Abstract
Thymoquinone (2-isopropyl-5-methylbenzo-1,4-quinone) is a major bioactive component of Nigella sativa, a plant used in traditional medicine to treat a variety of symptoms, including elevated blood glucose levels in type 2 diabetic patients. Normalization of elevated blood glucose depends on both glucose disposal by peripheral tissues and glucose-stimulated insulin secretion (GSIS) from pancreatic β-cells. We employed clonal β-cells and rodent islets to investigate the effects of thymoquinone (TQ) and Nigella sativa extracts (NSEs) on GSIS and cataplerotic metabolic pathways implicated in the regulation of GSIS. TQ and NSE regulated NAD(P)H/NAD(P)+ ratios via a quinone-dependent redox cycling mechanism. TQ content was positively correlated with the degree of redox cycling activity of NSE extracts, suggesting that TQ is a major component engaged in mediating NSE-dependent redox cycling. Both acute and chronic exposure to TQ and NSE enhanced GSIS and were associated with the ability of TQ and NSE to increase the ATP/ADP ratio. Furthermore, TQ ameliorated the impairment of GSIS following chronic exposure of β-cells to glucose overload. This protective action was associated with the TQ-dependent normalization of chronic accumulation of malonyl-CoA, elevation of acetyl-CoA carboxylase (ACC), fatty acid synthase, and fatty acid-binding proteins following chronic glucose overload. Together, these data suggest that TQ modulates the β-cell redox circuitry and enhances the sensitivity of β-cell metabolic pathways to glucose and GSIS under normal conditions as well as under hyperglycemia. This action is associated with the ability of TQ to regulate carbohydrate-to-lipid flux via downregulation of ACC and malonyl-CoA.
Keywords: insulin secretion, β-cells, thymoquinone, acetyl-CoA carboxylase, malonyl-CoA
nigella sativa, also known as black cumin, is a plant spice renowned for its ability to lower blood glucose and alleviate symptoms of type 2 diabetes (reviewed in Ref. 2). Normalization of elevated blood glucose levels in type 2 diabetics can be accomplished by regulating the efficiency of insulin-dependent signaling in peripheral tissues and/or insulin output by pancreatic β-cells; Nigella sativa extracts (NSEs) have been reported to modulate both pathways (4, 5, 18, 50). Thymoquinone (TQ) is one of the main bioactive constituents of the seed oil of Nigella sativa (21) and has been shown to stimulate insulin release from pancreatic β-cells (11). However, the molecular mechanisms of antidiabetic action for NSE and TQ have not been fully elucidated.
Pancreatic β-cells release insulin in response to a rise in blood glucose, and the level of released insulin is directly proportional to the degree of glucose metabolism inside these cells (33). Glucose metabolism leads to an increase in the ATP/ADP ratio, a trigger for insulin granule exocytosis via sequential closure of the KATP channels, plasma membrane depolarization, and Ca2+ influx (reviewed in Ref. 41). NADH and NADPH are generated by the reduction of NAD+ and NADP+ as a consequence of glucose metabolism (29). The NADPH/NADP+ redox pair is engaged primarily in reductive biosynthesis and maintenance of the glutaredoxin and thioredoxin system (29), whereas the NADH/NAD+ redox pair plays a critical role in oxidative metabolism (55), such as glycolysis, and is maintained at a low ratio (27). NADH produced by glycolysis must be continuously reoxidized back to NAD+, a substrate for the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase, to maintain glycolytic flux. Reoxidation of glycolytic NADH in β-cells is accomplished by mitochondrial shuttles (19) and the recently identified extramitochondrial pathway known as plasma membrane electron transport (PMET) (22). TQ, due to its conjugated double-bond system and resulting electrophilic nature, is also able to reoxidize NADH in the process of NAD(P)H-dependent redox cycling (34), providing an alternative pathway for the reoxidation of glycolytic NADH (7) and lowering cytosolic reductive poise inside the cell (32).
However, under chronic hyperglycemia [which can lead to the condition known as glucotoxicity (63)], the first two pathways for NADH reoxidation are saturated (22, 26, 65). A chronic excess of glucose is shunted into the formation of lipids by a mechanism that involves the activation of the acetyl-CoA carboxlase (ACC), an increase in malonyl-CoA levels and fatty acid synthesis, and an inhibition of fatty acid transport and oxidation (47). These changes ultimately result in lipid accumulation, β-cell dysfunction, and impairment of glucose-stimulated insulin secretion (GSIS), all of which accelerate the progression of type 2 diabetes (reviewed in Ref. 63).
In the current work, we describe the pleiotropic effect of Nigella sativa extract and its active component TQ (9) on multiple β-cell metabolic pathways relevant to GSIS. We demonstrate that cellular metabolism and reduction of TQ via the process of redox cycling facilitates NADH reoxidation. TQ, applied at high nanomolar doses, ameliorated the impairment of GSIS under chronic glucose overload. In parallel, TQ normalized levels of ACC, malonyl-CoA, fatty acid synthase (FAS), and fatty acid-binding proteins (FABPs), all of which were elevated under chronic glucose overload. This suggests that TQ regulates the switch between carbohydrate and fatty acid metabolism in pancreatic β-cells.
MATERIALS AND METHODS
Materials.
Collagenase was from Roche, and fetal calf serum was from Hyclone. Antibodies were from Cell Signaling Technology (Danvers, MA). d-[U-14C]glucose and [1-14C]palmitic acid were from Perkin-Elmer (Waltham, MA). All other chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
INS-1 832/13 cell and pancreatic islet preparation and culture.
Clonal INS-1 832/13 cells, provided by Dr. Christopher Newgard (Duke University), were maintained and cultured as described previously (28). Male CD-1 mice (Charles River Laboratories) were euthanized by halothane. All procedures were approved by the Institutional Animal Care and Use Committee at the Marine Biological Laboratory in compliance with US Public Health Service regulations. Pancreatic islets were isolated by collagenase (Roche, Indianapolis, IN) digestion, as described previously (25). After digestion and washing and separation by histopaque gradient centrifugation, islets were hand-picked and cultured overnight in a humidified incubator with 5% CO2. Islets were used after an overnight culture in RPMI supplemented with 10% fetal calf serum (Hyclone), penicillin-streptomycin, and 5 mM glucose.
Preparation of NSEs.
Air-dried ground seeds (2.0 kg) of Nigella sativa were obtained from Verdure Sciences (Noblesville, IN; lot no. VS-NSSP-001, kind courtesy of Blake Ebersole). The ground seed material was sequentially extracted with n-hexanes (4 liters × 3 times at 3 days/time period) followed by methanol (4 liters × 3 times at 3 days/time period) at room temperature. Solvent removal in vacuo afforded hexane (NSE_H, 0.37% TQ) and methanol (NSE_M, 0.09% TQ) NSEs. In addition, supercritical fluid extract (NSE_SP, 4.45% TQ), subcritical fluid extract (NSE_SB, 2.14% TQ), and petroleum ether extract (NSE_PE, 2.73% TQ) of Nigella sativa were obtained from Shenyang Longpower Technology (Shenyang, Liaoning, China; kind courtesy of Yingjun Mu). The TQ content of the extracts was quantified by a high-performance liquid chromatography method, as reported previously (21), using a Hitachi Elite LaChrom (Pleasanton, CA) system consisting of a L2130 pump, L-2200 autosampler, equipped with a L-2455 Diode Array Detector, all of which was operated by EZChrom Elite software with an Alltima C18 (5 mm, 4.6 × 250 mm) column with a flow rate of 0.75 ml/min at room temperature and injection volumes of 10 μl. A linear gradient solvent system consisting of solvent A (DI water containing 0.1% TFA) and solvent B (methanol) was used as follows: 0 min, 50% B; 20 min, 100% B.
Redox cycling activity (H2O2 production).
