Abstract
Patients with primary brain tumors such as malignant gliomas are highly symptomatic, often from the time of diagnosis. Signs and symptoms (signs/symptoms) can cause functional limitations that often worsen over the disease trajectory and may impact patient quality of life. It is recognized that standard measurements of tumor response do not adequately measure this impact or the impact that a therapy may have to mitigate these signs/symptoms and potentially have clinical benefit. Identifying a core set of signs/symptoms and functional limitations is important for understanding their clinical impact and is the first step to including clinical outcomes assessment in primary brain tumor clinical trials.
Keywords: clinical outcomes assessment, glioblastoma, glioma, outcomes, signs and symptoms
Primary brain tumors (PBTs), including malignant gliomas, are often associated with significant morbidity, and most are incurable. Neurologic signs and symptoms (signs/symptoms), such as headaches, seizures, neurocognitive dysfunction, and motor deficit, often lead to diagnosis and impact patients from the time of diagnosis. These signs/symptoms cause functional limitations and later in the disease trajectory may herald tumor recurrence. Similar to other solid tumors, treatment can also result in signs/symptoms that may compound tumor-associated signs/symptoms, further impacting patient function and quality of life (QOL).
There is increasing recognition that clinical studies evaluating tumor response and using only measurements of tumor size based on imaging or metrics (eg, overall survival [OS] or progression-free survival [PFS]) are inadequate in malignant glioma patients.1 In addition, patients not only want to live longer, they want to live comfortably and function normally for as long as they can. Unfortunately, too often clinical trials do not sufficiently include rigorous clinical outcomes assessments (COAs) as endpoints.
To help invigorate brain tumor drug development that aims to develop and approve drugs to both extend life and improve or maintain function, we need to advance the addition of a priority first-set of nonradiographic patient-centered endpoints that have clear value to patients and caregivers as well as sponsors, regulators, and researchers. In an effort to improve understanding of the clinical impact of malignant glioma, the FDA/ Jumpstarting Brain Tumor Drug Development Coalition (JSBTDDC) COA workshop was held in the fall of 2014, with the primary goal of creating a consensus for a brain-tumor clinical outcomes priority list to be included in clinical trials. This paper outlines the work of Panel 1, in which the practical objective was to provide a review of the literature describing the signs/symptoms and functional limitations in the primary brain tumor patient population in order to (i) identify important signs/symptoms and functional limitations as a first step in development of endpoints, (ii) measure clinically meaningful benefit, and (iii) create a core priority list for inclusion in clinical trials seeking OS and/or PFS as the primary endpoint. This panel was comprised of experts in neuro-oncology in the areas of patient care and treatment, patient-reported and neurocognitive outcomes, industry, and a representative from the patient community.
Background
Malignant gliomas are relatively uncommon, occurring in <30 000 people per year in the United States and representing 2% of all cancers.2 Despite advances over the last several decades in therapeutic techniques and availability, survival remains poor; overall life expectancy is <2 years for patients diagnosed with glioblastoma (GBM), and the 5-year survival rate is <20%.3 Although there are other cancers with similarly poor outcomes (eg, pancreatic cancer and small cell lung cancer), there is often a sense of treatment futility in patients with malignant gliomas that can influence perception of the risks versus the benefits of treatment. This sense of futility may be enhanced by the impact the disease can have from the time of diagnosis. Studies have shown that 80%–90% of patients with high-grade gliomas had symptoms beginning from the time of diagnosis that prevented their return to work.4–6 This is less common for patients with lower-grade tumors, where reports suggest that 40% of patients do not return to the work force.7 In addition, many patients report spending a significant portion of their lives feeling ill and being unable to perform their usual activities.8,9
Integration of state-of-the-art treatment does improve OS in malignant glioma;10 however, this improvement is often measured in months. For example, concurrent temozolomide and radiotherapy followed by adjuvant temozolomide has been established as the current standard of care for newly diagnosed GBM, based on an improved OS of 2.5 months compared with radiotherapy alone (median survival: 12.1 vs 14.6 mo).11 The improvement in survival at a population level may not be consistent in all, with a recent study indicating that elderly patients may be undertreated and not have the same survival benefit,10 and neither may those treated outside of an academic center.12
In addition to assessment of survival, imaging response has been a traditional endpoint for evaluating treatment effect; however, measurement of disease on MRI has multiple limitations. Differences in technique, variable interpretation, and the effects of therapy on imaging characteristics have all emerged as barriers to consistent measurement of progression and response.13 Targeted agents, particularly those that are cytostatic and do not necessarily result in tumor shrinkage, complicate the evaluation of disease response and control. Historically, clinical trials of new anticancer agents have used survival and objective response rate as measures of clinical benefit. Other endpoints capturing treatment effect on patients' signs/symptoms, and functioning have not been routinely or adequately assessed as potential indicators of clinical benefits, as demonstrated by a recent review indicating the paucity of studies exploring the burden of treatment on patients' abilities and deficits over time.14 These factors combined make evaluating efficacy challenging and result in debate about the accuracy and meaning of clinical trial outcomes.
Signs and Symptoms in Patients with Malignant Gliomas
People diagnosed with malignant gliomas experience a high symptom burden “typically characterized by an uncertain prognosis, rapid decline of physical function, and behavioral and neurocognitive changes that parallel tumor progression” (p1).15 Recently published studies have reported that patients with malignant gliomas have a unique illness trajectory, with rapid shifts in status16 that change over time.5,16 A systematic review of 25 studies exploring the impact of the diagnosis of primary gliomas documentss that patients often suffer from severe physical and neurological dysfunction throughout their illness that may reduce their ability to perform usual activities and meet occupational, financial, social, and family obligations.15 Others have found that the patients' symptoms and functional impairment led to changes in relationships and roles within the family, increased burden on relatives, and a sense of social isolation.14
Neurofunctional Anatomy and Malignant Gliomas
The most common locations for primary gliomas in adults are the frontal (23%) and temporal (17%) lobes, although tumors do not often respect anatomic boundaries and can involve multiple lobes. The majority of tumors occur in the cerebral hemispheres and recur and progress locally, within a few centimeters of the original tumor location. Knowledge of these mechanisms and underlying neuroanatomy are often used clinically to anticipate these recognized symptoms of focal versus generalized effects in evaluating patient and disease status.
Because of their location, malignant gliomas are often associated with neurologic and neurocognitive symptoms. Our brains are organized into dynamically changing, flexible modules of brain regions that are connected both structurally and functionally. Tumor association with specific signs/symptoms is based on established neuroanatomy and physiology as well as global brain connectivity. Malignant gliomas arise from the constitutive cellular elements of the brain and, unlike systemic tumors, tend to be unifocal and rarely metastasize outside the central nervous system. As a result, neurologic symptoms are determined by lesion size and location within the brain to a certain extent, whereas widespread loss of connectivity is reported to be associated with neurocognitive dysfunction.17 Although more basic brain functions like sensation and motor functioning have a more topographical predictability with the brain, studies using magnetoencephalography have shown that a localized lesion can give rise to widespread loss of connectivity in cerebral networks (including the apparently healthy contralateral hemisphere) on MRI imaging.
