Abstract
The origins of clinical genetics services vary throughout Europe with some emerging from paediatric medicine and others from an academic laboratory setting. In 2011, the cross-border patients’ rights directive recommended the creation of European Research Networks (ERNs) to improve patient care throughout EU. In 2013, the EU recommendation on the care for rare diseases came into place. The process of designating EU centres of expertise in rare diseases is being implemented to allow centres to enter ERNs. Hence, this is an opportune time to reflect on the current status of genetic services and research funding throughout Europe as 80 % of rare diseases have a genetic origin. Our aims were to determine (a) whether EU countries are prepared in terms of appropriate clinical genetic staffing to fulfil the European Union Committee of Experts on Rare Diseases (EUCERD) criteria that will allow national centres to be designated as centres of expertise, (b) which EU countries are successful in grant submissions to EU rare disease research funding and (c) country of origin of researchers from the EU presenting their research work as a spoken presentation at the European Society of Human Genetics annual conference. Our results show there is wide disparity of staffing levels per head of population in clinical genetics units throughout Europe. EU rare disease research funding is not being distributed equitably and the opportunity to present research is skewed with many countries not achieving spoken presentations despite abstract submissions. Inequity in the care of patients with rare diseases exists in Europe. Many countries will struggle to designate centres of expertise as their staffing mix and levels will not meet the EUCERD criteria which may prevent them from entering ERNs. The establishment of a small number of centres of expertise centrally, which is welcome, should not occur at the expense of an overall improvement in EU rare disease patient care. Caution should be observed to ensure that the inequity gap that already exists does not widen with the development of ERNs.
Keywords: Clinical genetics services, Equity, Europe, Rare disease research funding, ESHG conference
Introduction
Statistics from the World Health Organisation suggest that up to 70 % of birth defects have been prevented in high-income countries since the 1960s (http://www.who.int/topics/congenital_anomalies/en/). This was achieved by investment in a range of preventative measures including provision of a clinical genetics service (http://www.who.int/topics/congenital_anomalies/en/). WHO recommended provision of clinical genetics as a distinct speciality in the 1980s as one of the critical components to the prevention of birth defects (http://www.who.int/topics/congenital_anomalies/en/, http://www.dcp2.org/file/230/dcpp-twpcongenitaldefects_web.pdf, http://www.who.int/nutrition/publications/birthdefects_manual/en/). Indeed, primary prevention of congenital anomalies was identified as an important action in the field of rare diseases in the Communication from the Commission to the European Parliament, the European Council, the European Economic and Social Committee and the Committee of the Regions of 11 November 2008 (http://www.eurocat-network.eu/content/EUROCAT-EUROPLAN-Primary-Preventions-Recommendations.pdf).
Support from governments has varied with countries supporting primary and secondary prevention investing heavily. The economic argument is strong; the Irish Faculty of Paediatrics (2011) (http://www.efcni.org/fileadmin/Daten/Web/Brochures_Reports_Factsheets_Position_Papers/benchmarking_report/EFCNI_ireland_light.pdf_copyright.pdf) estimated that it costs >2000 € per day in a neonatal intensive care unit and Yi et al. (2011) noted that annual expenditure on a baby with spina bifida is six times that of a healthy baby in the first year of life and remains three times the normal rate at age 45 years (Yi et al. 2011) (http://www.efcni.org/fileadmin/Daten/Web/Brochures_Reports_Factsheets_Position_Papers/benchmarking_report/EFCNI_ireland_light.pdf_copyright.pdf).
