Abstract
Restless Legs Syndrome (RLS) is a common sleep disorder, but there is a paucity of large cohort studies examining the association of RLS with clinical outcomes including all-cause mortality, incident coronary heart disease (CHD), stroke and chronic kidney disease (CKD).
From a nationally representative prospective cohort of over 3 million US veterans (93% male, median follow-up time of 8.1 years (IQR: 7.0–8.5 years)) with baseline estimated glomerular filtration rate (eGFR) ≥60 ml/min/1.73m2, we created a propensity-matched cohort of 7,392 patients and examined the association between incident RLS and: 1) all-cause mortality, 2) incident CHD, 3) incident strokes, and 4) incident CKD defined as eGFR<60 ml/min/1.73m2. Associations were examined using Cox models.
The mean±SD age of the propensity-matched cohort at baseline was a 59±12 year, 89% and 8% of patients were white and black, respectively, 31% of the patients were diabetic and the mean baseline eGFR was 83.9±15.1 ml/min/1.73m2. Propensity matching resulted in a balanced cohort, with the disappearance in baseline differences in comorbidities. Compared to RLS negative patients, incident RLS was associated with 88% higher mortality risk, (hazard ratio (HR) and 95% confidence interval (CI): 1.88 (1.70–2.08)), and almost 4 times higher risk of CHD and stroke (HR: 3.97 (3.26–4.84) and 3.89 (3.07–4.94), respectively). The risk of incident CKD risk was also significantly higher in incident RLS patients (HR: 3.17 (2.74–3.66)) compared to RLS negative counterparts.
In this large and contemporary cohort of US veterans, incident RLS was associated with higher risk of mortality, incident CHD, stroke and CKD.
Keywords: coronary heart disease, chronic kidney disease, kidney function, mortality, restless legs syndrome, stroke
Introduction
Restless legs syndrome (RLS) was described in the late 17th century by Sir Thomas Willis and was named RLS by Ekbom in the middle of the last century.(Ekbom, 1960) RLS is characterized by an urge to move the legs that is often hard to resist and is usually but not always associated with disagreeable leg sensations. The symptoms occur during inactivity and may interfere with sleep, are worse in the evening and night with significant relief in the morning.(Ekbom, 1960) Clinical diagnostic criteria for RLS have been established by the International RLS Study Group (IRLSSG) and have recently been modified.(Allen et al., 2003) The prevalence of RLS is estimated to be between 0.1% and 15% in the general population.(Stiasny K, 2002, Nichols et al., 2003, Tan et al., 2001, Lavigne and Montplaisir, 1994, Phillips et al., 2000)
In the general population, four previous studies assessed the association between RLS and mortality and reported contradictory results.(Pollak et al., 1990, Li et al., 2013, Szentkiralyi et al., 2012b, Mallon et al., 2008) However, these studies were limited by relatively small number of events, short follow-up period and unbalanced characteristics of patients with and without RLS.(Pollak et al., 1990, Li et al., 2013, Szentkiralyi et al., 2012b, Mallon et al., 2008) The largest study, by Szentkiralyi et al., analyzed the results of four independently conducted prospective cohort studies and reported no association between RLS and mortality of any cause.(Szentkiralyi et al., 2012b) However, a recent paper from US reported 39% higher risk of mortality in diabetes, chronic kidney disease and arthritis-free US men during 8 years of follow-up.(Li et al., 2013)
Patients with RLS have also been reported to have a higher risk of stroke and heart disease than those without the disorder.(Elwood et al., 2006, Li et al., 2012) In the Nurses’ Health Study, the women who reported more than 3 years of RLS history had higher risk of coronary heart disease (CHD) and non fatal myocardial infarct (MI).(Li et al., 2012) However, this study was subject to recall bias. In a prospective population study of almost 2,000 men, and with 10-year follow-up, RLS was associated with a 67 % increase in the relative odds for stroke compared to subjects without RLS.(Elwood et al., 2006) Contrary to the findings from these studies, Winter et al. reported no association between RLS and CHD and MI in women.(Winter et al., 2012) In addition, Szentkiralyi et al. used two cohorts’ data and reported no association between RLS and CHD and stroke.(Szentkiralyi et al., 2013) However, most of these studies were limited by a low number of events.
In patients with chronic kidney disease (CKD) and end stage renal disease (ESRD) the predictors and outcomes of RLS are well documented,(Szentkiralyi et al., 2009, Molnar et al., 2007a, Molnar et al., 2007b, Mucsi et al., 2005, Molnar et al., 2005) but it is unclear if RLS itself is a predictor of new CKD in kidney disease-free populations. RLS-associated factors such as sleep disturbance, increased sympathetic activity, increased inflammatory markers, increased cytokines such as endothelin and some comorbid conditions, such as diabetes and hypertension, are well known risk factors of CKD.(Ferini-Strambi et al., 2014)
Given that the previous studies reported contradictory results and were limited by low numbers of events, we examined the associations of incident RLS with all-cause mortality, the incidences of coronary heart disease, ischemic stroke, and CKD in a large, nationally representative contemporary cohort of US veterans. Based on previous findings, we hypothesized that RLS is associated with higher risks of these adverse clinical outcomes.
