Scholar]
- 10.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Cawthon PM, Ewing SK, McCulloch CE, et al. Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2009;24(10):1728–1735. doi: 10.1359/JBMR.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ensrud KE, Taylor BC, Peters KW, et al. Implications of expanding indications for drug treatment to prevent fracture in older men in United States: cross sectional and longitudinal analysis of prospective cohort study. Bmj. 2014;349:g4120. doi: 10.1136/bmj.g4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11(7):984–996. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 15.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 16.Cawthon PM, Haslam J, Fullman R, et al. Methods and reliability of radiographic vertebral fracture detection in older men: The osteoporotic fractures in men study. Bone. 2014;67:152–155. doi: 10.1016/j.bone.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 18.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Suppl 3):1–37. doi: 10.4158/ep.16.s3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ettinger B, Ensrud KE, Blackwell T, et al. Performance of FRAX in a cohort of community-dwelling, ambulatory older men: the Osteoporotic Fractures in Men (MrOS) study. Osteoporos Int. 2013;24(4):1185–1193. doi: 10.1007/s00198-012-2215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schousboe J, McKiernan F, Fuehrer J, Binkley N. Use of a performance algorithm improves utilization of vertebral fracture assessment in clinical practice. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(3):965–972. doi: 10.1007/s00198-013-2519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie WD, Caetano PA, Macwilliam LR, Finlayson GS. Construction and validation of a population-based bone densitometry database. J Clin Densitom. 2005;8(1):25–30. doi: 10.1385/jcd:8:1:025. [DOI] [PubMed] [Google Scholar]
- 23.Leening MJ, Steyerberg EW, Van Calster B, D'Agostino RB, Sr, Pencina MJ. Net reclassification improvement and integrated discrimination improvement require calibrated models: relevance from a marker and model perspective. Stat Med. 2014;33(19):3415–3418. doi: 10.1002/sim.6133. [DOI] [PubMed] [Google Scholar]
- 24.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014;160(2):122–131. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
- 25.McCloskey EV, Oden A, Harvey NC, et al. Adjusting fracture probability by trabecular bone score. Calcif Tissue Int. 2015;96(6):500–509. doi: 10.1007/s00223-015-9980-x. [DOI] [PubMed] [Google Scholar]


