Abstract
Inflammation contributes to ANG II-associated impairment of renal autoregulation and microvascular P2X1 receptor signaling, but its role in renal autoregulation in mineralocorticoid-induced hypertension is unknown. Autoregulatory behavior was assessed using the blood-perfused juxtamedullary nephron preparation. Hypertension was induced in uninephrectomized control rats (UNx) by subcutaneous implantation of a DOCA pellet plus administration of 1% NaCl in the drinking water (DOCA-salt) for 3 wk. DOCA-salt rats developed hypertension that was unaltered by anti-inflammatory treatment with pentosan polysulfate (DOCA-salt+PPS) but was suppressed with “triple therapy” (hydrochlorothiazide, hydralazine, and reserpine; DOCA-salt+TTx). Baseline arteriolar diameters were similar across all groups. UNx rats exhibited pressure-dependent vasoconstriction with diameters declining to 69 ± 2% of control at 170 mmHg, indicating intact autoregulation. DOCA-salt treatment significantly blunted this pressure-mediated vasoconstriction. Diameters remained between 91 ± 4 and 98 ± 3% of control over 65–170 mmHg, indicating impaired autoregulation. In contrast, pressure-mediated vasoconstriction was preserved in DOCA-salt+PPS and DOCA-salt+TTx rats, reaching 77 ± 7 and 75 ± 3% of control at 170 mmHg, respectively. ATP is required for autoregulation via P2X1 receptor activation. ATP- and β,γ-methylene ATP (P2X1 receptor agonist)-mediated vasoconstriction were markedly attenuated in DOCA-salt rats compared with UNx (P < 0.05), but significantly improved by PPS or TTx (P < 0.05 vs. DOCA-salt) treatment. Arteriolar responses to adenosine and UTP (P2Y2 receptor agonist) were unaffected by DOCA-salt treatment. PPS and TTx significantly reduced MCP-1 and protein excretion in DOCA-salt rats. These results support the hypothesis that hypertension triggers inflammatory cascades but anti-inflammatory treatment preserves renal autoregulation in DOCA-salt rats, most likely by normalizing renal microvascular reactivity to P2X1 receptor activation.
Keywords: inflammation, afferent arteriole, hypertension, purinoceptors, triple therapy
normal kidneys possess an excellent capability to maintain stable renal blood flow and glomerular filtration rate by precisely adjusting preglomerular microvascular resistance in response to fluctuations in arterial pressure. Impairment of this intrinsic autoregulatory function would permit inappropriate transmission of elevated systemic arterial pressure to the glomerulus, leading to higher glomerular capillary hydrostatic pressure. Continuous transmission of high blood pressure to glomeruli could accelerate progression to glomerulosclerosis, proteinuria, and nephron loss. Studies have established that hypertensive patients with impaired renal autoregulatory capability are more likely to develop chronic kidney disease (1, 40). Protecting the kidney from autoregulatory dysfunction in hypertensive subjects would be an important intervention for prevention of glomerular hypertension.
Accumulating evidence suggests an important mechanistic linkage between inflammation and hypertensive renal injury (8, 18, 42, 46). We and others previously demonstrated that chronic ANG II infusion led to increases in renal interstitial fluid ATP concentrations and renal microvascular dysfunction characterized by renal microvascular hypertrophy, impaired renal autoregulation, and impaired P2X1 receptor signaling (9, 12, 14, 15, 24, 39, 53). Treatment with the nonselective anti-inflammatory agent pentosan polysulfate (PPS) or a selective lymphocyte proliferation inhibitor, mycophenolate mofetil (MMF), reduced kidney injury, preserved renal autoregulation, and normalized afferent arteriole reactivity to P2X1 receptor activation despite persistent hypertension (9, 14, 15), suggesting that locally or systemically released inflammatory factors play pivotal roles in renal microvascular dysfunction. ANG II is a potent vasoconstrictor, but it is also a potent proinflammatory mediator (18, 46). The question of whether inflammation-mediated hypertensive renal microvascular dysfunction is the result of elevated arterial pressure or a direct effect of ANG II has not been solved. To further investigate the contribution of inflammation in hypertension-associated renal vascular dysfunction, we determined the impact of anti-inflammatory treatment with PPS on renal microvascular function in deoxycorticosterone acetate (DOCA)-salt hypertensive rats, which is a low-renin/low-ANG II model of salt-sensitive hypertension.
Administration of DOCA plus a high-salt diet (DOCA-salt) and unilateral nephrectomy lead to hypertension via a salt-sensitive volume-overload and low-renin/low-ANG II mechanism (10). Early studies showed impaired renal autoregulation in rats (16, 19, 36) and in dogs (26) after DOCA-salt treatment. The mechanism responsible for the autoregulatory impairment is not well understood. The current study was designed to test the hypothesis that autoregulatory behavior and P2X1 receptor reactivity of afferent arterioles in DOCA-salt hypertensive rats is preserved with anti-inflammatory treatment or with suppression of hypertension development. We determined pressure-mediated autoregulatory behavior and P2X1 receptor reactivity in DOCA-salt hypertensive rats treated with PPS or with “triple therapy” (hydrochlorothiazide, hydralazine and reserpine, TTx).