Methods
Study Setting and Cohort Definition
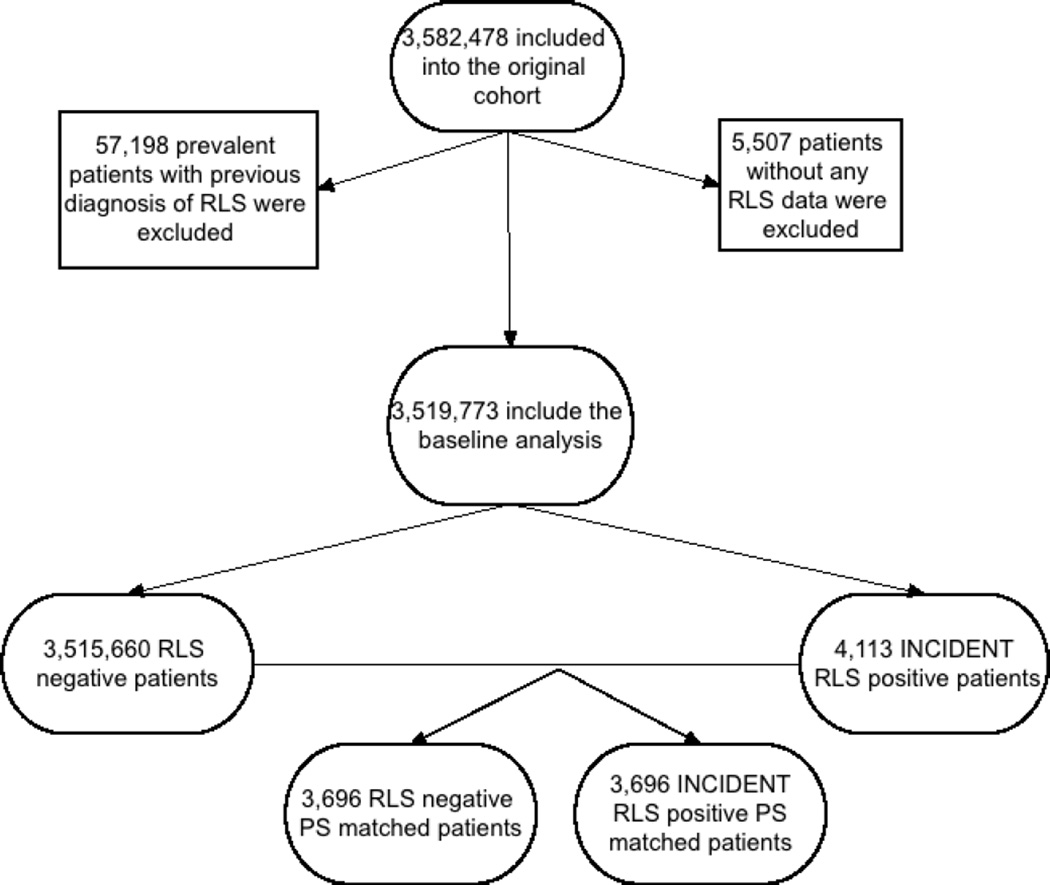
The institutional review committees at the Memphis and Long Beach Veterans Affairs Medical Centers approved the study. Given the large sample size, anonymity of the patients studied, and nonintrusive nature of the research, the requirement for written consent was waived. Data were obtained from the Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study, which examines risk factors in patients with incident CKD in US veterans, and which was previously described in detail.(Gosmanova et al., 2014, Molnar et al., 2014a) Presence of RLS was identified from the VA Inpatient and Outpatient Medical SAS Datasets using ICD-9-CM diagnostic codes (Table S1). The algorithm for cohort definition is shown in Figure 1. Patients were included in the study if they had a normal kidney functtion defined as a baseline estimated glomerular filtration rate (eGFR) ≥60 ml/min/1.73m2 and did not have a diagnosis of RLS at the first encounter in the inclusion period (October 1, 2004–September 30, 2006) and the follow-up period, while other comorbidities were listed during that encounter. The final cohort included 3,519,773 patients, 3,515,660 patients without RLS and 4,113 incident RLS patients. From this cohort, we created a 1:1 propensity score macthed cohort consisting of 3,696 patients in each group.
Figure 1.
Flow chart of patients’ selection
Exposure and Covariates
Incident RLS was defined as a new ICD9-CM code for RLS during the inclusion and follow-up period, without such a diagnosis at the first encounter.
Socio-demographic characteristics, comorbid conditions and laboratory characteristics were obtained, as previously described.(Molnar et al., 2014b, Kovesdy et al., 2012, Kovesdy et al., 2013, 2007, Molnar et al., 2014a) Information about age, gender and race were obtained through the VA Corporate Data Warehouse (CDW) and from Medicare through the VA-Medicare data merge project. Information about comorbidities was collected from the VA Inpatient and Outpatient Medical SAS Datasets using ICD-9-CM diagnostic and procedure codes and Current Procedural Terminology (CPT) codes (Table S2). Prevalent comorbidities were defined as those diagnosed during October 1, 2004–September 30, 2006.
Outcomes
We defined four different outcomes: 1) all-cause mortality, 2) incident CHD, 3) incident ischemic stroke, and 4) incidence of CKD.
Data on all-cause mortality was obtained from the VA Vital Status Files (VSF), which contain dates of death or last medical/administrative encounter from all sources in the VA system with sensitivity and specificity of 98.3% and 99.8%, respectively, as compared to the National Death Index.(Arnold N, 2006) Incident CHD was defined as the composite outcome of a first occurrence of an ICD-9-CM or CPT code for acute myocardial infarction, coronary artery bypass grafting, or percutaneous angioplasty after October 1, 2006 in patients without such diagnoses prior to this date. Incident stroke was defined as the first occurrence of ICD-9-CM codes for ischemic stroke after October 1, 2006 in patients without such diagnoses prior to this date (Table S3–S4). Incident CKD was defined as two consecutive eGFR levels <60 ml/min/1.73m2 separated by ≥90 days, and a >25% decrease from baseline eGFR. Estimated GFR was calculated from serum creatinine measurements using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation.(Levey et al., 2009) The diagnosis of RLS was always prior to the diagnosis of outcome. The follow-up time was calculated based on these dates (date of outcome – date of RLS).
Statistical Analysis
Data were summarized using proportions, means ± SD, or median (interquartile range (IQR)) as appropriate. Continuous variables were compared using the Student’s t-test and Mann-Whitney U test according to data type. Predictors of incident RLS were assessed using logistic regression analyses. The associations between RLS and outcomes were assessed using the Kaplan-Meier method, and Cox proportional hazard models.
The start of the follow-up period for mortality and incident CKD analyses was the date of the first eGFR ≥60 ml/min/1.73m2 during October 1, 2004–September 30, 2006. Patients diagnosed with RLS at a subsequent date were considered as part of the non-RLS group for the time period between cohort entry and diagnosis of RLS. Patients were followed until death or incident CKD, or were censored at the date of last healthcare or administrative visit, or on July 26, 2013.
In incident CHD and stroke analyses; incident CHD and stroke events were identified in patients without such diagnoses prior to this date; therefore, to avoid immortal time bias,(Liu et al., 2012) the start of the follow-up period for these end points was October 1, 2006. Patients diagnosed with RLS at a subsequent date were considered as part of the non-RLS group for the time period between October 1, 2006 and the diagnosis of RLS. Patients were followed until the first incident CHD/stroke event or were censored at the date of death, last healthcare or administrative visit, or on July 26, 2013.
The propensity score method was used to account for baseline differences arising from dissimilarities in clinical and demographic characteristics of patients with and without RLS. Variables associated with the presence of RLS were identified using logistic regression and were used to calculate propensity scores. STATA’s “psmatch2” command suite was used to generate the propensity score-matched cohorts by a 1-to-1 nearest neighbor matching without replacement. The following variables were included the logictis regression model to create the propensity score: age, gender, race/ethnicity, income, marital status, baseline eGFR, comorbidities at baseline (diabetes, hypertension, cardiovascular disease, heart failure, cerebrovascular disease, peripheral vascular disease, lung disease, dementia, rheumatic disease, malignancy, HIV/AIDS, depression, presence of Obstructive Sleep Apnea (OSA) and presence of Periodic Limb Movements in Sleep (PLMS)) and body mass index (BMI). All associations were examined in unadjusted models using our propensity-matched cohort of 7,392 patients. We performed subgroup analyses for all outcomes. Statistical analyses were performed using Stata MP version 12 (Stata Corporation, College Station, TX).