Following the EU recommendation on the care of patients with rare diseases, signature countries within the EU have committed to provide adequate care for their patients in terms of access to centres of expertise and development of centres of expertise using the European Union Committee of Experts on Rare Diseases (EUCERD) criteria (Rodwell and Aymé 2014) (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF), (Rodwell & Aymé S.Rare disease policies to improve care for patients in Europe. Biochim Biophys Acta 2015) (http://www.eucerd.eu/?post_type=document&p=1224). However, as no one is policing whether or not this is happening, we sought to estimate clinical genetics staffing to give an indication of how well-placed countries are to meet these obligations. Staffing requirements should be determined by (a) population size, (b) annual live-birth rates, (c) availability of termination of pregnancies for foetal abnormalities [if most affected pregnancies were being terminated, this would reduce the need for urgent neonatal consultations] and (d) prevalence of affected pregnancies and live-births [which would be influenced by factors such as consanguinity].
Termination of pregnancy is available for foetal abnormality in all countries except the Republic of Ireland, Northern Ireland and Malta. Consanguinity is not a common practice amongst native Europeans with only Roma gipsies and the Irish Traveller population being indigenous endogamous communities. European countries with a large Roma gypsy population (Bulgaria, Hungary and Slovakia) and countries with large migrant populations where consanguineous marriages are common and will have higher rates of recessive disorders. Hamamy (2012) estimated that European consanguinity levels are ~5 %(Hamamy 2012).
Most countries have not published recommended consultant clinical geneticist staffing levels, and those that have, differ: the UK and Germany have recommended 3 per million and 1 per 606,384 inhabitants, respectively (https://www.rcplondon.ac.uk/sites/default/files/clinical_genetics.pdf, https://www.g-ba.de/downloads/62-492-850/BPL-RL_2013-12-19_iK-2014-02-26.pdf). The EUCERD criteria do not specify clinical genetics staffing requirements but do seek information on multidisciplinary staffing levels and recommend timely access to specialist care (http://www.eucerd.eu/?post_type=document&p=1224).
In addition, the importance of research is an integral part of being designated a centre of expertise. The EU has funded numerous grant calls for patients with rare diseases. Larger numbers of affected cohorts lead to better research outcomes. The more countries that get involved the better as research not only improves the health of a nation but it also offers employment and other ancillary benefits. We sought to estimate how funding is being distributed across EU to determine which countries were most successful at securing EU rare disease research grants.
Career progression as a research scientist or clinical geneticist is enhanced by opportunities to present local research at international meetings. Spoken presentations foster collaboration and provide an impetus to cultivate further research and improve success rates in grant submissions. We sought to estimate whether the opportunity to present research as a spoken presentation was distributed equitably across researchers from all countries who participated by submitting abstracts to the European Society for Human Genetics (ESHG) conference.
Methods
Clinicians were identified throughout EU either by
Their country’s representative on the ESHG council
Their name being recommended by their ESHG representative
Their names being listed on Orphanet or
Prior knowledge of their involvement in local workforce planning
The following countries were emailed for information on staffing levels:
Austria, Belgium, Bulgaria, Czech Republic, Denmark, England, France, Germany, Italy, Malta, Netherlands, Northern Ireland, Norway, Poland, Portugal, Republic of Ireland, Romania, Spain, Scotland, Sweden and Wales. Information was received from 14 countries: Bulgaria, Czech Republic, England, France, Germany, Malta, Netherlands, Northern Ireland, Norway, Portugal, Republic of Ireland, Scotland, Sweden and Wales.
The information requested included (a) the population of their country; (b) the live-birth rate; (c) the number of whole time equivalent (WTE) clinical geneticists working in the public sector in their respective country; (d) the number of WTE doctors in training to be consultants or specialist registrars; (e) the number of WTE fully qualified non-medical genetic counsellors, with either nursing or science background; and (f) the number of WTE consultants doing research.
We sought live-born malformation rates through www.eurocat-network.eu/ and compared countries with access to termination of pregnancy to those without access (http://www.eurocat-network.eu/accessprevalencedata/prevalencetables).
We also sought to estimate participation by EU countries in those EU projects focusing on rare disease research in order to estimate the distribution of EU grant monies throughout the EU. Information was gleaned through a number of sources (http://www.orpha.net/orphacom/cahiers/docs/GB/Networks.pdf, (www.ec.europa.eu/budget/fts: eCORDA (External Common Research Datawarehouse) et al. 2014).
