Abstract
Patients with the acute respiratory distress syndrome (ARDS) have elevated levels of cell-free hemoglobin (CFH) in the air space, but the contribution of CFH to the pathogenesis of acute lung injury is unknown. In the present study, we demonstrate that levels of CFH in the air space correlate with measures of alveolar-capillary barrier dysfunction in humans with ARDS (r = 0.89, P < 0.001) and in mice with ventilator-induced acute lung injury (r = 0.89, P < 0.001). To investigate the specific contribution of CFH to ARDS, we studied the impact of purified CFH in the mouse lung and on cultured mouse lung epithelial (MLE-12) cells. Intratracheal delivery of CFH in mice causes acute lung injury with air space inflammation and alveolar-capillary barrier disruption. Similarly, in MLE-12 cells, CFH increases proinflammatory cytokine expression and increases paracellular permeability as measured by electrical cell-substrate impedance sensing. Next, to determine whether these effects are mediated by the iron-containing heme moiety of CFH, we treated mice with intratracheal hemin, the chloride salt of heme, and found that hemin was sufficient to increase alveolar permeability but failed to induce proinflammatory cytokine expression or epithelial cell injury. Together, these data identify CFH in the air space as a previously unrecognized driver of lung epithelial injury in human and experimental ARDS and suggest that CFH and hemin may contribute to ARDS through different mechanisms. Interventions targeting CFH and heme in the air space could provide a new therapeutic approach for ARDS.
Keywords: acute respiratory distress syndrome, air space inflammation, permeability, lung epithelium, hemoglobin, hemin
hemolysis leads to elevated plasma (PL) levels of cell-free hemoglobin (CFH) in several clinical conditions including sepsis, hemodialysis, sickle cell crisis, and extracorporeal circulation, and after red blood cell transfusion (9, 26, 32, 43, 46, 50, 64, 65). The effects of extracellular hemoglobin on the vascular endothelium and macrophages are particularly well studied (2, 13, 17, 18, 35, 40, 48, 59). In the circulation, hemoglobin-mediated nitric oxide scavenging precipitates vasoconstriction and pulmonary hypertension during sickle cell crisis (50) and accounts for some of the adverse effects of red blood cell transfusion (15, 26). In macrophages, hemoglobin acts as a damage-associated molecular pattern (DAMP) and results in activation of proinflammatory immune responses (8, 18). Many of the pathological effects of hemolysis are mediated by release of iron-carrying heme. For example, heme infusion increases mortality and organ damage in a mouse model of sepsis (39) and potentiates LPS-mediated cytokine production in macrophages and during endotoxemia (19). CFH also acts as a potent oxidant (9, 26, 47, 49, 54, 55), and elevated levels of CFH have been associated with markers of oxidative injury and with acute kidney injury in critically ill patients with sepsis and in postoperative patients (9, 33). Although these data demonstrate that CFH has numerous detrimental effects on macrophages and endothelial cells, its effects on epithelial cells are unknown. Furthermore, although the central pathogenic role for circulating CFH in systemic disease states has been well studied, there is little known about potential effects of CFH in the lung.
Alveolar hemorrhage is a common feature of the acute respiratory distress syndrome (ARDS) (3). Genetic variation in iron metabolism genes such as ferritin light chain and constitutive heme oxygenase 2 has been shown to affect risk of developing ARDS (38). In addition, levels of iron and iron-processing proteins are elevated in bronchoalveolar lavage (BAL) fluid of patients with ARDS (23, 27, 45). We previously reported that high levels of extracellular CFH were detected in pulmonary edema fluid (EF) samples from patients with ARDS and in BAL fluid from mice with experimental acute lung injury (6). Based on the known effects of CFH on endothelial cells and macrophages and the high levels of CFH in the air space during ARDS, we hypothesize that CFH is a driver of lung permeability and inflammation. The goal of the present study was to test the effects of CFH on the lung epithelium and to determine whether CFH in the air space can increase alveolar capillary barrier permeability during acute lung injury. We further aimed to test whether free heme in the air space, which can be released from CFH during degradation, could also mediate lung injury.
METHODS
Human sample collection and cell-free hemoglobin measurement.
Methods for collection of undiluted pulmonary EF and simultaneous PL samples from critically ill mechanically ventilated patients with ARDS have been previously described (7). All samples were centrifuged immediately after collection to remove the cellular fraction and the supernatant was stored at −80°C until thawed for analysis. Cell-free hemoglobin and total protein in EF and PL samples were measured spectrophtometrically by using HemoCue (Cypress, CA) and BCA assay (Pierce, Rockford, IL), respectively (6). Use of HemoCue for measurement in EF and PL was validated prior to experimental measurements (data not shown). The EF-to-PL ratio of total protein was calculated as a measurement of alveolar-capillary barrier permeability (66). Human studies were approved by the Vanderbilt Institutional Review Board (IRB no. 020260).
Experimental murine acute lung injury.
For ventilator-induced lung injury (VILI), adult C57Bl/6 mice were anesthetized with pentobarbital (5 mg/g mouse) and mechanically ventilated (MV) via tracheostomy with either low (6 ml/kg) or high (12 ml/kg) tidal volumes for 2 h. For some experiments, mice were treated with intratracheal (IT) instillation (6, 58) of 1 μg of lipopolysaccharide (LPS) in 100 μl PBS given 30 min prior to MV. Experimental pneumonia was induced by intranasal instillation of 2,000 CFU Klebsiella pneumoniae in 50 μl PBS to mice anesthetized with isoflurane (16). For CFH-induced or hemin-induced acute lung injury, CFH, hemin, and albumin (control) were prepared daily and filtered prior to use. Endotoxin-free CFH (Cell Sciences, Canton, MA) was diluted in PBS to a final concentration of 1 mg/ml (15 μM). This dose of CFH was based on the median levels of CFH measured in undiluted pulmonary edema fluid from patients with ARDS (6). Hemin (the chloride salt of ferric heme) (Sigma) was dissolved in 0.1 N NaOH and diluted in PBS to a final concentration of 60 μM (40 μg/ml), based on the molar equivalent of the dose of CFH since CFH contains four heme moieties. Albumin was diluted in PBS to 1 mg/ml as a protein control for CFH. Mice were anesthetized with isoflurane prior to direct IT instillation (53) of 100 μl of CFH, hemin, or albumin control. Samples were collected after 2 h for VILI, 24 h for pneumonia, and 24 h for IT CFH-mediated injury. Murine BAL fluid, flash frozen lungs, and formalin-fixed paraffin-embedded histological samples were collected as previously described (6) and stored at −80°C until analysis. If overt hemorrhage due to procedural complications occurred during sample collection, the BAL results from that animal were removed from analysis. All experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Measurement of indicators of acute lung injury in BAL.