Results
Baseline characteristics
The mean±SD age of the cohort at baseline was 59.8±14.3 years, 93% were male, 78% and 17% of patients were white and black, respectively, 23% of the patients were diabetic and the mean baseline eGFR was 83.8±15.5 ml/min/1.73m2. Baseline characteristics of patients categorized by RLS status are shown in Table 1. In the original cohort (n=3,519,773) patients with RLS were slightly younger, more likely to be white, divorced and to have lower income, and had higher body mass index (BMI), higher prevalence of hypertension, diabetes mellitus, CVD, congestive heart failure (CHF), cerebrovascular disease, peripheral artery disease, chronic lung disease, and depression. After propensity score matching these differences disappeared and the baseline characteristics of patients with and without RLS were balanced (Table 1).
Table 1.
Baseline characteristics of study population
| Before matching | After matching | |||
|---|---|---|---|---|
| RLS negative (n=3,515,660) |
RLS positive (incident) (n=4,113) |
RLS negative (n=3,696) |
RLS positive (incident) (n=3,696) |
|
| Age (years) | 60±14 | 59±11 | 59±13 | 59±11 |
| Gender (male) | 3,264,846 (93) | 3,816 (93) | 3,439 (93) | 3,446 (93) |
| Outcomes: | ||||
| Death | 726,539 (21) | 956 (23) | 782 (21) | 853 (23) |
| Incident CHD event* | 89,274 (3) | 456 (12) | 157 (4) | 425 (12) |
| Incident stroke event** | 61,811 (2) | 314 (8) | 104 (3) | 293 (8) |
| New CKD | 353,470 (10) | 886 (22) | 503 (14) | 787 (21) |
| Race: | ||||
| White | 2,471,180 (79) | 3,681 (91) | 3,222 (87) | 3,372 (91) |
| African-American | 543,365 (17) | 248 (6) | 399 (11) | 221 (6) |
| Hispanic | 72,386 (2) | 33 (1) | 35 (1) | 30 (1) |
| Other Race | 67,727 (2) | 80 (2) | 40 (1) | 73 (2) |
| Marital status: | ||||
| Married | 1,866,693 (56) | 2,090 (53) | 1,947 (53) | 1,946 (53) |
| Single | 375,896 (11) | 312 (8) | 401 (11) | 293 (8) |
| Divorced | 856,487 (26) | 1,242 (32) | 1,058 (29) | 1,195 (32) |
| Widow | 247,601 (7) | 283 (7) | 290 (8) | 262 (7) |
| Other sociodemographic: | ||||
| Mean per capita income (USD) | 22,955 (11,696–36,167) | 20,846 (12,029–31,300) | 20,473 (11,180–31,427) | 20,917 (12,091–31,315) |
| Baseline eGFR (ml/min./1.73m2) | 84±15 | 84±15 | 84±15 | 84±15 |
| BMI (kg/m2) | 28.8±5.2 | 29.7±5.5 | 29.6±5.6 | 29.7±5.5 |
| Comorbidities: | ||||
| Hypertension | 2,062,000 (59) | 2,642 (64) | 2,363 (64) | 2,367 (64) |
| Diabetes mellitus | 824,164 (23) | 1,278 (31) | 1,150 (31) | 1,134 (31) |
| Cardiovascular Disease*** | 396,243 (11) | 670 (16) | 574 (15) | 613 (17) |
| Congestive Heart Failure | 154,007 (4) | 300 (7) | 281 (8) | 273 (7) |
| Cerebrovascular Disease | 211,618 (6) | 327 (8) | 295 (8) | 301 (8) |
| Peripheral Arterial Disease | 189,796 (5) | 391 (10) | 368 (10) | 355 (10) |
| Chronic Lung Disease | 632,228 (18) | 1,295 (31) | 1,172 (32) | 1,160 (31) |
| Dementia | 29,443 (0.8) | 29 (0.7) | 38 (1) | 24 (1) |
| Rheumatologic Disease | 48,438 (1) | 76 (2) | 86 (2) | 68 (2) |
| Peptic ulcer disease | 64,471 (2) | 131 (3) | 77 (2) | 117 (3) |
| Liver Disease | 41,651 (1) | 74 (2) | 53 (1) | 71 (2) |
| All malignancies | 359,970 (10) | 413 (10) | 377 (10) | 377 (10) |
| AIDS/HIV | 21,466 (0.6) | 17 (0.4) | 12 (0.3) | 15 (0.4) |
| Depression | 316,188 (9) | 811 (20) | 735 (20) | 720 (19) |
| Obstructive Sleep Apnea | 432,871 (12) | 1693 (41) | 1536 (42) | 1503 (41) |
| Periodic Limb Movement in Sleep | 6352 (0.2) | 108 (3) | 82 (2) | 101 (3) |
Dichotomous/dummy variables are presented as number of patients and percentage; continous variables are presented as mean±SD or median (interquartile range, IQR)
in cardiovascular disease (see below) free patients at baseline
in stroke disease free patients at baseline
Cardiovascular Disease was defined as acute myocardial infraction, angina, coronary artery disease, previous coronary artery bypass grafting or percutaneous coronary intervention
Abbreviations: AIDS: Acquired immundeficiency syndrome; BMI: Body mass index; CHD: coronary heart disease; CKD: Chronic Kidney Disease; eGFR: Estimated glomerular filtration rate; HIV: Human Immundeficiency Virus; IQR: Interquartile range; RLS: Restless Legs Syndrome; SD: Standard deviation; USD: US dollars
Predictors of RLS
In our adjusted logistic regression model, female gender, white race, lower income, higher BMI and most of the comorbidities (such as diabetes, CVD, peripheral artery disease, chronic lung disease, depression, presence of OSA and presence of PLMS) were associated with a higher risk of incident RLS (Table 2).
Table 2.