METHODS
Animals
A total of 274 male Sprague-Dawley rats (250–275 g, Charles River Laboratories, Raleigh, NC) were used. All animals were treated according to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at Georgia Regents University or the University of Alabama at Birmingham.
Induction of DOCA-Salt Hypertension
All rats underwent unilateral nephrectomy (UNx; left kidney) under 2% isoflurane anesthesia. DOCA-salt hypertension was induced by implanting a 200-mg time release DOCA pellet (Innovative Research of America, Sarasota, FL) subcutaneously and administering 1% NaCl and 0.2% KCl in the drinking water for 3 wk. Some DOCA-salt rats were simultaneously treated with PPS (100 mg·kg−1·day−1, ELMIRON, Ortho-McNeil Pharmaceutical, Raritan, NJ) in the drinking water based on the previous day's water consumption (DOCA-salt+PPS). To determine whether the autoregulatory impairment arises from elevated arterial pressure, or directly from mineralocorticoid and salt treatment, we added an additional DOCA-salt group that also received “triple therapy” (DOCA-salt+TTx, hydralazine, hydrochlorothiazide, and reserpine at 30, 10, and 0.2 mg·kg−1·day−1, respectively) (Sigma, St. Louis, MO). Dosing was adjusted daily by recording the previous day's water consumption. UNx rats served as controls. All experiments were performed on day 20 or 21 of treatment. Systolic blood pressure (SBP) was monitored by tail-cuff plethysmography (IITC, Woodland Hills, CA) every 6–7 days in all rats.
Metabolic Cage Studies
Some rats were individually housed in metabolic cages for a period of 24 h on day 0 for baseline recording and this was repeated weekly after treatment. Urine (24 h) was collected, and food consumption, water consumption, and body weight were measured.
In Vitro Blood-Perfused Juxtamedullary Nephron Preparation
Juxtamedullary nephron preparation was used to directly assess afferent arteriolar autoregulatory behavior and microvascular reactivity to experimental manipulations as described previously (14, 15, 24, 53). Briefly, two identically treated rats (kidney donor and blood donor) were used for each experiment, and each kidney was used for only one experiment. Kidneys were perfused with the reconstituted blood (hematocrit of ∼33%). The vasoactive compounds tested were delivered to the kidney surface via superfusion of Tyrode's buffer containing 1% bovine serum albumin into the inner cortical surface of the kidney. The image of the kidney was displayed on a video monitor via a high-resolution NC-70 Newvicon video camera (DAGE-MTI) and recorded on a DVD for later analysis. Inner arteriolar diameter was measured every 12 s at a single site using a calibrated image-shearing monitor (model 908, Vista Electronics, Valencia, CA) and was calculated from the average of all diameter measurements collected during the final 2 min of each treatment period. Kidney weight data were obtained from decapsulated kidneys collected from the identically prepared blood donor rats. All data were pooled from the blood donors that were used for autoregulatory assessment, ATP, and β,γ-methylene ATP concentration response studies.
Experimental Design
Autoregulatory behavior of afferent arterioles.
Briefly, kidney perfusion was set at 100 mmHg after an equilibration period (>15 min). After a 5-min control period to establish baseline diameter, autoregulatory responses of afferent arterioles were assessed by measuring arteriolar diameter at a single site when perfusion pressure was reduced to 65 mmHg and then increased to 170 mmHg in 15-mmHg increments at 5-min intervals. At the end of each protocol, perfusion pressure was returned to 100 mmHg and norepinephrine (10−7 mol/l) was applied to examine arteriolar reactivity. Four groups (UNx, DOCA-salt, DOCA-salt+PPS, and DOCA-salt+TTx) were studied (n = 6–7/group).
Afferent arteriolar response to ATP.
ATP, an endogenous P2 receptor ligand, is involved in regulating afferent arteriolar tone and autoregulatory behavior (20). In separate groups, arteriolar reactivity to ATP was assessed in UNx, DOCA-salt, DOCA-salt+PPS, and DOCA-salt+TTx groups (n = 6/group). After a control period, kidneys were superfused with increasing concentrations of ATP from 10−8 to 10−4 mol/l at 5-min intervals while perfusion pressure was maintained at 100 mmHg.
Afferent arteriolar response to a selective P2X1 receptor agonist, β,γ-methylene ATP.