Following a 1-h preincubation in Krebs-Ringer bicarbonate (KRB) buffer (140 mM NaCl, 30 mM HEPES 7.4, 4.6 mM KCl,1 mM MgSO4, 0.15 mM Na2HPO4, 0.4 mM KH2PO4, 5 mM NaHCO3, 2 mM CaCl2, and 3 mM glucose), INS-1 832/13 cells (96-well plates) or intact islets (20 islets/microtube) were exposed to reaction mixes containing 25 μM Amplex Red (Invitrogen, Carlsbad, CA), 1 U/ml horseradish peroxidase, glucose (3 mM for basal insulin secretion, 16 mM for glucose-stimulated insulin secretion), and TQ or NSE in KRB buffer for 1 h. Increases in fluorescence (540 nm excitation, 595 nm emission) due to the conversion of Amplex Red to resorufin by horseradish peroxidase in the presence of H2O2 were monitored using a SpectraMax M5 multimode microplate reader (Molecular Devices, Sunnyvale, CA), as described previously (26). The rate of H2O2 formation, which directly correlates with the redox cycling activity, was calculated based on a standard curve constructed with H2O2 in a concentration range of 0.5–16 μM. For studies with subcellular fractions, cells washed and suspended in KRB buffer were subjected to sonication on ice with three 15-s pulses separated by cooling of samples for 1 min on ice. Cell lysates were centrifuged (3,000 g, 10 min, 4°C) to remove cellular debris. Precleared cell lysates were subjected to 10 min centrifugation at 12,000 g, and resulting cytosolic (supernatant) and mitochondrial (pellet) fractions were tested for redox cycling activity using the Amplex Red assay. Reactions were performed as above, with the addition of 250 μM NADPH or NADH.
Glucose and palmitate oxidation.
Glucose and palmitate oxidation were measured by the production of CO2 from [U-14C]glucose and [1-14C]palmitate, respectively. INS-1 832/13 cells, cultured in 25-cm flasks, were either untreated or chronically treated with 0.5 μM TQ for 48 h. The acute effects of TQ on glucose or palmitate oxidation were determined following a 1-h preincubation of untreated cells in KRB containing 3 mM glucose. Cells were then exposed for 2 h to 3 or 16 mM glucose in the presence or absence of 0.5 μM TQ and 0.2 μCi/ml of d-[U-14C]glucose (glucose oxidation) or [1-14C]palmitate plus 0.2 mM unlabeled palmitate complexed to 2% BSA and 1 mM carnitine (palmitate oxidation). During the 2-h incubation period, flasks were sealed with rubber stoppers, with an attached vial containing Whatman GF/B filter paper soaked with 300 μl of 5% KOH for CO2 trapping. At the end of the incubation period, metabolism was terminated by injecting perchloric acid (6% final concentration) via a syringe through the rubber stopper. KOH-trapped CO2 was measured by liquid scintillation counting. The chronic effect of TQ exposure on glucose and palmitate oxidation was determined as described above, except that cells were chronically treated for 48 h with 0.5 μM TQ, but TQ was absent during the preincubation and incubation periods.
Triglyceride content.
Triglyceride content was determined in freshly prepared extracts from INS-1 832/13 cells (grown in 6-well plates) treated for 48 h with 0.5 μM TQ, using the Triglyceride kit (Abcam, Cambridge, MA) according to the manufacturer's protocols.
Determination of nucleotides.
Adenine nucleotides were determined using NAD+/NADH, NADP+/NADPH, and ATP/ADP kits (Abcam) according to the manufacturer's protocols.
Determination of malonyl-CoA.
Malonyl-CoA levels were measured in freshly prepared INS-1 832/13 extracts using a rat malonyl-CoA ELISA kit (MyBiosource, San Diego, CA) according to the manufacturer's protocol.
Western blot analysis.
Following solubilization of cells in RIPA buffer, equal amounts of protein were resolved by gradient (4–20%) SDS-PAGE and electrotransferred to PVDF membranes. Following overnight incubation with primary antibodies (Cell Signaling Technology) in the presence of 5% nonfat dry milk, membranes were washed and probed with corresponding secondary antibodies, and proteins were visualized by enhanced chemiluminescence (Thermo Scientific, Rockford, IL).
Insulin secretion and insulin content.
INS-1 832/13 cells (24-well plates) and islets (20 islets/tube) were preincubated for 2 h in the presence of 3 mM glucose in KRB buffer (glucose starvation). Following 1 h of static incubation of cells or islets in KRB buffer supplemented with 3 (basal) or 16 mM (stimulatory) glucose, KRB buffer aliquots were briefly centrifuged at 1,000 g at 4°C to pellet out any cells prior to the determination of secreted insulin. The remaining cells were extracted with RIPA buffer for determination of protein and total insulin content. Insulin was determined using an ELISA kit (Alpco Diagnostics, Salem, NH). Protein content was determined by the Micro-BCA Protein Assay kit (Pierce, Rockford, IL).
NAD(P)H autofluorescence.
NAD(P)H fluorescence (340 nm excitation/460 nm emission) was measured in a suspension of INS-1 832/13 cells (106 cells/ml) using a fluorescence spectrophotometer (FluoroMax-3; Horiba Jobin Yvon). Cells placed in a cuvette positioned inside a temperature-controlled chamber were stirred during the experiment to ensure proper mixing. FCCP (1 μM) and rotenone (1 μM) were used to determine minimal and maximal reduction, respectively, of nicotinamide nucleotides.
Quantitative real-time RT-PCR.
Total RNA was extracted from islets of Langerhans using the RNeasy Micro Kit (Qiagen, Valencia, CA) and reverse-transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturers' protocols. Standard curves were generated using serial twofold dilutions from pooled cDNA samples to confirm ≥90% reaction efficiency for each primer set. Real-time PCR was performed using iQ SYBR Green Supermix on a MyIQ2 Real-Time PCR Detection System (Bio-Rad). All PCR primer sequences were generated using PrimerQuest (Integrated DNA Technologies, Coralville, IA). Primer sequences used were rat NQO1 (NCBI Reference Sequence: NM_017000.3; GGC CAT CAT TTG GGC AAG TCC ATT and ACT GAA AGC AAG CCA GGC AAA CTG), rat fatty acid synthase (NCBI Reference Sequence: NM_017332.1; GGC GAG TCT ATG CCA CTA TTC and GCT GAT ACA GAG AAC GGA TGA G), rat acetyl CoA synthase (NCBI Reference Sequence: NM_130739.1; GAT GCA GTC GGA AGA AGT AGA G and GCA TCG TCA TAG TAG TGG GTA), rat pyruvate kinase (NM_012624.3; TGT GGG TGA TCT GGT GAT TG and GGG AGA GGC ATT TCA GGA TAC), rat carbohydrate-responsive element-binding protein (FN_432819.1; GCT ATG CCG GGA CAA GAT T and GAG GAG TTA CGA AGC CAC ATA C), rat glycerol phosphate dehydrogenase 1 (NM_022215.2; TCA GCT CCC GGA GAC TAT TTA and GAT GGG AGT ATG CCT TGT ACT G), rat aldehyde dehydrogenase 2 (mitochondrial) (NM_032416.1; GAG ATC AGC CAA TCG GTA CAA and GAA CAA GGA GGA CGT AGA CAA G), rat AMP-activated protein kinase-α1 subunit (NM_019142.2; CGG GAT CCA TCA GCA ACT ATC and AGG TCA CGG ATG AGG TAA GA), rat AMP-activated protein kinase-β1 subunit (NM_031976.1; TAA GTC ACC AGG ACG AGA CTA T and CGT CTG GCT TGA TGG AGA AA), rat AMP-activated protein kinase-γ1 subunit (NM_013010.2; GAA CTG GAG GAG CAC AAG ATA G and CTG GAA GCC TGT GGA TCT TAT T), mouse NQO1 [NCBI Reference Sequence: NM_008706.5; AAG AGC TTT AGG GTC GTC TTG GCA and AGC CTC CTT CAT GGC GTA GTT GAA (mouse)], and β2-microglobulin (NCBI Reference Sequence NM_009735.3; ACC GGC CTG TAT GCT ATC CAG AAA and ATT TCA ATG TGA GGC GGG TGG AAC).
