Abstract
Screening for haemoglobin genotype was offered to senior school students in Manchester parish in south central Jamaica to test whether this knowledge would influence choice of partner and reduce births with sickle cell disease. Over six academic years, 15,539 students, aged mostly 15–19 years, were screened with voluntary compliance rising from 56 to 92 % over this period. All subjects were given permanent genotype cards and carriers of abnormal genes were offered counselling which explained the reproductive options but avoided recommendations. Prior to screening, all had been offered illustrated lectures on the genetics and clinical features of sickle cell disease. The current study, confined to females with the sickle cell trait, interviewed 763/845 (90.3 %) subjects seeking to assess retention of this knowledge and their response to subsequent boyfriends. Of those interviewed, 42 subjects were excluded (38 emigrated, one died, three received incorrect genotype cards) leaving 721 with complete information. Knowledge of genotype was retained in 95 %, the outcome of future offspring correctly recalled in 91 %, and haemoglobin genotype cards were still possessed by 89 %. A current ‘boyfriend’ was acknowledged in 403 (56 %) of whom the partner’s genotype was known in 88 (74 determined by the project laboratory; 14 by other laboratories) and unknown in 315 (78 %). Offers of free blood tests to all these partners were accepted by only 14 (4 %). Seventeen (2.4 %) were married but the husbands genotype was known in only five (four AA, one AS) of these. Most subjects retain knowledge of their genotype and of its significance for having affected children but the reluctance of partners to be tested was a major obstacle.
Keywords: Sickle cell disease, HbS, School screening, Genetic counselling
Introduction
Inheritance of the HbS gene from one parent and that for another interacting haemoglobin gene from the other results in a spectrum of conditions causing clinically significant sickle cell disease. Most common at birth is homozygous sickle cell (SS) disease resulting from the inheritance of HbS from both parents, the risk being 1 in 4 for each pregnancy. Other clinically significant forms of sickle cell disease result from the combined inheritance of HbS and the genes for HbC, HbD Punjab, HbO Arab, Hb Lepore or forms of beta thalassaemia. Inheritance of HbS along with the gene for the normal HbA results in the generally harmless sickle cell trait; so, selection by carriers of a partner with the AA genotype is one of the options for preventing affected births.
Sickle cell disease is the most common genetic disorder and the generally most severe form, SS disease, affects an estimated 240,000 births annually in sub-Saharan Africa (Piel et al. 2013). Births with sickle cell disease may be avoided by prenatal or pre-implantation diagnosis, but these are costly and often limited to developed societies. Prevention of the disease could also be achieved by identification of subjects with abnormal genes and education on their genetic significance empowering carriers to make informed decisions on their choice of partner. Although theoretically possible in sickle cell disease, attempts to investigate such an intervention have reached conflicting conclusions. In the Orchomenos Program, a farming community north of Athens, Greece with a 23 % prevalence of the sickle cell trait and characterised by arranged marriages in the mid 1960s, screening for the sickle cell gene was performed in 2300 families between 1966 and 1970 (Stammatoyannopoulos 1974). Review 7 years later indicated that although subjects correctly recalled their status and the genetics of sickle cell disease, four marriages had occurred between couples with the sickle cell trait compared with 4.5 marriages predicted from random mating. Of these four, two chose to marry while conscious of the risks and two had concealed their carrier status. It was concluded that the program had failed to achieve its goals although the small numbers limited the significance of this finding. Different conclusions were reached in the Kingdom of Bahrain, where voluntary premarital screening for the sickle cell gene reduced the prevalence of SS births from 2.0 to 0.9 % (Al Arrayed 2005a), and such screening became mandatory by Royal Decree in 2004 for both Bahrain (Al Arrayed 2005b) and for Saudi Arabia (Memish and Saeed 2011; Alswaidi et al. 2012). These programmes will reduce the frequency of affected births but Bahrain and Saudi Arabia are Islamic societies with traditions of arranged marriage. The effect of knowledge of haemoglobin genotype on subsequent partner selection in other societies is unknown and this question is now addressed by the Manchester project, centred in that parish in Jamaica. Over a 6-year period, identification of haemoglobin genotype was offered to the senior students of 15 secondary schools in the Parish and the screening of 15,539 students identified 846 females with the sickle cell trait. The current study sought to interview these former students, to determine their recall of genotype, its significance, whether they had had children, and knowledge of the haemoglobin genotype of partners.
