Abstract
Objective
Nuclear factor kappa-B (NF-κB) is a critical proinflammatory regulator that has been suggested to play a pivotal role in the pathogenesis and pathophysiology of endometriosis. In the present study, we aimed to evaluate whether the expression of NF-κB p65 subunit is increased in the eutopic endometrium and/or in the adenomyosis nodule of women with adenomyosis.
Methods
Thirty-three women with histologically confirmed adenomyosis after laparoscopic or transabdominal hysterectomy were recruited. Women with carcinoma in situ of uterine cervix without evidence of adenomyosis or endometriosis (n=32) served as controls. Formalin-fixed, paraffin-embedded archival tissues were sectioned and immunostained utilizing a monoclonal anti-human NF-κB p65 subunit antibody, and the immunoreactivity of NF-κB p65 subunit was compared between women with and without adenomyosis.
Results
The immunoreactivities of both the nuclear and the cytoplasmic NF-κB p65 subunit were significantly increased in the stromal cells in the eutopic endometrium as well as in the adenomyosis nodule of women with adenomyosis compared with controls, respectively. The nuclear expression of NF-κB p65 subunit was significantly higher in the glandular cells in the eutopic endometrium as well as the adenomyosis nodule of women with adenomyosis compared with controls, respectively.
Conclusion
The expression of NF-κB p65 is increased in the eutopic endometrium and adenomyosis nodule of women with adenomyosis, which strongly suggest that NF-κB plays a critical role in the pathogenesis and/or pathophysiology of adenomyosis.
Keywords: Adenomyosis, Endometriosis, Endometrium, NF-kappa B, p65 subunit
Introduction
Adenomyosis is a common benign gynecologic disease in which endometrial glands and stroma are present within myometrium, inducing myometrial inflammation and hypertrophy. Although some women with adenomyosis are asymptomatic, the condition often causes diffusely enlarged uterus, menorrhagia, dysmenorrhea, and subfertility [1,2]. Because the adenomyosis can be diagnosed with microscopic examination of the uterus after hysterectomy, the prevalence is not accurate. Although generally estimated to affect 20 percent of women, the incidence was approximately 65 percent in one study in which histopathological analysis of multiple myometrial sections was performed [3]. The pathogenesis of adenomyosis is poorly understood, but one hypothesis is that the basalis endometrium abnormally invades into the myometrium. The main mechanism of the pathogenesis of adenomyosis is disruption of the endometrial-myometrial borderline leading to the infiltration of endometrial cells, and survival of the endometrial cells within the myometrium [4]. Several studies have found out the differences between eutopic endometrium of women with adenomyosis and those without adenomyosis [5,6,7]. Several factors (inflammation, hormonal influences, immune factors, angiogenesis, and so on) have been suggested to be involved in in the development of adenomyosis [7,8,9,10,11].
Nuclear factor kappa-B (NF-κB) plays an important role as a key protein in stimulating inflammation and cell proliferation and also plays an important role in inhibiting apoptosis in various cell types [12]. The NF-κB is a family of peptides which is formed by five subunits: RelA or p65, RelB, c-Rel, p50/p105(NF-κB1), and p52/p100(NF-κB2), and these subunits bind forming different NF-κB dimers. These NF-κB dimers are held inactive by uniting with the NF-κB inhibitory protein (IκB). This protein blocks NF-κB target gene transcription by preventing NF-κB-DNA interaction [12,13]. The subunit p65 mainly participates in the active NF-κB complex. Proinflammatory cytokines can activate the canonical NF-κB pathway by IκB phosphorylation and subsequent ubiquitination. The NF-κB complex is degraded by the proteasome, then NF-κB free dimer binds to DNA for target gene transcription.
Based on the previous study showing that NF-κB plays a critical role in the pathogenesis of endometriosis [14], we hypothesized that NF-κB p65 subunit expression is also increased in adenomyosis. We aimed to determine whether the expression of NF-κB p65 subunit is increased in the eutopic endometrium and/or in the adenomyosis nodule of women with adenomyosis compared with the normal endometrium.