P2X1 receptors are prominently expressed in afferent arterioles, and their activation is postulated to mediate renal autoregulation (20, 53). β,γ-Methylene ATP is a selective P2X1 and P2X3 receptor agonist. Since P2X3 receptor expression is barely detected in preglomerular microvessels (17, 30), the arteriolar response to β,γ-methylene ATP is presumably via stimulating P2X1 receptors. Similar to the ATP concentration-response protocol, after a control period kidneys were superfused with increasing concentrations of β,γ-methylene ATP from 10−8 to 10−4 mol/l in UNx, DOCA-salt, DOCA-salt+PPS, and DOCA-salt+TTx groups (n = 6/group).
Afferent arteriolar response to the P2Y2 receptor agonist UTP.
Since activation of P2Y2 receptors by UTP causes potent afferent arteriolar vasoconstriction (20, 53), the blunted ATP-mediated vasoconstriction in DOCA-salt rats could reflect P2X and/or P2Y receptor signaling. Therefore, arteriolar reactivity to UTP was determined using increasing concentrations of UTP from 10−8 to 10−4 mol/l at 5-min intervals while perfusion pressure was maintained at 100 mmHg (n = 6/group).
Afferent arteriolar response to the P1 receptor agonist adenosine.
Adenosine is also suggested to mediate renal autoregulation via A1 receptors in afferent arterioles (5, 47). Similar to the UTP concentration response protocol, arteriolar reactivity to adenosine was determined using 10−8 to 10−4 mol/l adenosine at 5-min intervals while perfusion pressure was maintained at 100 mmHg (n = 6–7/group).
Monocyte Chemoattractant Protein-1 and Proteinuria
For evaluation of kidney injury and inflammatory factors, a weekly 24-h urine was collected before and after uninephrectomy in UNx, DOCA-salt, DOCA-salt+PPS, and DOCA-salt+TTx groups once a week (n = 7–12/group). Plasma and urinary monocyte chemoattractant protein-1 (MCP-1) concentration was measured by ELISA (BD Biosciences, San Jose, CA). Urinary protein was measured by Coomassie blue protein assay (Bio-Rad Laboratories, Hercules, CA).
Statistical Analysis
All values are expressed as means ± SE. Within-group analysis was conducted using ANOVA for repeated measures followed by post hoc analysis with Dunnett's multiple range test. Significant differences between groups, within each series, were determined using one-way ANOVA and Dunnett's multiple comparison test or Student's t-test where appropriate. P values <0.05 were considered to indicate significant differences.
RESULTS
Metabolic Parameters
Table 1 summarizes physiological parameters collected from metabolic cage studies over the 21-day treatment period. All baseline parameters (day 0) were similar across all groups. Body weight increased slowly in DOCA-salt and DOCA-salt+PPS groups compared with UNx, but there was no significant difference over treatments. Body weight declined initially in DOCA-salt+TTx rats but by day 21 was similar to the DOCA-salt group. Both DOCA-salt and DOCA-salt+TTx groups showed significant declines in food consumption on day 7 compared with day 0. With food intake increased, the body weights also climbed and showed no difference in UNx or DOCA-salt+PPS groups on day 21 (P > 0.05). Water intake and urine excretion were markedly increased in DOCA-salt and DOCA-salt+PPS rats over the 21-day treatment period compared with UNx rats (P < 0.05). Water intake and urine excretion were significantly lower in DOCA-salt+TTx rats than in DOCA-salt or DOCA-salt+PPS rats (P < 0.05) for days 7–21 of DOCA-salt treatment; however, compared with UNx rats, DOCA-salt+TTx rats had significant increases in urine output on days 14 and 21 (P < 0.05).
Table 1.
Comparison of metabolic parameters
| Day 0 |
Day 7 |
Day 14 |
Day 21 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UNx | DOCA | DOCA+PPS | DOCA+TTx | UNx | DOCA | DOCA+PPS | DOCA+TTx | UNx | DOCA | DOCA+PPS | DOCA+TTx | UNx | DOCA | DOCA+PPS | DOCA+TTx | |
| Body weight, g | 278 ± 4 | 288 ± 9 | 299 ± 6 | 291 ± 5 | 308 ± 4* | 294 ± 12 | 311 ± 9& | 240 ± 11*†# | 336 ± 6* | 315 ± 15* | 322 ± 9*& | 256 ± 12†# | 358 ± 9* | 321 ± 21 | 328 ± 11* | 302 ± 21 |
| Food intake, g | 27 ± 2 | 26 ± 2 | 25 ± 1 | 26 ± 1 | 21 ± 1 | 17 ± 3* | 23 ± 2& | 13 ± 3*# | 23 ± 2 | 22 ± 3 | 22 ± 3 | 21 ± 4 | 21 ± 3 | 25 ± 2 | 21 ± 2 | 31 ± 7 |
| Water intake, ml/day | 39 ± 2 | 43 ± 4 | 37 ± 2 | 40 ± 3 | 35 ± 2 | 138 ± 15*†& | 147 ± 13*†& | 23 ± 6 | 33 ± 3 | 178 ± 13*†& | 160 ± 21*†& | 61 ± 12 | 35 ± 4 | 161 ± 15*†& | 149 ± 21*†& | 38 ± 7# |
| Urine volume, ml/day | 14 ± 1 | 17 ± 3 | 13 ± 1 | 13 ± 1 | 17 ± 1 | 101 ± 15*†& | 113 ± 11*†& | 22 ± 7# | 18 ± 2 | 149 ± 12*†& | 130 ± 18*†& | 46 ± 8*†# | 19 ± 2 | 134 ± 15*†& | 122 ± 18*†& | 36 ± 8†# |
Values are means ± SE; n = 12, 9, 12, and 7 for uninephrectomized (UNx), DOCA plus 1% NaCl in the drinking water (DOCA-salt), DOCA-salt+pentosan polysulfate (PPS), and DOCA-salt+“triple therapy” (hydrochlorothiazide, hydralazine, and reserpine (TTx) rats, respectively. DOCA indicates DOCA-salt.