Cell counts and differentials were quantified by microscopic examination. After this, BAL fluid was centrifuged to remove leukocytes and red blood cells prior to quantification of CFH and other injury biomarkers. Cell-free hemoglobin was quantified by HemoCue and protein was measured in duplicate by BCA assay (Pierce) (6). Receptor for advanced glycation end products (RAGE) and cytokines were measured in duplicate by ELISA (R&D Systems, Minneapolis, MN) or a murine 7-plex proinflammatory cytokine assay that we have previously validated for use in mouse BAL (5) (Meso Scale Discovery, Gaithersburg, MD).
Histological assessment of lung injury.
Ten nonoverlapping ×20 fields of hematoxylin and eosin-stained lung tissue were assessed in a blinded manner as previously described (6, 21). Histological lung injury of each field was scored for inflammation, septal thickening, edema, and hemorrhage, and a composite total lung injury score was calculated.
Cultured epithelial cell model system.
Mouse lung epithelial cells (MLE-12) were maintained in HITES medium and then incubated with 15 μM CFH or 60 μM hemin in medium for 24 h. Conditioned medium was collected, centrifuged, and stored at −80°C until analysis. RNA was extracted from treated cells by use of the PureLink RNA extraction kit (Ambion, Grand Island, NY) and cytokine gene expression was measured by quantitative PCR. Protein, RAGE, and cytokine protein expression were measured in conditioned media by ELISA (R&D Systems). For resistance measurements, MLE-12 cells were grown for 24 h on 8W10E+ PC plates (Applied BioPhysics, Troy, NY) prior to treatment with CFH, hemin, or media control for an additional 24 h. Epithelial permeability was measured by resistance at 4,000 Hz via electrical cell-substrate impedance sensing (ECIS) (Applied BioPhysics). Data were normalized to the baseline resistance prior to addition of treatments. Viability of cells after CFH or hemin treatments was confirmed by manual assessment of Trypan blue exclusion and by MTS/PMS assay (Promega, Madison, WI) (data not shown).
Statistical analysis.
All statistical analysis was done with SPSS version 23 for Macintosh. Comparisons between three groups were performed by one-way ANOVA with post hoc Tukey's comparisons. Pairwise comparisons were performed by Student's t-tests. Correlations were tested by using Spearman's correlation coefficients. Nonnormally distributed data were natural log transformed prior to analysis. A P value <0.05 was considered statistically significant.
RESULTS
CFH is elevated in the air space in human ARDS and in murine experimental lung injury and correlates with increased alveolar-capillary barrier permeability.
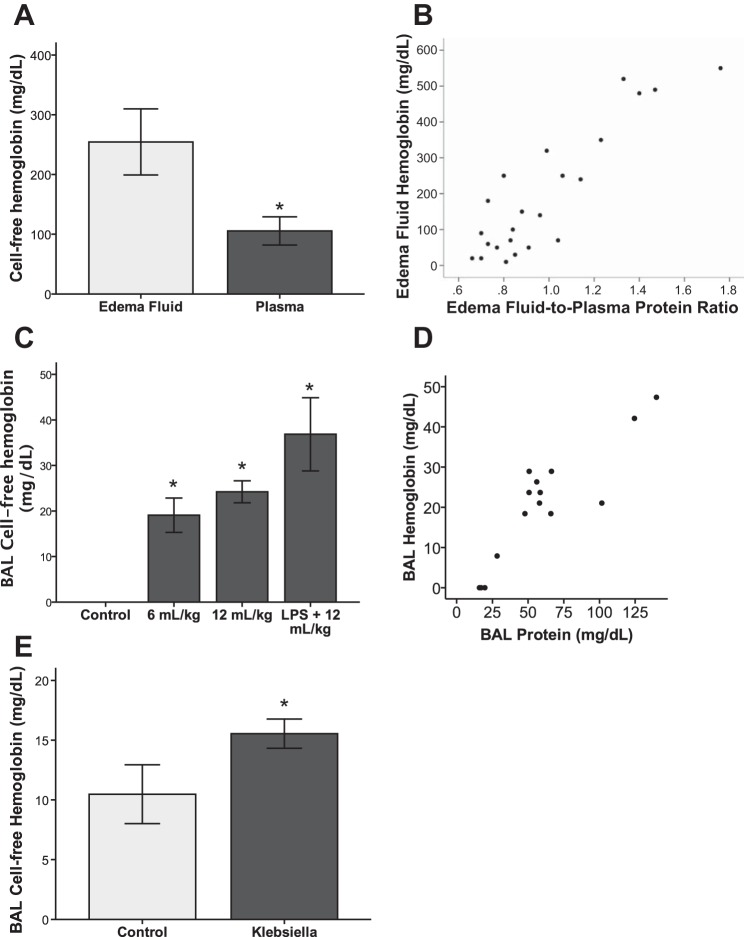
Although we previously reported that CFH levels were increased in pulmonary EF of patients with ARDS compared with control patients with hydrostatic pulmonary edema (6), it was not clear from that study what the relationship was between CFH levels in the air space compared with the circulation. To address this question, we compared simultaneous PL CFH levels to previously published levels of CFH in EF in 18 patients with ARDS (6). CFH levels in undiluted EF were significantly greater than simultaneous levels in undiluted PL (Fig. 1A). This finding suggests that air space CFH is generated from red blood cell breakdown within the alveolar space rather than by passive leakage from the circulation. In ARDS patients, EF CFH levels were tightly correlated with the EF-to-PL protein ratio, an index of alveolar-capillary barrier permeability (rho = 0.89, P < 0.001) (Fig. 1B). Total EF protein levels were not simply reflective of CFH levels in patients with ARDS; the mean EF total protein level was 4,314 ± 2,043 mg/dl and the mean CFH level was 227 ± 214 mg/dl, demonstrating that CFH accounted for 5–6% of the total protein present in EF.
Fig. 1.