Materials and methods
1. Tissue collection
Tissue samples of adenomyosis as well as their corresponding eutopic endometrium were collected from a total of 33 women with histological evidence of adenomyosis. We also obtained tissue samples of normal endometrium from a total of 32 women with carcinoma in situ of the uterine cervix, which were the same sections used as controls in our previous study [14]. All of the recruited women had regular menstrual cycles and had undergone hysterectomies by trans-abdominal or laparoscopic methods. We excluded women with endometrial abnormality, adenomyosis, or pelvic endometriosis from the control group. The date of the menstrual cycle was classified as proliferative (days 1 to 13), early secretory (days 14 to 19), mid secretory (days 20 to 23), and late secretory (days 24 to 28) phases by endometrial histology using the criteria of Noyes et al. [15]. The review board for human research in our hospital approved this project. We required no additional informed consent to use the specimens in this study, since only archived material was used.
2. Immunohistochemistry
Immunohistochemistry was performed as previously described [14]. Formalin-fixed paraffin-embedded samples were cut into 4 µm sections. After deparaffinization, slides were boiled in citrate buffer (10 mM, pH 6.0) for 15 minutes for antigen retrieval. Then sections were immersed in 3% hydrogen peroxide for 15 minutes to block endogenous peroxidase. Slides were then incubated in a humidified chamber with 5% blocking goat serum (Vector Laboratory, Burlingame, CA, USA) in tris-buffered saline for 30 minutes at room temperature. Afterwards, excess serum was drained and sections were incubated with rabbit polyclonal anti-human p65 antibody (Cell Signaling Technology, Beverly, MA, USA) for overnight at 4℃ in a humidified chamber. The primary antibody was omitted for verification of negative controls. The sections were washed three times for 5 minutes with tris-buffered saline, and then biotinylated goat anti-rabbit antibody (Vector Laboratories) was added as secondary antibody for 30 minutes at room temperature. The antigen-antibody complex was detected using an avidin-biotin-peroxidase kit (Vector Laboratories). Diaminobenzidine (Vector Laboratories) was used as the chromogen and sections were counterstained with hematoxylin.
The intensity for NF-κB p65 immunoreactivity was semiquantitatively evaluated using the following intensity categories: 0 (no staining), 1+ (weak but detectable staining), 2+ (moderate or distinct staining), 3+ (intense staining). For each tissue, a HSCORE value was derived by summing the percentages of cells that stained at each intensity category and multiplying that value by the weighted intensity of the staining, using the formula HSCORE=ΣPi(i+1), where i represents the intensity scores and Pi is the corresponding percentage of the cells. In each slide, five randomly selected areas were evaluated under a light microscope (×40 magnification), and the percentage of the cells for each intensity within these areas was determined at different times by 2 investigators. The average score was used for final analysis.
3. Statistical analysis
All of the data were normally distributed as assessed by Kolmogorov-Smirnov test. ANOVA and Fisher's least significant difference post hoc test for pairwise comparisons were used for statistical analysis for the HSCOREs obtained from immunohistochemistry. Statistical analyses were performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P<0.05.
Results
The clinical characteristics and menstrual phases of the controls and the adenomyosis group were summarized in the Table 1. The age and the number of deliveries were not different between the 2 groups. The indications for surgery in the adenomyosis group were dysmenorrhea (n=18), menorrhagia (n=11), or palpable mass (n=3).
Table 1. The clinical characteristics of patients and controls.
Values are presented as mean±standard deviation or number.