We reviewed abstract submission to the European Society of Human Genetics conference (https://www.eshg.org/95.0.html). We analysed two data sets: (1) the 276 spoken presentations given by young investigators who submitted abstracts for the years 2011–2015 and (2) the >4000 spoken and poster presentations, from submitted abstracts for years 2014 and 2015, to determine the country of origin of the research and the researcher (https://www.eshg.org/abstracts2015.0.html, https://www.eshg.org/home2014.0.html). We did not include invited speakers within these data sets.
Results
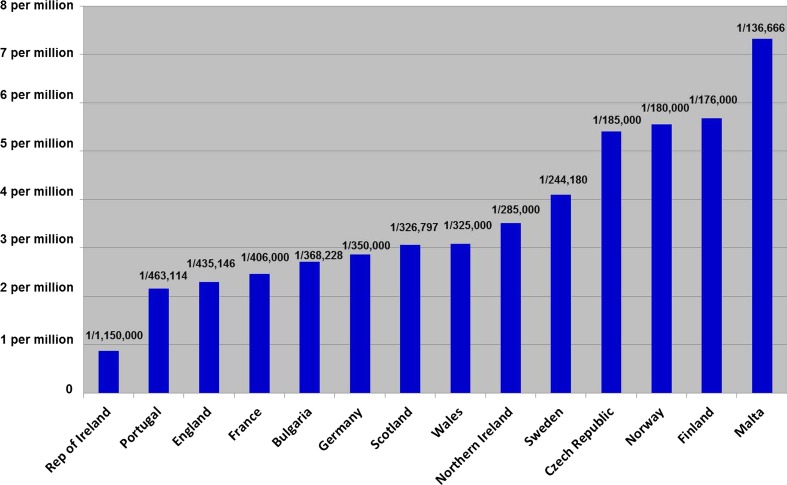
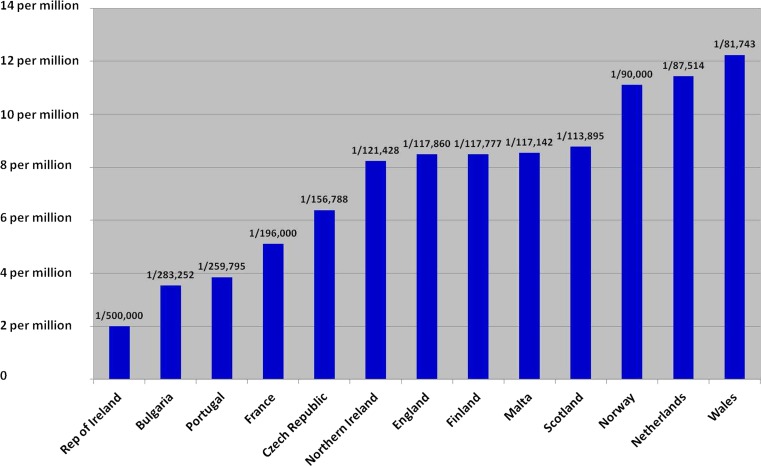
The results show a wide disparity of staffing levels (Figs. 1 and 2). Western European service provision was generally good with many countries having very optimal staffing. Long geographical distances play a factor in the high staffing levels within both Finland and Norway. Other countries, e.g. Malta, have good consultant staffing levels but have no other ancillary staff. Data from just two eastern European countries was forthcoming with good staffing levels in the Czech Republic but much poorer in Bulgaria.
Fig. 1.
Number of Clinical Genetics Consultants per capita across 14 European countries
Fig. 2.