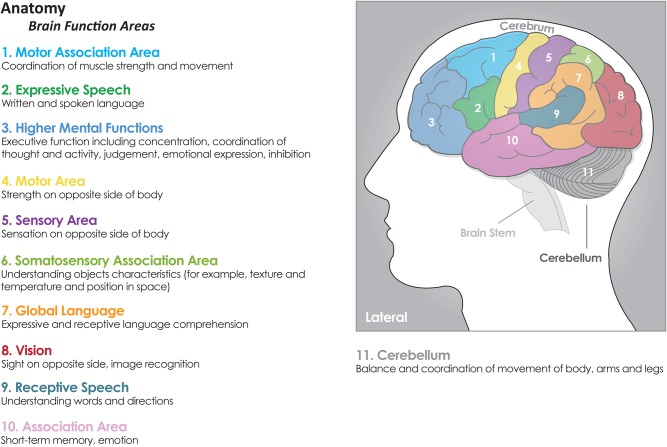
Malignant gliomas are thought to cause neurologic symptoms by multiple mechanisms that all can give rise to brain network changes in distinct ways including (i) invasion of brain parenchyma, (ii) brain compression, (iii) cerebrospinal fluid (CSF) obstruction (hydrocephalus), and (iv) herniation.4 Invasion and compression cause signs/symptoms referable to a particular region of the brain (ie, signs referable to a particular region of the brain) as shown in the Fig. 1 These include signs/symptoms such as changes in vision, motor and sensory function, balance, seizures, speech, concentration, neurocognitive functioning, and personality based on lesion location. Obstruction of CSF flow and herniation cause symptoms related to increased intracranial pressure (eg, headache, nausea, vomiting, altered neurocognitive functioning, and reduced level of consciousness). As stated earlier, neurocognitive functioning is represented in widespread cerebral networks but can also be related to brain regions to a certain extent.18
Fig. 1.
Signs referable to a particular region of the brain. Reprinted with permission from www.cern-foundation.org.
Identifying Signs and Symptoms
The individual patient experiences symptoms as deficits characterized by their frequency and severity but also as a self-shaped perception influenced by interaction among demographic, individual, and disease characteristics.1 For example, a recent study exploring predictors of distress and poorer QOL in high-grade glioma patients during treatment indicated that loss of employment and the resulting financial impact, poorer functional status, and lower education predicted higher distress and poorer QOL.19 These factors can impact an individual's perception and response to symptoms that occur and support the dynamic and interconnectedness of symptoms and their associated impact on daily life.
Several groups have worked to identify symptoms relevant to the diagnosis and management of patients with malignant gliomas including exploration of the content validity of instruments designed to allow patient self-report of symptoms. Two of these instruments, the EORTC QLQ-BN20 and the FACT-Br, were designed to measure the broader construct of QOL; however, signs/symptoms are also subsumed and measured. The third instrument, the MD Anderson Symptom Inventory-Brain Tumor (MDASI-BT), was designed to measure symptom burden and the interference of symptoms in daily life.
The development of the EORTC QLQ-BN20 included interviews with patients and caregivers as well as expert health care professionals, with a list of more than 80 issues reviewed (and redundant items removed); a final total of 28 items were found to be most relevant and were included in the final scale.20 The development of the FACT-Br was outlined by Weitzner21 and also included interviews with 17 sets of patients and caregivers and review by expert clinicians. During the development of the MDASI-BT, Armstrong et al22,23 identified domains of symptoms that occur in primary brain tumor patients and included those that represent increased intracranial pressure, focal neurologic signs/symptoms, and those related to therapy. Items identified by review of the literature were then reviewed by an expert panel that included clinicians, patients, and caregivers. Content validity index (CVI) scores were calculated and used for further evaluation and validation of items. Dr. Lai et al recently refined symptoms included in the FACT-BR; they used a symptom index developed by identifying priority symptoms for a panel of 50 patients enrolled through NCCN-designated cancer centers. The patients reported symptoms that were important, resulting in a symptom index.24 In the development of all of these measures, exploration of the prevalence, severity, and association with other measures (eg, disease and performance status) were evaluated, lending further support for these signs/symptoms for this population.25 Tables 1 and 2 provide a summary of symptoms and functional limitations and include all 3 groups. In addition, the occurrence of these symptoms and their importance was rated by participants in the JSBTDDC survey of brain tumor patients. The final signs/symptoms identified by these groups and further instrument validation work (provided in Tables 1 and 2), lend additional support that these signs/symptoms are relevant to the malignant glioma population.
Table 1.
Common signs and symptoms
| EORTC QLQ C30 | BN20 | MDASI-BT | NCCN/FACT-BR | NBTS Survey Symptoms Lead to Diagnosis | NBTS Survey Symptoms Want to See Treatment Impact | |
|---|---|---|---|---|---|---|
| SYMPTOMS | Rank Order (%) | 0-5 Scale of Importance | ||||
| Headache/pain | X | X | X | X | 1 (57%) | X (4.14) |
| Short-term memory | X | X | X | 4 (25%) | X (4.33) | |
| Expressive aphasia/speech | X (2 items) | X | X (2 items) | 3 (26%) | X (3.9) | |
| Receptive aphasia | x | x | 8 (9%) | |||
| Concentration | X | X | X | 4 (25%) | X (4.33) | |
| Drowsiness | x | x | ||||
| Hemiparesis/hemiplegia | X | X | X | X | 7 (13%) | X (4.12) |
| Change in body sensation | x | 6 (14%) | ||||
| Vision | x (3 items) | x | ||||
| Seizures | X | X | X (2 items) | 2 (40%) | X (4.16) | |
| Walking | x (2 items) | x | 5 (23%) | X (4.63) | ||
| Coordination/ataxia | X (2 items) | x | 5 (23%) | |||
| Incontinence | x |
Table 2.
Common functional domains and psychiatric symptoms
| EORTC QLQ C30 | BN20 | MDASI-BT | NCCN/FACT-BR | NBTS Survey Symptoms Leading to Diagnosis | NBTS Survey Symptoms Want to See Treatment Impact | |
|---|---|---|---|---|---|---|
| FUNCTION | X | X | X | X (4.26)* | ||
| Basic tasks/dressing, bathing, caring for self | X | X (4.63) | ||||
| Working | x | x | ||||
| Family life | x | x | x | |||
| Enjoyment | x | x | ||||
| Mood/P\personality change | x | x | 3 (26%) | X (4.33) | ||
| Distress | x | |||||
| Irritability/tension | x | x | ||||
| Worry/anxiety | x | x | ||||
| Sadness/depression | x | x | ||||
| Fear | ||||||
| Uncertainty | x | |||||
| Frustration | x | |||||
| Hopelessness | x |
Context of Signs and Symptoms in Malignant Gliomas
Symptoms at Diagnosis in Patients with Malignant Gliomas
Patients with malignant gliomas typically present with either an acute neurologic event or a more protracted course of worsening symptoms. The literature describing signs/symptoms of primary brain tumors at the time of diagnosis is primarily limited to retrospective data. Three seminal papers26–28 (containing clinical information from as early as 1924) have been used historically to define the overall incidence of symptoms in the malignant glioma population.4 The antiquity of the data is important because all studies are commonly cited as references, even in contemporary reports. Thereby, several reports on this subject include antiquated information derived from data captured in the era before modern imaging with CT and MRI, which have allowed earlier diagnosis of brain tumors.
