Abstract
Processing speed is an important human cognitive capability that might underlie differences in other cognitive skills and their aging. We aimed to test aging-related processing speed differences using a novel cross-sectional design that adjusted for cognitive ability tested in youth. We examined aging differences on three different ways of assessing processing speed: psychometric, experimental, and psychophysical. We compared large narrow-age cohorts of 70- and 83-year-old people who were matched for cognitive ability in childhood. There were decrements of substantial effect size in all processing speed assessments in the older group that were not accounted for by prior cognitive ability, health, or fitness differences, though these factors also contributed to processing speed differences. These findings confirm age-related cognitive slowing using an unusual research design, and provide evidence against recent theories characterizing aging-related cognitive decline as a myth.
Keywords: Processing speed, IQ, Aging, Health
Highlights
-
•
We tested processing speed differences in 70- and 83-year-olds adjusting for cognitive ability at age 11.
-
•
We tested processing speed using psychometric, experimental, and psychophysical methods.
-
•
There were age decrements of substantial effect size in all processing speed assessments.
-
•
Processing speed decrements were not accounted for by prior cognitive ability, health, or fitness differences.
1. Introduction
The present study asks an old question: how does age affect processing speed? It uses a novel design, by comparing varied assessments of processing speed between narrow-age cohorts of 70-year-olds and 83-year-olds who are matched on intelligence test scores gathered at age 11 years.
The perennial psychological construct of processing speed captures the idea that there are measurable limits to the rate at which humans can correctly complete simple psychological tasks. Processing speed tests typically assess how efficiently people can complete mental tasks that, if there were no time pressure, would rarely be answered incorrectly. Although there is that similarity among them, the tests that attract the epithet processing speed are heterogeneous. They include paper-and-pencil psychometric tests, reaction time tests from experimental psychology, and psychophysical tests (Deary, I. J., 2001a, Deary, I. J., 2001b). In psychology, interest in human processing speed is long-lived, important, and continuing (Boring, E. G., 1950, Deary, I. J., 2001b, Deary, I. J. and Ritchie, S. J., 2014, Verhaeghen, P., 2014). Assessments of processing speed appeared in the earliest batteries of human mental tests (Cattell, J. M., 1890, Galton, F., 1884, Galton, F., 1890). In addition to being one among many domains of cognitive function, processing speed has also been promoted by some as a primus inter pares among cognitive domains, in that it has been suggested as a foundation for competence in other cognitive abilities and an influence on how well or badly they age (Jensen, A. R., 2006, Salthouse, T. A., 1996, Verhaeghen, P., 2014).
Given the widespread interest in processing speed test scores as indices of brain efficiency, it is important to understand how they age, not least because research into understanding cognitive aging differences has risen to high priority in aging societies (Lindenberger, 2014). Cross-sectional studies using psychometric and experimental tests find that processing speed declines steadily from early adulthood (e.g. Der, G. and Deary, I. J., 2006, Salthouse, T. A., 2004). Longitudinal studies also show declines in processing speed (e.g. Ritchie, S. R., et al., 2014, Wilson, R. S., et al., 2002). When longitudinal studies pair processing speed with other mental tests there is often a substantial correlation between the amount of decline in processing speed and the decline in other cognitive domains (e.g. Ritchie, S. R., et al., 2014, Verhaeghen, P., 2014, Wilson, R. S., et al., 2002, Zimprich, D. and Martin, M., 2002though see Sternäng, Wahlin, & Nilsson, 2008). Some studies suggest that processing speed substantially mediates the influence of age on other aspects of cognitive performance, though others do not (Deary, I. J., et al., 2009, Finkel, D., et al., 2007, Robitaille, A., et al., 2013, Verhaeghen, P., 2014).
No design is perfect in assessing how age affects processing speed. Cross-sectional studies can suffer differential selection into their different age groups. Longitudinal studies have problems that include practice effects (Salthouse, 2014) and non-random attrition (Newman, 2003). Most studies use a single test, or tests of a similar type, to assess processing speed. Almost no studies take account of the fact that variation in processing speed test performance in older age is substantially associated with intelligence differences in childhood (Deary, Johnson, & Starr, 2010). The present cross-sectional study uses a novel approach by assessing how processing speed differs in generally healthy older people of 70 versus 83 years after adjusting for, or matching on, their childhood intelligence. It uses several methods to assess processing speed, and controls for potentially confounding variables.
2. Method
2.1. Participants
Participants were members of the Lothian Birth Cohorts of 1921 and 1936 (LBC1921 and LBC1936, respectively). At recruitment in older age they were generally healthy older people, most of whom resided in the area around the City of Edinburgh in Scotland (the Lothian Region). Most had taken part in the Scottish Mental Surveys of 1932 or 1947 (Scottish Council for Research in Education [SCRE], 1933; Scottish Council for Research in Education [SCRE], 1949). The tracing, recruitment and testing of the LBC1921 are described in detail elsewhere (Deary, Whiteman, Starr, Whalley, & Fox, 2004b), as they are for the LBC1936 (Deary et al., 2007). Both cohorts are also described in a cohort profile article (Deary, Gow, Pattie, & Starr, 2012). For the present study, we selected participants who had data on childhood intelligence and cognitive test scores in older age, who had no diagnosis of dementia, who had adequate corrected visual acuity to complete the processing tests, who had a Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) score of 24 or more (and so were unlikely to have significant cognitive pathology), and who had a total score of 16 or more out of 20 on the two longest inspection time durations (150 ms and 200 ms; see below).