Release of cell-free hemoglobin into the air space is a feature of human and experimental acute lung injury. A: cell-free hemoglobin (CFH) levels are significantly higher in pulmonary edema fluid (EF) compared with plasma (PL) of patients with acute respiratory distress syndrome (ARDS), n = 18; *P = 0.011 vs. control. The levels of CFH in EF from these patients was previously published and are reported here for direct comparison to PL CFH levels (6). B: in patients with ARDS, EF CFH correlates strongly with EF-to-PL protein ratio, an index of alveolar-capillary barrier permeability, rho = 0.89, P < 0.001 by Spearman's correlation coefficient. C: CFH is elevated in bronchoalveolar lavage (BAL) fluid of mice treated with mechanical ventilation ± intratracheal LPS; *P < 0.029 for each group vs. control, n = 3–5 per group. D: BAL CFH correlates strongly with BAL protein in mice with ventilator induced lung injury, rho = 0.89, P < 0.001 by Spearman's correlation. E: BAL CFH is increased during experimental bacterial pneumonia compared with control animals, *P = 0.05, n = 5–9 per group.
To determine whether release of CFH into the air space is also a feature of experimental acute lung injury, we measured CFH in BAL from mice with two types of experimental acute lung injury (42, 56). Mice with ventilator-induced acute lung injury using low (6 ml/kg) or high (12 ml/kg) tidal volume ventilation with or without the addition of IT LPS 30 min prior to mechanical ventilation had increased CFH levels in BAL fluid compared with control mice treated with IT PBS (Fig. 1C). Similar to findings in humans with ARDS, levels of CFH in the BAL correlated strongly with BAL protein, an index of alveolar-capillary barrier permeability (rho = 0.89, P < 0.001) (Fig. 1D). In addition, BAL CFH was elevated in a clinically relevant model of gram-negative pneumonia caused by Klebsiella pneumoniae (16) (Fig. 1E). These data demonstrate that elevated intra-alveolar CFH is a feature of both human ARDS and experimental acute lung injury.
CFH is sufficient to cause acute lung injury.
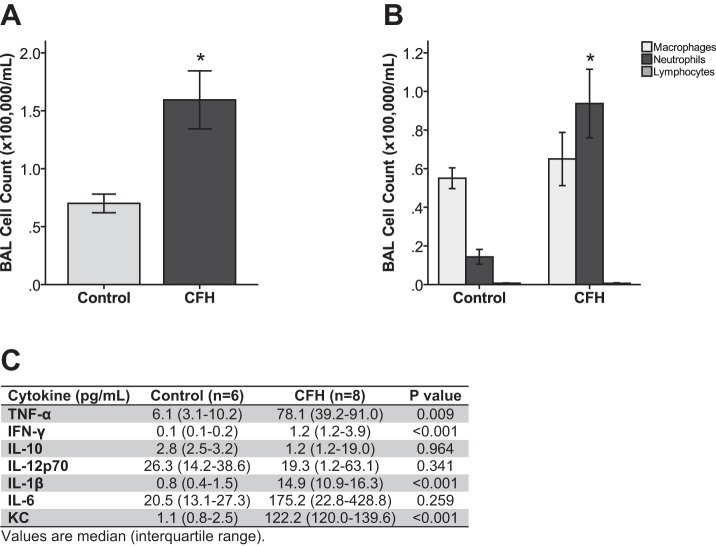
To define the specific contribution of intra-alveolar CFH to acute lung injury, wild-type C57Bl/6 mice were treated IT with 100 μl of 1 mg/ml CFH (total dose 100 μg per mouse) or albumin control (100 μg per mouse) and studied at 24 h. This dose of CFH was chosen based on the median levels of CFH measured in EF from patients with ARDS. Compared with controls, mice treated with CFH had increased lung inflammation (Fig. 2A) with neutrophil predominance (Fig. 2B). In addition, BAL levels of the proinflammatory cytokines TNF-α, IL-1β, IL-6, CXCL-1/KC, and IL12p70 were elevated in response to CFH (Fig. 2C).
Fig. 2.
Cell-free hemoglobin induces acute air space inflammation in mice. Mice were treated with 100 μg endotoxin-free CFH or 100 μg albumin control and sampled after 24 h. CFH increases total BAL fluid cell counts (*P = 0.032 vs. albumin control) (A) and trends toward increased neutrophils in the air space (*P = 0.09 vs. albumin control) (B) compared with control mice. There is no significant difference in numbers of macrophages or lymphocytes between treatments. C: CFH increases proinflammatory cytokine concentrations in BAL fluid; n = 6–8 per group.
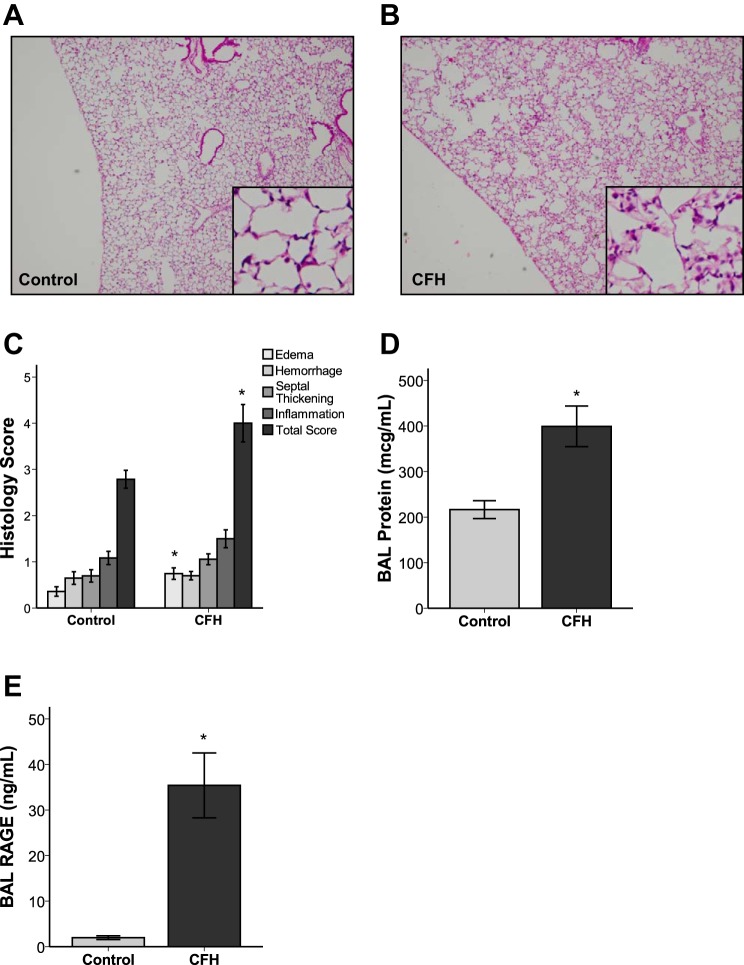
Assessment of acute lung injury by histological analysis demonstrated that CFH-treated mice had increased edema and total lung injury scores (Fig. 3, A–C). To further examine whether lung permeability was altered by CFH, BAL levels of total protein were measured. CFH-treated mice had significantly increased BAL protein (Fig. 3D), consistent with disruption of the alveolar-capillary barrier. The mean BAL protein concentration in CFH treated mice was 399 ± 126 μg/ml, exceeding the total amount of CFH administered. In addition, CFH-treated mice had elevated BAL levels of RAGE, a marker of lung epithelial cell injury (62) (Fig. 3E). Together, these data demonstrate that IT CFH recapitulates the key features of ARDS even in the absence of other injurious stimuli, causing significant air space inflammation, damage to the lung epithelium, and disruption of the alveolar-capillary barrier.