Several of the more recent reports of symptoms in patients with brain tumors have similar limitations and focus on solitary symptoms or limited histologic subtype. Krouwer et al published a retrospective review of presenting symptoms in 52 patients with oligoastrocytomas. In their small sample, seizures were the most common (71%) sympton, with most other symptoms occurring in <20% of patients.29 Yeh et al reported that the most common presenting symptoms in GBM were headache (69%) and weakness (55%), with all other symptoms occurring in <25% of patients.30 An additional study reported the occurrence of multiple deficits in persons with PBT and other secondary brain tumors.30 Another paper reported that 75% of patients with primary and secondary brain tumors had ≥3 concurrent neurologic deficits, and 40% had ≥5 deficits, thus supporting the need to assess multiple symptoms in the PBT population.31 Commonly reported symptoms in this study include weakness, sensory and visual changes, and problems with speech.
The Glioma Outcome Study included 565 malignant glioma patients and reported that the most common symptoms at presentation were headaches (56%), memory loss (36%), neurocognitive changes (34%), motor issues (34%), language disturbances (33%), seizures (31%), and personality changes (23%). Predictably, a statistically significant difference in presenting symptoms, which was dependent on lesion location in the dominant hemisphere (DH) or nondominant hemisphere (NDH), was described. Language deficit was higher in the DH group (59.7% compared with 7.9% in the NDH group, P = .0001), whereas motor deficits were seen more commonly in the NDH group (42.1% compared with 28.8% in the DH group, P = .02). Other less localized symptoms at presentation occurred equally in the DH and the NDH groups, including headache (56.1% and 54.7%, respectively) and altered level of consciousness (17.3% and 15.8%, respectively).32,33
More recently, Collins et al34 conducted a retrospective review of data from 2003–2009 and reported presenting symptoms for those patients with malignant glioma stratified by those living less than or more than 120 days. Out of 2011 patients evaluated, 46% were admitted through the ER at the time of diagnosis, with 48% arriving by ambulance. These figures highlight the acuity of presenting symptoms in this patient population. For those patients living longer than 120 days (n = 678), the most common presenting symptoms were paresis/weakness (15%), speech/communication or swallowing difficulties (14%), seizures (14%), and neurocognitive/behavioral difficulties (12%). Nearly two-thirds of patients who died during the initial diagnostic admission had ≥2 symptoms at diagnosis, the most common being neurocognitive or behavioral difficulties (41%), paresis (35%), incontinence (33%), speech communication and swallowing difficulties (32%), and seizures (21%). For those who survived this initial hospitalization but died during first 120 days, paresis (20%), neurocognitive or behavioral difficulties (19%), speech, communication or swallowing difficulties (17%), seizures (13%), and incontinence (12%) were the most prevalent symptoms.
Additional data come from an effort to further refine symptoms reported by patients with malignant gliomas during the modern era; the JSBTDDC conducted an online survey asking patients to report symptoms at presentation, symptoms most important to respondents, and symptoms they would like treatment to improve. Full details related to this survey are provided in the Panel 4 paper in this same supplement. Respondents to the JSBTDDC survey indicated that the 5 most common symptoms at presentation for high-grade astrocytomas were headache (57%), seizures (40%), changes in speech, vision, or hearing (25%), changes in mood or personality (25%), problems with memory or ability to concentrate (25%), and problems with balance or walking (24%).
In contrast to symptom reports, Tucha et al35 conducted neurocognitive testing at the time of diagnosis and before neurosurgical resection of tumor on 161 consecutively admitted adults with tumor of the frontal or temporal region. Upon neurological examination of this sample, personality changes were apparent in 13%, slight to moderate hemiparesis/paresis was elicited in 12%, sensory disturbance was detected in 1%, visual impairments were found in 8%, and 52% exhibited no abnormalities on neurologic exam. However, based on the results obtained from standardized neuropsychological testing, 91% of patients displayed impairment of neurocognitive function; for 71% of patients impairment was evident in ≥3 areas, and for 27% of patients impairment was evident in ≥8 areas. Impairment was most common in the domains of executive function (78%) and memory (64%).
In summary, symptoms at diagnosis include those related to increased intracranial pressure and focal deficits based on lesion location. The most common symptoms reported in the literature and by patient survey include headache, seizures, problems with walking or strength, problems with memory or ability to concentrate, difficulty with speech, and changes in personality. In addition, objective neurocognitive testing supports a high incidence of impaired executive function and memory.
Trajectory of Signs/Symptoms
Signs/symptoms occurring at the time of diagnosis can improve, worsen, or be fixed over time and may be related to disease progression, other neurologic events (seizures, infection, stroke), concomitant medications, or comorbidities. As symptoms evolve, patients with malignant gliomas and their caregivers often make adjustments to their lives to accommodate functional limitations. These include physical alterations to the home, introducing regular exercise to their lives, and using complementary therapies, all which may modify the impact of symptoms on the patient's functional status.36
Studies exploring the trajectory and severity of symptoms are limited. Molassiotis, Wilson, Brunton et al37 reported symptoms in patients with primary gliomas longitudinally over the first year after diagnosis. Ongoing issues included fatigue, memory problems, and lack of independence, specifically inability to drive due to seizures or other deficits. Tiredness/fatigue was a reoccurring theme and was reported as the most severe of multiple symptoms in almost all participants. Fatigue was also reported to become more debilitating over time and was the only symptom that negatively affected their QOL and overall well-being. In another study, Sundararajan et al reported that in longer-term survivors (>120 days), symptoms accumulated over time with 18% of patients having ≥2 symptoms at diagnosis, 26% at 120 days to death, and 32% at the time of death admission. Taphoorn et al applied the EORTC QLQ-BN20 to data from 841 patients from 2 completed phase 3 studies to measure symptoms at baseline and post radiation. They determined that seizures had the lowest mean score with <15% patients reporting them at any time point (which may be a consequence of asking about occurrence in the last week); future uncertainty had the highest score, and most symptoms were stable over the reporting period.38
In summary, the trajectory of symptoms over time is variable, with intercurrent illness, concomitant medications, and disease progression impacting both the number and severity of symptoms. Patients commonly report fatigue, which may compound other symptoms. There is evidence for an increased number of symptoms and worsening severity occurring over the course of the disease.