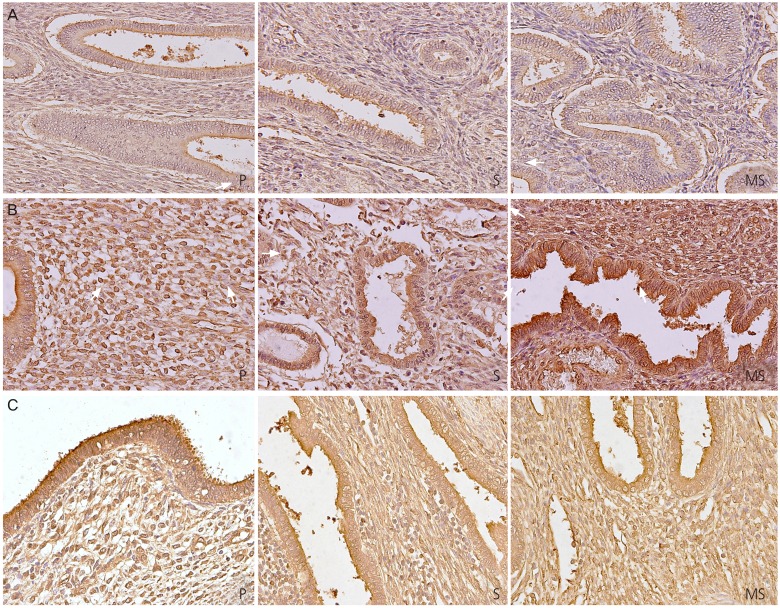
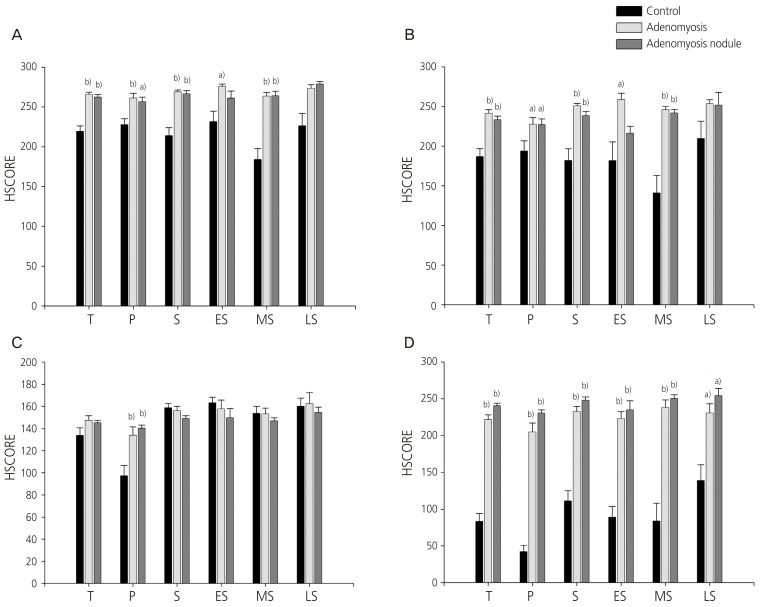
Fig. 1 shows the representative micrographs of NF-κB p65 subunit immunohistochemical staining of the eutopic endometrium of the control (Fig. 1A), the eutopic endometrium of the adenomyosis patient (Fig. 1B), and adenomyosis nodule of the adenomyosis patient (Fig. 1C). When we compare the immunoreactivity of NF-κB p65 subunit in the stromal cells among the 3 groups, we could see obvious increases of NF-κB p65 subunit expression in both the cytoplasm (Fig. 2A) and the nucleus (Fig. 2B) in the eutopic endometrium of the adenomyosis group as well as in the adenomyosis nodule of the adenomyosis group compared to the eutopic endometrium of the controls during total menstrual phase put together (P<0.005, P<0.005 in the cytoplasm; P<0.005, P<0.005 in the nucleus), the proliferative phase (P<0.005, P<0.05 in the cytoplasm; P<0.05, P<0.05 in the nucleus), the secretory phase (P<0.005, P<0.005 in the cytoplasm; P<0.005, P<0.005 in the nucleus), respectively. Dividing the secretory phase into 3 sub-phases, the increase of NF-κB p65 subunit was observed mainly in the mid-secretory phase (P<0.005, P<0.005 in the cytoplasm; P<0.005, P<0.005 in the nucleus, respectively) (Fig. 2A, B). In the glandular cells, NF-κB p65 subunit expression was significantly increased in the cytoplasm only in the proliferative phase in the eutopic endometrium of the adenomyosis group (P<0.005) as well as in the adenomyosis nodule of the adenomyosis group (P<0.005) compared to the eutopic endometrium of the controls (Fig. 2C). However, we could see obvious increases of nuclear NF-κB p65 subunit expression in the glandular cells in the eutopic endometrium of the adenomyosis group as well as in the adenomyosis nodule of the adenomyosis group compared to the eutopic endometrium of the controls during total menstrual phase put together (P<0.005, P<0.005), the proliferative phase (P<0.005, P<0.005), the secretory phase (P<0.005, P<0.005), early secretory phase (P<0.005, P<0.005), mid-secretory phase (P<0.005, P<0.005), and late secretory phase (P<0.05, P<0.05), respectively (Fig. 2D).