Number of Clinical Genetic staff (non-laboratory) including clinical researchers and Genetic counsellors per capita in 13 European countries
Some countries (notably France) have other specialists “providing clinical genetics services for specific conditions”. These were not included. We also noted, but have not included, consultants working in the private sector. Some countries, notably the Czech Republic, have a high number of consultants who work in the private sector. In those countries where research clinician posts existed, most clinicians had job descriptions with part-time sessions reserved for research time only. The Netherlands have excellent staffing levels but were unable to distinguish consultant research from consultant clinical sessional commitment and so only appear on the research graph.
One western European country demonstrated very poor provision of services, notably the Republic of Ireland (ROI) whose staffing (1 consultant per 1.15 million) is in stark contrast to its neighbour Northern Ireland (1 consultant per 277,000) despite having the highest birthrate across Europe (15.6 per 1000 births) (http://ec.europa.eu/eurostat/web/population-demography-migration-projections/births-fertitily-data).
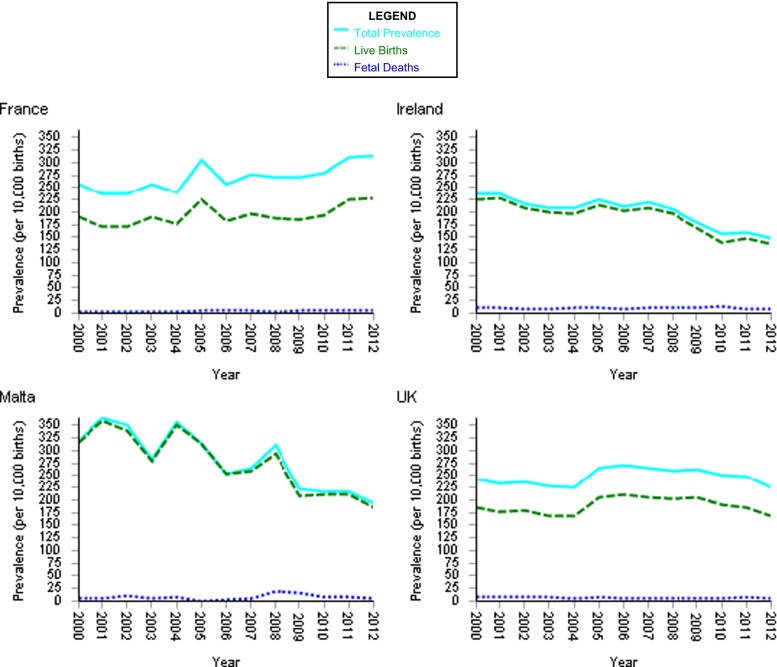
There is evidence of a higher live-born malformation rate in countries without access to termination of pregnancy (EUROCAT 2015), further compounding service provision (see Fig. 3).
Fig. 3.
EUROCAT prevalence data tables. Prevalence per 10,000 births of all anomalies for the following registries: France, Ireland, Malta and UK from 2000–2012. Data shows that the liveborn malformation rate in countries with no access to termination of pregnancy is higher than in those countries where access if available
The distribution of EU rare disease research grants is skewed towards larger wealthier countries with well-established genetics services (see Table 1 and Fig. 4). The coordinators of 213 European clinical networks funded through the EU, come from 17 countries (http://www.orpha.net/orphacom/cahiers/docs/GB/Networks.pdf) (see Table 1). These 17 countries (total population 410 million) participate in a further 1172 networks. As a rule, a coordinating country receives more funding to cover costs incurred in the administration of the grant. In contrast, a further 16 European countries who have no coordinators participate in just 207 networks—one fifth that of the 17 countries detailed above despite their populations comprising circa 120 million (one third that of the coordinator groups).
Table 1.