Impact of Therapy
During treatment, patients experience symptoms that can be associated with disease, therapy, or both. Therapy includes treatment designed to control tumor growth, reduce brain edema, or prevent seizures and thus improve neurological functioning. In most cases, maximal safe resection is performed at the time of initial surgery. Recent surgical advances (eg, functional preoperative and intraoperative mapping and monitoring of motor and sensory function, language, visual fields, visuospatial orientation, calculation, and reading abilities) are postulated to improve safety and efficacy of surgical resection and thus improve symptomatic outcomes post surgery.39,40
After surgery, most patients are treated with radiation, chemotherapy, or both. Sutton et al, using a phenomenological approach, described treatment impact on QOL for patients with brain tumors on a research trial involving new anticancer agents.41 This study identified the theme of a complex symptom profile; with many of the patients experiencing symptoms attributable to either the drug or the cancer itself, thus making it difficult to isolate the cause. This suggests that perspective, cancer, and treatment are inextricably linked from the patient's perspective. A wide variety of symptoms were described including deficits related to fatigue, mobility, and the consequent reduction in independence.
Both the short-term and long-term impact of chemotherapy on symptoms has not been fully elucidated. A recent report exploring the impact of PCV in low-grade glioma patients reported no impact on cognition using the MMSE up to 5 years post treatment.42 In patients with GBM, overall symptom severity and symptom interference with activity are worse in those who receive dose-intense temozolomide compared with standard dose.43 In a separate study, bevacizumab was associated with worse neurocognitive function by objective testing and patient report, as well as worse overall symptoms burden and both treatment and generalized symptoms using the MDASI-BT. In addition, patients reported greater impact on the interference of symptoms with both activity and mood over time.44,3 Interestingly, a recent small pilot study in patients with high-grade gliomas demonstrated that prolonged treatment with bevacizumab is associated with brain atrophy in the contralateral hemisphere,45 while a mouse study suggested that bevacizumab may mitigate the development of radiation necrosis.46 At recurrence, the use of bevacizumab was associated with stability or improvement of function in those without progression.47
The use of corticosteroids to reduce morbidity and mortality by controlling peritumoral edema is well established.48,49 However, their use may be associated with significant adverse effects, thus indicating the importance of reducing use as a measure of clinical benefit.50 In clinical practice, corticosteroids are used primarily as adjuncts to tumor surgery, radiation, and chemotherapy, contributing to both radiologic and symptomatic response. The magnitude and duration of their contribution is thought to be variable depending on the context of use.50 Although corticosteroid use is common, there are limited studies on the frequency and severity of their side effects in this patient population. Two retrospective studies reported that approximately half of the patients had at least one steroid toxicity, and nearly 20% required hospitalization due to complications.51 Another study emphasized the importance of the starting dose at initiation of therapy, with doses <16 mg/day being associated with a 65% incidence of a single toxicity, and higher doses having an almost 91% incidence of at least one toxicity.52 Interindividual differences in toxicity occur, and a study of primary brain tumor patients reported that one-third of patients experienced toxicity within the first 3 weeks of treatment.51 More recently, Armstrong et al53 reported that 35% of patients reported increased appetite, difficulty with sleep, and difficulty with standing associated with corticosteroid dose, whereas other symptoms were not associated with the dose of corticosteroids prescribed. This suggests that dose and duration alone can't predict which patients will develop toxicity.50
In summary, treatment-associated symptoms and disease-related symptoms are inextricably linked. Corticosteroids may mitigate symptoms but are also variably associated with side effects that may be independent of dose and duration.
Symptoms, Imaging, and Survival Endpoints
Signs/Symptoms at Recurrence
It is recognized that symptoms often worsen or new symptoms develop as brain tumors progress. A study of patients with malignant glioma demonstrated that progressive deterioration in information-processing capacity, psychomotor speed, and attentional function may precede progression of disease on MRI.54 In these patients, tests of memory and fine motor coordination were the most sensitive to decline. In another study, overall symptom burden and interference with activities such as working and ability to walk or initiate activities were self-reported (using the MDASI-BT) as significantly worse in those patients with progression on MRI.55
Signs/Symptoms and Survival Endpoints
A meta-analysis of studies completed by the EORTC in a variety of cancers, including brain tumors, suggests that self-reported symptoms and neurocognitive deficits identified by neurocognitive testing are predictive of OS.56 Additional data from 2 recently completed phase 3 studies conducted by the RTOG support this finding.3,43 In RTOG 0525, baseline (post surgery but prior to starting other treatment) neurocognitive function tests and self-report of physical functioning were associated with OS and PFS. After concurrent chemoradiotherapy, deterioration of self-reported neurocognitive function and motor dysfunction and objective testing of recall and visuospatial processing speed were associated with shorter OS time.43 In addition, a recent analysis showed no association with QOL using the FACT-G or FACT-Br with survival, but did find that self-report of fatigue was a strong predictor of survival and provided incremental prognostic value to traditional markers of prognosis in recurrent high-grade glioma.57
In summary, signs/symptoms including objective neurocognitive functioning and self-report of neurologic deficits, interference with functional status (difficulty walking, working, and starting and completing tasks), and fatigue appear to be correlated with both disease progression on imaging and survival endpoints.
Concurrent and Specific Symptoms
Concurrent Symptoms
As noted in the section describing symptoms at diagnosis, patients often do not experience symptoms in isolation, and multiple concurrent symptoms can influence the experience of any one symptom. Data from 617 patients asked to report (using the MDASI-BT) symptoms and interference of symptoms were reviewed to assess symptom prevalence, severity, and occurrence as they relate to disease status and tumor grade.53 The results indicated that >50% of patients had at least 10 symptoms, and >40% had at least 3 moderate-severe symptoms. However, the data indicated variability in the reported symptoms, with most symptoms being reported as moderate-severe by <20% of the sample. The most severe symptoms reported were fatigue, drowsiness, difficulty remembering, difficulty with sleep, and distress. For those with GBM, weakness on one side of the body and difficulty speaking were also counted as severe. Patients with newly diagnosed malignant gliomas described problems with concentration and irritability as moderate-severe. Functional interference of concurrent symptoms with daily activities, including working, walking, and ability to perform activities, were also reported by 25% of patients with malignant glioma.