Statistical analysis.
Data are expressed as means ± SE. Significance was determined for multiple comparisons using one-way analysis of variance (ANOVA), followed by the Bonferroni post hoc analysis (43). A P value of <0.05 was considered significant.
RESULTS
Synthetic quinones were previously shown to modulate insulin secretion by MacDonald (38) via an unknown mechanism. Later work suggested that quinones undergo redox cycling in β-cells, producing H2O2 (26), which at low levels may act as a coupling factor in GSIS (37, 45). In the current work, we evaluated the capacity of NSE and its major bioactive component TQ to modulate GSIS and investigated its effects on the underlying β-cell metabolic pathways.
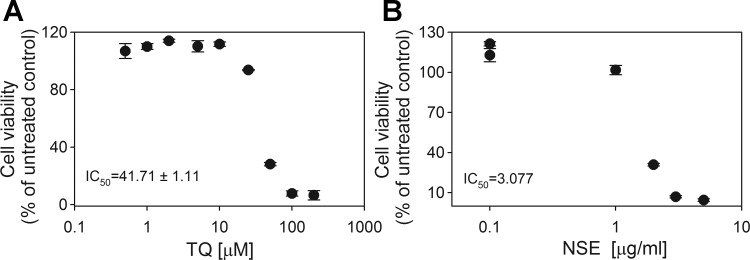
Concentrations of TQ and NSE_SP (the extract of Nigella sativa used for the majority of the functional studies) were between 0.1–5 μM and 0.1–1 μg/ml respectively, doses at which no deleterious effects to the cells were observed (Fig. 1). Acute (1 h) coexposure to TQ and stimulatory (16 mM) glucose caused an additional 20 and 25% increase in GSIS in clonal β-cells (Fig. 2A) and isolated mouse islets (Fig. 3A), respectively. Similarly, NSE increased insulin secretion at stimulatory glucose in INS-1 832/13 cells (Fig. 2B). To evaluate how chronic exposure to TQ or NSE affected metabolism and function, cells were exposed to TQ and NSE for 48–72 h, and GSIS was measured after the removal of these compounds. Chronic pretreatment with TQ or NSE caused a 25–35% increase in GSIS in INS-1 832/13 (Fig. 2, C and D) and pancreatic islets (Fig. 3B), respectively. However, the effective dose (0.1 μM) required to affect GSIS following chronic treatment was as much as 20 times lower than the dose required to affect GSIS due to an acute treatment. The fact that chronic TQ treatment elicited an effect on GSIS despite the absence of TQ during the preincubation (2 h) and the secretory incubation (1 h) period suggests that chronic treatment involves changes in the expression and/or activity of the metabolic enzymes involved in the regulation of GSIS. Together, these results indicate both an immediate and long-term effect of TQ on the insulin secretory capacity of β-cells and that these effects likely involve different mechanisms.
Fig. 1.
Thymoquinone (TQ)-dependent cell viability. INS-1 832/13 cells were exposed to various doses of TQ (A) or Nigella sativa extract (NSE; 4.45% TQ content; B) for 48 h, and cell viability was determined by the CellTiter-Blue Viability Assay kit (Promega, Madison, WI) as per the manufacturer's instructions. ID50 was calculated using a least square fit with variable slope with GraphPad Prism 5.04. ●, Individual data points.
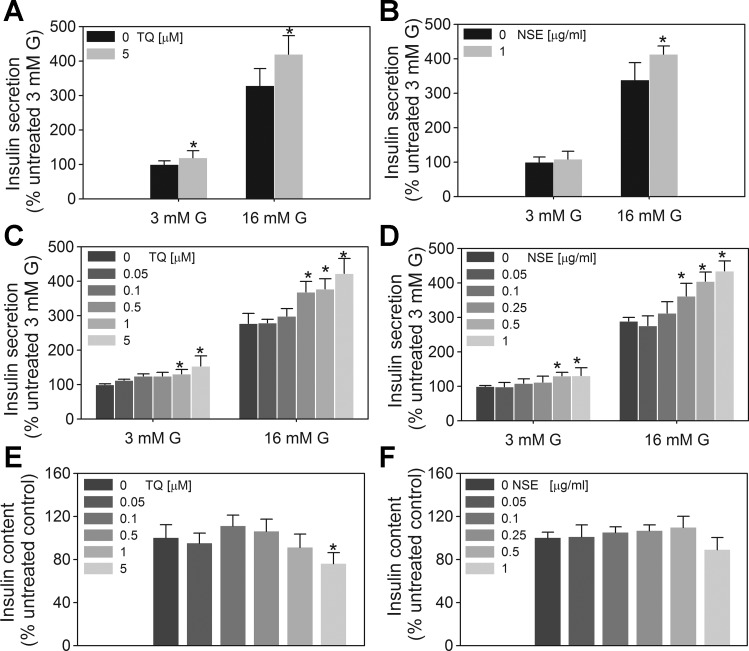
Fig. 2.
Effect of TQ and NSE on insulin secretion in INS-1 832/13 cells. A and B: acute exposure. Following a 2-h preincubation period in Krebs-Ringer bicarbonate (KRB) buffer, cells were exposed to 3 or 16 mM glucose (G) for 1 h in the presence or absence of the indicated concentrations of TQ or NSE (4.45% TQ). C and D: chronic exposure. Cells were treated with 0.05–5 μM TQ or 0.05–1 μg/ml NSE (corresponding to 0.0135–0.27 μM TQ content) for 48 h, after which growth medium was removed and cells were washed with and preincubated in KRB buffer containing 3 mM glucose for 2 h. E and F: the amount of released insulin and total insulin content was determined as described in materials and methods following 1-h incubation of cells with 3 or 16 mM G. TQ and NSE were absent during the preincubation and stimulation periods. Basal insulin secretion (defined as secretion in the presence of 3 mM glucose) was 1.56 ± 0.4 ng insulin·mg protein−1·h−1. Data are means ± SE from 2 to 4 independent experiments performed in quadruplicate measurements. *P < 0.05 when compared with corresponding untreated control value at 3 or 16 mM G.
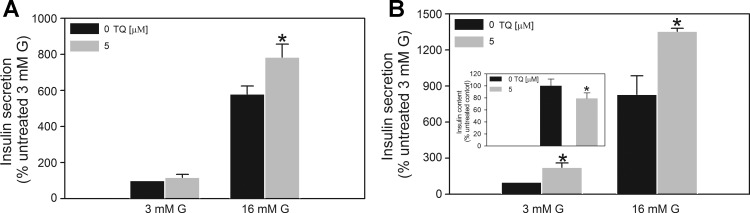
Fig. 3.
Effect of TQ on insulin secretion in mouse islets. A: acute exposure. Following a 2-h preincubation period in 3 mM G KRB buffer, islets were exposed to 3 or 16 mM G in the presence or absence of 5 μM TQ for 1 h. B: chronic exposure. Islets cultured in the presence TQ for 48 h were washed with and preincubated in KRB buffer with 3 mM glucose for 2 h. The amount of released insulin and total insulin content (inset) was determined as described in materials and methods following a 1-h incubation of islets with 3 or 16 mM G. TQ was absent during the preincubation and stimulation periods. Basal insulin secretion (defined as secretion in the presence of 3 mM G) was 34.75 ± 6.25 pg insulin·20 islets−1·h−1. Data are means ± SE from 2 to 3 independent experiments performed in quadruplicate measurements. *P < 0.05 when compared with corresponding control value.