P < 0.05 vs. day 0 in the same group.
P < 0.05 vs. UNx group during the same collection period.
P < 0.05 vs. DOCA-salt+PPS group during the same collection period.
P < 0.05 vs. DOCA-salt+TTx group during the same collection period.
SBP
Figure 1 shows SBP measured in conscious rats from the metabolic studies. UNx rats had normal SBP throughout the 21-day period and averaged 111 ± 3 to 117 ± 5 mmHg (n = 12) over that period. In contrast, SBP increased significantly from 110 ± 4 to 180 ± 7 mmHg in DOCA-salt rats (n = 9). The magnitude and progression of hypertension were unchanged by PPS treatment (177 ± 3 mmHg, n = 12, P > 0.05 vs. DOCA-salt). DOCA-salt rats receiving TTx remained normotensive, with SBP averaging 131 ± 3 and 121 ± 4 mmHg on day 0 and treatment day 21, respectively (n = 7, P > 0.05).
Fig. 1.
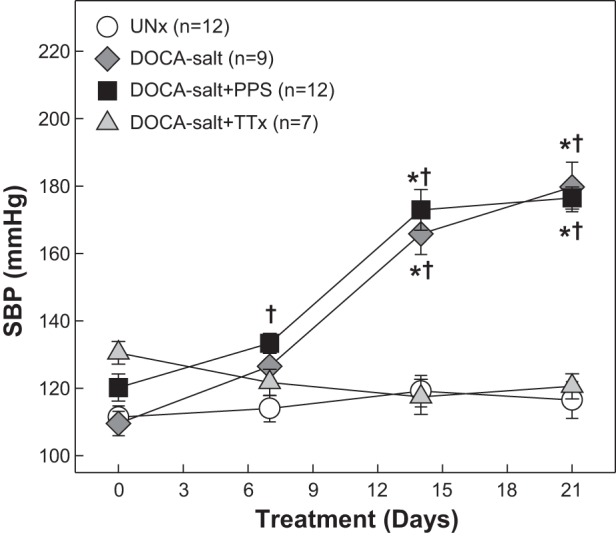
Systolic blood pressure (SBP) in DOCA-salt hypertensive rats. SBP was measured by tail-cuff plethysmography in uninephrectomized rats (UNx; circles), DOCA-salt rats (diamonds), DOCA-salt rats treated with pentosan polysulfate (PPS; DOCA-salt+PPS; 100 mg·kg−1·day−1, squares), and DOCA-salt rats treated with triple therapy (DOCA-salt+TTx; hydralazine, hydrochlorothiazide, and reserpine, triangles) from the metabolic studies. PPS did not alter the progression or magnitude of hypertension in DOCA-salt rats through the 21-day treatment period. TTx treatment suppressed the development of hypertension in DOCA-salt rats. Values are means ± SE. *P < 0.05 vs. SBP on day 0. †P < 0.05 vs. UNx.
Kidney Weights
After 21 days of DOCA-salt treatment, kidney weights obtained from identically treated blood donor rats for autoregulatory assessment, ATP, and β,γ-methylene ATP concentration response studies increased significantly in DOCA-salt (2.77 ± 0.05 g, n = 19) compared with UNx rats (1.77 ± 0.06 g, n = 18, P < 0.05) but were significantly lower in DOCA-salt+PPS rats (2.30 ± 0.07 g, n = 18, P < 0.05 vs. DOCA-salt) and DOCA-salt+TTx rats (2.19 ± 0.09 g, n = 19, P < 0.05 vs. DOCA-salt).