Predictors of incident RLS using logistic regression analysis in the entire cohort (n=3,519,773)
| Odds Ratio (OR) | 95% confidence interval of OR |
|
|---|---|---|
| Age (+10 year) | 1.01 | 0.98 – 1.04 |
| Gender: female vs male (ref.) | 1.37 | 1.19 – 1.56 |
| Race: | ||
| White (ref.) | 1.00 | 1.00-1.00 |
| African-American | 0.28 | 0.24 – 0.32 |
| Hispanic | 0.29 | 0.20 – 0.42 |
| Other Race | 0.77 | 0.61 – 0.97 |
| Income (+1 log) | 0.91 | 0.88 – 0.94 |
| Marital status: Unmarried vs married (ref.) | 1.10 | 1.03 – 1.18 |
| Baseline eGFR (+10 ml/min./1.73m2) | 1.03 | 1.00 – 1.05 |
| Presence of diabetes vs absence of diabetes (ref.) | 1.22 | 1.13 – 1.32 |
| Presence of hypertension vs absence of hypertension (ref.) | 1.04 | 0.96 – 1.12 |
| Presence of Cardiovascular Disease vs absence of Cardiovascular Disease (ref.) | 1.13 | 1.03 – 1.24 |
| Presence of Congestive Heart Failure vs absence of Congestive Heart Failure (ref.) | 1.14 | 0.99 – 1.30 |
| Presence of Cerebrovascular Disease* vs absence of Cerebrovascular Disease* (ref.) | 1.09 | 0.96 – 1.23 |
| Presence of Peripheral Arterial Disease vs absence of Peripheral Arterial Disease (ref.) | 1.43 | 1.27 – 1.60 |
| Presence of Chronic Lung Disease vs absence of Chronic Lung Disease (ref.) | 1.52 | 1.42 – 1.64 |
| Presence of Dementia vs absence of dementia (ref.) | 0.71 | 0.47 – 1.07 |
| Presence of Rheumatologic Disease vs absence of Rheumatologic Disease (ref.) | 1.14 | 0.90 – 1.45 |
| Presence of Malignancy vs absence of malignancy (ref.) | 0.98 | 0.88 – 1.09 |
| Presence of AIDS/HIV vs absence of AIDS/HIV (ref.) | 0.85 | 0.51 – 1.42 |
| Presence of depression vs absence of depression (ref.) | 1.70 | 1.57 – 1.85 |
| Body mass index (+1 kg/m2) | 0.98 | 0.98 – 0.99 |
| Presence of OSA vs absence of OSA (ref.) | 4.28 | 3.98 – 4.61 |
| Presence of PLMS vs absence of PLMS (ref.) | 4.83 | 3.94 – 5.92 |
Cardiovascular Disease was defined as acute myocardial infraction, angina, coronary artery disease, previous coronary artery bypass grafting or percutaneous coronary intervention