European collaborative research projects funded by DG Research and by E-Rare in the field of rare diseases and European clinical networks funded by DG Sanco and contributing to clinical research in the field of rare diseases (www.orpha.net/orphacom/cahiers/docs/GB/Networks.pdf)
| Country | Number of projects that country coordinates | Number of projects that country participates in |
|---|---|---|
| Austria | 8 | 39 |
| Belgium | 10 | 69 |
| Bulgaria | 0 | 6 |
| Croatia | 0 | 5 |
| Cyprus | 0 | 8 |
| Czech Republic | 0 | 30 |
| Denmark | 6 | 50 |
| Estonia | 0 | 9 |
| Finland | 2 | 29 |
| France | 48 | 150 |
| Germany | 39 | 164 |
| Greece | 2 | 26 |
| Great Britain | 23 | 127 |
| Hungary | 1 | 21 |
| Iceland | 1 | 7 |
| Ireland | 0 | 26 |
| Italy | 27 | 137 |
| Latvia | 0 | 6 |
| Lithuania | 0 | 5 |
| Luxembourg | 1 | 4 |
| Netherlands | 8 | 96 |
| Norway | 2 | 31 |
| Poland | 0 | 28 |
| Portugal | 0 | 32 |
| Romania | 0 | 8 |
| Slovenia | 0 | 13 |
| Slovakia | 0 | 5 |
| Spain | 14 | 82 |
| Sweden | 8 | 74 |
| Switzerland | 2 | 66 |
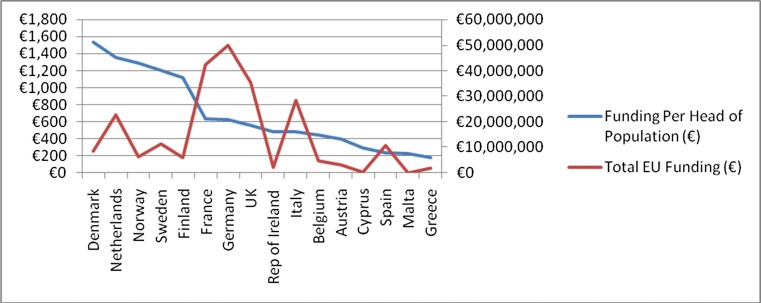
Fig. 4.
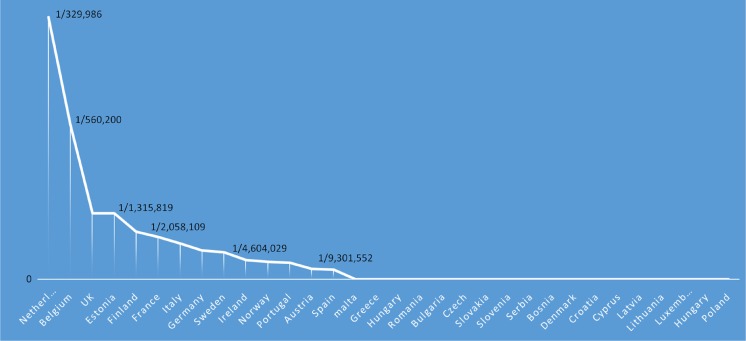
Data from DG SANCO and DG RTD depicting per capita which countries have received EU FP7 grant funding in the field of rare disease research for the period 2007 to 2014 (eCORDA [External Common Research Datawarehouse], European Commission, as of 2014/06/20
Additionally, the FP7 projects covering the thematic activities of the health programme in “rare diseases” namely External Common Research Datawarehouse [eCORDA], European Commission as of 2014/06/20, were identified (www.ec.europa.eu/budget/fts: eCORDA (External Common Research Datawarehouse) et al. 2014) (see Fig. 4). Some EU countries were not recipients of any grants (Croatia, Latvia, Luxembourg and Slovenia). A further 7/24 (30 %) participated in one grant only. In total therefore, 11/28 EU member states (40 %) demonstrated minimal to no participation.
The country of origin of the research group for the ESHG young investigator who were selected for spoken presentations between 2011 and 2015 is represented in Table 2 (https://www.eshg.org/95.0.html). The Netherlands represented the most successful individual country group (65/276 = 24 %) followed by Germany and the UK (38/276 = 14 % each), France (25/276 = 9 %), Belgium (23/276 = 8 %) and Italy (17/276 = 6 %). There was poor representation from elsewhere in Europe. However, 73/276 (26 %) researchers came from countries other than their country of work/research, and 34/73 (47 %) of these originated from countries that were otherwise not represented in a spoken slot.