Effect of PPS Treatment on Afferent Arteriolar Autoregulatory Behavior
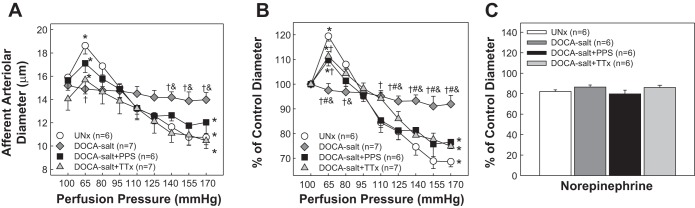
Figure 2 presents autoregulatory behavior of afferent arterioles in response to changes in renal perfusion pressure. The control diameter (Fig. 2A) was similar among UNx, DOCA-salt, DOCA-salt+PPS, and DOCA-salt+TTx and averaged 15.9 ± 0.4, 15.2 ± 0.4, 15.6 ± 0.8, and 14.0 ± 1.0 μm (P > 0.05), respectively. UNx rats exhibited normal autoregulatory responses similar to our previous reports in normal Sprague-Dawley rats (14, 15, 21, 22). The diameter increased to 18.6 ± 0.7 μm (119 ± 2% of control, Fig. 2B, P < 0.05) when perfusion pressure was decreased from 100 to 65 mmHg, and then the diameter decreased in a stepwise manner as perfusion pressure was increased. The arteriole diameter averaged 10.7 ± 0.6 μm or 69 ± 2% of control at 170 mmHg (Fig. 2, P < 0.05). In contrast, DOCA-salt rats exhibited impaired autoregulation as shown by the markedly blunted pressure-mediated vasoconstriction. Increasing perfusion pressure to 170 mmHg only reduced diameters to 92 ± 4% of control (Fig. 2B, P < 0.05 vs. UNx). Simultaneous treatment with DOCA-salt+PPS preserved normal autoregulatory reactivity in DOCA-salt rats. The pressure-diameter relationship in DOCA-salt+PPS rats was similar to that of UNx rats (Fig. 2). The arteriole diameter increased to 110 ± 2% of control (P < 0.05) when perfusion pressure was reduced from 100 to 65 mmHg and then declined to 78 ± 6% of control at 170 mmHg (Fig. 2B, P < 0.05). This response is significantly different from the response in DOCA-salt rats, indicating intact autoregulation in DOCA-salt+PPS rats. Autoregulation was also preserved in DOCA-salt+TTx rats (Fig. 2). The arteriolar diameter increased to 111 ± 2% of control (P < 0.05) as perfusion pressure was decreased from 100 to 65 mmHg and followed by vasoconstriction to 75 ± 3% of control as perfusion pressure was stepped up from 65 to 170 mmHg (P < 0.05). This pressure-diameter profile resembled that in UNx and DOCA-salt+PPS rats (P > 0.05 vs. UNx or DOCA-salt+PPS) but was markedly different from the profile in DOCA-salt alone rats (P < 0.05).
Fig. 2.
Autoregulatory behavior of afferent arterioles in DOCA-salt hypertensive rats. A: afferent arteriolar response to alterations in renal perfusion pressure was measured in UNx (circles), DOCA-salt (diamonds), DOCA-salt+PPS (100 mg·kg−1·day−1, squares), or DOCA-salt+TTx (triangles). B: data are normalized as percentage of the control diameter at 100 mmHg. C: afferent arteriolar response to norepinephrine at the end of each experiment except that one arteriole was not assessed from the DOCA-salt or DOCA-salt+TTx group due to insufficient perfusate blood. Values are means ± SE. *P < 0.05 vs. control diameter in the same group. †P < 0.05 vs. UNx rats at the same perfusion pressure. #P < 0.05 vs. DOCA-salt+PPS at the same perfusion pressure. &P < 0.05 vs. DOCA-salt+TTx at the same perfusion pressure.
When possible, we applied norepinephrine (10−7 mol/l) at the end of the autoregulatory experiments to verify vasoreactivity of afferent arterioles. Data were collected successfully in all groups except for one kidney in both DOCA-salt and DOCA-salt+TTx due to insufficient perfusate blood. As shown in Fig. 2C, norepinephrine-induced vasoconstriction was similar across all groups. The arteriolar diameter decreased to 82 ± 2, 86 ± 2, 80 ± 2, and 86 ± 2% of their respective controls in UNx, DOCA-salt, DOCA-salt+PPS and DOCA-salt+TTx, respectively (P > 0.05), indicating that the contractile apparatus in afferent arterioles is intact in DOCA-salt rats.