Approval was granted for the study by the Multi-Centre Research Ethics Committee for Scotland (MREC/01/0/56) and the Lothian Research Ethics Committee (LREC/2003/2/29). Written informed consent was obtained from each participant before testing.
3. Measures
3.1. Intelligence tested in childhood
In both cohorts, the measure used to assess intelligence in childhood was a version of the Moray House Test No. 12 (Scottish Council for Research in Education [SCRE], 1933; Scottish Council for Research in Education [SCRE], 1949). This is a group-administered test with a time limit of 45 min and a maximum score of 76. The same test and instructions were used in the 1932 and 1947 Scottish Mental Surveys. The test has a preponderance of verbal reasoning items, and also some numerical and other items. In both Surveys, the Moray House Test scores correlated about r = .8 with individually-administered Stanford Binet test scores on Ns of about 1000 (Scottish Council for Research in Education,, 1933, Scottish Council for Research in Education,, 1949).
3.2. Intelligence tested in older age
The Moray House Test No. 12 that participants took at about age 11 years was administered again in older age using the same instructions. For the LBC1921 this was given about four years before (at mean age 79.1 years) all of the other tests used here (at mean age 83.3 years). For the LBC1936 this was given on the same occasion as the other tests used here (at mean age 69.5 years).
Participants from both cohorts took the National Adult Reading Test (Nelson & Willison, 1991) on the same occasion as the processing speed and other older-age assessments. This test requires the participant to pronounce 50 words that are irregular in grapheme–phoneme correspondence and/or stress. It is often used to estimate people's peak prior intelligence, for which it has been validated (Dykiert & Deary, 2013). Here, it was used as an additional variable to use as a control for the cognitive background of the participants in the two cohorts. That is, when comparing participants for processing speed variables we were able to adjust for measured childhood intelligence and estimated peak prior intelligence in adulthood.
3.3. Processing speed
The participants took four tests of processing speed. The same tests were given to both cohorts. The processing speed tests were at three different levels, i.e. psychometric, experimental, and psychophysical, as described by Deary, I. J., 2001a, Deary, I. J., 2001b.
3.3.1. Digit Symbol
This is a psychometric, paper-and-pencil test of processing speed. The Digit Symbol Substitution subtest was used from the Wechsler Adult Intelligence Scale-IIIUK (Wechsler, 1998). Using a code that is given on the same page as the test, the participant writes symbols below numbers as quickly as possible. The score is the number of correct symbols entered in 2 min.
3.3.2. Simple reaction time
This and the choice reaction time test are experimental-type tests of processing speed. Simple and choice reaction time were tested using the same self-contained box that we described elsewhere (Deary, Der, & Ford, 2001). It was designed for the UK's Health and Lifestyle Study by Batvale Electronics (Cambridge, United Kingdom; Cox, 1987, Appendix A, p. 153). It has been used in large general population studies in the United Kingdom (Deary, I. J., et al., 2001, Der, G. and Deary, I. J., 2006). For simple reaction time, the participant pressed a key on the box as soon as possible after a zero appeared on the LCD display on the box. The inter-stimulus interval varied between 1 and 3 s. There were 8 practice trials and 20 experimental trials. We used the mean of the experimental trials as the measure of simple reaction time.
3.3.3. Choice reaction time
For choice reaction time the participant placed the index and middle fingers of both hands over keys on the box marked 1, 2, 3, and 4. The participant pressed the appropriate key on the box as soon as possible after the digit 1, 2, 3 or 4 appeared on the LCD on the box. The inter-stimulus interval varied between 1 and 3 s. There were 8 practice trials and 40 experimental trials. We used the mean of the correct experimental trials as the measure of choice reaction time.
3.3.4. Inspection time
This is a psychophysical test of visual processing speed which we have described in detail elsewhere (Deary, I. J., et al., 2004a, Deary, I. J., et al., 2007). Importantly, it does not require any fast or co-ordinated physical reaction. In each trial, the participant is presented on a computer screen with a shape composed of two parallel vertical lines of markedly different lengths that are joined at the top with a horizontal line. In 50% of the trials the long line is on the left and in 50% it is on the right. The participant presses a computer key at their leisure to indicate the position of the long line. No response time is taken—just whether the answer to each item is correct or wrong—and fast responses are discouraged, including by disallowing responses until after the offset of the backward mask. The stimulus is preceded by a cue. A backward pattern mask appears immediately at stimulus offset. The method of constant stimuli is used. There were 15 durations between 6 and 200 ms, each of which was repeated 10 times. Durations for each trial were selected at random. Prior to the experimental session there were five practice sessions with 10 trials each. The experimental sessions were divided into blocks of 30 trials, and a short rest could be taken after each block. The score used was the total number of correct trials out of 150. To ensure that participants had performed consistently throughout, we selected for analyses only those subjects who scored 16 or more out of 20 when the two easiest durations (150 ms and 200 ms) were summed. Given that durations appear at random, this score means that the subjects reliably were able to make correct discriminations during the whole task.