Psychiatric Symptoms
Neurobehavioral symptoms, including changes in personality and behavior, mood issues (eg, depression, anger, and anxiety), hallucinations, and psychosis are reported to occur throughout the trajectory of illness.58 There are conflicting data on the prevalence of depression among patients with malignant glioma. Several recent reports indicated that these patients have symptoms indicative of depression with varying prevalence, whereas other studies describe a relatively low prevalence. While data derived from formal psychiatric interviews (using DSM-IV criteria) indicated the prevalence of depression to be 15%–28%, estimates from patients' self-reporting were higher at 35%–93%.59–62 Mood and behavioral changes can be global in nature but can also be correlated with brain anatomy. Depression is associated with left-sided63 and frontal tumors,64 obsession with left anterior tumors,61,65 and anger and indifference with temporal lobe lesions.66 Resection of tumors does not appear to improve depressive symptoms, and data from the largest study to date described these symptoms as actually increasing after surgery.60
It is more difficult to estimate the incidence of changes in personality or behavior because most studies include case reports or qualitative studies.67 Symptoms that have been reported include changes in behavior, anger, indifference, or loss of emotional control.66,68–71 Some quantitative studies have indicated that patients with frontal tumors are more likely to have these symptoms, although they are also reported in patients with lesions outside of the frontal region. It is also reported that patients may have limited awareness of personality changes, thus making self-report difficult. Finally, although the exact incidence is not known, hallucinations and psychosis may also occur; patients with temporal lobe lesions or temporal lobe seizures are more at risk, as are patients on corticosteroids.72,73
Neurocognitive Function
Neurocognitive deficits are commonly observed in GBM patients during every stage of their disease and remain or worsen in the course of the disease.74,75 In addition, a recent study indicated that 38% of those with grade IV tumors may lack the mental capacity to give informed consent; this may be underestimated by clinicians.76 Preoperative deficits in ≥1 neurocognitive domain such as language, memory, and executive functions have been reported in ∼80% of patients based on individual tests and 24%–38% if based on domains of function.77 Subjectively, glioma patients often complain about word-finding problems, short-term memory deficits, or completing complex, multistep tasks. Patients with grade III and grade IV tumors appear to have similar neurocognitive deficits; Kayl and Meyers reported no difference in domain scores between these patients after controlling for tumor volume.78 Recent studies have demonstrated comprehensive neurocognitive assessment and reported that left-sided tumors affected attentional and executive functioning, verbal fluency, and verbal learning,63,79,80 thus supporting the link between left hemisphere tumors and impaired verbal functioning as previously described.81,82
Tumor treatment and concomitant medications may also impact cognition. Resective tumor surgery may aggravate or induce neurological compromise and/or neurocognitive deficits, which usually resolve within 3 months following surgery.83 Studies do report that use of corticosteroids is associated with better recognition memory and that some antiepileptics reduce working memory capacity, while it may improve with others.63,84 Other treatment can also impact neurocognitive function, with reports indicating that radiation therapy and chemotherapy are all also associated with alterations in neurocognitive functioning.85
Subclinical neurocognitive impairment can have a large impact on the daily life of patients and often precedes reductions in their QOL.86,87 Reduced neurocognitive function in malignant gliomas has been associated with reduced independence and difficulty maintaining roles in the home and work environments.88
Fatigue
Fatigue is one of the most common and most severe symptoms in patients with solid tumors, including those with malignant gliomas.89,90 Cross-sectional studies have indicated that 40%–70% of PBT patients have fatigue throughout the illness trajectory,62,91–93 and fatigue has been reported as frequent (48%) and significant at post surgery baseline prior to the administration of anticancer therapy.94 Fatigue has also been reported to be the most troublesome symptom to patients,95 with a high percentage of patients reporting “very low” energy levels92 or rating fatigue as moderate to severe on Likert scales.93
Fatigue is a prominent symptom when QOL data are collected as part of randomized controlled treatment trials.96–99 Two studies investigated fatigue in relation to cranial irradiation.92,100 One study reported somnolence syndrome in 16 of 19 patients after treatment and recorded increased fatigue at diagnosis that increased significantly after radiotherapy and correlated negatively with QOL.100 Another study reinforced the impression that fatigue is common and correlates negatively with QOL and depressive symptoms.59
Seizures
The reported incidence of seizures in patients with GBM is between 30% and 62%, with two-thirds occurring at presentation and one-third during the course of the disease,101–103 although a recent report indicated a higher incidence during the follow-up period (48%).104 Seizures are most commonly associated with tumors of the frontal, temporal, and parietal lobes.105 Focal seizures can occur in 38% of patients with GBM, with 40% of patients having focal onset seizures with secondary generalization.101 It is estimated that nearly a quarter of patients have both partial and generalized seizures, and status epilepticus may occur in >10% of patients.101 Following initial tumor resection, three-quarters of those with GBM are seizure-free during the first year of follow-up,101,106 while ∼15% of patients continue to have seizures despite different therapeutic regimens.101,107
Seizure recurrence following a period of longstanding postoperative seizure control or worsening of seizure control is associated with tumor progression following first-line treatment.106 Interestingly, both administration of radiation and chemotherapy with either PCV or temozolomide have been reported to be associated with seizure reduction in low-grade gliomas.108,109 Prolonged seizure control is associated with improved functional status.102
Conclusions
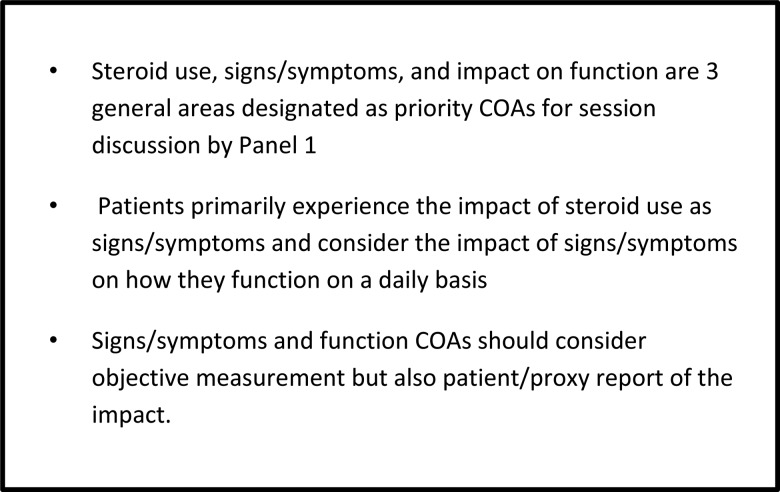
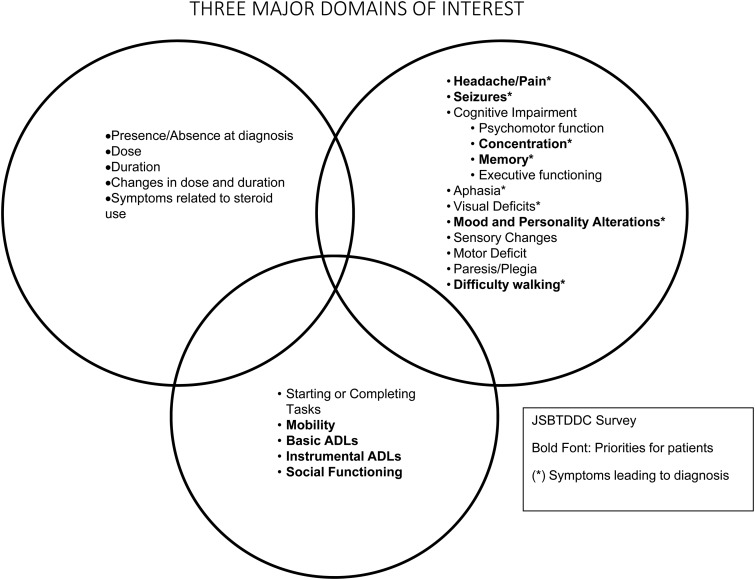
As indicated in the review above, patients with malignant glioma often suffer from multiple symptoms and have a variable course over time. These symptoms can be impacted by the disease and by concomitant therapy and medications and can have a significant impact on patient cognitive and physical functioning. Panel 1 assertions resulting from this review are listed in Fig. 2. Both objective neurocognitive testing and self-report of symptoms and functional limitations have been shown to be associated with tumor progress and survival endpoints. Based on the review of the literature and considering the input from the complete survey and the panel, assertions related to the collection of data in clinical studies were identified (Fig. 2) and a preliminary list of priority signs/symptoms (Fig. 3) was also outlined for discussion at the FDA/JSBTDDC COA workshop. This list was further refined and evaluation of assessment measures to address these priority areas that could be added to treatment studies as a measure of clinical benefit were reviewed. The Panel 4 paper in this supplement provides a finalized list resulting from this meeting and the next steps in terms of evaluating signs/symptoms for inclusion in clinical trials.