Effect of PPS Treatment on Afferent Arteriolar Response to ATP
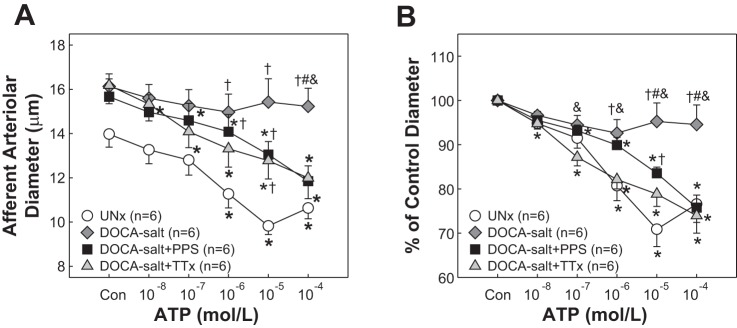
The control diameter of afferent arterioles averaged 14.0 ± 0.6, 16.1 ± 0.6, 15.7 ± 0.8, and 16.2 ± 0.8 μm for UNx, DOCA-salt, DOCA-salt+PPS, and DOCA-salt+TTx (Fig. 3A, P > 0.05), respectively. Superfusion of ATP elicited concentration-dependent arteriolar vasoconstriction in UNx rats. The diameter declined to 13.3 ± 0.6, 12.8 ± 0.7, 11.3 ± 0.6, 9.8 ± 0.4, and 10.6 ± 0.5 (Fig. 3A) in response to successive increases in ATP concentration, corresponding to 95 ± 1, 91 ± 2, 81 ± 3, 71 ± 4, and 77 ± 4% of the control diameter, respectively (Fig. 3B). ATP-induced vasoconstriction was almost completely abolished in DOCA-salt rats with the diameter decreasing to only 97 ± 1, 94 ± 2, 93 ± 3, 95 ± 4 and 95 ± 4% of control over the same concentration range (P < 0.05 vs. UNx). In contrast, ATP-induced vasoconstriction was significantly improved in DOCA-salt+PPS rats as shown by a greater vasoconstriction compared with the responses in DOCA-salt rats (Fig. 3B, P < 0.05), but was not completely normal compared with UNx rats (84 ± 1 vs. 71 ± 4% of the control diameter at 10−5 mol/l of ATP, P < 0.05). Suppression of high blood pressure development with TTx treatment, however, maintained normal vasoconstriction to ATP in DOCA-salt+TTx rats. The maximal vasoconstriction was 74 ± 4% of control at 10−5 mol/l of ATP, similar to in the UNx group (Fig. 3B, P > 0.05) but was significantly greater than the response in DOCA-salt alone rats (P < 0.05).
Fig. 3.
Afferent arteriolar response to ATP in DOCA-salt hypertensive rats. A: afferent arteriolar response to superfusion of ATP was assessed in UNx (circles), DOCA-salt (diamonds), DOCA-salt treated with PPS (DOCA-salt+PPS, 100 mg·kg−1·day−1, squares), or DOCA-salt treated with triple therapy (DOCA-salt+TTx, hydralazine, hydrochlorothiazide, and reserpine, triangles). B: data are normalized as percentage of the control diameter at 100 mmHg. Values are means ± SE. *P < 0.05 vs. control diameter in the same group. †P < 0.05 vs. UNx at the same concentration. #P < 0.05 vs. DOCA-salt+PPS at the same concentration. &P < 0.05 vs. DOCA-salt+TTx at the same concentration.
Effect of PPS Treatment on Afferent Arteriolar Response to β,γ-Methylene ATP
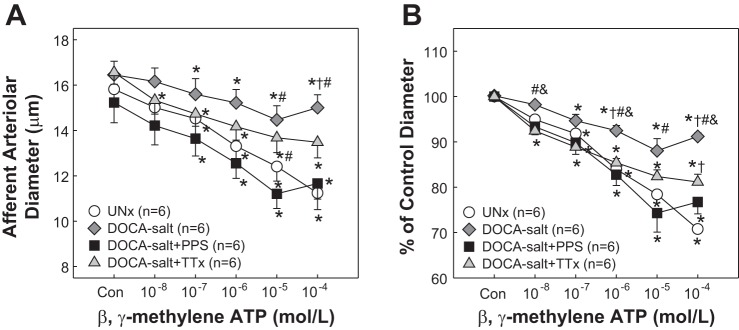
As P2X1 receptor signaling participates in autoregulation, the afferent arteriolar response to the P2X1 receptor agonist β,γ-methylene ATP was assessed. Similar to ATP responses, arterioles from UNx rats showed a concentration-dependent vasoconstriction to β,γ-methylene ATP (Fig. 4). The diameter decreased from 15.8 ± 0.6 to 11.2 ± 0.7 (Fig. 4A), corresponding to 71 ± 2% of control at 10−4 mol/l (Fig. 4B, P < 0.05). β,γ-Methylene ATP-mediated vasoconstriction was markedly blunted in DOCA-salt rats. The diameter decreased to only 88 ± 3% of control at 10−5 mol/l (Fig. 4B, P < 0.05 vs. UNx), but the blunted vasoconstriction was normalized by PPS treatment. The magnitude of vasoconstriction in DOCA+PPS rats was almost identical to the responses in UNx rats. The diameter declined to 74 ± 4 and 77 ± 3% of control at 10−5 and 10−4 mol/l, respectively (Fig. 4B, P > 0.05 vs. UNx). DOCA-salt rats treated with TTx also exhibited a concentration-dependent vasoconstriction to β,γ-methylene ATP that was similar to UNx rats from 10−8 to 10−5 mol/l despite an attenuated vasoconstriction at 10−4 mol/l (Fig. 4B, 81 ± 2%, P < 0.05). In contrast to the response in DOCA-salt alone rats, however, the β,γ-methylene ATP-mediated vasoconstriction was significantly greater in DOCA-salt+TTx rats, with a maximal reduction of diameter at 81 ± 2 vs. 91 ± 2% at 10−4 mol/l in DOCA-salt rats (P < 0.05).