Table 2.
ESHG conference young investigator spoken presentations 2011–2015
| Country | 2011 | 2012 | 2013 | 2014 | 2015 | Total |
|---|---|---|---|---|---|---|
| Netherlands | 112 | 11 | 115 | 12 | 15 | 65 |
| Germany | 3 | 16 | 7 | 6 | 6 | 38 |
| UK | 5 | 3 | 9 | 7 | 14 | 38 |
| France | 4 | 2 | 11 | 2 | 6 | 25 |
| Belgium | 7 | 3 | 1 | 5 | 7 | 23 |
| Italy | 1 | 2 | 2 | 9 | 3 | 17 |
| Switzerland | 2 | 3 | 4 | 3 | 2 | 14 |
| USA | 1 | 2 | 4 | 4 | 3 | 14 |
| Finland | 1 | 2 | 1 | 1 | 1 | 6 |
| Canada | 4 | 1 | 5 | |||
| Spain | 1 | 4 | 5 | |||
| Denmark | 2 | 2 | 4 | |||
| Israel | 1 | 2 | 1 | 4 | ||
| Portugal | 1 | 1 | 1 | 1 | 4 | |
| Ireland | 2 | 1 | 3 | |||
| Austria | 1 | 1 | 2 | |||
| Estonia | 1 | 1 | 2 | |||
| Sweden | 1 | 1 | 2 | |||
| Australia | 1 | 1 | ||||
| Brazil | 1 | 1 | ||||
| Hong Kong | 1 | 1 | ||||
| Iceland | 1 | 1 | ||||
| Japan | 1 | 1 | ||||
| Total | 44 | 50 | 59 | 56 | 67 | 276 |
Collectively, the 2014 and 2015 conferences received >4000 abstracts (https://www.eshg.org/abstracts2015.0.html, https://www.eshg.org/home2014.0.html). Overall, the largest number of submitted abstracts was received by the two host countries for those years: Italy in 2014 (>300 for years 2014 and 2015) and the UK in 2015 (>230 for 2014 and 2015). Thereafter, the countries contributing most abstracts for 2014 and 2015 inclusive included France, The Netherlands and Germany (>200 each), Iran, Russia, Spain and USA (~150), Turkey (>140), Czech Republic (>120), Belgium (>90), Poland (~80), Canada (>60), Israel, Portugal and Switzerland (>50 each). Data from the 2014 and 2015 sessions, namely “Concurrent” and “What’s New” sessions [both sessions reflect submitted abstracts that are reviewed and selected for spoken presentations] reveal that the countries with most success at securing spoken presentations from overall abstract submission were The Netherlands (~25 % of all abstracts), Belgium (~22 %), UK (~20 %), France (16 %), Switzerland (~17 %), Canada (~16 %), Germany and the USA (~13 %), see Fig. 5.
Fig. 5.
Number of spoken presentations at ESHG per capita for the years 2014-2015
Discussion
A clinical geneticist would argue that families benefit from attendance at a genetics counselling service whatever the outcome of the consultation. The role of the geneticist is not to effect action but more to inform and allow the family to make their own decisions. However, this is very difficult to quantify, and specifically, costing the value of a genetic consultation is complex. Prevention is not instantaneous; the value may not be apparent until years have passed and may not be possible to calculate. Furthermore, focusing on a preventative economic argument ignores patient empowerment that results from the information gleaned from a consultation. Furthermore, estimating adequate clinical genetics staffing levels is compounded by the changing roles of geneticists highlighted by Hennekam and Biesecker (2012) and by the UK Clinical Genetics society (2015) (http://www.clingensoc.org/media/954931/theevolvingroleoftheclinicalgeneticist_29.07.15.pdf).