Subjects and methods
Manchester is a parish in south central Jamaica with an area of 830 km2 stretching from the coast in the south to an altitude of approximately 3000 ft in the north, a population of 192,000 in 2008 (Statistical Institute of Jamaica), and the parish capital Mandeville is 100 km west of the country capital Kingston. An educational programme on sickle cell disease previously offered to senior classes of secondary schools throughout the island, identified the student’s need for haemoglobin genotype determination. This project offered screening to 15 secondary schools in the parish, but one declined to participate after 2 years believing that this information could be obtained from their private physicians.
Study population
The target population was the 5th and 6th forms (grades 11–13; aged mostly 15–19 years) of whom 15,539 (8750 or 56.3 % females) were screened over six academic years (2007/8 to 2012/13). The sickle cell trait occurred in 846 (9.7 %) females but one subject much older than the others was excluded leaving a study group of 845 females.
Screening procedure
At pre-arranged times, a team of a physician, clerical assistants, and 3–4 experienced phlebotomists visited a school site selected by the teaching staff. The students were invited to attend by the form teachers, completed a data form of basic demography (class, date of birth, address, family and contact details), and one 5 ml EDTA sample was taken from each student by venepuncture. The average turn-round time for individuals was about 20 min and depending on local factors, 150–250 students could have blood samples taken within a 2–2½ h period. Most schools required at least two visits and larger schools received 4–5 visits to satisfy the demand for screening.
Laboratory procedures
The technical methods are published (Mason et al. 2015) and the distribution of genotypes is shown in Table 1.
Table 1.
Haemoglobin genotypes by gender
| Genotype | Male (%) | Female (%) | Total (%) |
|---|---|---|---|
| AA | 5766 (84.91) | 7436 (84.94) | 13,202 (84.93) |
| AS | 663 (9.78) | 846 (9.65) | 1509 (9.72) |
| AC | 231 (3.39) | 307 (3.52) | 538 (3.46) |
| β thalassaemia trait | 68 (1.00) | 74 (0.85) | 142 (0.91) |
| HPFH trait | 24 (0.35) | 28 (0.35) | 52 (0.35) |
| SS disease | 8 (0.12) | 16 (0.18) | 24 (0.15) |
| SC disease | 14 (0.21) | 20 (0.23) | 34 (0.22) |
| Sβ+ thalassaemia | 2 (0.03) | 7 (0.08) | 9 (0.06) |
| Sβo thalassaemia | 0 | 0 | 0 |
| S/HPFH | 2 (0.03) | 1 | 3 (0.03) |
| CC disease | 3 (0.04) | 4 (0.05) | 7 (0.05) |
| Cβo thalassaemia | 1 | 0 | 1 |
| Variant trait | 7 (0.10) | 10 (0.11) | 17 (0.11) |
| S/variant | 0 | 1 | 1 |
| Total | 6789 | 8750 | 15,539 |
HPFH indicates the trait for classic hereditary persistence of fetal haemoglobin
Distribution of results and counselling
All tested students received laminated cards bearing personal details (name, school, date of birth), laboratory ID#, and the haemoglobin genotype with interpretation. Normal subjects (AA genotype) received a card stating that they were not at risk of a baby with sickle cell disease. Carriers of abnormal genes received a card stating the genotype, for example AS with designation sickle cell trait and the notation that ‘This will not affect your health but you could pass it on to your children. If your partner is normal, you cannot have a child with sickle cell disease’. The reverse side of the card for those with abnormal genes had a simple pedigree as an example and contact information for the screening laboratory. The normal genotype cards were distributed by the teacher or guidance counsellor and students with abnormal genotypes were contacted by the screening team, given the cards and offered genetic counselling which could be taken either individually or in small groups as preferred. Care was taken to avoid ‘directive counselling’ as the intention was to observe the responses and decisions of this ‘informed cohort’. All carriers of abnormal genes were also given explanation sheets along with pedigrees for common potential partner genotypes and information on how to get their partner tested.
Details of current review
The present review focused on pregnancy outcome and so was confined to females, the protocol requiring participation of at least 90 % of the 845 females with the sickle cell trait over the period June 2013–September 2014. The median interval between screening and interview was 31 months (range 12–74 months). Interviews were conducted by phone but 93 required home visits. Interviews conducted by phone took 5–10 min depending on the questions and level of understanding of the respondent. Students were asked whether they recalled their genotype, the significance of the genotype, whether they still possessed their genotype cards, if they had a ‘boyfriend’, did they know his genotype, had they become pregnant, and whether they were married. For the purposes of this study, boyfriend was defined as a regular partner which usually, but not always, implied sexual activity. Almost all interviews were conducted by a female member of staff who had been intimately involved in the school screening and in the associated laboratory work.