Fig. 3.
Cell-free hemoglobin causes histological lung injury and disrupts the alveolar-capillary barrier. A–C: CFH increases the total lung injury score and edema score as assessed by histological examination (*P = 0.033 for edema score and P = 0.002 for total lung injury score compared with PBS control). D: CFH increases BAL protein, consistent with disruption of the alveolar-capillary barrier (*P = 0.013). E: CFH induced injury to epithelial cells as measured by concentration of receptor of advanced glycation end products (RAGE) in BAL fluid (*P = 0.001 vs. control); n = 6–8 in each group.
CFH induces cytokine production and increases permeability in cultured lung epithelial cells.
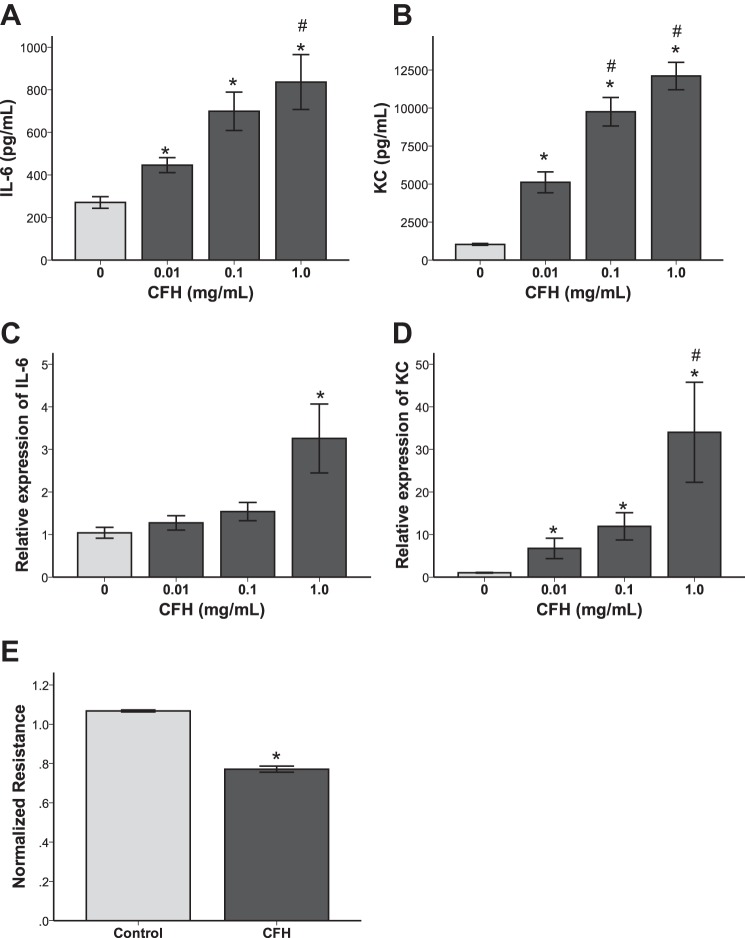
Because the lung epithelium is known to be a critical regulator of permeability and inflammation in the air space (28, 41) and because of evidence of lung epithelial injury after IT CFH, we next tested the direct effects of CFH on cultured lung epithelial cells to better define the cellular mechanisms of CFH effects in the lung. MLE-12 cells treated with increasing doses of CFH for 24 h had dose-dependent increases in expression of the inflammatory mediators IL-6 (Fig. 4, A and C) and KC (Fig. 4, B and D) at both the protein (Fig. 4, A and B) and mRNA (Fig. 4, C and D) level. MLE-12 cells did not increase TNF-α production in response to CFH (data not shown). In addition, CFH (1 mg/ml) reduced transepithelial resistance of MLE-12 cell monolayers as measured by ECIS over 24 h (Fig. 4E), suggesting increased lung epithelial paracellular permeability. There were no effects of CFH on cell viability over 24 h at any of the doses utilized in the in vitro experiments (not shown). These data show that the lung epithelium is directly affected by CFH in vitro and increases proinflammatory cytokines and disrupts permeability.
Fig. 4.
Cell-free hemoglobin induces cytokine production and permeability defects in cultured mouse lung epithelial cells. MLE-12 lung epithelial cells produce higher levels of IL-6 (A) and CXCL-1/KC (B) in response to increasing doses of CFH in vitro (*P < 0.05 vs. control, #P < 0.05 vs. 0.01 mg/ml dose). MLE-12 cells increase IL-6 (C) and KC (D) transcription in response to CFH exposure (*P < 0.05 vs. control, #P < 0.05 vs. 0.01 mg/ml). E: CFH reduces epithelial permeability as measured by electric cell-substrate impedance sensing (ECIS) over 24 h (P < 0.001 vs. control); n = 4–8 in each group.
The heme moiety of CFH increases permeability, but does not affect air space inflammation in vivo.