Our research demonstrates that clinical genetics staffing levels across European clinical genetics units vary hugely, reflecting individual country’s health policies rather than needs (see Figs. 1 and 2).
It is in the interest of all EU citizens that we optimise participation in EU rare disease research, but our work shows that many EU citizens are being disenfranchised because of poor participation of many countries (see Table 1). Countries that have a broader participation rate benefit a wider patient group. In total, 38 % of member states participated in one project only (www.ec.europa.eu/budget/fts: eCORDA (External Common Research Datawarehouse), European Commission, as of 2014/06/20), suggesting limited access for many patient groups within these countries (see Table 1 and Fig. 4).
The barriers to successful grant applications to the EU are mainly infrastructural ones. Navigating through large EU grant applications is a difficult process. Successful countries employ project coordinators to help steer through the numerous online forms that are required before being considered eligible to apply. In addition, other local support including database managers, statistical support and advice from other successful local researchers is often lacking in poorer EU countries. Finding collaborators, an essential element for successful applications, may also be a barrier for a new researcher from a country with a poor track record in research. Lastly, researchers may lack confidence and consider these grants as being the right preserve of the richer countries only.
It would seem reasonable to develop a core office of experienced staff within the EU specifically to help applicants navigate the process. Smaller countries could benefit by accessing the necessary support through this resource. The council of the European Society for Human Genetics could help in this regard by promoting equity in research.
Wider representation at ESHG meetings from investigators working throughout the EU and elsewhere would be welcome. Currently, the ESHG scientific committee consists of six individuals from the UK, three individuals from France and the Netherlands, two from Finland, Germany, Italy, Spain and Belgium and one each from Norway, Portugal, Slovenia, Sweden and Switzerland (https://www.eshg.org/spc.0.html). As >2000 abstracts are received every year, selection for spoken presentation is an onerous task. The ESHG conference (and other conferences in the same research field) provide an essential opportunity to further research and improves the grant application success rate for applicants who can show they have secured a platform presentation. As such, delegates are entitled to some transparency with regard to selection of abstracts for platform presentation.
It is mandatory for conference organisers to receive feedback on the quality of the presentations from the participants. This puts pressure on the scientific committee to minimise risk of poor presentations. It is possible that this inadvertently results in a tendency to choose abstracts from known research units (committee members are given the authors’ names and affiliations (Jerome del Picchia, personal communication)) that are deemed safe and the avoidance of choosing an abstract from a completely unknown research centre. More than 70 countries submitted abstracts to both the 2014 and 2015 meetings. The ESHG website states that <10 % of all submitted abstracts achieve a spoken presentation. However, 6/28 (21 %) EU countries secured approximately 72 % of all spoken slots over the 2 years [The Netherlands, UK, France, Germany, Italy and Belgium]. Clearly, countries that invest in cutting-edge research will be rewarded for their efforts and investment, and this is to be commended and encouraged. However, the conference organisers may wish to consider an anonymised abstract submission system to improve transparency and confidence in the process.
Some countries, notably the Czech Republic, Russia, Turkey and Poland did not achieve any spoken presentation despite high numbers (>100) of submitted abstracts. Of course it is possible (but unlikely) that all the researchers from these countries requested poster only in their submissions. One encouraging feature is the number of young investigators originating from less affluent countries, who were succeeding in their research in larger centres, indicating opportunities for high-quality trainees to access research abroad. Perhaps, the scientific programme committee could consider publishing clear guidelines of what they expect to be of good scientific/clinical value worthy of being presented at a conference of the scale of ESHG. This might encourage disadvantaged centres to seek collaborations with good centres, in order to boost their research and hence to improve their research standards. In addition, the committee could consider recommending studies in topics where there is a paucity of data and that are not costly (e.g. natural history studies in rare disorders). This would direct those researchers in these countries into studies of value to us all. The TEDEX workshop in the 2015 meeting went some way towards inclusivity for researchers from countries without a track record in research being able to present their work. Perhaps, this could be expanded to allow each national genetics society to nominate a good local researcher to present their research as a spoken presentation; this would be a positive move towards inclusivity. Without this opportunity, it must seem disheartening knowing that if you are from a small unrecognised unit, the chance of achieving a spoken presentation is very slim.