Fig. 2.
Panel 1 assertions related to the use of clinical outcomes assessments in patients with malignant gliomas.
Fig. 3.
Panel 1 preliminary list of priority signs and symptoms.
Funding
There was no funding support for the preparation of this manuscript.
Conflict of interest statement. Terri Armstrong: consultant for ABBvie, Immunocellular Therapeutics, and Tocagen.
Acknowledgments
The authors would like to thank Drs Terri Armstrong, Mark Gilbert, Patrick Wen and Jennifer Helfer, and David Arons for their efforts to coordinate and lead the Jumpstarting Brain Tumor Drug Development Coalition and FDA Clinical Trials Clinical Outcome Assessment Endpoints Workshop on October 15, 2014; Drs Paul Kluetz, Joohee Sul, and Virginia Kwitkowski for their expert advice during the preparation of the Clinical Outcome Assessment Endpoints Workshop.
References
- 1.Armstrong TS, Gilbert MR. Patient reported endpoints for measuring clinical benefit in (high grade glioma) primary brain tumor patients. Curr Treat Options Oncol. 2014;15(4):519–528. [DOI] [PubMed] [Google Scholar]
- 2.Quinn T, Ostrom HG, Liao P et al. . CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert MR, Dignam JJ, Armstrong TS et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong TS, Cohen MZ, Eriksen LR, Hickey JV. Symptom clusters in oncology patients and implications for symptom research in people with primary brain tumors. J Nurs Scholarsh. 2004;36(3):197–206. [DOI] [PubMed] [Google Scholar]
- 5.Bradley S, Sherwood PR, Donovan HS et al. . I could lose everything: understanding the cost of a brain tumor. J Neurooncol. 2007;85(3):329–338. [DOI] [PubMed] [Google Scholar]
- 6.Fobair P, Mackworth N, Varghese A, Prados M. Quality of life issues among 200 brain tumor patients treated at the University of California in San Francisco, interviewed 1988. Paper presented at: Brain Tumor Conference: a living resource guide San Francisco, California, March 3–5, 1990. [Google Scholar]
- 7.Armstrong TS, Vera-Bolanos E, Gilbert MR. Clinical course of adult patients with ependymoma: results of the Adult Ependymoma Outcomes Project. Cancer. 2011;117(22):5133–5141. [DOI] [PubMed] [Google Scholar]
- 8.Strang S, Strang P. Spiritual thoughts, coping and ‘sense of coherence’ in brain tumour patients and their spouses. Palliat Med. 2001;15(2):127–134. [DOI] [PubMed] [Google Scholar]
- 9.Salander P, Spetz A. How do patients and spouses deal with the serious facts of malignant glioma? Palliat Med. 2002;16(4):305–313. [DOI] [PubMed] [Google Scholar]
- 10.Woehrer A, Bauchet L, Barnholtz-Sloan JS. Glioblastoma survival: has it improved? Evidence from population-based studies. Curr Opin Neurol. 2014;27(6):666–674. [DOI] [PubMed] [Google Scholar]
- 11.Stupp R, Mason WP, van den Bent MJ et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 12.Robin AM, Walbert T, Mikkelsen T et al. . Through the patient's eyes: the value of a comprehensive brain tumor center. J Neurooncol. 2014;119(3):465–472. [DOI] [PubMed] [Google Scholar]
- 13.Ellingson BM, Bendszus M, Boxerman J et al. . Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell B, Collins A, Dally M et al. . Living longer with adult high-grade glioma: setting a research agenda for patients and their caregivers. J Neurooncol. 2014;120(1):1–10. [DOI] [PubMed] [Google Scholar]
- 15.Moore G, Collins A, Brand C et al. . Palliative and supportive care needs of patients with high-grade glioma and their carers: a systematic review of qualitative literature. Patient Educ Couns. 2013;91(2):141–153. [DOI] [PubMed] [Google Scholar]
- 16.McConigley R, Halkett G, Lobb E, Nowak A. Caring for someone with high-grade glioma: a time of rapid change for caregivers. Palliat Med. 2010;24(5):473–479. [DOI] [PubMed] [Google Scholar]
- 17.Kinno R, Ohta S, Muragaki Y, Maruyama T, Sakai KL. Left frontal glioma induces functional connectivity changes in syntax-related networks. Springerplus. 2015;4:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cipolotti L, Healy C, Chan E et al. . The impact of different aetiologies on the cognitive performance of frontal patients. Neuropsychologia. 2015;68:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halkett GK, Lobb EA, Rogers MM et al. . Predictors of distress and poorer quality of life in High Grade Glioma patients. Patient Educ Couns. 2015;98(4):525–532. [DOI] [PubMed] [Google Scholar]
- 20.Osoba D, Aaronson NK, Muller M et al. . The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5(1):139–150. [DOI] [PubMed] [Google Scholar]
- 21.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–1161. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong TS, Cohen MZ, Eriksen L, Cleeland C. Content validity of self-report measurement instruments: an illustration from the development of the Brain Tumor Module of the M.D. Anderson Symptom Inventory. Oncol Nurs Forum. 2005;32(3):669–676. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong TS, Mendoza T, Gning I et al. . Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 24.Lai JS, Jensen SE, Beaumont JL et al. . Development of a symptom index for patients with primary brain tumors. Value Health. 2014;17(1):62–69. [DOI] [PubMed] [Google Scholar]
- 25.Mauer M, Bottomley A, Taphoorn MJ. Evaluating health-related quality of life and symptom burden in brain tumour patients: instruments for use in experimental trials and cilnical practice. Curr Opin Neurol. 2008;21(6):745–753. [DOI] [PubMed] [Google Scholar]
- 26.Frankel SA, German WJ. Glioblastoma multiforme; review of 219 cases with regard to natural history, pathology, diagnostic methods, and treatment. J Neurosurg. 1958;15(5):489–503. [DOI] [PubMed] [Google Scholar]
- 27.Jelsma R, Bucy PC. The treatment of glioblastoma multiforme of the brain. J Neurosurg. 1967;27(5):388–400. [DOI] [PubMed] [Google Scholar]
- 28.Roth JG, Elvidge AR. Glioblastoma multiforme: a clinical survey. J Neurosurg. 1960;17:736–750. [DOI] [PubMed] [Google Scholar]
- 29.Krouwer HG, van Duinen SG, Kamphorst W, van der Valk P, Algra A. Oligoastrocytomas: a clinicopathological study of 52 cases. J Neurooncol. 1997;33(3):223–238. [DOI] [PubMed] [Google Scholar]
- 30.Yeh SA, Leung SW, Sun LM, Wang CJ, Fang FM, Chen HC. Postoperative radiotherapy for supratentorial malignant gliomas. J Neurooncol. 1999;42(2):183–187. [DOI] [PubMed] [Google Scholar]
- 31.Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil. 2001;80(5):346–350. [DOI] [PubMed] [Google Scholar]
- 32.Polin RS, Marko NF, Ammerman MD et al. . Functional outcomes and survival in patients with high-grade gliomas in dominant and nondominant hemispheres. J Neurosurg. 2005;102(2):276–283. [DOI] [PubMed] [Google Scholar]