Ethical issues
The programme was approved by the Jamaican Ministry of Health and the Ministry of Education and was preceded by discussions with the school staff, Parent-Teachers Associations, and illustrated lectures on sickle cell disease and its genetics to the students. All students were given an information letter for their parents outlining the objectives of the study, the possible advantages, and the option of declining for their child to be tested (the opt-out approach having been approved by the Ministry of Health). The Ministry of Health considered that written informed consent was not necessary or logistically feasible under the circumstances especially since this programme was evolved in response to the request for haemoglobin genotype and the voluntary nature of participation in screening.
Results
Compliance with screening
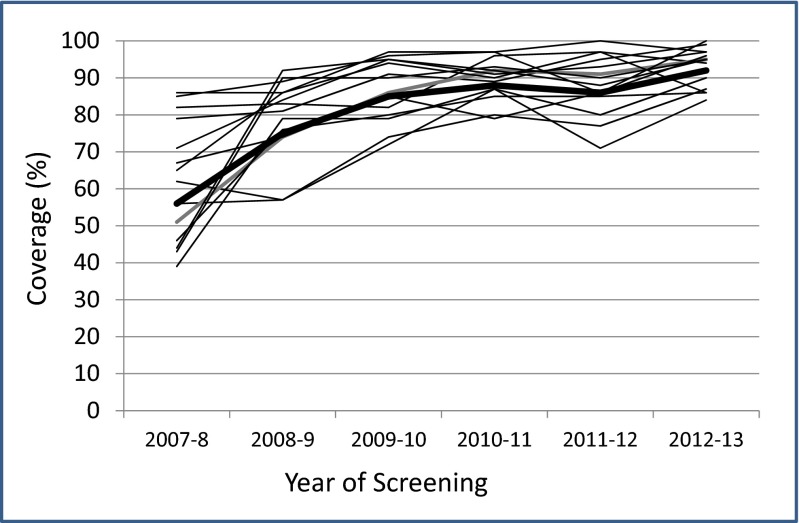
Voluntary compliance with testing increased over the 6-year screening period from 56 to 92 % (Fig. 1). Of the 763 subjects contacted, 42 had incomplete data (38 moved abroad, one died, three had received incorrect genotype cards due to clerical errors) leaving a study group of 721.
Fig. 1.
Compliance with testing in 14 schools over the screening period. Heavy black line represents mean values for each year
Recall of genotype
Haemoglobin genotype was correctly recalled in 686 (95.1 %) and one third of the 35 students failing to recall correctly had missed the lectures or the genotype card distribution. There was no obvious pattern regarding site of school or year of screening but many students especially in rural areas have irregular school attendance.
Recall of significance
The significance of the trait in terms of the risk of a baby with sickle cell disease according to their choice of partner was correctly recalled in 653 (90.6 %) without any obvious pattern for year of screening or site of school (rural vs urban).
Possession of genotype card
The cards were retained by 644 (89.2 %) and some students who had lost their cards requested replacements indicating a desire to retain the information. The proportion of lost cards increased with the interval since screening, rising from 4 % in the most recent screening year to 15 % in the first screening year.
Knowledge of boyfriend’s genotype
A current boyfriend was acknowledged in 403 (56 %) subjects and the genotype was known in 88 (22 %) partners, 74 tested in the project laboratory (64 AA, four AS, four AC, one SS disease, and one with Hb Shimonoseki trait) and another 14 in other laboratories. A further 74 had assumed themselves to be normal because of no symptoms or lack of family history of sickle cell disease. Of the 315 (78 %) partners with unknown genotype, all were offered free testing at the project laboratory through their girlfriends or directly with the girlfriends’ acquiescence but only 14 (4 %) accepted this offer.
Genotype of offspring
There were 212 pregnancies among 190 mothers with the sickle cell trait, twins occurring in first pregnancies of two mothers and in the second pregnancy of one mother. The phenotypes in 188 singleton first pregnancies were 89 AA, 93 AS, one AC, one SC, and four SS.
Marriages
There were 17 (2.4 %) marriages consistent with a common Jamaican pattern of marriage at a later age: the husbands genotypes were known in five marriages (four AA, one AS) and unknown in 12.