Clearly, there are limitations to our study; we did not capture staffing data from all EU countries; hence, there are data gaps, particularly from eastern Europe. Our study focused on one conference only, but as the main European conference for rare diseases, we feel it does reflect what is happening in rare disease research across Europe. Much of our research funding data came from European websites e.g. EurCAT and the European Commission Financial transparency system (FTS) and so was gleaned through websites rather than publications.
We had to rely on website data mainly because this topic has not previously been tackled by individual authors, and hence, not many publications were available for referencing.
Europe remains a very heterogeneous place in its clinical genetics practices, as undeniably reflected in its genetic health care provision. The establishment of centres of expertise and ERNs may help alleviate some of the inequity. Concerns over whether and how this will happen has been voiced by Azzopardi-Muscat and Brand, and we would echo their sentiments (Azzopardi-Muscat and Brand 2015). Basic local clinical genetics service provision is indispensable as the diagnosis of a rare disease will be made locally [no one will be allowed to travel without a diagnosis], and many patients with rare diseases will not be in a position to travel. Our study highlights inequities which may not be solved by the establishment of ERNs.
Acknowledgments
We thank Mr. Chris Russell and Dr. Jillian Casey for their assistance. We thank Jörg Schmidtke for the information on German recommendations on Clinical Genetic staffing requirements. We thank the following for contributing their country’s data: Veselina Gadancheva (Bulgaria), Milan Macek (Czech Republic), Helena Kaarinen (Finland), Stanislas Lyonnet (France), Christine Scholz (Germany), Marleen Kets (Netherlands), Gunnar Houge (Norway), Jorge Sequeiros (Portugal), Ulf Kristoffersson (Sweden), UK data: Carol Gardiner (Scotland), AlexMurray (Wales), Kay Metcalfe (England), Fiona Stewart (Northern Ireland) and Mark Longmuir (UK genetic counsellors).
Compliance with ethical standards
Conflict of interest
Dr. Lynch declares no conflict of interest. Dr. Borg declares no conflict of interest.
References
- Azzopardi-Muscat N, Brand H. Will European Reference Networks herald a new era of care for patients with rare and complex diseases? Eur J Public Health. 2015;25(3):362–3. doi: 10.1093/eurpub/cku144. [DOI] [PubMed] [Google Scholar]
- EUROCAT (2015) EUROCAT prevalence tables. Available at EUROCAT Website Database: http://www.eurocat-network.eu/ACCESSPREVALENCEDATA/PrevalenceTables (data uploaded 06/01/2015)
- Hamamy H. Consanguineous marriages; preconception consultation in primary health care settings. J Community Genet. 2012;3:185–192. doi: 10.1007/s12687-011-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekam RC, Biesecker LG. Next-generation sequencing demands next-generation phenotyping. Hum Mutat. 2012;33(5):884–6. doi: 10.1002/humu.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell C, Aymé S (2014) Report on the state of the art of rare disease activities in Europe 2014. http://www.eucerd.eu/upload/file/Reports/2014ReportStateofArtRDActivities.pdf
- Rodwell C, Aymé S.Rare disease policies to improve care for patients in Europe. Biochim Biophys Acta. 2015 Feb 25. pii: S0925-4439(15)00059-9. doi: 10.1016/j.bbadis.2015.02.008. [DOI] [PubMed]
- www.ec.europa.eu/budget/fts: eCORDA (External Common Research Datawarehouse), European Commission, as of 2014/06/20
- Yi Y, Lindemann M, Colligs A, Snowball C. Economic burden of neural tube defects and impact of prevention with folic acid: a literature review. Eur J Pediatr. 2011;170:1391–400. doi: 10.1007/s00431-011-1492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]