Abbreviations: AIDS: Acquired immundeficiency syndrome; eGFR: Estimated glomerular filtration rate; HIV: Human Immundeficiency Virus; OSA: Obstructive Sleep Apnea; PLMS: Periodic Limb Movements in Sleep; RLS: Restless Legs Syndrome
Mortality
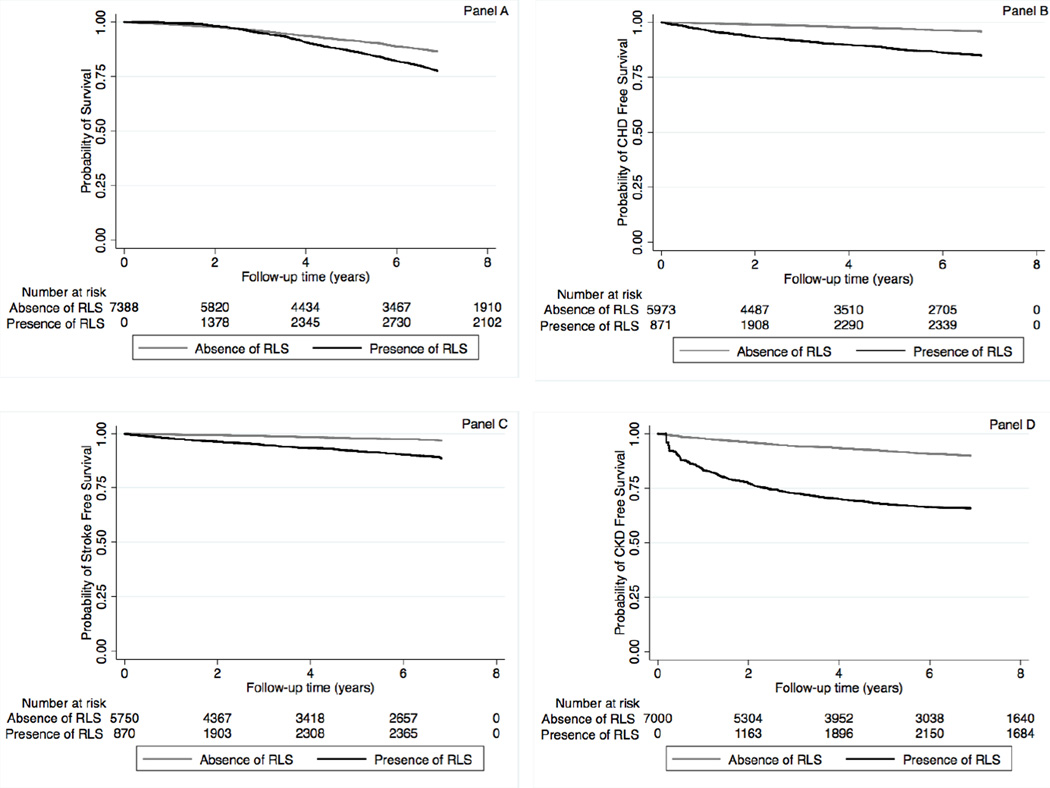
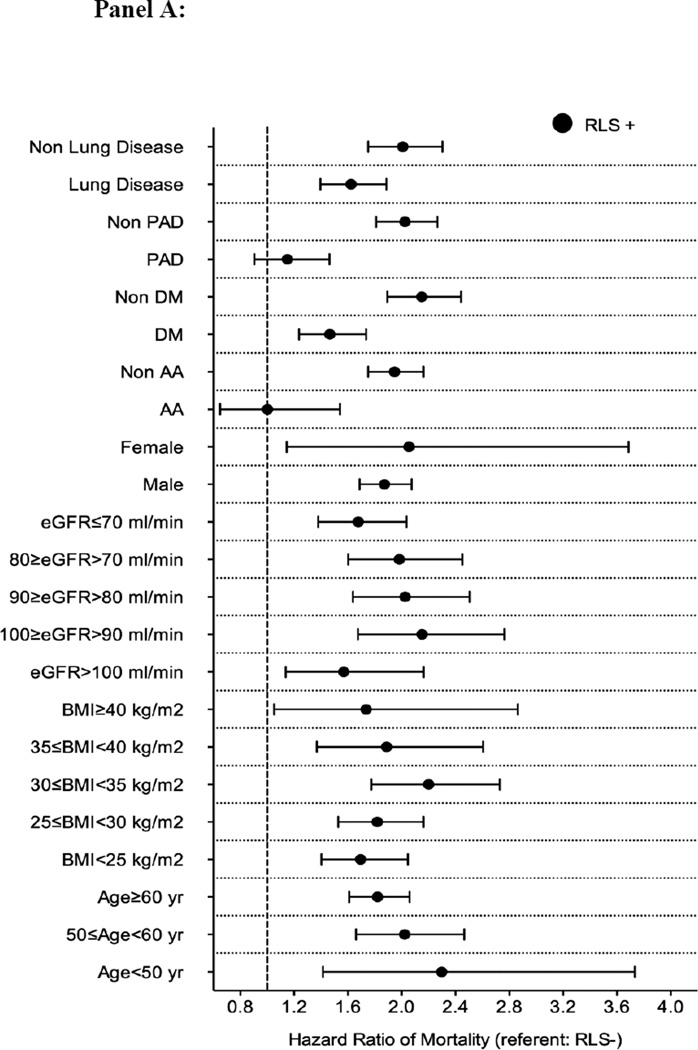
The median follow-up time was 8.1 years (IQR: 7.0–8.5 years). There were 782 deaths (21%, mortality rate 20.7 [19.3–22.2]/1000 patient-years) in the RLS negative group, and 853 deaths (23%, 51.4 [48.0–54.9]/1000 patient-years) in the RLS positive group in the propensity-matched cohort. Figure 2 panel A shows the associations between incident RLS and mortality in the propensity-matched cohort. Incident RLS was associated with higher risk of mortality (hazard ratio (HR): 1.88, 95% confidence interval (CI): 1.70–2.08) compared to RLS negative patients (Table 3). Similar results were found in all subgroups, except in patients with peripheral artery disease and in African-American patients (Figure 3 panel A). Similar association was found when we adjusted for insomnia in our sensitivity analysis (Table S5).
Figure 2.
Association between incident restless legs syndrome and outcomes (panel A: mortality; panel B: CHD free survival; panel C: ischemic stroke event free survival and panel D: CKD free survival) using Kaplan-Meiers curves in the propensity matched cohort
Abbreviations: CHD: coronary heart disease; CKD: Chronic Kidney Disease; RLS: Restless Legs Syndrome
Table 3.
Association between incident restless legs syndrome and outcomes using Cox regression models in the propensity-matched cohort (n=7,392)
| Outcomes | Hazard Ratio (HR) | 95% Confidence Interval of HR |
|---|---|---|
| All-cause mortality | 1.88 | 1.70–2.08 |
| Incident cardiovascular disease | 3.97 | 3.26–4.84 |
| Incident stroke | 3.89 | 3.07–4.94 |
| Incident chronic kidney disease | 3.17 | 2.74–3.66 |
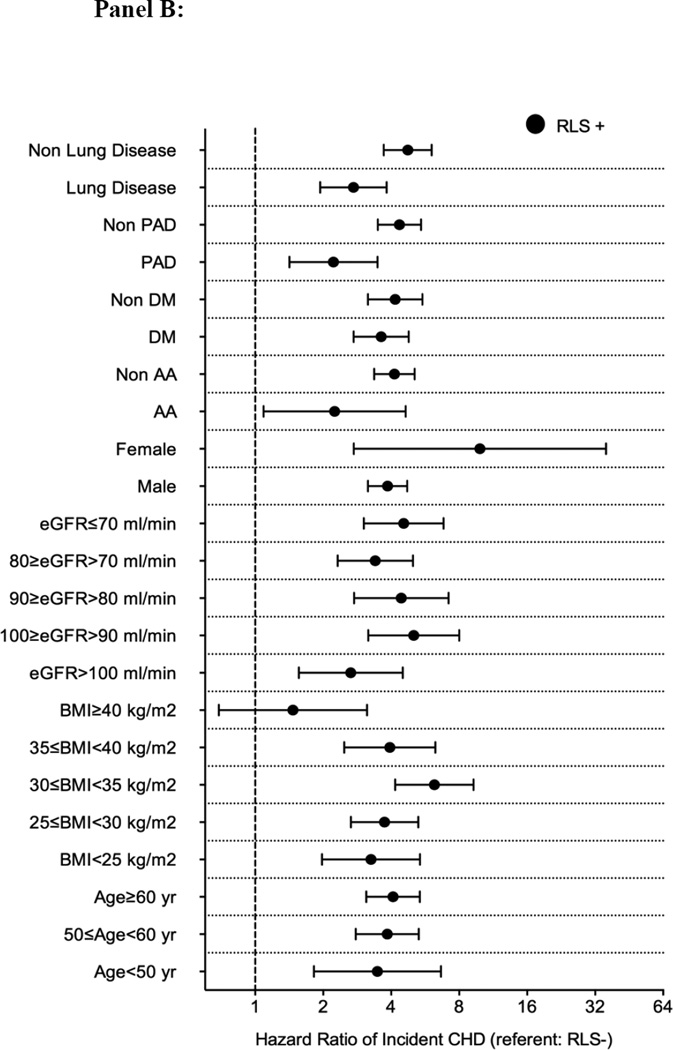
Figure 3.