3.4. Health and fitness
A structured medical interview took place at the same time as the cognitive testing. Participants were asked about their histories of hypertension, diabetes, cardiovascular disease, and cerebrovascular disease. These were each recorded as yes or no.
Grip strength of the hands was measured using a North Coast Medical Hydraulic Hand Dynamometer (Jamar). The best of three attempts of the dominant hand was used as the score and was recorded in kg. Grip strength is a good marker of current and future health (Cooper, Kuh, & Hardy, 2010).
4. Results
The LBC1921 and LBC1936 had similar proportions of men and women (Table 1). There were more people in the older cohort with hypertension and cardiovascular disease, and their grip strength was weaker. The cohorts had similar and non-significantly different scores on the Moray House Test at age 11, and on the National Adult Reading Test in older age. Therefore, they were of similar levels of measured and estimated prior intelligence. The small (non-significant) advantage in Moray House Test score in the LBC1936 is about the same as the whole-population difference between the Scottish Mental Surveys of 1947 and 1932; that is, in the whole-of-Scotland population comparison (N = 87,498 for the 1932 Survey and N = 70,805 for the 1947 Survey) the 1947 Survey participants' mean was 2.3 points (Cohen's d = 0.14) higher (Scottish Council for Research in Education, 1949, p. 85). Here, the LBC1936 scored 1.6 points higher than the LBC1921, and the Cohen's d was 0.14.
Table 1.
Demographic, health, cognitive, and processing speed data on the Lothian Birth Cohorts of 1921 and 1936.
| Lothian Birth Cohort 1921 | Lothian Birth Cohort 1936 | Mean comparison (p-value) |
Cohen's d | Variance comparison (p-value) |
|
|---|---|---|---|---|---|
| Sex: n male, n female | 112, 132 | 481, 469 | .20 | – | – |
| Hypertension: n (%) | 121 (49.6) | 370 (38.9) | .003 | – | – |
| Diabetes: n (%) | 16 (6.6) | 74 (7.8) | .59 | – | – |
| Cardiovascular disease: n (%) | 39 (16.2) | 222 (23.4) | .018 | – | – |
| Cerebrovascular disease: n (%) | 17 (7.0) | 44 (4.6) | .14 | – | – |
| Grip strength (kg) | 25.2 (9.1) | 29.8 (10.1) | < .001 | 0.48 | .004 |
| Mini-Mental State Examination score | 28.3 (1.5) | 28.9 (1.2) | < .001 | 0.44 | < .001 |
| Moray House Test, age 11 (out of 76) | 47.7 (11.4) | 49.3 (11.7) | .065 | 0.14 | .78 |
| Age when sat Moray House Test, age 11 | 10.9 (0.29) | 10.9 (0.28) | .177 | 0.00 | .377 |
| Moray House Test in older age (out of 76) | 61.5 (9.3) | 64.9 (8.0) | < .001 | 0.39 | < .001 |
| Age when sat Moray House Test in older age | 79.1 (0.60) | 69.5 (0.84) | – | – | – |
| Age at all other assessments in older age | 83.3 (0.53) | 69.5 (0.84) | – | – | – |
| National Adult Reading Test (out of 50) | 35.4 (7.6) | 34.7 (8.0) | .21 | 0.09 | .22 |
| Wechsler Digit Symbol Substitution score | 42.8 (11.9) | 57.5 (12.4) | < .001 | 1.21 | .51 |
| Simple reaction time mean (ms) | 306.2 (65.9) | 273.8 (53.1) | < .001 | 0.54 | < .001 |
| 4-Choice reaction time mean (ms) | 780.2 (128.4) | 636.0 (80.5) | < .001 | 1.35 | < .001 |
| Inspection time total score (out of 150) | 104.8 (10.4) | 112.9 (10.0) | < .001 | 0.79 | .15 |
| General speed | − 1.01 (1.03) | 0.26 (0.81) | < .001 | 1.28 | < .001 |
Table 2 reports the correlations among the four speed tests for both cohorts. All of the tests correlated positively together (correlations ranged from r = .17 to .55), and on that basis we extracted a ‘general speed’ factor—the first, unrotated principal component from a principal components analysis of all four tests. This explained 54% of the variance across the four tests in the LBC1921, and 50% of the variance in the LBC1936. The principal component was used as a variable in the subsequent analyses.
Table 2.