- 33.Laws ER, Parney IF, Huang W et al. . Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. [DOI] [PubMed] [Google Scholar]
- 34.Collins A, Sundararajan V, Brand CA et al. . Clinical presentation and patterns of care for short-term survivors of malignant glioma. J Neurooncol. 2014;119(2):333–341. [DOI] [PubMed] [Google Scholar]
- 35.Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324–333; discussion 333–324. [DOI] [PubMed] [Google Scholar]
- 36.Molassiotis A, Wilson B, Brunton L, Chaudhary H, Gattamaneni R, McBain C. Symptom experience in patients with primary brain tumours: a longitudinal exploratory study. Eur J Oncol Nurs. 2010;14(5):410–416. [DOI] [PubMed] [Google Scholar]
- 37.Johansson E, Wilson B, Brunton L, Tishelman C, Molassiotis A. Symptoms before, during, and 14 months after the beginning of treatment as perceived by patients with lymphoma. Oncol Nurs Forum. 2010;37(2):E105–E113. [DOI] [PubMed] [Google Scholar]
- 38.Taphoorn MJ, Claassens L, Aaronson NK et al. . An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 39.Ottenhausen M, Krieg SM, Meyer B, Ringel F. Functional preoperative and intraoperative mapping and monitoring: increasing safety and efficacy in glioma surgery. Neurosurg Focus. 2015;38(1):E3. [DOI] [PubMed] [Google Scholar]
- 40.Freyschlag CF, Duffau H. Awake brain mapping of cortex and subcortical pathways in brain tumor surgery. J Neurosurg Sci. 2014;58(4):199–213. [PubMed] [Google Scholar]
- 41.Sutton K. The impact on quality of life for people with brain tumours of entering a research trial involving new anti-cancer agents. Eur J Oncol Nurs. 2013;17(4):396–401. [DOI] [PubMed] [Google Scholar]
- 42.Prabhu RS, Won M, Shaw EG et al. . Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: secondary analysis of RTOG 98–02. J Clin Oncol. 2014;32(6):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong TS, Wefel JS, Wang M et al. . Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taphoorn MJ, Henriksson R, Bottomley A et al. . Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol. 2015;3319:2166–2175. [DOI] [PubMed] [Google Scholar]
- 45.Bag AK, Kim H, Gao Y et al. . Prolonged treatment with bevacizumab is associated with brain atrophy: a pilot study in patients with high-grade gliomas. J Neurooncol. 2015;122(3):585–593. [DOI] [PubMed] [Google Scholar]
- 46.Jiang X, Engelbach JA, Yuan L et al. . Anti-VEGF antibodies mitigate the development of radiation necrosis in mouse brain. Clin Cancer Res. 2014;20(10):2695–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wefel JS, Cloughesy T, Zazzali JL et al. . Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(6):660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galicich JH, French LA. Use of dexamethasone in the treatment of cerebral edema resulting from brain tumors and brain surgery. Am Pract Dig Treat. 1961;12:169–174. [PubMed] [Google Scholar]
- 49.Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet. 1961;81:46–53. [PubMed] [Google Scholar]
- 50.Ryan R, Booth S, Price S. Corticosteroid-use in primary and secondary brain tumour patients: a review. J Neurooncol. 2012;106(3):449–459. [DOI] [PubMed] [Google Scholar]
- 51.Weissman DE, Dufer D, Vogel V, Abeloff MD. Corticosteroid toxicity in neuro-oncology patients. J Neurooncol. 1987;5(2):125–128. [DOI] [PubMed] [Google Scholar]
- 52.Sturdza A, Millar BA, Bana N et al. . The use and toxicity of steroids in the management of patients with brain metastases. Support Care Cancer. 2008;16(9):1041–1048. [DOI] [PubMed] [Google Scholar]
- 53.Armstrong TS, Vera-Bolanos E, Acquaye AA, Gilbert MR, Ladha H, Mendoza T. The symptom burden of primary brain tumors: evidence for a core set of tumor and treatment related symptoms. Neuro Oncol. 2016;182:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro Oncol. 2003;5(2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong TS, Vera-Bolanos E, Gning I et al. . The impact of symptom interference using the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT) on prediction of recurrence in primary brain tumor patients. Cancer. 2011;117(14):3222–3228. [DOI] [PubMed] [Google Scholar]
- 56.Quinten C, Coens C, Mauer M et al. : EORTC Clinical Groups. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual m=patient data from EORTC clinical trials. Lancet Oncol. 2009;109:865–871. [DOI] [PubMed] [Google Scholar]
- 57.Peters KB, West MJ, Hornsby WE et al. . Impact of health-related quality of life and fatigue on survival of recurrent high-grade glioma patients. J Neurooncol. 2014;120(3):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boele FW, Rooney AG, Grant R, Klein M. Psychiatric symptoms in glioma patients: from diagnosis to management. Neuropsychiatr Dis Treat. 2015;11:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57(1):41–49. [DOI] [PubMed] [Google Scholar]
- 60.Litofsky NS, Farace E, Anderson F Jr, Meyers CA, Huang W, Laws ER Jr. Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery. 2004;54(2):358–366; discussion 366–357. [DOI] [PubMed] [Google Scholar]
- 61.Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Depression and functional outcome in patients with brain tumors: a population-based 1-year follow-up study. J Neurosurg. 2005;103(5):841–847. [DOI] [PubMed] [Google Scholar]
- 62.Acquaye AA, Vera-Bolanos E, Armstrong TS, Gilbert MR, Lin L. Mood disturbance in glioma patients. J Neurooncol. 2013;113(3):505–512. [DOI] [PubMed] [Google Scholar]
- 63.Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55(4):992–999. [DOI] [PubMed] [Google Scholar]
- 64.Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11(3):230–238. [DOI] [PubMed] [Google Scholar]
- 65.Mainio A, Hakko H, Niemela A, Salo J, Koivukangas J, Rasanen P. Level of obsessionality among neurosurgical patients with a primary brain tumor. J Neuropsychiatry Clin Neurosci. 2005;17(3):399–404. [DOI] [PubMed] [Google Scholar]
- 66.Andrewes DG, Kaye A, Murphy M et al. . Emotional and social dysfunction in patients following surgical treatment for brain tumour. J Clin Neurosci. 2003;10(4):428–433. [DOI] [PubMed] [Google Scholar]
- 67.Boele FW, Douw L, Reijneveld JC et al. . Health-related quality of life in stable, long-term survivors of low-grade glioma. J Clin Oncol. 2015;33(9):1023–1029. [DOI] [PubMed] [Google Scholar]
- 68.Cavers D, Hacking B, Erridge SE, Kendall M, Morris PG, Murray SA. Social, psychological and existential well-being in patients with glioma and their caregivers: a qualitative study. CMAJ. 2012;184(7):E373–E382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janda M, Steginga S, Dunn J, Langbecker D, Walker D, Eakin E. Unmet supportive care needs and interest in services among patients with a brain tumour and their carers. Patient Educ Couns. 2008;71(2):251–258. [DOI] [PubMed] [Google Scholar]