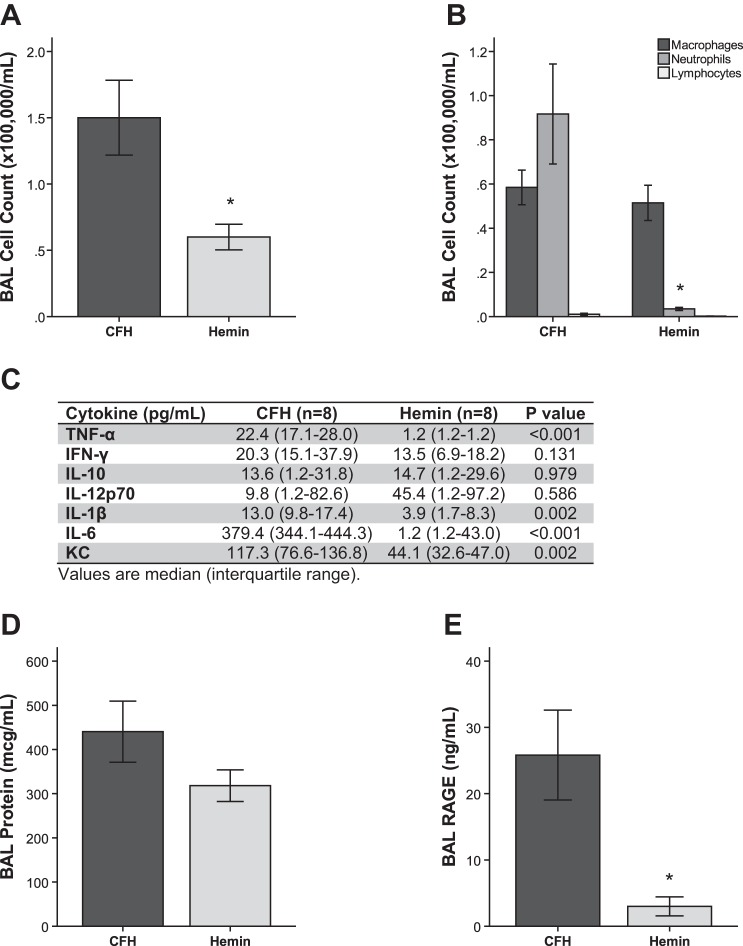
Heme dissociates from CFH in the extracellular space and may mediate the effects of CFH in the lung. We tested whether the isolated heme moiety of CFH could induce acute lung injury by treating C57Bl/6 mice with IT hemin (60 μM in 100 μl PBS), which is the chloride salt of heme, compared with equimolar CFH (15 μM). After 24 h, mice treated with hemin had no evidence of inflammatory cell recruitment into the air space (Fig. 5A), no increase in air space neutrophils (Fig. 5B), and no significant elevations in a panel of proinflammatory cytokines in BAL (Fig. 5C). Compared with CFH, IT hemin resulted in similar increases in alveolar-capillary permeability (Fig. 5D) as measured by BAL protein levels. However, in contrast to after CFH, there was no evidence of hemin-dependent epithelial cell injury in the lung as measured by RAGE release into BAL (Fig. 5E). Overall, these data suggest that isolated free heme is sufficient to disrupt the alveolar-capillary barrier but insufficient to cause alveolar inflammation.
Fig. 5.
The heme moiety of cell-free hemoglobin affects permeability but not inflammation during acute lung injury. C57Bl/6 mice were treated with intratracheal CFH (100 μg) or hemin (60 μM), the chloride salt of heme, for 24 h prior to sample collection. Hemin causes less alveolar inflammation as measured by bronchoalveolar cell counts (*P = 0.037) (A) with fewer neutrophils in the air space (*P = 0.009) (B). C: hemin induces less proinflammatory cytokines in BAL compared with CFH. BAL protein (D) was equivalent between CFH and hemin (P = 0.178). Hemin caused less release of BAL RAGE (E) compared with CFH (*P = 0.006); n = 8 in each group.
The heme moiety of CFH increases permeability but fails to induce inflammation in cultured lung epithelial cells.
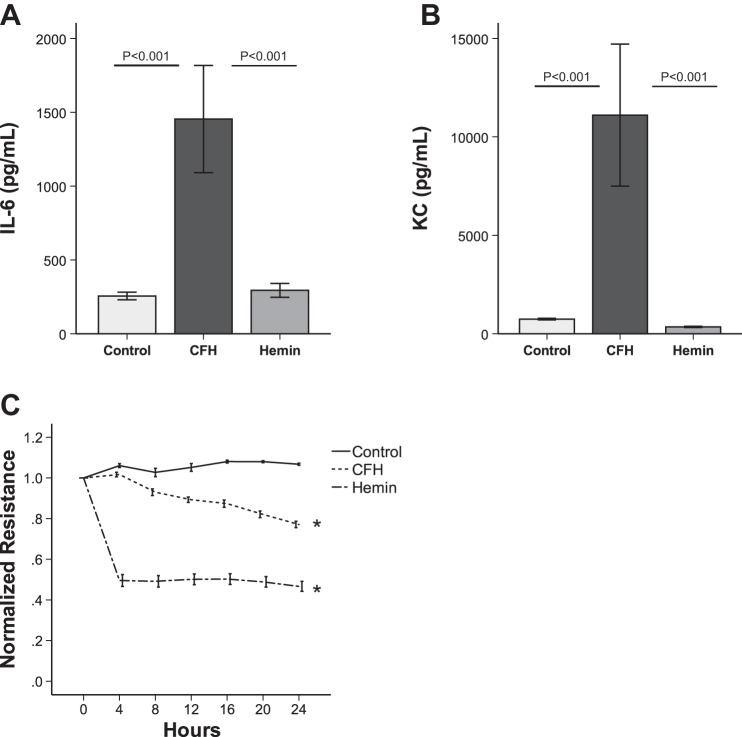
To test whether the heme moiety of CFH had distinct effects on lung epithelial cells in vitro compared with intact CFH, we incubated MLE-12 cells with hemin (60 μM) or CFH (15 μM) for 24 h. Hemin did not increase protein expression of IL-6 or KC in vitro (Fig. 6, A and B). Hemin reduced the resistance of MLE-12 cells as measured by ECIS (Fig. 6C), consistent with the in vivo finding of increased permeability in response to hemin. In vitro, hemin disrupted paracellular permeability more rapidly and to a greater degree than CFH. These data indicate that the CFH and the heme moiety have distinct effects on cultured lung epithelial cells.
Fig. 6.
The hemin moiety of cell-free hemoglobin affects permeability but not inflammation in cultured lung epithelial cells. MLE-12 cells were incubated with media control, CFH (1 mg/ml), or hemin (60 μM) for 24 h. Expression of IL-6 (A) and KC (B) protein were measured by ELISA. C: permeability was measured by ECIS over time (*P < 0.001 vs. control at 24 h); n = 4–8 replicates for each measurement.
DISCUSSION
In this study, we show for the first time that instillation of CFH into the air space is sufficient to recapitulate the main features of human ARDS in an experimental murine acute lung injury model. When delivered directly to the air space, CFH induces inflammation, causes lung epithelial cell injury, and increases alveolar-capillary permeability. These changes are also seen in cultured lung epithelial cells, suggesting that the lung epithelium is a major target for the pathological effects of CFH in vivo. Furthermore, the instillation of the heme moiety of CFH induces similar permeability changes but fails to induce inflammation or release of RAGE. These findings identify CFH and its heme moiety as novel mediators during acute lung injury.