Fig. 1. Representative micrographs (×400) of nuclear factor kappa-B p65 subunit immunostaining in the eutopic endometrium of the control patient (A), the eutopic endometrium of the adenomyosis patient (B), and adenomyosis nodule of the adenomyosis patient (C). P, proliferative phase; S, secretory phase; MS, mid-secretory phase.
Fig. 2. Graphs showing HSCORES of nuclear factor kappa-B p65 subunit immunostaining in the cytoplasm (A,C) and the nucleus (B,D) of the stromal cells (A,B) and glandular cells (C,D). T, total menstrual stages put together; P, proliferative phase; S, secretory phase; ES, early secretory phase; MS, mid-secretory phase; LS, late secretory phase. Data of HSCORES are expressed as mean±SEM. a)P <0.05 vs. control, b)P <0.005 vs. control.
Discussion
In the present study, we compared the immunoreactivity of NF-κB p65 subunit in the eutopic endometrium and/or adenomyosis nodule of histologically confirmed adenomyosis with the eutopic endometrium of the controls, and demonstrated the followings: (1) the immunoreactivity of both the nuclear and cytoplasmic NF-κB p65 subunit was significantly increased in the stromal cell of the eutopic endometrium as well as the adenomyosis nodule of women with adenomyosis compared with the controls, respectively; (2) the nuclear expression of NF-κB p65 subunit significantly increased in the glandular cells of the eutopic endometrium as well as the adenomyosis nodule of women with adenomyosis compared with the controls, respectively. Invasion into myometrium following disruption of basal endometrium is considered as a main mechanism of the pathogenesis of adenomyosis, and several factors have been reported to play a pivotal role in the establishment of adenomyosis through increased invasion of endometrium into myometrial layer. Inflammation, hormonal, growth factors and enzymes are thought to be involved in the development of adenomyosis [16,17]. Several studies on adenomyosis showed that the eutopic and ectopic endometria have increased proliferation, angiogenesis, estrogen-dependency and abnormal cytokine levels with molecular and metabolic abnormalities, which might lead to acceleration of the endometrial invasion to the uterine junctional zone and cause the growth of endo-metrial cell into the myometrium layer [18,19].
Although the pathogenesis and/or pathophysiology of the adenomyosis is uncertain, it was suggested that the NF-κB plays an important role in inflammation, proliferation, angiogenesis, and invasion that might be involved in the pathogenesis of adenomyosis [20]. In our previous study, we found that the expression of P21-activated kinases 1 (Pak1) was increased in the eutopic endometrium as well as in the adenomyosis nodule of women with adenomyosis compared to the controls [21]. We suggested that blunting of progesterone-induced down-regulation of Pak1 might lead to establishment of adenomyosis as well as endometriosis, based on our previous findings showing that Pak1 is down-regulated by progesterone along with its decreased expression in endometriosis [22]. In a recent study, we also showed an increased expression of Pak4 in adenomyosis and demonstrated that the Pak4 expression is NF-κB–dependent and that Pak4 can activate MMP (matrix metalloproteinase)-2 and -9 in endometrial cells, which eventually leads to the infiltration of endometrial cells into myometrial layer [23].