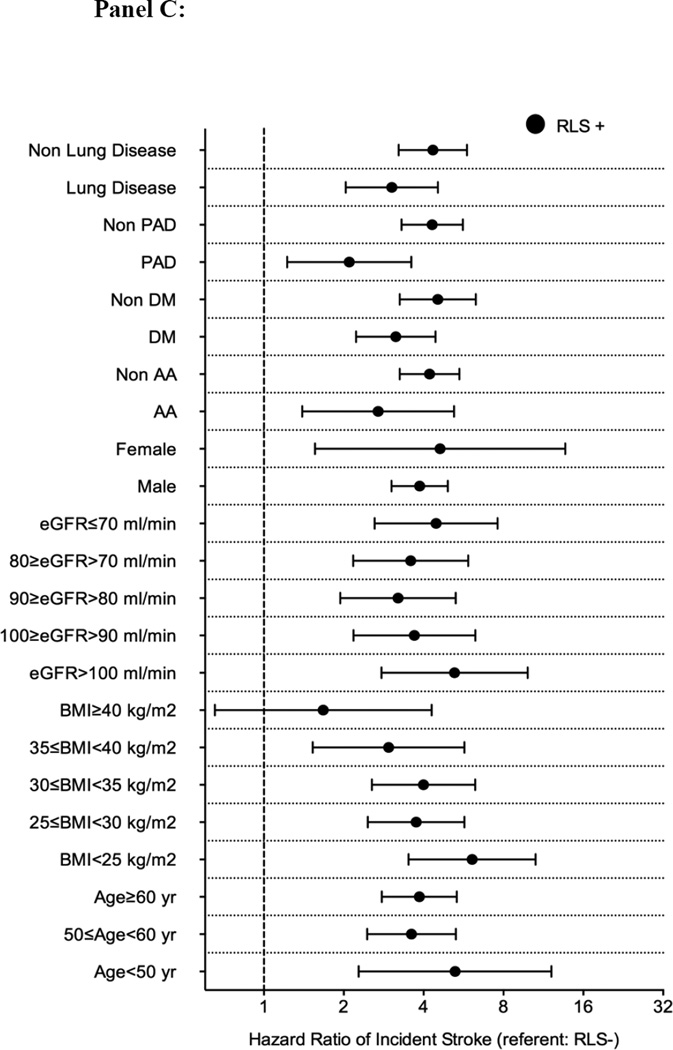
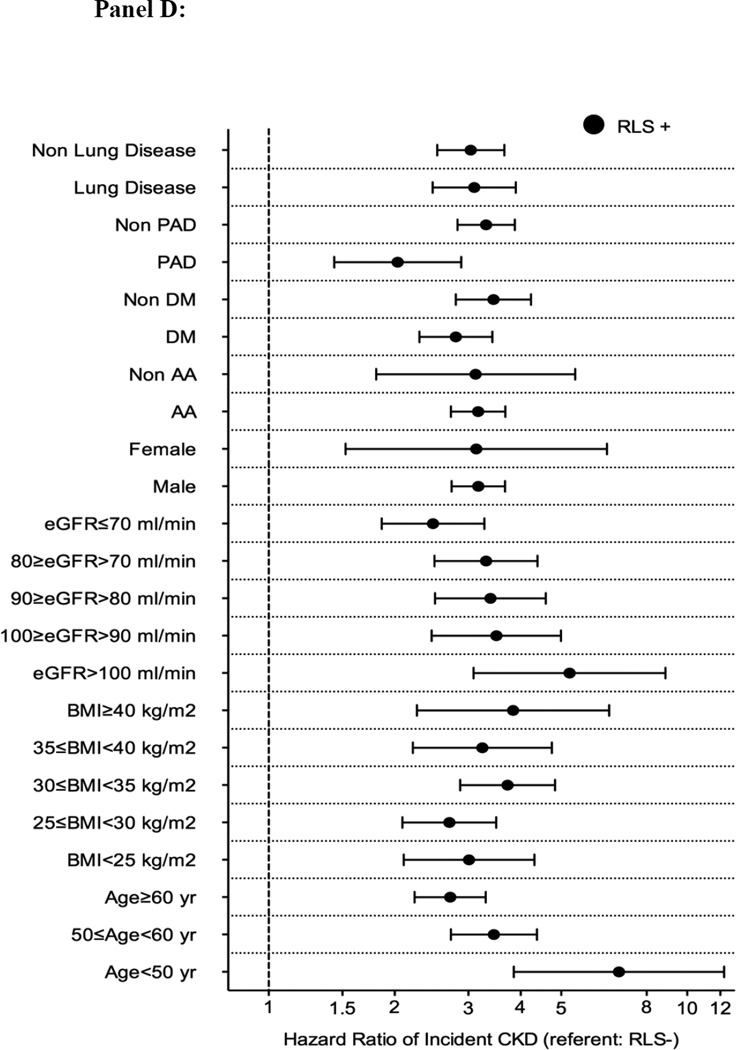
Association between incident restless legs syndrome and outcomes in different subgroups of patients (panel A: mortality; panel B: incident CHD; panel C: incident ischemic stroke event and panel D: CKD) in the propensity matched cohort
Abbreviations: AA: African-American; BMI: Body mass index; CHD: coronary heart disease; CKD: Chronic Kidney Disease; DM: Diabetes mellitus; eGFR: Estimated glomerular filtration rate; PAD: Peripheral artery disease; RLS: Restless Legs Syndrome
Incident CHD
There were 157 incident CHD events (4%, event rate 5.9 [5.1–6.9]/1000 patient-years) in the RLS negative group, and 425 incident CHD events (12%, 22.6 [20.2–25.3]/1000 patient-years) in the RLS positive group in the propensity-matched cohort. Figure 2 panel B shows the associations between incident RLS and incident CHD in the propensity-matched cohort. Incident RLS was associated with higher risk of incident CHD (HR: 3.97, 95%CI: 3.26–4.84) (Table 3). Similar results were found in all subgroups, except in patients with BMI≥40 kg/m2 (Figure 3 panel B). Similar association was found when we adjusted for insomnia in our sensitivity analysis (Table S5).
Incident stroke
There were 104 incident stroke events (3%, event rate 4.0 [3.3–4.9]/1000 patient-years) in the RLS negative group, and 293 incident stroke events (9%, 16.6 [14.5–18.9]/1000 patient-years) in the RLS positive group in the propensity-matched cohort. Figure 2 panel C shows the associations between incident RLS and incident stroke in the propensity-matched cohort. Incident RLS was associated with higher risk of incident stroke (HR: 3.89, 95%CI: 3.07–4.94) (Table 3). Similar results were found in all subgroups, except patients with BMI ≥40 kg/m2 (Figure 3 panel C). Similar association was found when we adjusted for insomnia in our sensitivity analysis (Table S5).
Incidence of eGFR <60 ml/min/1.73m2
There were 503 incident CKD events (14%, event rate 14.8 [13.5–16.1]/1000 patient-years) in the RLS negative group, and 787 incident CKD events (21%, 29.8 [27.0–32.8]/1000 patient-years) in the RLS positive group in the propensity-matched cohort. Figure 2 panel D shows the associations between incident RLS and incident CKD in the propensity-matched cohort. Incident RLS was associated with higher risk of incident CKD events (HR: 3.17, 95%CI: 2.74–3.66) (Table 3). Similar results were found in all subgroups (Figure 3 panel D). Similar association was found when we adjusted for insomnia in our sensitivity analysis (Table S5).
Discussion
In a large cohort of US veterans with baseline eGFR ≥60 ml/min/1.73m2, we examined the association of incident RLS with higher risk of all-cause mortality, incident CHD and stroke, and incident eGFR <60 ml/min/1.73m2. Incident RLS was associated with substantially higher risk of all examined clinical outcomes in this well-balanced propensity-matched cohort.