The potential pathogenic consequences of extracellular CFH in the air space have not been well studied, despite intra-alveolar hemorrhage being a frequent feature of ARDS (3). One possible reason for this may be that prior studies showed that delivery of intact red blood cells into the air space was protective in a hyperoxia model of ARDS (63) through a mechanism requiring red blood cell glutathione, a potent antioxidant. In addition, whereas alveolar hemorrhage is a well-described feature of ARDS, release of CFH into the air space in the setting of alveolar hemorrhage was only recently recognized (6). The present studies demonstrate that the injurious effects of CFH in the air space are distinct from the protective effects of intact red blood cells.
Our study identifies the alveolar epithelium as a crucial target for the injurious effects of CFH during acute lung injury. In the presence of CFH, MLE-12 lung epithelial cells have increased paracellular permeability, a previously unrecognized consequence of CFH exposure. These data are confirmed during acute lung injury in vivo with evidence of alveolar-capillary barrier disruption measured by increased BAL protein and with type I epithelial cell damage as evidenced by increased BAL RAGE (62). In addition to effects on epithelial permeability, CFH at concentrations found in the air space during human ARDS also induces proinflammatory cytokine expression in lung epithelial cells both in vivo and in vitro. These results are consistent with and expand upon one previous study of the inflammatory effects of CFH on lung epithelial cells in which Mumby et al. (44) showed that CFH induced expression of IL-8 and MCP-1 through activation of NF-κB in the A549 human lung epithelial cell line. Additional studies are needed to determine the specific molecular mechanisms of CFH-mediated effects on inflammation and epithelial cell damage in the lung. Specifically, whether NF-κB or Toll-like receptor 4 (TLR4), a key mediator of the proinflammatory effects of heme on macrophages (20), contributes to CFH-mediated acute lung injury needs to be investigated. Whether the impact of purified hemoglobin differs in the presence of other injurious stimuli such as LPS also warrants further study. Furthermore, that CFH can induce epithelial permeability in vitro in the absence of other cell types suggests that CFH has direct effects on the lung epithelium that are not dependent on the presence of inflammatory cells in the air space.
Our studies highlight important distinctions between the effects of intact CFH and the heme component of CFH in the lung. Instillation of the heme component of CFH into the air spaces was sufficient to increase alveolar permeability, but lung epithelial injury and increased inflammation required the intact CFH molecule. Previous work has demonstrated that administration of intravenous hemin resulted in acute lung injury with increased permeability in a model of sickle cell disease (25). We extend these findings to demonstrate that hemin can also disrupt epithelial permeability when administered IT in vivo and to lung epithelial cells in vitro. Surprisingly, IT hemin did not increase BAL RAGE as was seen with IT CFH. It may be that CFH and hemin have different molecular mechanisms of permeability, an idea supported by our in vitro results showing that hemin disrupts permeability more quickly and robustly than CFH. Alternately, elevated RAGE may occur in response to elevated CFH as a protective measure to sequester CFH to limit its toxicity. Our in vivo data also suggest that the heme moiety of CFH is not responsible for the inflammatory response to CFH. One potential explanation of this finding could be that free heme fails to reach the distal air spaces because of the avidity of binding to its scavenger protein hemopexin; however, this is less likely because of the significant alveolar permeability defect seen after IT hemin. The lack of inflammation in response to hemin is particularly interesting since circulating heme has been reported to be an important mediator of systemic inflammation (19, 34, 51). For example, iron is a key factor for oxidation of low-density lipoproteins and for membrane damage by CFH in endothelial cells and triggers neutrophil extracellular trap formation during sickle cell disease (14, 36). Furthermore, released iron is a stimulus for inflammation in the lung, in part through production of reactive oxygen species (22, 24, 37). Why heme has such different effects in mediating inflammation systemically compared with in the air space is unknown but has important implications for therapeutic targeting of CFH in ARDS and will require further study.
Based on the present studies, we propose that the release of CFH in the air space could be a novel therapeutic target for ARDS. Importantly, since CFH levels in the air space exceed concentrations of CFH in the circulation and because CFH has direct toxicity to the lung epithelium, therapies aimed at CFH will need to be targeted to the air space rather than given systemically. Augmentation of the endogenous mechanisms for CFH removal may limit the adverse effects of CFH in the air space. In the healthy state, CFH in the circulation is bound by haptoglobin and free heme is bound by hemopexin prior to uptake by monocytes via CD163 and CD91, respectively (10, 11, 60, 61). Based on our results, we would expect that haptoglobin would bind CFH, thereby improving barrier integrity and reducing inflammation, whereas hemopexin may only attenuate permeability with limited impact on inflammation. In support of this treatment approach, mice that overexpress haptoglobin in alveolar macrophages have reduced air space hemoglobin during acute lung injury, suggesting that rapid binding of CFH in the lung may limit tissue injury (67, 68). Systemic haptoglobin also reduces blood pressure, vascular injury, and renal dysfunction in animal models of hypertension (4, 11, 31) and reduces iron accumulation and vascular remodeling in a model of CFH-induced pulmonary hypertension (31). Similarly, intraperitoneal hemopexin administration improved lung permeability after bromine inhalation (1) and intravenous hemopexin improved survival and reduced liver and kidney toxicity in an animal model of sepsis (39). Because CFH and free heme have different effects in the air space compared with the circulation, air space targeting of CFH and heme in ARDS will need to be explored.
Although the current findings advance our understanding of the role of intra-alveolar CFH in the pathogenesis of ARDS, unanswered questions remain. For example, the mechanisms leading to CFH accumulation in the lung are unknown. Based on the human data showing that CFH in EF is disproportionately elevated compared with simultaneous levels in PL, we propose that CFH is actively released from red blood cells within the air space rather than passively leaking into the lung from the circulation. Increased red blood cell fragility in the injured air space may also play a role in CFH production, similar to the mechanisms of hemolysis of circulating red blood cells in patients with sepsis (52, 57). Despite the possibility that red blood cell lysis could also release antioxidants (63), previous studies have demonstrated very low levels of the antioxidants glutathione, ascorbate, and urate in pulmonary EF sampled from the distal air spaces of patients with ARDS (12). As discussed above, it is also uncertain how CFH is removed from the air space. Further studies are needed to understand the balance between CFH generation in the air space and potential mechanisms of clearance. In addition, although we have specifically evaluated the effect of CFH on the lung epithelium, there are other cells in the air space that may modulate the effects of CFH. In particular, alveolar macrophages may play a key role in CFH-mediated acute lung injury because of their role in innate immunity and their hypothesized role in clearance of CFH from the air space. In fact, alveolar macrophages upregulate heme oxygenase-1, a key enzyme for heme degradation, and increase reactive oxygen species production in response to hemin exposure (29, 30).