Li et al. [20] proposed that increased NF-κB expression by stimulation of proinflammatory cytokines can effectively suppress the progesterone receptor isoform B, which lead to establishment and progression of endometriosis through progesterone resistance, prosurviaval, proinflammation, proangiogenesis, and invasion. Although there are many similarities in estrogen dependence between adenomyosis and endometriosis, symptomology, and molecular aberrations, they have been considered as a separate disease entity. Proinflammatory cytokines such as tumor necrosis factor-α and interleukin 1 could stimulate NF-κB p65 subunit activation, and lead to increased expression of cyclooxygease-2, vascular endothelial growth factor, and tissue factor in adenomyotic stromal cells, which might cause dysmenorrhea and menorrhagia in women with adenomyosis [20].
Previously, only one study suggested an increased expression of NF-κB p65 subunit in adenomyosis [24]. However, the data of the previous study [24] has to be confirmed by another study, since the control group was made up of small number of patients (n=18) with very heterogeneous diseases and the data were missing on each menstural sub-phase as well as detailed semi-quantitative scoring of HSCOREs. To the best of our knowledge, this study is the first study comparing the nuclear expression of NF-κB p65 subunit as a primary end point between the adenomyosis and controls with a single disease entity during various menstrual sub-phases. We could support the findings of the previous study of ours [23] as well as others [20,24] by clearly showing an increased in vivo nuclear expression of NF-κB p65 subunit in patients with adenomyosis.
The findings of the present study seem to be very consistent with our previous study showing increased nuclear expression of NF-κB p65 subunit in the eutopic endometrium of women with endometriosis [14], which suggests that many molecular alterations observed in adenomyosis are similar to those in endometriosis. Considering that NF-κB dimers are held inactive by uniting with IκB in cytoplasm and NF-κB target gene transcription is induced by nuclear NF-κB-DNA interaction, it is the nuclear NF-κB p65 subunit expression that may play critical role in the pathogenesis of adenomyosis. In the present study, the nuclear expression of NF-κB p65 subunit was increased consistently during almost all of the menstrual sub-phases compared to the controls in the stromal cells as well as glandular cells, whereas there were some discrepancies in the cytoplasmic expression of NF-κB p65 subunit between the 2 cells among various menstrual cycles. We could not see an obvious increase of NF-κB p65 subunit expression in the stromal cells in the eutopic and ectopic endometrial tissues from adenomyosis patients during the late secretory phase. Although we do not have enough data to explain the lack of difference only during the late secretory phase, it is highly likely to be due to low number of samples as well as high standard deviation in the control group.
The present study has several limitations as follows. Because it is extremely hard to take endometrium from disease-free controls, we used endometrial tissues from the patients with carcinoma in situ of the uterine cervix who had been confirmed histologically as having no endometrial abnormality. Although we have shown the increased nuclear expression of NF-κB p65 subunit in adenomyosis, we could not determine exactly whether NF-κB-DNA binding is also increased in the present study. We could not compare the HSCOREs of NF-κB p65 subunit among the adenomyosis patients according to their severity of symptoms such as dysmenorrhea or menorrhagia.
Despite these limitations, the findings of the present study strongly support the hypothesis that activation of NF-κB pathway possibly by proinflammatory stimuli can be a crucial pathway leading to establishment and progression of adenomyosis by regulating various down-stream signaling pathways that might include Pak, vascular endothelial growth factor, cyclooxygease-2, and tissue factor. Further studies are necessary to identify which factor might be the most critical one to activate the NF-κB pathway in adenomyosis.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2008247).