Pearson correlation matrix for all speed measures in the Lothian Birth Cohort of 1921 (below diagonal) and the Lothian Birth Cohort of 1936 (above diagonal).
| Wechsler Digit Symbol Substitution | Simple reaction time | Choice reaction time | Inspection time | General speed | |
|---|---|---|---|---|---|
| Wechsler Digit Symbol Substitution | – | .26 | .49 | .27 | .73 |
| Simple reaction time | .35 | – | .44 | .17 | .65 |
| Choice reaction time | .55 | .48 | – | .34 | .84 |
| Inspection time | .35 | .21 | .34 | – | .59 |
| General speed | .79 | .70 | .83 | .60 | – |
Note: all correlations significant at p < .001; general speed = first unrotated principal component from the four speed tests. Simple and choice reaction time scores reversed so that higher scores indicate better (faster) performance.
The LBC1921 had a lower older-age Moray House Test score than the LBC1936, with an effect size of d = 0.39. We note that this—for the LBC1921—was the only test that was not completed on the same occasion as the other tests. It was completed at age 79.1 instead of 83.3 years, and so this probably underestimates the within-older-age cognitive ability difference between the two cohorts on this test.
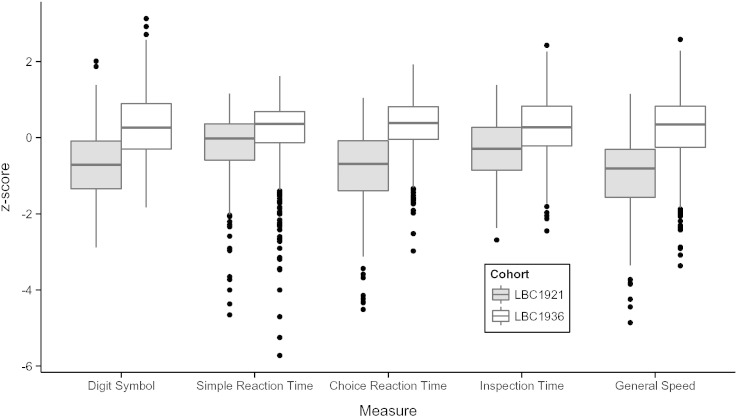
The LBC1921 scored significantly less well than the LBC1936 on all four processing speed tasks and on the ‘general speed’ component (Table 1). The effect sizes (Cohen's d) were as follows: Digit Symbol = 1.21; simple reaction time mean = 0.54; choice reaction time mean = 1.35; inspection time = 0.79; and general speed = 1.28). The result for inspection time is noted for being a large effect in the one test that does not require a speeded and co-ordinated physical reaction. The LBC1921 showed significantly greater variance than the LBC1936 in simple and choice reaction time mean, and in general speed. Mean results for all speed tests are illustrated in Fig. 1.
Fig. 1.
Boxplots comparing the (standardized) scores for all four speed tests and the ‘general speed’ component between the LBC1921 and LBC1936 cohorts. Simple and choice reaction time tests are rescaled so that higher scores indicate better (faster) performance.
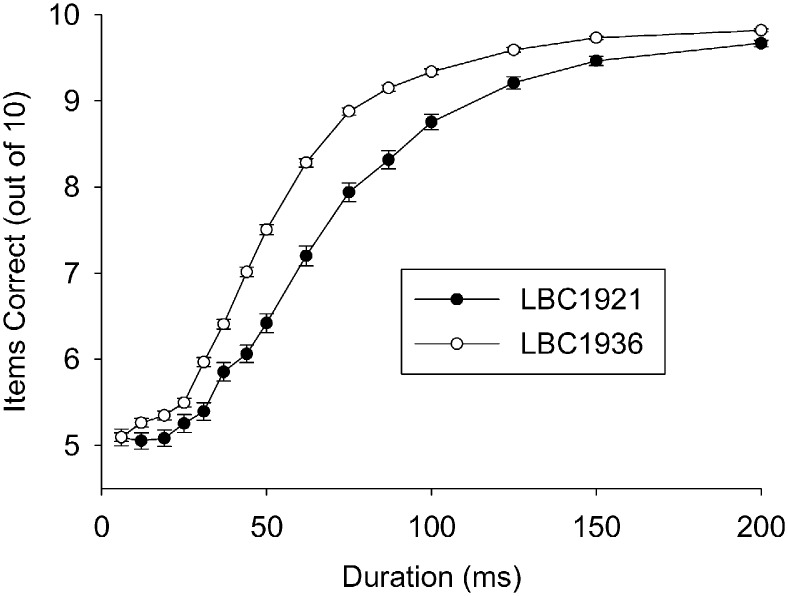
The inspection time difference between the LBC1921 and LBC1936 cohorts is described in more detail in Fig. 2. The LBC1921 cohort scored notably lower on all durations that were longer than those that were associated with near-to-chance responding, i.e. at durations from 31 ms and longer. The LBC1921 psychometric curve is markedly shifted to the right. Note that the closing-in of the two cohorts in the longest two durations is an artifact of our selection of subjects who scored 16 or more out of 20 on the sum of these durations.
Fig. 2.
Mean inspection time results per stimulus duration for Lothian Birth Cohorts 1921 (LBC1921) and 1936 (LBC1936). Only subjects who scored 16 out of 20 or better on the two longest durations (150 ms and 200 ms) were included. Error bars represent 1 standard error of the mean.