We hypothesized that the capacity to modulate GSIS is linked to the ability of TQ and NSE to alter the β-cell redox state. This hypothesis was rationalized by our earlier findings demonstrating the ability of menadione, a prototypical quinone, to engage in glucose-dependent redox cycling in pancreatic β-cells (26), a process that converts NAD(P)H to NAD(P)+ and produces H2O2, a putative coupling factor for GSIS (45). Indeed, both TQ and NSE supported redox cycling in intact clonal INS-1 832/13 cells and islets (Fig. 4). TQ content was correlated with the degree of redox cycling activity of the various NSE extracts (Fig. 4A), suggesting that TQ is a major component engaged in mediating NSE-dependent redox cycling. In contrast to the quinone menadione, whose redox cycling in β-cells increased with increasing glucose concentrations (see Fig. 4C and Ref. 26), TQ- and NSE-dependent redox cycling in islets and INS-1 832/13 cells decreased in response to increasing glucose concentrations (Fig. 4, C–E). To determine the mechanism responsible for this difference between menadione and TQ, in vitro studies using INS-1 832/13 subcellular fractions were performed. Both cytosolic and mitochondrial fractions from INS-1 832/13 cells were capable of facilitating redox cycling of TQ, and both NADH and NADPH were able to support redox cycling with similar Km and Vmax values (Table 1). The similarity of these values to those for redox cycling of menadione (data not shown) suggests that the same enzymes are capable of supporting redox cycling of menadione and TQ. The lack of a glucose-dependent increase in TQ redox cycling seen in live INS-1 832/13 cells may be explained by the differential partitioning of TQ (as compared with menadione) to subcellular compartments and/or selective use of different cellular pools of reducing equivalents. However, the theoretical partition coefficient (LogP) values for both compounds are similar (2.044 for menadione vs. 1.9 for TQ), suggesting that enzymatic specificity is more likely to contribute to differences in NADH/NADPH utilization than localization of the compounds. Future studies will be required to determine the mechanism for the difference between menadione and TQ redox cycling responses to glucose in β-cells.
Fig. 4.
Acute effect of TQ and NSE on redox cycling activity in INS-1 832/13 cells and islets. Redox cycling activity in intact INS-1 832/13 cells (A, B, D, and E) and islets (C) was measured as the production of H2O2 using the Amplex Red/horseradish peroxidase assay, as described in materials and methods. NSE-dependent redox cycling activity is TQ content (A) and dose dependent (B). TQ-dependent redox cycling activity decreases with glucose concentration in both islets (C) and INS-1 832/13 cells (D and E). Data are means ± SE from 2 to 3 independent experiments performed in duplicate or triplicate measurements. *P < 0.05 when compared with corresponding control value at 3 or 16 mM G. MEN, menadione.
Table 1.
Kinetic properties of TQ-dependent redox cycling by cytosolic and mitochondrial fractions of INS-1 832.13 cells in the presence of NADPH and NADH
| Cytosol |
Mitochondria |
|||
|---|---|---|---|---|
| Pyrimidine | Km, μM | Vmax, μM TQ/min | Km, μM | Vmax, μM TQ/min |
| NADPH | 2.678 | 164.8 | 3.165 | 179.9 |
| NADH | 2.061 | 127.5 | 1.158 | 165.8 |
TQ, thymoquinone.
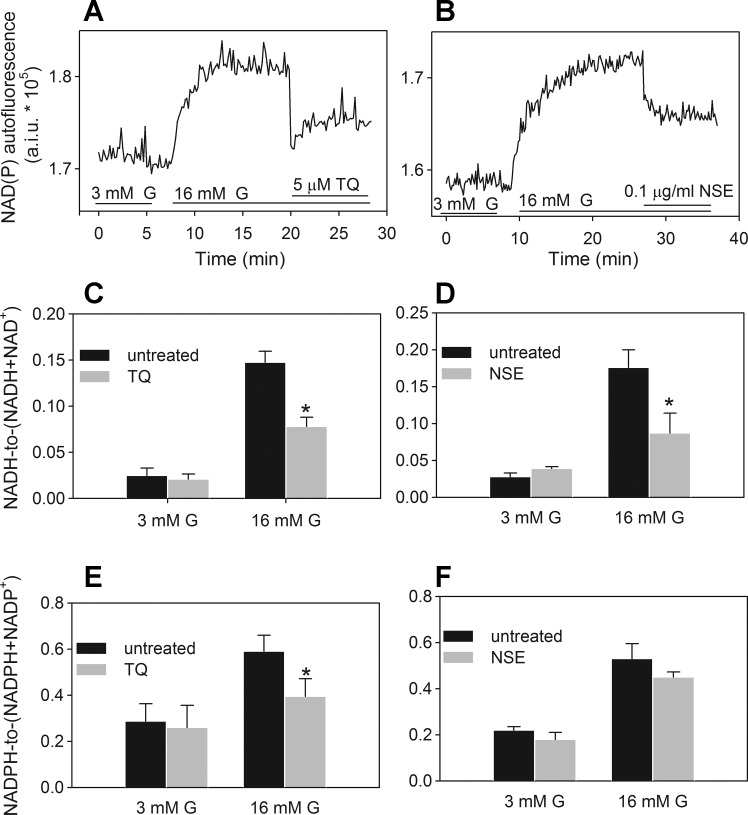
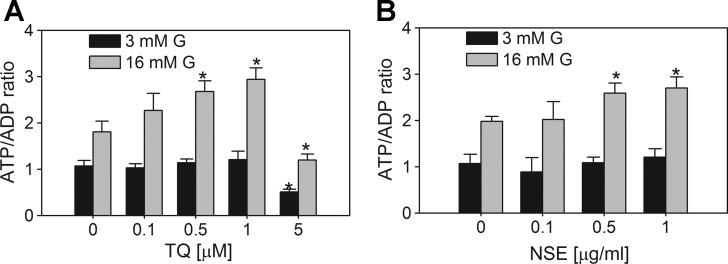
Evidence for the redox cycling-dependent conversion of NAD(P)H to NAD(P)+ was supported by a decrease in the NAD(P)H autofluorescence in INS-1 832/13 cells following the application of TQ and NSE (Fig. 5, A and B). However, the identical spectral properties of NADH and NADPH precluded us from determining whether NADH, NADPH, or both nucleotides were oxidized. In cells other than β-cells, it is well accepted that both NADH and NADPH can support redox cycling (17, 49, 64), although there may be preferential utilization of NADH or NADPH, depending on the enzymes involved and the relative abundance of NADH and NADPH. To determine preference for NADH or NADPH in β-cells, we measured intracellular TQ- and NSE-dependent consumption of NADH and NADPH separately. Both TQ (5 μM) and NSE (0.1 μg/ml) lowered NADH and NADPH levels by about 45 and 30% and 25 and 20%, respectively (Fig. 5, C–F). Reoxidation of NADH by TQ and NSE will provide NAD+, a substrate for glycolysis, while decreasing the cellular reductive poise. Since the NADH/NAD+ ratio also contributes to the regulation of mitochondrial oxidative phosphorylation, we measured the effect of TQ and NSE on the ATP/ADP ratio. Both TQ and NSE increased the glucose-dependent ATP/ADP ratio (Fig. 6). This suggests that TQ increases the sensitivity of glucose-dependent oxidative catabolic pathways. The ATP/ADP ratio was also elevated following chronic exposure of cells to doses of TQ <1 μM (Fig. 6A), at which neither significant redox cycling activity nor changes in the NAD(P)H autofluorescence were observed (data not shown). This further supports the idea that when TQ is applied at levels <1 μM, there are TQ-dependent changes in the expression or activity of enzymes that regulate the ATP/ADP ratio and GSIS, which are likely redox independent. A comprehensive analysis of TQ's effects on glycolytic and mitochondrial pathways involved in the regulation of ATP production will be the topic of future studies.
Fig. 5.