Discussion
Options to avoid births with sickle cell disease include the voluntary avoidance of relationships between carriers of abnormal genes, early detection of affected pregnancies allowing termination (prenatal diagnosis) and pre-implantation genetic diagnosis. The first involving population screening and counselling could be implemented with limited technical resources whereas the latter require increasing cost and technical expertise. The choice depends to some extent upon the perceived severity of the disease which is quite different between families at risk for transfusion-dependent beta thalassaemia and those for homozygous sickle cell (SS) disease.
In transfusion-dependent beta thalassaemia, where survival requires blood transfusion every 3–4 weeks from early life and regular chelation, there is a powerful stimulus to avoid such births (Cousens et al. 2010). The clinical picture is totally different in families at risk of a baby with SS disease, where the outcome varies from death in the first year of life to survival to the age of 80 years and a median survival in Jamaica of 55 years (Wierenga et al. 2001). Faced with this variable clinical course, 50–70 % of mothers with an affected fetus chose to continue the pregnancy (Alter 1987; Wang et al. 1994) although termination of an affected fetus may be becoming more acceptable (Wonkam et al. 2015) especially with the use of non-invasive parental testing (Hill et al. 2014). Fetal diagnosis is now simpler and more accurate but with the social and cultural concerns regarding termination of affected pregnancies, the family desires information on the likely clinical course which is currently almost impossible to predict. Pre-implantation genetic diagnosis (Xu et al. 1999) requires facilities for in vitro fertilisation which are limited in high-risk areas, are expensive and have success rates of 40–50 %, although its acceptability for sickle cell disease is expanding in the USA (Kuliev et al. 2011). However, these technologies are not widely available in developing societies and neither prenatal diagnosis nor pre-implantation genetic diagnosis is currently offered in Jamaica.
These limitations do not apply to population screening and premarital screening has successfully reduced births with beta thalassaemia in Cyprus (Angastiniotis and Hadjiminas 1981) and Sardinia (Cao et al. 1996) and has led to mandatory premarital screening in Iran (Karimi et al. 2007), Bahrain (Al Arrayed 2005b), Saudi Arabia (Al Hamdan et al. 2007), the Palestinian territories (Tarazi et al. 2007), and Cyprus (Cowan 2009) as reviewed by Giordano et al (2014). Although contrary to the WHO guidelines (WHO 1998) on compulsory genetic testing, these programmes seem to have been well accepted. The advent of mandatory premarital screening programmes in both Bahrain and Saudi Arabia since 2004 will certainly reduce the number of affected births. Data from the Saudi programme for 2009 indicated that of nearly 300,000 engagements, marriage cancellations occurred in 52 % of the 1171 families receiving ‘incompatible certificates’ (Memish and Saeed 2011). Most published reports have occurred in communities with relatively low frequencies of the sickle cell gene and often high-technology resources offering prenatal diagnosis and termination such as the Cuban programme (Granda et al. 1991) but logistical problems of follow-up and of monitoring pregnancy outcome have limited assessment of their impact.
Jamaica is ideal for such studies since with a population of 2.8M of predominantly West African origin and abnormal haemoglobins occurring in 14.5 % of the population, it can be predicted that sickle cell disease would affect 6–7/1000 births or 270 of the 40,000 annual births. Furthermore, the geographical features of an island with good communications and adequate roads allow follow-up of virtually all births. It was against this background that the Manchester project offered haemoglobin genotype identification to the senior students of high schools selected because these represented an intelligent, motivated group, the majority of whom had not yet formed serious attachments and might factor their haemoglobin genotype into subsequent decisions. Potential disadvantages were that these students carried a greater likelihood of pursuing tertiary education and delaying child-bearing although this concern seemed unfounded as 190 (26.4 %) females with the sickle cell trait have had a total of 212 pregnancies. The procedures of screening were well tolerated and compliance with this voluntary process increased to 92 % over the 6-year period suggesting that students wanted to learn their carrier status. The genetic significance of the trait for future children was recalled in 91 % but of the 403 subjects, the genotype was unknown in 315 (78 %) current sexual partners. It was not possible to reliably assess the frequency with which girls disclosed their haemoglobin genotype to their boyfriends or the level of pressure which they used to persuade their boyfriends to be tested. With the frailty of many relationships, there may have been a reluctance to embark on such discourse but in direct contact with the boyfriends by the project staff with the acquiescence of the female partner, only 14 (4 %) visited the laboratory despite the offer of free, definitive blood tests. Such testing for genetic counselling purposes requires the greatest precision (Giordano 2013), and detection of the beta thalassaemia trait with estimations of HbA2 and HbF and possibly gene sequencing may be difficult to justify in communities such as Jamaica with a 1 % frequency of beta thalassaemia trait. However, despite a theoretical understanding of the risks of an offspring with sickle cell disease, the great majority were not aware of their partners’ haemoglobin genotype. It is currently unknown whether this reluctance of male partners to be tested is related to fear of the result or the desire to avoid the mild discomfort of the venepuncture but this reluctance compromises the identification of ‘at-risk’ couples limiting the options of possible interventions.