We ran regression models with each of the four processing speed variables to examine the extent of cohort differences and how these altered when potentially confounding variables were entered. For each speed variable, we ran four models: first, the baseline, only including sex and age 11 Moray House Test IQ score; a second including the cohort effect; a third including as an additional covariate the NART score; and a fourth also including the health and grip strength variables. With only sex and Moray House Test IQ score in the model, the standardized betas for the cohort effects on speed variables ranged from −.519 for choice reaction time, through .496 (general speed), .419 (Digit Symbol) and .297 (inspection time) to −.219 (simple reaction time; see Table 3). The two subsequent models had little difference in their cohort betas or their adjusted R2. These results are summarized in Table 3, and fuller results of all models for each of the individual processing speed variables are given in Supplementary Tables 1–5. Although the principal interest was in the LBC1921 versus LBC1936 difference, the multivariate models in Supplementary Tables 1–5 also provide additional information about the contributions to processing speed differences in older age. Women performed significantly better than men on Digit Symbol and simple reaction time. Cognitive ability from age 11 was associated significantly with all four processing speed measures (as we reported in Deary et al. [2010] for the LBC1936 cohort) and with general speed, and NART contributed to all except inspection time. The presence of cerebrovascular disease was associated with cognitive slowing in all measures except simple reaction time. Stronger hand grip was significantly associated with faster processing speed on all four measures.
Table 3.
Summary results from linear regression models of four processing speed tests; the effects presented describe the comparison of the Lothian Birth Cohorts of 1921 (LBC1921; mean age 83) and 1936 (LBC1936; mean age 70). Standardized betas (with adjusted R2 in parentheses) are shown for the four processing speed tests and for general speed in three models that cumulatively control for childhood intelligence, estimated peak prior intelligence (National Adult Reading Test score), and health and fitness.
| Digit Symbol | Simple reaction time | Choice reaction time | Inspection time | General speed | |
|---|---|---|---|---|---|
| Model including sex and age 11 Moray House Test IQ: Standardized beta for LBC1921 vs. LBC1936 (Adjusted R2) |
.419 (.317) |
.219 (.096) |
.519 (.313) |
.297 (.131) |
.496 (.347) |
| Model including sex, age 11 Moray House Test IQ, and NART score: Standardized beta for LBC1921 vs. LBC1936 (Adjusted R2) |
.431 (.332) |
.229 (.106) |
.525 (.316) |
.302 (.133) |
.507 (.360) |
| Model including sex, age 11 Moray House Test IQ, NART score, and health/grip strength: Standardized beta for LBC1921 vs. LBC1936 (Adjusted R2) |
.411 (.348) |
.197 (.118) |
.506 (.331) |
.275 (.147) |
.474 (.385) |
Note: NART = National Adult Reading Test. For beta values for all covariates, see Tables S1–S5 in the Supplemental Material. Simple and choice reaction time tests are rescaled so that higher scores indicate better performance. For each measure, positive beta values indicate faster performance in the LBC1936.
4.1. Propensity score matching analysis
As a final analysis, we used propensity score matching to ensure that we were comparing the LBC1921 participants to a well-matched subsample of the (larger) LBC1936. We calculated propensity scores based on sex, age 11 Moray House Test IQ score, and NART score (that is, we matched the participants for sex and for pre-existing cognitive ability; analysis was run using the ‘nonrandom’ package for R; Stampf, 2014). For each of the 234 LBC1921 participants who had data on all the matching variables, we selected three LBC1936 participants. Matches were made within 0.2 standard deviations of the logit of the propensity score. We then compared the 243 LBC1921 participants to the resulting 729 matched LBC1936 participants. Comparing Table 4 to Table 2, it can be seen that the between-cohort differences on the pre-existing cognitive ability tests (the Moray House Test and the NART) were substantially smaller. There was also no significant difference in the groups' sex ratios (χ2(1) = 0.09, p = 0.76). For the four speed tests, the between-cohort effect sizes were near-identical to those in the regression analyses reported above (Table 4). Thus, with a more stringent matching criterion, large cohort differences in the speed tasks were still readily apparent.
Table 4.
Comparison of all cognitive tests between the Lothian Birth Cohorts of 1921 and 1936 (LBC1921 and LBC1936) after the propensity score matching procedure.
| Category | Measure | Sample mean (SD) |
Mean comparison |
Variance comparison |
||
|---|---|---|---|---|---|---|
| LBC1921 | LBC1936 | p-value | Cohen's d | p-value | ||
| Matching variables | Age 11 MHT (out of 76) | 47.71 (11.5) | 47.95 (12.0) | .79 | 0.02 | .43 |
| NART score (out of 50) | 35.44 (7.6) | 35.12 (8.0) | .58 | 0.04 | .30 | |
| Speed variables | Wechsler Digit Symbol Substitution score | 42.8 (11.86) | 57.5 (12.44) | <.001 | 1.22 | .37 |
| Simple reaction time (ms) | 306.4 (66.0) | 274.2 (53.4) | <.001 | 0.51 | <.001 | |
| Choice reaction time (ms) | 779.3 (127.8) | 637.3 (80.09) | <.001 | 1.21 | <.001 | |
| Inspection time score (out of 150) | 104.8 (10.3) | 112.7 (9.8) | <.001 | 0.77 | .33 | |
| General speed | − 0.92 (1.01) | 0.31 (0.78) | <.001 | 1.28 | <.001 | |
Note: MHT = Moray House Test; NART = National Adult Reading Test.