Acute effect of TQ and NSE on NADH/(NADH + NAD+) and NADPH/(NADPH + NADP+) ratios in INS-1 832/13 cells. A and B: examples of the TQ (A) and NSE (4.45% TQ; B) effect on the real-time NAD(P)H autofluorescence in a population of INS-1 832/13 cells. C–F: traces are representatives of 3–5 independent experiments. Effect of TQ (5 μM) and NSE (0.1 μg/ml) on the NADH/NAD+ (C and D) and NADPH/NADP+ ratios (E and F) were determined following a 1-h exposure of cells to 3 or 16 mM G in the absence or presence of these agents. Data are means ± SE from 2 independent experiments performed in duplicate measurements. *P < 0.05 when compared with corresponding untreated control value at 3 or 16 mM G.
Fig. 6.
Effect of TQ and NSE on ATP/ADP ratio in INS-1 832/13 cells. Following 48-h treatment in the presence of TQ (μM; A) or NSE 4.45% TQ content (μg/ml; B), cells were preincubated for 2 h in 3 mM glucose KRB. Measurements of ATP/ADP ratios, performed as described in materials and methods, were done following a 1-h exposure of cells to 3 or 16 mM G. Data are means ± SE from 2 to 3 independent experiments performed in duplicate measurements. *P < 0.05 when compared with corresponding untreated 3 or 16 mM G controls.
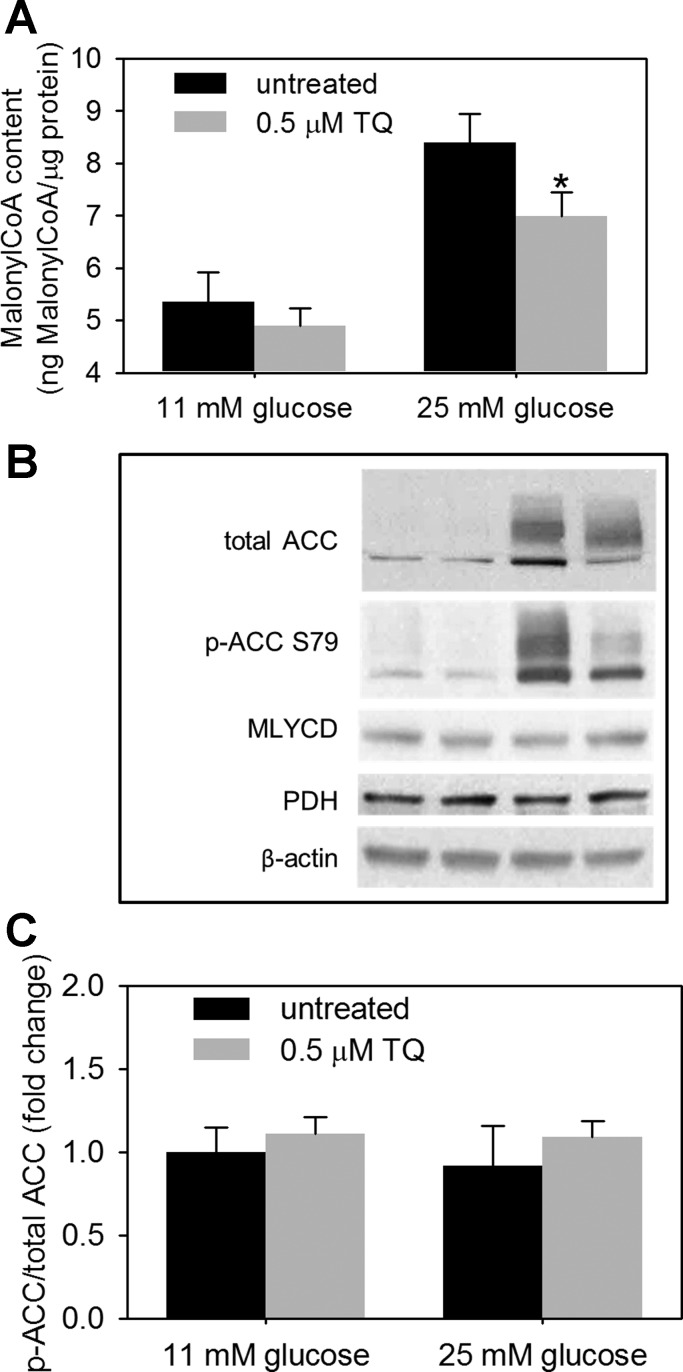
Normal glucose metabolism following an acute glucose rise supports GSIS via the generation of a battery of transient secretory signals, one of which is malonyl-CoA (48). However, chronic exposure of β-cells to glucose overload leads to the steady-state elevation in the malonyl-CoA pool, promoting the conversion of excess of carbohydrates to lipid stores, which will negatively affect β-cell function and GSIS (6, 51). Both TQ and NSE ameliorated the impairment in GSIS in INS-1 832/13 cells exposed to 25 mM glucose (Fig. 7), suggesting that TQ and NSE counteract metabolic changes associated with the glucotoxic environment and increase the sensitivity of β-cells to glucose. To investigate the mechanism responsible for this protective TQ-dependent action, pathways involved in the carbohydrate and lipid metabolism were examined in cells chronically exposed to both 11 (control) and 25 mM [glucose overload (GO)] glucose in the absence and presence of nanomolar doses of TQ. The GO-dependent increase in malonyl-CoA, a metabolic switch that directs excess carbohydrates into lipid stores, was decreased following TQ exposure (Fig. 8A). TQ-dependent decreases in malonyl-CoA levels were paralleled by the TQ-dependent decrease in ACC, a malonyl-CoA-producing enzyme, without any effect on the levels of the malonyl-CoA-degrading enzyme malonyl-CoA decarboxylase (Fig. 8B). When normalized for the ACC protein, ACC phosphorylation status was not significantly affected by TQ (Fig. 8C). Indeed, levels of the ACC regulatory enzyme AMP-activated protein kinase (Table 2) and its phosphorylation status (data not shown) were not affected by TQ treatment.
Fig. 7.
TQ treatment normalizes glucose-stimulated insulin secretion following chronic exposure of β-cells to 25 mM glucose. INS-1 832/13 cells were cultured for period of 48 h in the presence of 11 (control) or 25 mM G [glucose overload (GO)] in the absence or presence of TQ (A) or NSE (4.45% TQ content; B). Following a 2-h preincubation period in the KRB buffer, insulin secretion at 3 and 16 mM G and total insulin content (C and D) were measured as described in the Fig. 1 legend. Data are means ± SE from 3 independent experiments performed in triplicate measurements. *P < 0.05 when GO treatment was compared with corresponding TQ or NSE treatment.
Fig. 8.
TQ regulates levels of malonyl-CoA and acetyl-CoA carboxylase (ACC). INS-1 832/13 cells were cultured for a period of 48 h in complete medium containing 11 (control) or 25 mM G (GO) in the absence or presence of 0.5 μM TQ. Malonyl-CoA (A) and protein levels of ACC, phospho (p)-ACC, malonyl-CoA decarboxylase (MLCYD), pyruvate dehydrogenase (PDH), and β-actin (B and C) were determined as described in materials and methods. A and C: data are means ± SE from 3 independent experiments performed in triplicate measurements (A). *P < 0.05 (A and B). B: Western blot images representative of 3–4 independent experiments are shown. Quantification of Western blots was performed using ImageJ, and the level of ACC phosphorylation was normalized to total protein ACC level. Results are presented as fold change in ACC phosphorylation vs. untreated control at 11 mM G.
Table 2.