In this large cohort study, incident RLS was associated with an 88% higher risk of mortality during a median 8-year follow-up period. Higher mortality associated with RLS was reported in several previous studies, however, most of these studies used smaller cohorts of RLS patients and the number of events was also small.(Mallon et al., 2008, Li et al., 2013) Contrary to the findings from these studies and to our results, Szentkiralyi et al. did not find an association between incident RLS and mortality in an analysis of four cohorts; however, only one out of the four cohorts included more than one hundred patients with RLS.(Szentkiralyi et al., 2012b) There is extensive literature about potential explanations of why RLS can contribute to increased mortality.(Ferini-Strambi et al., 2014) First, the sleep disturbance, which is observed in 85% of RLS patients,(Ferini-Strambi et al., 2014) can contribute to increased mortality directly and/or via different comorbidities (such as CHD, stroke, hypertension, depression and diabetes), which are themselves strong predictors of RLS.(Szentkiralyi et al., 2014) Second, RLS and its sister-disorder periodic limb movements in sleep (PLMS) increases blood pressure and heart rate and increase the risk of a non-dipping blood pressure pattern.(Manconi et al., 2011, Siddiqui et al., 2007, Erden et al., 2012) Finally, RLS and PLMS are associated with increased systemic inflammation;(Trotti et al., 2012) however it is not clear whether systemic inflammation is a cause or a consequence of this disorder.(Weinstock et al., 2012)
The incident CHD and stroke risk were 4 times higher in incident RLS patients compared to patients without RLS in our cohort. Earlier studies reported strong associations between RLS and the presence of CHD and stroke.(Elwood et al., 2006, Li et al., 2012) However, Szentkiralyi et al. proposed that hypertension, MI, or stroke significantly predict the onset of RLS and proposed an alternative explanation due to different time-sequence in these cross-sectional observations.(Szentkiralyi et al., 2013) In our study, we analyzed the association of incident RLS with incident CHD and stroke in a cohort of patients who were CHD-free and stroke-free in the inclusion period. Our results confirm previous observations implicating RLS with incident CHD and stroke. In addition to the abovementioned pathophysiological mechanisms, there are other potential explanations for the association of RLS with incident CHD and stroke. There is evidence that brain iron metabolism disturbances in RLS arise from an increase in the activity of the hypoxia response pathway, specifically hypoxia-inducible factor 1 (HIF-1).(Patton et al., 2011) HIF activation is critical for carotid body mediated responses to chronic intermittent hypoxia, and it might play a role in inducing disturbances in brain iron metabolism and ischemic events in patients with RLS.(Ferini-Strambi et al., 2014) Another interesting observation is the significantly increased diastolic left ventricular diameter and mass in patients with RLS/PLMS compared to controls, both in dialysis patients and in those without ESRD and advanced heart failure.(Giannaki et al., 2013, Mirza et al., 2013) However, both these studies have identified changes in LV morphology that appear not to result in any functional changes in cardiac output. Further studies may reveal whether these changes eventually lead to functional cardiac abnormalities in patients with RLS and PLMS, or if the reverse is true.(Ferini-Strambi et al., 2014)
There is an extensive literature describing an increasing prevalence of RLS with decreasing kidney function, and the higher prevalence of this disease in patients with ESRD.(Szentkiralyi et al., 2009, Molnar et al., 2007a, Molnar et al., 2007b, Molnar et al., 2005, Mucsi et al., 2005) We report strong associations of incident RLS with incident low eGFR for the first time in a population with normal eGFR at baseline. As discussed above, RLS can cause increased systemic/renal sympathetic activity, intermittent hypoxia, hypertension, accelerated atherosclerosis, depression, production of pro-inflammatory cytokines, endothelial dysfunction, which could all contribute to the development and progression of CKD.(Ferini-Strambi et al., 2014)
Our study is notable for its large sample size and event numbers, and for it being representative of veterans in the entire US. To our knowledge, this is the first large study using a propensity score matched approach to balance measured confounders. In addition, this is the first study to find substantial associations between RLS and incident decrease in eGFR. This study also has several limitations that need to be acknowledged. This being an observational study, we can only report associations, and we cannot claim that incident RLS was indeed the cause of the worse clinical outcomes. Additionally, a propensity score method can only account for the effects of known confounders. Therefore, we cannot rule out residual confounding. Our study is limited by the use of diagnostic codes to define RLS. The diagnostic performance of these codes is not known. However, the significant predictors of incident RLS in our study were similar to those found in previous studies.(Szentkiralyi et al., 2012a) We were unable to assess the associations between the severity of RLS with various outcomes. We also have no data about the treatment of RLS. The study population consisted of mostly male patients; hence, the results may not be generalizable to females, although 7% of our propensity-matched cohort consisted of females. Because we did not have information about causes of death, we could not analyze associations with cause-specific mortality. Additionally, we did not have data about albuminuria in our database; consequently we cannot assess the association with properly defined incident CKD.
Conclusions
In our large and contemporary cohort of US veterans, incident RLS was associated with higher risk of mortality, incident CHD, stroke and CKD. Improvement of the diagnostics and early detection as well the effect of proper therapy of RLS on preventing these clinical events needs to be tested in clinical trials.
Supplementary Material
Acknowledgments
None.
Funding Sources
This study is supported by grant 1R01DK096920 from the NIH to CPK and KKZ, and by resources from the US Department of Veterans Affairs. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Footnotes
Conflict of interest
None.
Disclosures
CPK and KKZ are employees of the Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or part.
The contribution of the authors is detailed as follows:
Miklos Z Molnar contributed to data collection, contributed to analysis of the data, interpretation of data and wrote the manuscript.
Jun L Lu contributed to interpretation of data and writing the manuscript.
Kamyar Kalantar-Zadeh contributed to interpretation of data and writing the manuscript.
Csaba P Kovesdy contributed to data collection, contributed to analysis of the data, interpretation of data and wrote the manuscript.