5. Discussion
Because of the limitations of cross-sectional and longitudinal designs, it is not straightforward to gauge the effect that age has on the key mental quality of processing speed. The present study found that 83-year-olds were substantially slower compared with 70-year-olds on psychometric, experimental and psychophysical assessments of processing speed. Importantly and unusually, the two groups were matched on childhood IQ scores. The differences persisted in effect size after taking into account, in addition to childhood IQ, estimated peak adult intelligence (NART score), health, and grip strength. They were also evident after an alternative, propensity score-matching procedure where only closely-matched participants were selected for analysis. In addition to the cohorts' age difference, stronger grip and the absence of cerebrovascular disease were associated with faster processing speed across the different measures.
Strengths of the study included these large older-age samples having taken the same well-validated intelligence test at age 11, being tested on four processing speed tasks of different types, and having additional health and fitness data. A weakness of the study is that, although visual acuity was assessed in both cohorts, it was not done in precisely the same way and so acuity could not be used as a covariate. However, we judge that acuity was not a limiting factor in the tasks, for several reasons. First, subjects were tested with corrected vision. Second, all subjects completed the practice parts of all four processing speed tests successfully. Third, all subjects performed well on the longer inspection time durations, indicating that stimulus discrimination was not problematic when exposure was sufficiently long. The visual angle of the high contrast inspection time stimuli is large; inspection time stimuli are designed so that stimulus duration, and not contrast or visual angle, provides the variation in task difficulty.
It is not new to report that older people are slower than younger people. What is new here is the unusual design that matches an older sample with a younger sample on intelligence at age 11 years. However, the LBC1921 are not just older than the LBC1936; they were born 15 years earlier and lived through different experiences. Schaie (2013) notes that this cohort difference is a critical confound in cross-sectional studies, but also that full confounding can be avoided if there is “evidence to suggest that older cohorts performed at the same level as younger cohorts at equivalent ages” (p. 20). Our matching on childhood ability partly provides this evidence, so long as one also notes the following differences between the cohorts. Some of the LBC1921 served in, and all experienced aspects of, World War II, whereas the LBC1936 were children at the time. The United Kingdom's National Health Service started in 1948, and so the LBC1921 had 27 years of non-free health care, and the LBC1936 only 12 years. These factors, which are not easily adjusted-for, may have confounded the estimates we report. Overall, the LBC1921 spent about a year less at school than the LBC1936; we did not include education as a control variable in our models because of these differences (and because we have shown previously that differences in educational duration in both cohorts do not contribute significantly to variation in later-life processing speed; Ritchie, Bates, Der, Starr, & Deary, 2013). A similar argument applies to our not including measures of social status as control variables: because of different patterns of social mobility across the relevant period of the 20th Century, these measures are not directly comparable across the two cohorts.
Though the effect was much smaller than that of the processing speed and Moray House Test scores' differences in older age, some mention should be made of how the LBC1921 versus LBC1936 difference in Moray House Test score at age 11 might affect the results here. The effect size (Cohen's d = 0.14) was the same as has been found in the whole population. Therefore, in the original population surveys (Scottish Council for Research in Education,, 1933, Scottish Council for Research in Education,, 1949) there was an early example of what later became known as the Flynn Effect (e.g. Pietschnig & Voracek, 2015). However, some research has suggested that the Flynn Effect might not apply to tests of processing speed, and not to inspection time in particular (Nettelbeck & Wilson, 2004). In our main regression models, we added Moray House Test at age 11 as a covariate; this will have had the effect of our assuming that the slightly higher mean childhood Moray House Test score of the LBC1936 was real, and that the processing speed difference in older age should in effect be adjusted down slightly. However, if the Flynn Effect applied to the Moray House Test scores and not to processing speed test scores, we might have slightly under-estimated the age-70 versus age-83 difference. Our secondary analysis, using propensity scores, ensured that there were no significant differences between the groups in Moray House Test (or NART) score; we found similarly-sized differences in all the speed measures, indicating that the analysis type was not of critical importance for finding the effects we describe.
There have been relatively few studies of grip strength and processing speed in older people. Grip strength is a useful general indicator of physical fitness in older age. It tracks chronological age well, and lower levels are associated with higher mortality (Cooper, R., et al., 2010, Dodds, R. M., et al., 2014). Other studies have found grip strength to be associated with processing speed in older age, including among those in their 80s, and have also indicated that some of the association is related to genetic factors (Otaga, S., et al., 2014, Sternäng, O., et al., 2015, Takema, D. G., et al., 2012). In all of these studies, though, processing speed was assessed using Digit Symbol-type psychometric tests. These require repeated fast and co-ordinated movements. Here, we used psychometric tests in addition to reaction time (an experimental test) and inspection time (a psychophysical test). Of these, the association between inspection time and grip strength is the most informative, because it does not involve the speeded co-ordinated movement that the others require, and which might be helped by a better grip strength.