Effect of TQ on gene expression following chronic exposure (72 h) of INS 832/13 cells to 11 or 25 mM glucose
| Gene | 11G | 11G/0.1 TQ | 11G/0.5 TQ | 25G | 25G/0.1 TQ | 25G/0.5 TQ |
|---|---|---|---|---|---|---|
| ACSL6 | 1.00 ± 0.29 | 1.07 ± 0.29 | 0.91 ± 0.21 | 0.8 ± 0.06 | 0.55 ± 0.1 | 0.65 ± 0.11 |
| PK | 1.00 ± 0.45 | 1.04 ± 0.22 | 1.04 ± 0.37 | 0.89 ± 0.14 | 1.39 ± 0.4 | 0.90 ± 0.13 |
| ChREBP2 | 1.00 ± 0.46 | 1.19 ± 0.33 | 1.25 ± 0.46 | 1.07 ± 0.05 | 0.90 ± 0.30 | 0.80 ± 0.17 |
| GPD1 | 1.00 ± 0.36 | 1.23 ± 0.57 | 0.95 ± 0.38 | 1.10 ± 0.48 | 1.82 ± 0.42 | 1.48 ± 0.16 |
| GAPDH | 1.00 ± 0.12 | 0.98 ± 0.14 | 1.16 ± 0.22 | 1.25 ± 0.23 | 0.91 ± 0.19 | 1.00 ± 0.27 |
| ALDH2 | 1.00 ± 0.23 | 1.43 ± 0.30 | 1.21 ± 0.56 | 2.72 ± 1.00* | 1.33 ± 0.31 | 1.58 ± 0.52 |
| AMPKα1 | 1.00 ± 0.35 | 1.10 ± 0.35 | 0.91 ± 0.14 | 0.95 ± 0.03 | 1.06 ± 0.21 | 0.99 ± 0.33 |
| AMPKβ1 | 1.00 ± 0.12 | 1.04 ± 0.11 | 1.06 ± 0.20 | 0.69 ± 0.14 | 0.84 ± 0.10 | 0.69 ± 0.05 |
| AMPKγ1 | 1.00 ± 0.11 | 1.02 ± 0.05 | 1.06 ± 0.11 | 0.83 ± 0.11 | 0.99 ± 0.05 | 0.88 ± 0.11 |
| ACC | 1.00 ± 0.48 | 0.58 ± 0.11 | 0.60 ± 0.16 | 2.25 ± 0.78 | 1.61 ± 0.55 | 0.98 ± 0.20 |
| FASN | 1.00 ± 0.37 | 1.01 ± 0.41 | 1.07 ± 0.12 | 1.77 ± 0.24 | 0.99 ± 0.24 | 1.01 ± 0.25 |
| FABP3 | 1.00 ± 0.22 | 1.06 ± 0.25 | 1.07 ± 0.23 | 2.07 ± 0.63* | 1.1 ± 0.07 | 1.57 ± 0.38 |
| FABP5 | 1.00 ± 0.27 | 0.99 ± 0.17 | 0.98 ± 0.33 | 1.98 ± 0.63* | 1.92 ± 0.42 | 1.43 ± 0.38 |
Values are means ± SE.
11G, 11 mM glucose; 25G, 25 mM glucose; ACSL6, acetyl-CoA synthase 6; PK, pyruvate kinase; ChREBP2, carbohydrate-responsive element-binding protein 2; GPD1, glycerol phosphate dehydrogenase 1; ALDH2, aldehyde dehydrogenase 2 (mitochondrial); AMPK, AMP-activated protein kinase; ACC, acetyl-CoA carboxylase, FASN, fatty acid synthase; FABP, fatty acid-binding protein.
P < 0.05, significant differences from 11G control as determined by 1-way ANOVA, followed by postanalysis comparison with the 11G control group.
Glucose-derived malonyl-CoA inhibits carnitine palmitoyltransferase 1, which regulates the transport of fatty acids into the mitochondria for their oxidation. We measured glucose and palmitate oxidation under acute and chronic TQ (0.5 μM) exposure in INS-1 832/13 under control conditions or under chronic GO. Under acute TQ exposure, where TQ was present only during the glucose and palmitate stimulation, glucose oxidation was enhanced (Fig. 9A). Under chronic exposure, where cells were cultured in the presence of TQ for 48 h and TQ was removed during the stimulatory period, palmitate oxidation, but not glucose oxidation, was enhanced (Fig. 9, B and C). The acute TQ-dependent enhancement of glucose oxidation is consistent with TQ-dependent lowering of the NADH/NAD+ ratio to promote glycolytic flux via the provision of NAD+ for the glycolytic glyceraldehyde-3-phosphate dehydrogenase. TQ-dependent enhancement of palmitate oxidation (which is depressed under acute 16 mM glucose exposure and also following GO treatment) is consistent with the TQ-dependent lowering of malonyl-CoA levels. Consistent with the TQ stimulatory effect on fatty acid oxidation and inhibition of fatty acid esterification to triglycerides (Fig. 9D), TQ also decreased the GO-dependent elevation in FAS and FABPs as well as aldehyde dehydrogenase (Table 2), an enzyme traditionally known for its role in the detoxification of reactive aldehydes (57) but recently suggested to display broader function in regulating intermediary metabolism (66).
Fig. 9.
TQ effect on glucose and fatty acid oxidation and triglyceride content. INS-1 832/13 cells were either untreated (to determine the acute effect of TQ on glucose oxidation; A) or cultured for a period of 48 h in complete medium containing 11 (control) or 25 mM G (to determine the chronic effect of TQ on glucose oxidation, palmitate oxidation, and triglyceride synthesis; B, C, and D, respectively). Glucose and palmitate oxidation and determination of triglyceride content were performed as described in materials and methods. Data are means ± SE from 2 independent experiments performed in quadruplicate measurements. *P < 0.05 when compared with corresponding TQ-untreated controls at 3 or 16 mM G.
DISCUSSION
Pancreatic β-cells function as glucose sensors to ensure adequate insulin output in response to incremental postprandial increases in the blood glucose level. To facilitate this function, glucose is rapidly equilibrated across the β-cell plasma membrane by high-capacity GLUT2 transporters (41) and undergoes metabolism via both cataplerotic and anaplerotic routes to produce ATP and other metabolic factors that mediate GSIS (35–37, 40, 41). Efficient glycolysis is central to β-cell metabolism and GSIS (16). Under physiological glucose levels, more than 90% of nearly all incoming glucose undergoes glycolysis and is oxidized to CO2 (53), which enables β-cells to function as glucose sensors (reviewed in Ref. 39).
Reoxidation of glycolytically derived NADH, which is crucial to the maintenance of the glycolytic flux, is accomplished by mitochondrial shuttles and by the induction of the PMET pathway, which transfers electrons from cytosolic NAD(P)H across the plasma membrane (15). Our previous work demonstrated that in β-cells, PMET activity is increased in response to an increase in glucose and when mitochondrial shuttle activity is inhibited (22). However, under exposure to chronically elevated levels of glucose, these pathways are not sufficient to accommodate NADH reoxidation (22, 65). This is shown by the accumulation of cytosolic NADH (24), enhanced lipid accumulation (47), and subsequent impairment in β-cell function and survival under glucose overload (reviewed in Refs. 6 and 58). Here, we have provided a comprehensive evaluation of the antidiabetic action of TQ on INS-1 832/13 cells and isolated mouse islets by examining the effects of TQ on several pathways relevant to GSIS.
First, we have explored an alternative pathway for NADH reoxidation and its pharmacological manipulation by the redox cycling agent TQ. Our previous work demonstrated that treatment of β-cells with the synthetic redox cycling agent menadione provided an alternate mechanism to alleviate NADH accumulation in the β-cells (26); by consuming NADH via redox cycling, menadione is enzymatically reduced to a semiquinone, and subsequently, it reacts chemically with oxygen to regenerate the parent compound (26). However, menadione exhibits significant toxicity mediated by direct oxidation of protein thiols (14), direct arylation (20), and ERK phosphorylation (44). In the current work, we investigated a natural dietary quinone compound, TQ, that retains the ability to decrease cellular reductive poise but lacks cytotoxic properties even at doses exceeding severalfold those that were utilized in this study (1). TQ is present at high levels in Nigella sativa (black cumin) seeds, and traditional medicinal practices use Nigella sativa to treat diabetes (9). Indeed, one published study demonstrated that TQ doses as high as 0.03% in drinking water (corresponding to 90 mg·kg body wt−1·day−1) for 90 days showed no toxicity but did cause a significant decrease in fasting plasma glucose (3). The resulting calculated whole body concentrations of 0.751 (according to a 1-compartment model) or 9.39 mM (assuming distribution entirely to the blood) are well above the concentrations applied in vitro in our study.