Fig. 4.
Afferent arteriolar response to β,γ-methylene ATP in DOCA-salt hypertensive rats. A: afferent arteriolar response to superfusion of β,γ-methylene ATP was assessed in UNx (circles), DOCA-salt (diamonds), DOCA-salt treated with PPS (DOCA-salt+PPS, 100 mg·kg−1·day−1, squares), or DOCA-salt treated with triple therapy (DOCA-salt+TTx, hydralazine, hydrochlorothiazide, and reserpine, triangles). B: data are normalized as percentage of the control diameter at 100 mmHg. Values are means ± SE. *P < 0.05 vs. control diameter in the same group. †P < 0.05 vs. UNx at the same concentration. #P < 0.05 vs. DOCA-salt+PPS at the same concentration. &P < 0.05 vs. DOCA-salt+TTx at the same concentration.
Afferent Arteriolar Response to P2Y2 and P1 Receptor Activation
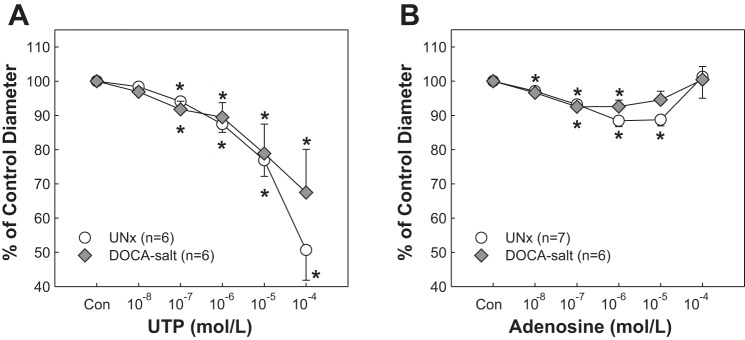
As shown in Fig. 5A, activation of P2Y2 receptors by UTP evoked similar concentration-dependent vasoconstriction of afferent arterioles in both UNx and DOCA-salt rats. The control diameter averaged 15.6 ± 1.0 and 14.8 ± 0.9 μm in UNx and DOCA-salt rats, respectively (P > 0.05). Successive increases in UTP concentration reduced the diameter from 98 ± 1 to 51 ± 9% of control in UNx rats and 97 ± 2 to 57 ± 9% of control in DOCA-salt rats, respectively (P > 0.05). Similar to the response to UTP, arteriolar responses to adenosine (P1 receptor agonist) were also indistinguishable between UNx and DOCA-salt rats (Fig. 5B, P > 0.05).
Fig. 5.
Afferent arteriolar response to UTP and adenosine in DOCA-salt hypertensive rats. A: afferent arteriolar response to superfusion of UTP was assessed in UNx (circles) and DOCA-salt (diamonds). B: afferent arteriolar response to superfusion of adenosine was assessed in UNx (circles) and DOCA-salt (diamonds). All data are expressed as percentage of the control diameter at 100 mmHg. Values are means ± SE. *P < 0.05 vs. control diameter in the same group.
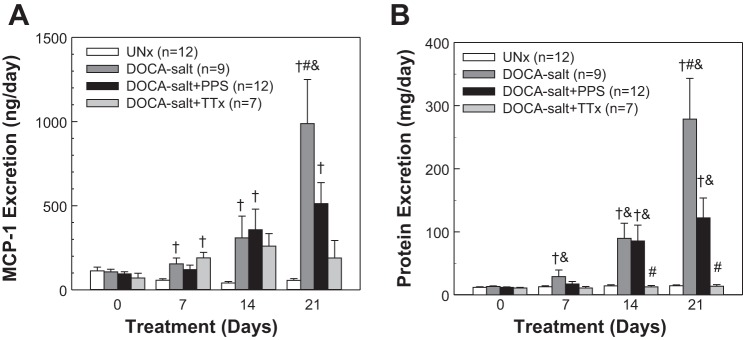
Effect of PPS Treatment on MCP-1 Excretion
Urinary MCP-1 excretion (Fig. 6A) was similar across the four groups on day 0. Compared with UNx rats, MCP-1 excretion was significantly elevated in DOCA-salt rats on day 7 (P < 0.05) and continued to increase through day 21 (988 ± 262 ng/day, P < 0.05). MCP-1 increased significantly to 512 ± 125 ng/day in DOCA-salt+PPS rats on day 21 or ∼50% of that seen in the DOCA-salt group (P < 0.05). MCP-1 excretion was elevated in DOCA-salt+TTx rats on day 7 (190 ± 33 ng/day, P < 0.05 vs. UNx) and then stabilized through day 21. At day 21, MCP excretion was 189 ± 104 ng/day, which was only 19% of that in DOCA-salt rats (P < 0.05). Plasma MCP-1 concentration, however, was not different across the four groups on day 21 (17 ± 1, 20 ± 2, 17 ± 2, and 26 ± 5 ng/ml in UNx, DOCA-salt, DOCA-salt+PPS, and DOCA-salt+TTx rats, respectively, P > 0.05).