Age-related cognitive decline has recently been called a “myth”, and it has been suggested that “older adults' performance reflects increased knowledge, not cognitive decline” (Ramscar, Hendrix, Shaoul, Milin, & Baayen, 2014, p. 34). Given the present study's results, where 83-year-olds performed substantially worse on very simple, knowledge-free tests of processing speed than matched 70-year-olds, we would suggest that this position is difficult to maintain.
Footnotes
We thank the Lothian Birth Cohort 1921 and 1936 teams for data collection, collation and checking. We thank the Scottish Council for Research in Education for access to the Scottish Mental Surveys of 1932 and 1947. The Lothian Birth Cohort 1921 data collection was supported by the Biotechnology and Biological Sciences Research Council (BBSRC; 15/SAG09977) and by a Royal Society-Wolfson Award to Ian Deary. The Lothian Birth Cohort 1936 data collection was supported by Age UK (Disconnected Mind project). The work was undertaken by The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative, which supports Ian Deary and Stuart Ritchie (MR/K026992/1). Funding from the BBSRC and Medical Research Council (MRC) is gratefully acknowledged.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.intell.2016.01.002.
Appendix A. Supplementary data
Supplementary tables.
References
- Boring E.G. 2nd ed. Prentice Hall; Upper Saddle River, NJ: 1950. A history of experimental psychology. [Google Scholar]
- Cattell J.M. Mental tests and measurements. Mind. 1890;15:373–380. [Google Scholar]
- Cooper R., Kuh D., Hardy R. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. British Medical Journal. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B.D. Health Promotion Research Trust; London, UK: 1987. The health and lifestyle survey. [Google Scholar]
- Deary I.J. Human intelligence differences: Towards a combined experimental-differential approach. Trends in Cognitive Sciences. 2001;5:164–170. doi: 10.1016/s1364-6613(00)01623-5. [DOI] [PubMed] [Google Scholar]
- Deary I.J. Oxford University press; Oxford, UK: 2001. Looking down on human intelligence: From psychometrics to the brain. [Google Scholar]
- Deary I.J., Ritchie S.J. 10 quick questions about processing speed. British Academy Review. 2014;24:6–9. [Google Scholar]
- Deary I.J., Simonotto E., Meyer M., Marshall A., Marshall I., Goddard N., Wardlaw J.M. The functional anatomy of inspection time: An event-related fMRI study. NeuroImage. 2004;22:1466–1479. doi: 10.1016/j.neuroimage.2004.03.047. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Whiteman M.C., Starr J.M., Whalley L.J., Fox H.C. The impact of childhood intelligence on later life: Following up the Scottish Mental Surveys of 1932 and 1947. Journal of Personality and Social Psychology. 2004;86:130–147. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Allerhand M., Der G. Smarter in middle age, faster in old age: A cross-lagged panel analysis of reaction time and cognitive ability over 13 years in the West of Scotland Twenty-07 Study. Psychology and Aging. 2009;24:40–47. doi: 10.1037/a0014442. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Der G., Ford G. Reaction times and intelligence differences: A population-based cohort study. Intelligence. 2001;29:389–399. [Google Scholar]
- Deary I.J., Gow A.J., Pattie A., Starr J.M. Cohort profile: The Lothian Birth Cohorts of 1921 and 1936. International Journal of Epidemiology. 2012;41:1576–1584. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Gow A.J., Taylor M.D., Corley J., Brett C., Wilson V.…Starr J.M. The Lothian Birth Cohort 1936: A study to examine influences on cognitive aging from age 11 to age 70 and beyond. BMC Geriatrics. 2007;7:28. doi: 10.1186/1471-2318-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I.J., Johnson W., Starr J.M. Are processing speed tasks biomarkers of cognitive ageing? Psychology and Aging. 2010;25:219–228. doi: 10.1037/a0017750. [DOI] [PubMed] [Google Scholar]
- Der G., Deary I.J. Age and sex differences in reaction time in adulthood: Results from the United Kingdom Health and Lifestyle Study. Psychology and Aging. 2006;21:62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- Dodds R.M., Sydall H.E., Cooper R., Benzeval M., Deary I.J., Dennison E.M. Grip strength across the life course: Normative data from twelve British studies. PloS One. 2014;9 doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykiert D., Deary I.J. Retrospective validation of WTAR and NART scores as estimators of prior cognitive ability using the Lothian Birth Cohort 1936. Psychological Assessment. 2013;25:1361–1366. doi: 10.1037/a0033623. [DOI] [PubMed] [Google Scholar]
- Finkel D., Reynolds C.A., McArdle J.J., Pedersen N.L. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galton F. William Clowes and Sons Ltd.; London, UK: 1884. Anthropometric laboratory. [Google Scholar]
- Galton F. Remarks on ‘Mental tests and measurements’ by J. McK. Cattell. Mind. 1890;15:380–381. [Google Scholar]