A limitation of the current study was the omission of interviews with male students but the information would have been difficult to assess in view of an impaternity rate exceeding 10 % (unpublished results) and the unpracticality of DNA confirmation of paternity. There may also be concerns on the extent to which Jamaican data are applicable elsewhere. Although the population is predominantly of West African origin with over 14 % carrying abnormal genes, its cultural and social history differs from many more conservative populations of African origin and may limit extrapolation of these data to other societies.
The objective of this programme was to establish an informed cohort of students with knowledge of their haemoglobin genotype who was not yet in established relationships, providing the option of factoring this information into their choice of partner. This objective has been compromised by the reluctance of male partners to be tested but the outcome of subsequent births remains a valid question and must await a greater number of pregnancies. In the meantime, it is clear that greater education and awareness on the genetics of sickle cell disease are necessary and should be incorporated in all biology and health-related educational programmes at school and in the public domain. If such population screening fails to achieve a reduction in affected births, the development of more widespread prenatal diagnosis facilities, despite the concerns detailed above, may become necessary.
Acknowledgments
This work was supported by the National Health Fund of Jamaica, the Chase Fund of Jamaica, and the Alcoa Foundation. Support from the Southern Regional Health Authority and the staff of the hospitals operating in south and west Jamaica is gratefully acknowledged.
Compliance with ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. The Ministry of Health and the Ministry of Education of the Jamaican Government approved the study and believing that the advantages of testing far outweighed the potential disadvantages of blood tests, approved the ‘opt-out’ procedure for parental consent.
Conflict of interest
The authors declare that they have no competing interests.
References
- Al Arrayed S. Campaign to control genetic blood diseases in Bahrain. Community Genet. 2005;8:52–55. doi: 10.1159/000083340. [DOI] [PubMed] [Google Scholar]
- Al Arrayed SS (2005b) Premarital genetic counseling: a new law in the Kingdom of Bahrain. J Health Soc Environ Issues, Middlesex University 6:31–4
- Al Hamdan NA, Al Mazrou YY, Al Swaidi FM, Choudary AJ. Premarital screening for thalassemia and sickle cell disease in Saudi Arabia. Genet Med. 2007;9:372–377. doi: 10.1097/GIM.0b013e318065a9e8. [DOI] [PubMed] [Google Scholar]
- Alswaidi FM, Memish ZA, O’Brien SJ, Al-Hamdan NA, Al-Enzy FM, Alhayani OA, Al-Wadey AM. At-risk marriages after compulsory premarital testing and counselling for β-thalassemia and sickle cell disease in Saudi Arabia, 2005–6. J Genet Couns. 2012;21:243–255. doi: 10.1007/s10897-011-9395-4. [DOI] [PubMed] [Google Scholar]
- Alter BP. Prenatal diagnosis of hematologic diseases, 1986 update. Acta Haematol. 1987;78:137–141. doi: 10.1159/000205863. [DOI] [PubMed] [Google Scholar]
- Angastiniotis MA, Hadjiminas MG. Prevention of thalassaemia in Cyprus. Lancet. 1981;1:369–371. doi: 10.1016/S0140-6736(81)91682-2. [DOI] [PubMed] [Google Scholar]
- Cao A, Rosatelli MC, Galanello R. Control of beta-thalassaemia by carrier screening, genetic counselling and prenatal diagnosis: the Sardinian experience. Ciba Found Symp. 1996;197:137–151. doi: 10.1002/9780470514887.ch8. [DOI] [PubMed] [Google Scholar]
- Cousens NE, Gaff CL, Metcalfe SA, Delatycki MB. Carrier screening for betao thalassaemia: a review of international practice. Eur J Hum Genet. 2010;18:1077–1083. doi: 10.1038/ejhg.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RS. Moving up the slippery slope: mandated genetic screening in Cyprus. Am J Med Genet C: Semin Med Genet. 2009;151C:95–103. doi: 10.1002/ajmg.c.30202. [DOI] [PubMed] [Google Scholar]