- 70.Lucas MR. Psychosocial implications for the patient with a high-grade glioma. J Neurosci Nurs. 2010;42(2):104–108. [DOI] [PubMed] [Google Scholar]
- 71.Sterckx W, Coolbrandt A, Dierckx de Casterle B et al. . The impact of a high-grade glioma on everyday life: a systematic review from the patient's and caregiver's perspective. Eur J Oncol Nurs. 2013;17(1):107–117. [DOI] [PubMed] [Google Scholar]
- 72.Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 73.Shah AH, Gordon CE, Bregy A, Shah N, Komotar RJ. Considering iatrogenic psychosis after malignant glioma resection. BMJ Case Rep. 2014. doi:10.1136/bcr-2013-201318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Habets EJ, Kloet A, Walchenbach R, Vecht CJ, Klein M, Taphoorn MJ. Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir (Wien). 2014;156(8):1451–1459. [DOI] [PubMed] [Google Scholar]
- 75.Gehrke AK, Baisley MC, Sonck AL, Wronski SL, Feuerstein M. Neurocognitive deficits following primary brain tumor treatment: systematic review of a decade of comparative studies. J Neurooncol. 2013;115(2):135–142. [DOI] [PubMed] [Google Scholar]
- 76.Kerrigan S, Erridge S, Liaquat I, Graham C, Grant R. Mental incapacity in patients undergoing neuro-oncologic treatment: a cross-sectional study. Neurology. 2014;83(6):537–541. [DOI] [PubMed] [Google Scholar]
- 77.Talacchi A, Santini B, Savazzi S, Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103(3):541–549. [DOI] [PubMed] [Google Scholar]
- 78.Kayl AE, Meyers CA. Does brain tumor histology influence cognitive function? Neuro Oncol. 2003;5(4):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein M, Taphoorn MJ, Heimans JJ et al. . Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19(20):4037–4047. [DOI] [PubMed] [Google Scholar]
- 80.Bosma I, Vos MJ, Heimans JJ et al. . The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hom J, Reitan RM. Neuropsychological correlates of rapidly vs. slowly growing intrinsic cerebral neoplasms. J Clin Neuropsychol. 1984;6(3):309–324. [DOI] [PubMed] [Google Scholar]
- 82.Scheibel RS, Meyers CA, Levin VA. Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neurooncol. 1996;30(1):61–69. [DOI] [PubMed] [Google Scholar]
- 83.Klein M, Duffau H, De Witt Hamer PC. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J Neurooncol. 2012;108(2):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Groot M, Douw L, Sizoo EM et al. . Levetiracetam improves verbal memory in high-grade glioma patients. Neuro Oncol. 2013;15(2):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267–275. [DOI] [PubMed] [Google Scholar]
- 86.Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 87.Mitchell AJ, Kemp S, Benito-Leon J, Reuber M. The influence of cognitive impairment on health-related quality of life in neurological disease. Acta Neuropsychiatr. 2010;22(1):2–13. [Google Scholar]
- 88.Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 2005;76(4):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Catt S, Chalmers A, Fallowfield L. Psychosocial and supportive-care needs in high-grade glioma. Lancet Oncol. 2008;9(9):884–891. [DOI] [PubMed] [Google Scholar]
- 90.Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;14(Suppl 4):iv65–iv72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin L, Acquaye AA, Vera-Bolanos E, Cahill JE, Gilbert MR, Armstrong TS. Validation of the Mishel's uncertainty in illness scale-brain tumor form (MUIS-BT). J Neurooncol. 2012;110(2):293–300. [DOI] [PubMed] [Google Scholar]
- 92.Lovely MP, Miaskowski C, Dodd M. Relationship between fatigue and quality of life in patients with glioblastoma multiformae. Oncol Nurs Forum. 1999;26(5):921–925. [PubMed] [Google Scholar]
- 93.Armstrong TS, Cron SG, Bolanos EV, Gilbert MR, Kang DH. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715. [DOI] [PubMed] [Google Scholar]
- 94.Valko PO, Siddique A, Linsenmeier C, Zaugg K, Held U, Hofer S. Prevalence and predictors of fatigue in glioblastoma: a prospective study. Neuro Oncol. 2015;17(2):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powell C, Guerrero D, Sardell S et al. . Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumours: a prospective study. Radiother Oncol. 100(1):131–136. [DOI] [PubMed] [Google Scholar]
- 96.Osoba D, Brada M, Prados MD, Yung WK. Effect of disease burden on health-related quality of life in patients with malignant gliomas. Neuro Oncol. 2000;2(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osoba D, Brada M, Yung WK, Prados M. Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme. J Clin Oncol. 2000;18(7):1481–1491. [DOI] [PubMed] [Google Scholar]
- 98.Brown PD, Jensen AW, Felten SJ et al. . Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. [DOI] [PubMed] [Google Scholar]
- 99.Brown PD, Ballman KV, Rummans TA et al. . Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76(3):283–291. [DOI] [PubMed] [Google Scholar]
- 100.Faithfull S, Brada M. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin Oncol (R Coll Radiol). 1998;10(4):250–254. [DOI] [PubMed] [Google Scholar]
- 101.Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia. 2013;54(suppl 9):12–17. [DOI] [PubMed] [Google Scholar]
- 102.Kerkhof M, Dielemans JC, van Breemen MS et al. . Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol. 2013;15(7):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 104.Luchi T, Hasegawa Y, Kawasaki K, Sakaida T. Epilepsy in patients with gliomas: incidence and control of seizures. J Clin Neursci. 2015;22(1):87–91. [DOI] [PubMed] [Google Scholar]
- 105.Michelucci R, Pasini E, Meletti S et al. . Epilepsy in primary cerebral tumors: the characteristics of epilepsy at the onset (results from the PERNO study–Project of Emilia Romagna Region on Neuro-Oncology). Epilepsia. 2013;54:86–91. [DOI] [PubMed] [Google Scholar]
- 106.Chaichana KL, Parker SL, Olivi A, Quinones-Hinojosa A. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg. 2009;111(2):282–292. [DOI] [PubMed] [Google Scholar]
- 107.Armstrong TS, Kanusky JT, Gilbert MR. Seize the moment to learn about epilepsy in people with cancer. Clin J Oncol Nurs. 2003;7(2):163–169. [DOI] [PubMed] [Google Scholar]
- 108.Koekkoek JA, Kerkhof M, Dirven L, Heimans JJ, Reijneveld JC, Taphoorn MJ. Seizure outcome after radiotherapy and chemotherapy in low-grade glioma patients: a systematic review. Neuro Oncol. 2015;177:924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruda R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14:(suppl 4):iv55–iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]