REFERENCES
- 1.VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006–2007. Hines, IL, U.S. Department of Veterans Affairs. VA Information Resource Center. 2007 [Google Scholar]
- 2.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 3.Arnold NSM, Maynard C. Hynes Dm VIReC Technical Report 2: VA-NDI Mortality Data Merge Project. In: Center VIR, editor. Hines, IL. 2006. [Google Scholar]
- 4.Ekbom KA. Restless legs syndrome. Neurology. 1960;10:868–873. doi: 10.1212/wnl.10.9.868. [DOI] [PubMed] [Google Scholar]
- 5.Elwood P, Hack M, Pickering J, Hughes J, Gallacher J. Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J Epidemiol Community Health. 2006;60:69–73. doi: 10.1136/jech.2005.039057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erden EC, Erden I, Turker Y, Sivri N, Dikici S, Ozsahin M. Incremental effects of restless legs syndrome on nocturnal blood pressure in hypertensive patients and normotensive individuals. Blood Press Monit. 2012;17:231–234. doi: 10.1097/MBP.0b013e32835b5a39. [DOI] [PubMed] [Google Scholar]
- 7.Ferini-Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. J Neurol. 2014;261:1051–1068. doi: 10.1007/s00415-013-7065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannaki CD, Zigoulis P, Karatzaferi C, et al. Periodic limb movements in sleep contribute to further cardiac structure abnormalities in hemodialysis patients with restless legs syndrome. J Clin Sleep Med. 2013;9:147–153. doi: 10.5664/jcsm.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gosmanova EO, Lu JL, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64:951–957. doi: 10.1161/HYPERTENSIONAHA.114.03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovesdy CP, Lott EH, Lu JL, et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Lott EH, Lu JL, et al. Outcomes associated with microalbuminuria: effect modification by chronic kidney disease. J Am Coll Cardiol. 2013;61:1626–1633. doi: 10.1016/j.jacc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–743. [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126:1689–1694. doi: 10.1161/CIRCULATIONAHA.112.112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of restless legs syndrome and mortality among men. Neurology. 2013;81:52–59. doi: 10.1212/WNL.0b013e318297eee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Weinhandl ED, Gilbertson DT, Collins AJ, St Peter WL. Issues regarding 'immortal time' in the analysis of the treatment effects in observational studies. Kidney Int. 2012;81:341–350. doi: 10.1038/ki.2011.388. [DOI] [PubMed] [Google Scholar]
- 17.Mallon L, Broman JE, Hetta J. Restless legs symptoms with sleepiness in relation to mortality: 20-year follow-up study of a middle-aged Swedish population. Psychiatry Clin Neurosci. 2008;62:457–463. doi: 10.1111/j.1440-1819.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 18.Manconi M, Ferri R, Zucconi M, et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12:47–55. doi: 10.1016/j.sleep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Mirza M, Shen WK, Sofi A, et al. Frequent periodic leg movement during sleep is associated with left ventricular hypertrophy and adverse cardiovascular outcomes. J Am Soc Echocardiogr. 2013;26:783–790. doi: 10.1016/j.echo.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C virus infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2014a doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. ACE Inhibitor and Angiotensin Receptor Blocker Use and Mortality in Patients with Chronic Kidney Disease. J Am Coll Cardiol. 2014b;63:650–658. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molnar MZ, Novak M, Ambrus C, et al. Restless Legs Syndrome in patients after renal transplantation. Am J Kidney Dis. 2005;45:388–396. doi: 10.1053/j.ajkd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Molnar MZ, Novak M, Szeifert L, et al. Restless legs syndrome, insomnia, and quality of life after renal transplantation. J Psychosom Res. 2007a;63:591–597. doi: 10.1016/j.jpsychores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Molnar MZ, Szentkiralyi A, Lindner A, et al. Restless legs syndrome and mortality in kidney transplant recipients. Am J Kidney Dis. 2007b;50:813–820. doi: 10.1053/j.ajkd.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Mucsi I, Molnar MZ, Ambrus C, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005;20:571–577. doi: 10.1093/ndt/gfh654. [DOI] [PubMed] [Google Scholar]
- 26.Nichols DA, Allen RP, Grauke JH, et al. Restless legs syndrome symptoms in primary care: a prevalence study. Arch Intern Med. 2003;163:2323–2329. doi: 10.1001/archinte.163.19.2323. [DOI] [PubMed] [Google Scholar]
- 27.Patton SM, Ponnuru P, Snyder AM, Podskalny GD, Connor JR. Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur J Neurol. 2011;18:1329–1335. doi: 10.1111/j.1468-1331.2011.03397.x. [DOI] [PubMed] [Google Scholar]
- 28.Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160:2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 29.Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990;15:123–135. doi: 10.1007/BF01321316. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–1930. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Stiasny KOW, Trenkwalder C. Clinical symptomatology and treatment of restless legs syndrome and periodic limb movement disorder. Sleep Med Rev. 2002;6:253–265. doi: 10.1053/smrv.2001.0193. [DOI] [PubMed] [Google Scholar]
- 32.Szentkiralyi A, Fendrich K, Hoffmann W, Happe S, Berger K. Socio-economic risk factors for incident restless legs syndrome in the general population. J Sleep Res. 2012a;21:561–568. doi: 10.1111/j.1365-2869.2012.01001.x. [DOI] [PubMed] [Google Scholar]
- 33.Szentkiralyi A, Molnar MZ, Czira ME, et al. Association between restless legs syndrome and depression in patients with chronic kidney disease. J Psychosom Res. 2009;67:173–180. doi: 10.1016/j.jpsychores.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Szentkiralyi A, Volzke H, Hoffmann W, Happe S, Berger K. A time sequence analysis of the relationship between cardiovascular risk factors, vascular diseases and restless legs syndrome in the general population. J Sleep Res. 2013;22:434–442. doi: 10.1111/jsr.12040. [DOI] [PubMed] [Google Scholar]
- 35.Szentkiralyi A, Volzke H, Hoffmann W, Trenkwalder C, Berger K. Multimorbidity and the risk of restless legs syndrome in 2 prospective cohort studies. Neurology. 2014;82:2026–2033. doi: 10.1212/WNL.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 36.Szentkiralyi A, Winter AC, Schurks M, et al. Restless legs syndrome and all-cause mortality in four prospective cohort studies. BMJ Open. 2012b:2. doi: 10.1136/bmjopen-2012-001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan EK, Seah A, See SJ, Lim E, Wong MC, Koh KK. Restless legs syndrome in an Asian population: A study in Singapore. Mov Disord. 2001;16:577–579. doi: 10.1002/mds.1102. [DOI] [PubMed] [Google Scholar]
- 38.Trotti LM, Rye DB, De Staercke C, Hooper WC, Quyyumi A, Bliwise DL. Elevated C-reactive protein is associated with severe periodic leg movements of sleep in patients with restless legs syndrome. Brain Behav Immun. 2012;26:1239–1243. doi: 10.1016/j.bbi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstock LB, Walters AS, Paueksakon P. Restless legs syndrome--theoretical roles of inflammatory and immune mechanisms. Sleep Med Rev. 2012;16:341–354. doi: 10.1016/j.smrv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Winter AC, Schurks M, Glynn RJ, et al. Restless legs syndrome and risk of incident cardiovascular disease in women and men: prospective cohort study. BMJ Open. 2012;2:e000866. doi: 10.1136/bmjopen-2012-000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.