TQ exhibited several notable differences from that of menadione. First, the more negative redox potential of TQ (−589 vs. −206 mV for menadione) indicates that the reduction of TQ is less favorable than that for menadione, suggesting that this compound may have a lower capacity to undergo redox cycling (8). Indeed, our published data show that, at all concentrations tested, the TQ-dependent production of H2O2 is lower than that for menadione (see the third figure in Ref. 26). Second, whereas the redox cycling of menadione increased in response to an increase in glucose concentrations, the redox cycling of TQ decreased in response to increasing glucose concentrations (Fig. 4, C and D). Both cytosolic and mitochondrial fractions of INS-1 832/13 cells supported NADH and NADPH-dependent redox cycling of TQ (Table 1), suggesting the involvement of multiple oxidoreductase enzymes in this process. These data also showed that, despite the largely negative redox potential, TQ was capable of undergoing redox cycling in vitro in a manner similar to that of menadione. Although a similar group of oxidoreductases may facilitate redox cycling of both quinones (due to the similar Km and Vmax values for menadione and TQ for both NADH and NADPH), differences in the cellular localization of these quinones or selective use of intracellular nicotinamide pools may be responsible for the observed lack of glucose dependency of TQ. The detailed mechanism(s) responsible for the differences in glucose-stimulated redox cycling and subcellular localization of these compounds requires further investigation.
Lowering of the cellular reductive poise via lowering of the NAD(P)H/NAD(P)+ ratio is the result of TQ-dependent redox cycling. A lowered NADH/NAD+ ratio may sensitize the β-cell's oxidative metabolic pathway by increasing substrate flux to the mitochondria, as shown by the enhanced glucose-dependent ATP/ADP ratio (Fig. 6), GSIS (Fig. 2, 3, and 7), and increased glucose oxidation in the presence of TQ (Fig. 9A). Redox cycling of TQ may also slow NADH accumulation in the mitochondrial matrix and the resulting excessive formation of the reactive oxygen intermediates inside the mitochondria via complex I (42), which occurs in β-cells chronically exposed to high levels of glucose, a condition that can cause glucotoxicity (46, 56). Indeed, increased oxidation of the reduced NADH pool in mitochondria by expression of a yeast NADH dehydrogenase has been shown to decrease superoxide production in mammalian mitochondria (54).
The notion that some quinones may be beneficial to cellular health and function is in agreement with previous reports demonstrating that TQ, at concentrations <5.0 μM, mitigated oxidative and metabolic stress as well as the suppressive effect of nelfinavir (an anti-HIV agent) on GSIS in pancreatic β-cells (10). Furthermore, in vivo application of the quinone β-lapachone improved energy metabolism and fatty acid oxidation in peripheral tissues of rodents with metabolic syndrome (30). The reported stimulatory effect of the quinone β-lapachone on fatty acid oxidation (30) is in agreement with our data demonstrating the regulatory role of TQ on the ACC, malonyl-CoA formation, and fatty acid oxidation and triglyceride synthesis under glucose overload (Figs. 8 and 9 and Table 2). Chronic glucose excess has been proposed to impair glucose sensitivity and GSIS via malonyl-CoA-dependent increased provision of glucose carbon for fatty acid synthesis and lipid deposition via inhibition of β-oxidation of fatty acids (13, 23, 46, 52, 59–62). Here, we demonstrate that TQ decreases glucose-dependent formation of malonyl-CoA via regulation of ACC, whereas it enhances fatty acid oxidation and consequently decreases its esterification to form triglycerides. These in vitro data are supported by our ongoing in vivo study in which TQ administration (10 mg·kg body wt−1·day−1) ameliorated the weight gain and fat accumulation in mice fed a high-fat diet (Heart E and Gray J, unpublished data). Furthermore, TQ decreased expression of FAS, FABP3, and FABP5 (31), whereas it did not significantly affect genes of carbohydrate metabolism (pyruvate kinase, glycerol phosphate dehydrogenase, and glyceraldehyde-3-phosphate dehydrogenase). The precise molecular mechanisms by which TQ regulates ACC, as well as aldehyde dehydrogenase, an enzyme traditionally known to play a key role in reactive aldehyde removal (12), require further investigation.
In summary, we have demonstrated that NSE and TQ applied at μM to nM doses can enhance the sensitivity of β-cell metabolic pathways and GSIS under normal conditions as well as under glucose overload. Higher TQ doses (5 μM) were required to elicit enhanced GSIS response, and H2O2, a previously proposed mediator of GSIS, was likely responsible. However, under chronic TQ treatment, where lower TQ doses (100–500 nM) were used and shown to be effective to ameliorate impairment associated with chronic glucose overload, H2O2 production is negligible. Rather, increased fuel oxidation and decrease in glucose-dependent triglyceride content is responsible for the TQ-dependent effect at nM doses and is at least in part mediated by the ability of TQ to regulate malonyl-CoA and ACC.
GRANTS
The work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-098747 and American Diabetes Association Grant No. 7-12-BS-073 (E. A. Heart).
DISCLOSURES
J. P. Gray is a Professor at the US Coast Guard Academy. The views presented here are his own and not necessarily those of the Academy or the US Government. The authors report no competing interests.
AUTHOR CONTRIBUTIONS
J.P.G. and E.A.H. conception and design of research; J.P.G., D.Z.B., T.Y., N.S., R.R., R.F., and E.A.H. performed experiments; J.P.G., D.Z.B., and E.A.H. analyzed data; J.P.G. and E.A.H. interpreted results of experiments; J.P.G. and E.A.H. prepared figures; J.P.G. and E.A.H. drafted manuscript; J.P.G. and E.A.H. edited and revised manuscript; J.P.G. and E.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Christopher Benton and Malcolm Johns for their donation of a PCR machine and other equipment via the Department of Homeland Security Homeland Defense Equipment Reuse program to support Cadet research at the US Coast Guard Academy (J. P. Gray). We thank Sarah Carter, recipient of the Biological Discovery in Woods Hole Research Experience for Undergraduate Program, for helpful technical assistance. We are forever indebted to the intellectual input of the late Prof. M. Meow and Prof. L. Dracek for their relentless support in the preparation of this manuscript.