- Jensen A.R. Elsevier; Amsterdam, The Netherlands: 2006. Clocking the mind: Mental chronometry and individual differences. [Google Scholar]
- Lindenberger U. Human cognitive aging: Corriger la fortune? Science. 2014;342:572–578. doi: 10.1126/science.1254403. [DOI] [PubMed] [Google Scholar]
- Nelson H.E., Willison J.R. NFER-Nelson; Windsor, UK: 1991. National Adult Reading Test (NART) Test Manual (Part II) [Google Scholar]
- Nettelbeck T., Wilson C. The Flynn effect: Smarter not faster. Intelligence. 2004;32:85–93. [Google Scholar]
- Newman D. Longitudinal modeling with randomly and systematically missing data: A simulation of ad hoc, maximum likelihood, and multiple imputation techniques. Organizational Research Methods. 2003;6:328–362. [Google Scholar]
- Otaga S., Kato K., Honda C., Hayakawa K. Common genetic factors influence hand strength, processing speed, and working memory. Journal of Epidemiology. 2014;24:31–38. doi: 10.2188/jea.JE20130070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschnig J., Voracek M. One century of global IQ gains: A formal meta-analysis of the Flynn Effect (1909–2013) Perspectives on Psychological Science. 2015;10:282–306. doi: 10.1177/1745691615577701. [DOI] [PubMed] [Google Scholar]
- Ramscar M., Hendrix P., Shaoul C., Milin P., Baayen H. The myth of cognitive decline: Non-linear dynamics of lifelong learning. Topics in Cognitive Science. 2014;6:5–42. doi: 10.1111/tops.12078. [DOI] [PubMed] [Google Scholar]
- Ritchie S.J., Bates T.C., Der G., Starr J.M., Deary I.J. Education is associated with higher later life IQ scores, but not with faster cognitive processing speed. Psychology and Aging. 2013;28:515–521. doi: 10.1037/a0030820. [DOI] [PubMed] [Google Scholar]
- Ritchie S.R., Tucker-Drob E.M., Deary I.J. A strong link between speed of visual discrimination and cognitive ageing. Current Biology. 2014;24:R681–R683. doi: 10.1016/j.cub.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille A., Piccinin A.M., Muniz-Terrera G., Hoffman L., Johansson B., Deeg D.J.…Hofer S.M. Longitudinal mediation of processing speed on age-related change in memory and fluid intelligence. Psychology and Aging. 2013;28:887–901. doi: 10.1037/a0033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T.A. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse T.A. Localizing age-related individual differences in a hierarchical structure. Intelligence. 2004;32:541–561. doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T.A. Why are there different age relations in cross-sectional and longitudinal comparisons of cognitive functioning? Current Directions in Psychological Science. 2014;23:252–256. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie K.W. 2nd ed. Oxford University Press; Oxford, UK: 2013. Developmental influences on adult intelligence: The Seattle Longitudinal Study. [Google Scholar]
- Scottish Council for Research in Education . University of London Press; London, UK: 1933. The intelligence of Scottish children. [Google Scholar]
- Scottish Council for Research in Education . University of London Press; London, UK: 1949. The trend of Scottish intelligence: A comparison of the 1947 and 1932 surveys of the intelligence of eleven-year-old pupils. [Google Scholar]
- Stampf S. Nonrandom: Stratification and matching by propensity score. R package (v.1.42) 2014. http://CRAN.R-project.org/package=nonrandom
- Sternäng O., Reynolds C.A., Finkel D., Ernsth-Bravell M., Pedersen N.L., Dahl Aslan A.K. Grip strength and cognitive abilities: Associations in old age. Journals of Gerontology Series B. 2015 doi: 10.1093/geronb/gbv017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternäng O., Wahlin Å., Nilsson L.G. Examination of the processing speed account in a population-based longitudinal study with narrow age cohort design. Scandinavian Journal of Psychology. 2008;49:419–428. doi: 10.1111/j.1467-9450.2008.00663.x. [DOI] [PubMed] [Google Scholar]
- Takema D.G., Ling C.H.Y., Kurrle S.E., Cameron I.D., Meskers C.G.M., Blauw G.J.…Maier A.B. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age and Ageing. 2012;41:506–512. doi: 10.1093/ageing/afs013. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. Oxford University Press; Oxford, UK: 2014. The elements of cognitive aging: Meta-analyses of age-related differences in processing speed and their consequences. [Google Scholar]
- Wechsler D. Psychological Corporation; London, UK: 1998. Wechsler Adult Intelligence Scale-IIIUK administration and scoring manual. [Google Scholar]
- Wilson R.S., Beckett L.A., Barnes L.L., Schneider J.A., Bach J., Evans D.A., Bennett D.A. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Zimprich D., Martin M. Can longitudinal changes in processing speed explain longitudinal age changes in fluid intelligence? Psychology and Aging. 2002;17:690–695. doi: 10.1037/0882-7974.17.4.690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.