In summary, we have shown for the first time that elevated levels of CFH are a consistent feature of human and experimental ARDS and that CFH delivered IT to mice causes robust acute lung injury independent of any other stimulus. Intact CFH results in increased inflammation, loss of alveolar capillary barrier integrity, and epithelial cell injury. In contrast, the isolated heme moiety of CFH fails to induce significant inflammation or epithelial cell injury, despite causing a similar disruption in lung epithelial permeability to CFH. These studies identify distinct effects of intact CFH and its heme moiety in the air space and suggest that CFH and heme may be specific targets for novel therapeutic agents for ARDS patients with elevated CFH in the air space.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL090785, HL117676, HL103836, HL126671, and HL087738; by an American Heart Association (AHA) Established Investigator Award and an AHA Mentored Clinical Research Award; and by Vanderbilt CTSA award RR024975 and UL1TR000445 from the National Center for Advancing Translational Sciences. Funding was also provided by Courtney's Race for the ARDS Cure and the Courtney Charneco Family.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.M.S., D.R.J., S.I.D., L.B.W., and J.A.B. conception and design of research; C.M.S., C.P.U., B.S.G., N.P., N.W., S.I.D., L.B.W., and J.A.B. performed experiments; C.M.S., C.P.U., B.S.G., N.P., N.W., S.I.D., L.B.W., and J.A.B. analyzed data; C.M.S., D.R.J., B.S.G., N.W., S.I.D., L.B.W., and J.A.B. interpreted results of experiments; C.M.S., L.B.W., and J.A.B. prepared figures; C.M.S., L.B.W., and J.A.B. drafted manuscript; C.M.S., D.R.J., S.I.D., L.B.W., and J.A.B. edited and revised manuscript; C.M.S., C.P.U., D.R.J., B.S.G., N.P., N.W., S.I.D., L.B.W., and J.A.B. approved final version of manuscript.
REFERENCES
- 1.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. Heme attenuation ameliorates irritant gas inhalation induced acute lung injury. Antioxid Redox Signal 24: 99–112, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med 15: 452–460, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 2: 319–323, 1967.4143721 [Google Scholar]
- 4.Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122: 1444–1458, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastarache JA, Koyama T, Wickersham NE, Ware LB. Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples. J Immunol Methods 408: 13–23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastarache JA, Sebag SC, Clune JK, Grove BS, Lawson WE, Janz DR, Roberts JL 2nd, Dworski R, Mackman N, Ware LB. Low levels of tissue factor lead to alveolar haemorrhage, potentiating murine acute lung injury and oxidative stress. Thorax 67: 1032–1039, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastarache JA, Wang L, Geiser T, Wang Z, Albertine KH, Matthay MA, Ware LB. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax 62: 608–616, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belcher JD, Nath KA, Vercellotti GM. Vasculotoxic and proinflammatory effects of plasma heme: cell signaling and cytoprotective responses. ISRN Oxidative Med 2013: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billings FT 4th, Ball SK, Roberts LJ 2nd, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med 50: 1480–1487, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boretti FS, Buehler PW, D'Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 119: 2271–2280, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boretti FS, Buehler PW, D'Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 119: 2271–2280, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowler RP, Velsor LW, Duda B, Chan ED, Abraham E, Ware LB, Matthay MA, Day BJ. Pulmonary edema fluid antioxidants are depressed in acute lung injury. Crit Care Med 31: 2309–2315, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Buttari B, Profumo E, Businaro R, Saso L, Capoano R, Salvati B, Rigano R. Oxidized haemoglobin-driven endothelial dysfunction and immune cell activation: novel therapeutic targets for atherosclerosis. Curr Med Chem 20: 4806–4814, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 123: 3818–3827, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 124: 465–476, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dulek DE, Newcomb DC, Goleniewska K, Cephus J, Zhou W, Reiss S, Toki S, Ye F, Zaynagetdinov R, Sherrill TP, Blackwell TS, Moore ML, Boyd KL, Kolls JK, Peebles RS Jr. Allergic airway inflammation decreases lung bacterial burden following acute Klebsiella pneumoniae infection in a neutrophil- and CCL8-dependent manner. Infect Immun 82: 3723–3739, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci USA 111: E4110–E4118, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutra FF, Bozza MT. Heme on innate immunity and inflammation. Front Pharmacol 5: 115, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez PL, Dutra FF, Alves L, Figueiredo RT, Mourao-Sa D, Fortes GB, Bergstrand S, Lonn D, Cevallos RR, Pereira RM, Lopes UG, Travassos LH, Paiva CN, Bozza MT. Heme amplifies the innate immune response to microbial molecules through spleen tyrosine kinase (Syk)-dependent reactive oxygen species generation. J Biol Chem 285: 32844–32851, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, Bozza MT. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem 282: 20221–20229, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 165: 242–249, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Ghio AJ, Carter JD, Richards JH, Brighton LE, Lay JC, Devlin RB. Disruption of normal iron homeostasis after bronchial instillation of an iron-containing particle. Am J Physiol Lung Cell Mol Physiol 274: L396–L403, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Ghio AJ, Carter JD, Richards JH, Richer LD, Grissom CK, Elstad MR. Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome. Crit Care Med 31: 395–400, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Ghio AJ, Turi JL, Madden MC, Dailey LA, Richards JD, Stonehuerner JG, Morgan DL, Singleton S, Garrick LM, Garrick MD. Lung injury after ozone exposure is iron dependent. Am J Physiol Lung Cell Mol Physiol 292: L134–L143, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Adisa OA, Chappa P, Tan F, Jackson KA, Archer DR, Ofori-Acquah S. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest 123: 4809–4820, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol 16: 515–523, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Gutteridge JM, Mumby S, Quinlan GJ, Chung KF, Evans TW. Pro-oxidant iron is present in human pulmonary epithelial lining fluid: implications for oxidative stress in the lung. Biochem Biophys Res Commun 220: 1024–1027, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol 151: 1–15, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Hualin C, Wenli X, Dapeng L, Xijing L, Xiuhua P, Qingfeng P. The anti-inflammatory mechanism of heme oxygenase-1 induced by hemin in primary rat alveolar macrophages. Inflammation 35: 1087–1093, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Huffman LJ, Miles PR, Shi X, Bowman L. Hemoglobin potentiates the production of reactive oxygen species by alveolar macrophages. Exp Lung Res 26: 203–217, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Irwin DC, Hyen Baek J, Hassell K, Nuss R, Eigenberger P, Lisk C, Loomis Z, Maltzahn J, Stenmark KR, Nozik-Grayck E, Buehler PW. Hemoglobin-induced lung vascular oxidation, inflammation, and remodeling contribute to the progression of hypoxic pulmonary hypertension and is attenuated in rats with repeated-dose haptoglobin administration. Free Radic Biol Med 82: 50–62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janz DR, Bastarache JA, Peterson JF, Sills G, Wickersham N, May AK, Roberts LJ 2nd, Ware LB. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med 41: 784–790, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janz DR, Bastarache JA, Rice TW, Bernard GR, Warren MA, Wickersham N, Sills G, Oates JA, Roberts LJ 2nd, Ware LB; Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis Study Group. Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: the Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis Trial. Crit Care Med 43: 534–541, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 100: 879–887, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol 84: 618–625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kono M, Saigo K, Takagi Y, Takahashi T, Kawauchi S, Wada A, Hashimoto M, Minami Y, Imoto S, Takenokuchi M, Morikawa T, Funakoshi K. Heme-related molecules induce rapid production of neutrophil extracellular traps. Transfusion 54: 2811–2819, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Lagan AL, Melley DD, Evans TW, Quinlan GJ. Pathogenesis of the systemic inflammatory syndrome and acute lung injury: role of iron mobilization and decompartmentalization. Am J Physiol Lung Cell Mol Physiol 294: L161–L174, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Lagan AL, Quinlan GJ, Mumby S, Melley DD, Goldstraw P, Bellingan GJ, Hill MR, Briggs D, Pantelidis P, du Bois RM, Welsh KI, Evans TW. Variation in iron homeostasis genes between patients with ARDS and healthy control subjects. Chest 133: 1302–1311, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepulveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2: 51ra71, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Spolarics Z. Methemoglobin is a potent activator of endothelial cells by stimulating IL-6 and IL-8 production and E-selectin membrane expression. Am J Physiol Cell Physiol 285: C1036–C1046, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer C, Heiss C, Drexhage C, Kehmeier ES, Balzer J, Muhlfeld A, Merx MW, Lauer T, Kuhl H, Floege J, Kelm M, Rassaf T. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol 55: 454–459, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Mumby S, Ramakrishnan L, Evans TW, Griffiths MJ, Quinlan GJ. Methemoglobin-induced signaling and chemokine responses in human alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 306: L88–L100, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mumby S, Upton RL, Chen Y, Stanford SJ, Quinlan GJ, Nicholson AG, Gutteridge JM, Lamb NJ, Evans TW. Lung heme oxygenase-1 is elevated in acute respiratory distress syndrome. Crit Care Med 32: 1130–1135, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 299: 2304–2312, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsson MG, Allhorn M, Bulow L, Hansson SR, Ley D, Olsson ML, Schmidtchen A, Akerstrom B. Pathological conditions involving extracellular hemoglobin: molecular mechanisms, clinical significance, and novel therapeutic opportunities for alpha(1)-microglobulin. Antioxid Redox Signal 17: 813–846, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Polimeni M, Valente E, Aldieri E, Khadjavi A, Giribaldi G, Prato M. Haemozoin induces early cytokine-mediated lysozyme release from human monocytes through p38 MAPK- and NF-kappaB-dependent mechanisms. PLoS One 7: e39497, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal 13: 1087–1123, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121: 1276–1284, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharte M, Fink MP. Red blood cell physiology in critical illness. Crit Care Med 31: S651–S657, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Shaver CM, Grove BS, Putz N, Clune JK, Lawson WE, Carnahan RH, Mackman N, Ware LB, Bastarache JA. Lung epithelial tissue factor regulates alveolar pro-coagulant activity and permeability in direct acute lung injury. Am J Respir Cell Mol Biol 53: 719–727, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva G, Jeney V, Chora A, Larsen R, Balla J, Soares MP. Oxidized hemoglobin is an endogenous proinflammatory agonist that targets vascular endothelial cells. J Biol Chem 284: 29582–29595, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva TB, Spulber M, Kocik MK, Seidi F, Charan H, Rother M, Sigg SJ, Renggli K, Kali G, Bruns N. Hemoglobin and red blood cells catalyze atom transfer radical polymerization. Biomacromolecules 14: 2703–2712, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Standiford LR, Standiford TJ, Newstead MJ, Zeng X, Ballinger MN, Kovach MA, Reka AK, Bhan U. TLR4-dependent GM-CSF protects against lung injury in Gram-negative bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol 302: L447–L454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starzyk D, Korbut R, Gryglewski RJ. The role of nitric oxide in regulation of deformability of red blood cells in acute phase of endotoxaemia in rats. J Physiol Pharmacol 48: 731–735, 1997. [PubMed] [Google Scholar]
- 58.Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol 175: 2598–2605, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Tanabe S, Gottschalk M, Grenier D. Hemoglobin and Streptococcus suis cell wall act in synergy to potentiate the inflammatory response of monocyte-derived macrophages. Innate Immun 14: 357–363, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal 12: 305–320, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal 12: 305–320, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173: 1008–1015, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Asbeck BS, Hoidal J, Vercellotti GM, Schwartz BA, Moldow CF, Jacob HS. Protection against lethal hyperoxia by tracheal insufflation of erythrocytes: role of red cell glutathione. Science 227: 756–759, 1985. [DOI] [PubMed] [Google Scholar]
- 64.Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg 142: 1–11, 2011. [DOI] [PubMed] [Google Scholar]
- 65.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int 77: 913–920, 2010. [DOI] [PubMed] [Google Scholar]
- 66.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J 35: 331–337, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang F, Ghio AJ, Herbert DC, Weaker FJ, Walter CA, Coalson JJ. Pulmonary expression of the human haptoglobin gene. Am J Respir Cell Mol Biol 23: 277–282, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Yang F, Haile DJ, Berger FG, Herbert DC, Van Beveren E, Ghio AJ. Haptoglobin reduces lung injury associated with exposure to blood. Am J Physiol Lung Cell Mol Physiol 284: L402–L409, 2003. [DOI] [PubMed] [Google Scholar]