REFERENCES
- 1.Al-Ali A, Alkhawajah AA, Randhawa MA, Shaikh NA. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad 20: 25–27, 2008. [PubMed] [Google Scholar]
- 2.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17: 299–305, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Badary OA, Al-Shabanah OA, Nagi MN, Al-Bekairi AM, Elmazar MM. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res 44: 56–61, 1998. [Google Scholar]
- 4.Benhaddou-Andaloussi A, Martineau L, Vuong T, Meddah B, Madiraju P, Settaf A, Haddad PS. The In Vivo Antidiabetic Activity of Nigella sativa Is Mediated through Activation of the AMPK Pathway and Increased Muscle Glut4 Content. Evid Based Complement Alternat Med 2011: 538671, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benhaddou-Andaloussi A, Martineau LC, Vallerand D, Haddad Y, Afshar A, Settaf A, Haddad PS. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab 12: 148–157, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Bensellam M, Laybutt DR, Jonas JC. The molecular mechanisms of pancreatic beta-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol 364: 1–27, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol 13: 135–160, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Butler J, Hoey BM. The one-electron reduction potential of several substrates can be related to their reduction rates by cytochrome P-450 reductase. Biochim Biophys Acta 1161: 73–78, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Butt MS, Sultan MT. Nigella sativa: reduces the risk of various maladies. Crit Rev Food Sci Nutr 50: 654–665, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Chandra S, Mondal D, Agrawal KC. HIV-1 protease inhibitor induced oxidative stress suppresses glucose stimulated insulin release: protection with thymoquinone. Exp Biol Med (Maywood) 234: 442–453, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Chandra S, Murthy SN, Mondal D, Agrawal KC. Therapeutic effects of Nigella sativa on chronic HAART-induced hyperinsulinemia in rats. Can J Physiol Pharmacol 87: 300–309, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev 94: 1–34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen K, Jin P, He HH, Xie YH, Xie XY, Mo ZH. Overexpression of Insig-1 protects beta cell against glucolipotoxicity via SREBP-1c. J Biomed Sci 18: 57, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comporti M. Glutathione depleting agents and lipid peroxidation. Chem Phys Lipids 45: 143–169, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Crane FL, Low H, Morre DJ. Historical Perspective. In: Oxidoreduction at the Plasma Membrane: Relation to Growth and Transport, edited by Crane FL, Morre DJ, and Low H. Boca Raton, FL: CRC, 1990, p. 1–27. [Google Scholar]
- 16.Deeney JT, Prentki M, Corkey BE. Metabolic control of beta-cell function. Semin Cell Dev Biol 11: 267–275, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Dicker E, Cederbaum AI. Requirement for iron for the production of hydroxyl radicals by rat liver quinone reductase. J Pharmacol Exp Ther 266: 1282–1290, 1993. [PubMed] [Google Scholar]
- 18.El-Dakhakhny M, Mady N, Lembert N, Ammon HP. The hypoglycemic effect of Nigella sativa oil is mediated by extrapancreatic actions. Planta Med 68: 465–466, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, Kasai H, Yazaki Y, Kadowaki T. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283: 981–985, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Gant TW, Rao DN, Mason RP, Cohen GM. Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem Biol Interact 65: 157–173, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Ghosheh OA, Houdi AA, Crooks PA. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.). J Pharm Biomed Anal 19: 757–762, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Gray JP, Eisen T, Cline GW, Smith PJ, Heart E. Plasma membrane electron transport in pancreatic β-cells is mediated in part by NQO1. Am J Physiol Endocrinol Metab 301: E113–E121, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmon JS, Gleason CE, Tanaka Y, Poitout V, Robertson RP. Antecedent hyperglycemia, not hyperlipidemia, is associated with increased islet triacylglycerol content and decreased insulin gene mRNA level in Zucker diabetic fatty rats. Diabetes 50: 2481–2486, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Hayden MR, Tyagi SC. Islet redox stress: the manifold toxicities of insulin resistance, metabolic syndrome and amylin derived islet amyloid in type 2 diabetes mellitus. JOP 3: 86–108, 2002. [PubMed] [Google Scholar]
- 25.Heart E, Cline GW, Collis LP, Pongratz RL, Gray JP, Smith PJ. Role for malic enzyme, pyruvate carboxylation, and mitochondrial malate import in glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 296: E1354–E1362, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heart E, Palo M, Womack T, Smith PJ, Gray JP. The level of menadione redox-cycling in pancreatic beta-cells is proportional to the glucose concentration: role of NADH and consequences for insulin secretion. Toxicol Appl Pharmacol 258: 216–225, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedeskov CJ, Capito K, Thams P. Cytosolic ratios of free [NADPH]/[NADP+] and [NADH]/[NAD+] in mouse pancreatic islets, and nutrient-induced insulin secretion. Biochem J 241: 161–167, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol 228: 121–128, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Holmgren A, Johansson C, Berndt C, Lonn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans 33: 1375–1377, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, Yoo SK, Park MK, Kwak TH, Kho YL, Han J, Choi HS, Lee SH, Kim JM, Lee I, Kyung T, Jang C, Chung J, Kweon GR, Shong M. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes 58: 965–974, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyder A, Zenhom M, Klapper M, Herrmann J, Schrezenmeir J. Expression of fatty acid binding proteins 3 and 5 genes in rat pancreatic islets and INS-1E cells: regulation by fatty acids and glucose. Islets 2: 174–184, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxid Redox Signal 9: 931–942, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53: 1019–1032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khader M, Bresgen N, Eckl PM. In vitro toxicological properties of thymoquinone. Food Chem Toxicol 47: 129–133, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab 5: 253–264, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leloup C, Casteilla L, Carriere A, Galinier A, Benani A, Carneiro L, Penicaud L. Balancing mitochondrial redox signaling: a key point in metabolic regulation. Antioxid Redox Signal 14: 519–530, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 58: 673–681, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald MJ. Stimulation of insulin release from pancreatic islets by quinones. Biosci Rep 11: 165–170, 1991. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 360: 2211–2225, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402: 685–689, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev 2: 163–214, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neter J, Wasserman W, Kutner M. Applied Linear Statistical Models. Chicago, IL: Irwin, 1990. [Google Scholar]
- 44.Osada S, Sakashita F, Hosono Y, Nonaka K, Tokuyama Y, Tanaka H, Sasaki Y, Tomita H, Komori S, Matsui S, Takahashi T. Extracellular signal-regulated kinase phosphorylation due to menadione-induced arylation mediates growth inhibition of pancreas cancer cells. Cancer Chemother Pharmacol 62: 315–320, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56: 1783–1791, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 143: 339–342, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes 51, Suppl 3: S405–S413, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 267: 5802–5810, 1992. [PubMed] [Google Scholar]
- 49.Rashba-Step J, Cederbaum AI. Generation of reactive oxygen intermediates by human liver microsomes in the presence of NADPH or NADH. Mol Pharmacol 45: 150–157, 1994. [PubMed] [Google Scholar]
- 50.Rchid H, Chevassus H, Nmila R, Guiral C, Petit P, Chokaïri M, Sauvaire Y. Nigella sativa seed extracts enhance glucose-induced insulin release from rat-isolated Langerhans islets. Fundam Clin Pharmacol 18: 525–529, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 53, Suppl 1: S119–S124, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Roche E, Farfari S, Witters LA, Assimacopoulos-Jeannet F, Thumelin S, Brun T, Corkey BE, Saha AK, Prentki M. Long-term exposure of beta-INS cells to high glucose concentrations increases anaplerosis, lipogenesis, and lipogenic gene expression. Diabetes 47: 1086–1094, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem 272: 18572–18579, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Seo BB, Marella M, Yagi T, Matsuno-Yagi A. The single subunit NADH dehydrogenase reduces generation of reactive oxygen species from complex I. FEBS Lett 580: 6105–6108, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Slater EC, de Vijlder JJ, Boers W. The binding of NAD + and NADH to glyceraldehydephosphate dehydrogenase. Vitam Horm 28: 315–327, 1970. [DOI] [PubMed] [Google Scholar]
- 56.Teodoro JS, Gomes AP, Varela AT, Duarte FV, Rolo AP, Palmeira CM. Uncovering the beginning of diabetes: the cellular redox status and oxidative stress as starting players in hyperglycemic damage. Mol Cell Biochem 376: 103–110, 2013. [DOI] [PubMed] [Google Scholar]
- 57.Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics 2: 138–143, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallace M, Whelan H, Brennan L. Metabolomic analysis of pancreatic beta cells following exposure to high glucose. Biochim Biophys Acta 1830: 2583–2590, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci 118: 3905–3915, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Maechler P, Antinozzi PA, Herrero L, Hagenfeldt-Johansson KA, Bjorklund A, Wollheim CB. The transcription factor SREBP-1c is instrumental in the development of beta-cell dysfunction. J Biol Chem 278: 16622–16629, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Weir GC. Non-insulin-dependent diabetes mellitus: interplay between B-cell inadequacy and insulin resistance. Am J Med 73: 461–464, 1982. [DOI] [PubMed] [Google Scholar]
- 62.Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes 50, Suppl 1: S154–S159, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab 11, Suppl 4: 82–90, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Witschi H, Kacew S, Hirai KI, Cote MG. In vivo oxidation of reduced nicotinamide-adenine dinucleotide phosphate by paraquat and diquat in rat lung. Chem Biol Interact 19: 143–160, 1977. [DOI] [PubMed] [Google Scholar]
- 65.Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res 2014: 137919, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Babcock SA, Hu N, Maris JR, Wang H, Ren J. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3beta and mitochondrial function. BMC Med 10: 40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]