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:511–521. doi: 10.1016/j.bpobgyn.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:603–616. doi: 10.1016/j.bpobgyn.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 3.McElin TW, Bird CC. Adenomyosis of the uterus. Obstet Gynecol Annu. 1974;3:425–441. [PubMed] [Google Scholar]
- 4.Curtis KM, Hillis SD, Marchbanks PA, Peterson HB. Disruption of the endometrial-myometrial border during pregnancy as a risk factor for adenomyosis. Am J Obstet Gynecol. 2002;187:543–544. doi: 10.1067/mob.2002.124285. [DOI] [PubMed] [Google Scholar]
- 5.Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod. 1997;57:514–519. doi: 10.1095/biolreprod57.3.514. [DOI] [PubMed] [Google Scholar]
- 6.Ulukus M, Ulukus EC, Seval Y, Cinar O, Zheng W, Arici A. Expression of interleukin-8 receptors in patients with adenomyosis. Fertil Steril. 2006;85:714–720. doi: 10.1016/j.fertnstert.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Li H, Yang Z, Du X, Cui M, Wen Z. Expression of interleukin-10 in patients with adenomyosis. Fertil Steril. 2009;91:1681–1685. doi: 10.1016/j.fertnstert.2008.02.164. [DOI] [PubMed] [Google Scholar]
- 8.Huang TS, Chen YJ, Chou TY, Chen CY, Li HY, Huang BS, et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med. 2014;18:1358–1371. doi: 10.1111/jcmm.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streuli I, Dubuisson J, Santulli P, de Ziegler D, Batteux F, Chapron C. An update on the pharmacological management of adenomyosis. Expert Opin Pharmacother. 2014;15:2347–2360. doi: 10.1517/14656566.2014.953055. [DOI] [PubMed] [Google Scholar]
- 10.Ota H, Igarashi S, Hatazawa J, Tanaka T. Is adenomyosis an immune disease. Hum Reprod Update. 1998;4:360–367. doi: 10.1093/humupd/4.4.360. [DOI] [PubMed] [Google Scholar]
- 11.Zhou S, Yi T, Liu R, Bian C, Qi X, He X, et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteomics. 2012;11:M112. doi: 10.1074/mcp.M112.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Ramos R, Defrere S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520–528. doi: 10.1016/j.fertnstert.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Ihm HJ, Oh YS, Chae HD, Kim CH, Kang BM. Increased nuclear expression of nuclear factor kappa-B p65 subunit in the eutopic endometrium and ovarian endometrioma of women with advanced stage endometriosis. Am J Reprod Immunol. 2013;70:497–508. doi: 10.1111/aji.12161. [DOI] [PubMed] [Google Scholar]
- 15.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 16.Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98:572–579. doi: 10.1016/j.fertnstert.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Vercellini P, Vigano P, Somigliana E, Daguati R, Abbiati A, Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006;20:465–477. doi: 10.1016/j.bpobgyn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update. 2014;20:386–402. doi: 10.1093/humupd/dmt052. [DOI] [PubMed] [Google Scholar]
- 19.Benagiano G, Brosens I. The endometrium in adenomyosis. Womens Health (Lond Engl) 2012;8:301–312. doi: 10.2217/whe.12.8. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Chen M, Liu X, Guo SW. Constitutive and tumor necrosis factor-α-induced activation of nuclear factor-κB in adenomyosis and its inhibition by andrographolide. Fertil Steril. 2013;100:568–577. doi: 10.1016/j.fertnstert.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Kim SR, Kim SH, Lee HW, Chae HD, Kim CH, Kang BM. Increased expression of p21-activated kinase in adenomyosis. Fertil Steril. 2010;94:1125–1128. doi: 10.1016/j.fertnstert.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Lee HW, Kim YH, Koo YH, Chae HD, Kim CH, et al. Down-regulation of p21-activated kinase 1 by progestin and its increased expression in the eutopic endometrium of women with endometriosis. Hum Reprod. 2009;24:1133–1141. doi: 10.1093/humrep/den484. [DOI] [PubMed] [Google Scholar]
- 23.Yi KW, Kim SH, Ihm HJ, Oh YS, Chae HD, Kim CH, et al. Increased expression of p21-activated kinase 4 in adenomyosis and its regulation of matrix metalloproteinase-2 and -9 in endometrial cells. Fertil Steril. 2015;103:1089–1097.e2. doi: 10.1016/j.fertnstert.2014.12.124. [DOI] [PubMed] [Google Scholar]
- 24.Nie J, Lu Y, Liu X, Guo SW. Immunoreactivity of progesterone receptor isoform B, nuclear factor kappaB, and IkappaBalpha in adenomyosis. Fertil Steril. 2009;92:886–889. doi: 10.1016/j.fertnstert.2009.01.084. [DOI] [PubMed] [Google Scholar]