- Giordano PC. Strategies for basic laboratory diagnostics of the hemoglobinopathies in multi-ethnic societies: interpretation of results and pitfalls. Int J Lab Hematol. 2013;35:465–479. doi: 10.1111/ijlh.12037. [DOI] [PubMed] [Google Scholar]
- Giordano PC, Harteveld CL, Bakker E. Genetic epidemiology and preventive healthcare in multiethnic societies: the hemoglobinopathies. Int J Environ Res Public Health. 2014;11:6136–6146. doi: 10.3390/ijerph110606136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granda H, Gispert S, Dorticós A, Martín M, Cuadras Y, Calvo M, Martínez G, Zayas MA, Oliva JA, Heredero L. Cuban programme for prevention of sickle cell disease. Lancet. 1991;337:152–153. doi: 10.1016/0140-6736(91)90810-C. [DOI] [PubMed] [Google Scholar]
- Hill M, Compton C, Karunaratna M, Lewis C, Chitty L. Client views and attitudes to non-invasive prenatal diagnosis for sickle cell disease, thalassaemia and cystic fibrosis. J Genet Couns. 2014;23:1012–1021. doi: 10.1007/s10897-014-9725-4. [DOI] [PubMed] [Google Scholar]
- Karimi M, Jamalian N, Yarmohammadi H, Askarnejad A, Afrasiabi A, Hashemi A. Premarital screening for β-thalassaemia in Southern Iran: options for improving the programme. J Med Screen. 2007;14:62–66. doi: 10.1258/096914107781261882. [DOI] [PubMed] [Google Scholar]
- Kuliev A, Pakhalchuk T, Verlinsky O, Rechitsky S. Pre-implantation genetic diagnosis for hemoglobinopathies. Hemoglobin. 2011;35:547–555. doi: 10.3109/03630269.2011.608457. [DOI] [PubMed] [Google Scholar]
- Mason K, Gibson F, Higgs D, Fisher C, Thein SL, Clark B, et al. Haemoglobin variant screening in Jamaica: meeting student’s request. Br J Haematol. 2015 doi: 10.1111/bjh.13531. [DOI] [PubMed] [Google Scholar]
- Memish ZA, Saeed MY. Six-year outcome of the national premarital screening and genetic counseling program for sickle cell disease and b-thalassemia in Saudi Arabia. Ann Saudi Med. 2011;31:229–235. doi: 10.4103/0256-4947.81527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, Temperley WH, Williams TN, Weatherall DJ, Hay SI. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stammatoyannopoulos G (1974) Problems of screening and counselling in the hemoglobinopathies in birth defects In: Motulsky AG, Lenz W (eds) Excerpta Medica Int Congr Ser, pp 268–76
- Tarazi I, Al Najjar E, Lulu N, Sirdah M. Obligatory premarital tests for β-thalassaemia in the Gaza Strip: evaluation and recommendations. Int J Lab Hematol. 2007;29:111–118. doi: 10.1111/j.1751-553X.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Seaman C, Paik M, Chen T, Bank A, Piomelli S. Experience with 500 prenatal diagnoses of sickle cell diseases: the effect of gestational age on affected pregnancy outcome. Prenat Diagn. 1994;14:851–857. doi: 10.1002/pd.1970140914. [DOI] [PubMed] [Google Scholar]
- Wierenga KJJ, Hambleton IR, Lewis NA. Survival estimates for patients with homozygous sickle-cell disease in Jamaica: a clinic-based population study. Lancet. 2001;357:680–683. doi: 10.1016/S0140-6736(00)04132-5. [DOI] [PubMed] [Google Scholar]
- Wonkam A, Ngo Bitoungui VJ, Ngogang J. Perspectives in genetics and sickle cell disease prevention in Africa: beyond the preliminary data from Cameroon. Public Health Genomics. 2015;18:237–241. doi: 10.1159/000431020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1998) Proposed international guidelines on ethical issues in medical genetics and genetic services [PubMed]
- Xu K, Shi ZM, Veeck LL, Hughes MR, Rosenwaks Z. First unaffected pregnancy using preimplantation genetic diagnosis for sickle cell anemia. JAMA. 1999;281:1701–1706. doi: 10.1001/jama.281.18.1701. [DOI] [PubMed] [Google Scholar]