Fig. 6.
Monocyte chemoattractant protein-1 (MCP-1) and proteinuria in DOCA-salt hypertensive rats. Urine (24 h) was collected on days 0, 7, 14, and 21. A: MCP-1 excretion. B: protein excretion. Values are means ± SE. †P < 0.05 vs. UNx in the same period of urine collection. #P < 0.05 vs. DOCA-salt+PPS in the same period of urine collection. &P < 0.05 vs. DOCA-salt+TTx in the same period of urine collection.
Effect of PPS Treatment on Proteinuria
Protein excretion was similar in UNx, DOCA-salt, DOCA-salt+PPS, and DOCA-salt+TTx rats and averaged 12 ± 1, 13 ± 1, 12 ± 1, 11 ± 1 mg/day, respectively, on day 0 (Fig. 6B). Protein excretion began to increase in DOCA-salt rats on day 7 and continued to rise over 21 days (279 ± 65 vs. UNx at 15 ± 1 mg/day, P < 0.05). Proteinuria was also increased in DOCA-salt+PPS rats but was significantly alleviated by PPS treatment (122 ± 32 mg/day on day 21, P < 0.05 vs. DOCA-salt). In contrast, protein excretion in DOCA-salt+TTx rats was not different from UNx rats over the 21-day treatment period (14 ± 3 mg/day on day 21, P > 0.05 vs. UNx).
DISCUSSION
The novel findings of the current study demonstrate that anti-inflammatory treatment prevents renal microvascular dysfunction in a low-renin/low-ANG II hypertensive rat model. DOCA-salt treatment led to a markedly blunted pressure-mediated vasoconstriction of afferent arterioles and attenuated the vasoconstrictor responses to exogenous P2 receptor ligands ATP and P2X1 receptor activation with β,γ-methylene ATP. The vasoconstriction to A1, P2Y2, and adrenergic receptor activation, however, was unaltered. Anti-inflammatory treatment with PPS, or suppression of hypertension development with triple therapy (TTx) treatment, preserved the autoregulatory behavior of afferent arterioles and significantly improved vasoconstriction to ATP or P2X1 receptor activation. The improvement in function in the DOCA-salt+PPS rats occurred despite the persistent hypertension. Furthermore, PPS or TTx treatment provided significant benefit to MCP-1 excretion and proteinuria. These results demonstrate that hypertension per se leads to the renal microvascular dysfunction observed in DOCA-salt hypertensive rats characterized by impaired renal autoregulation and preglomerular microvascular reactivity to P2X1 receptor activation, possibly relating to activating inflammatory cascades involving elevated MCP-1.
Growing evidence indicates that the ATP-P2 receptor system plays an important role in controlling renal vascular resistance and regulating renal hemodynamics and tubular transport function (13, 34). Recent studies also show a close link between P2 receptor signaling pathways, hypertension, and hypertensive renal injury (8, 13, 34, 42). Our previous studies revealed the coexistence of impaired renal autoregulation and impaired P2X1 receptor signaling in ANG II-infused hypertensive rats, but the impairments were prevented by anti-inflammatory treatment with PPS or MMF despite the hypertension (14, 15). The DOCA-salt model of hypertension is a low-renin/low-ANG II model compared with ANG II-infused hypertensive rats (10). Therefore, this model is ideal for distinguishing between the direct effects of ANG II and other factors. Early studies showed impaired autoregulation in rats (16, 19, 36) or dogs (26) receiving chronic DOCA-salt treatment. Furthermore, evidence shows renoprotective effects of anti-inflammatory treatment on DOCA-salt hypertensive rats (4, 6, 49), but the major vascular component of autoregulation, afferent arteriolar function, has never been directly evaluated in the DOCA-salt hypertensive model. In the current study, we demonstrate that afferent arterioles from DOCA-salt rats exhibit impaired autoregulatory behavior, as evidenced by a fairly flat pressure-diameter relationship. DOCA-salt rats receiving PPS treatment maintained normal afferent arteriole autoregulatory behavior, leading to a pressure-diameter relationship that was markedly better than in DOCA-salt rats despite the fact that DOCA-salt+PPS rats had a similar progression and magnitude of hypertension. This indicates that renal autoregulatory behavior can be protected by anti-inflammatory treatment and that the functional improvement with PPS is independent of changes in the hypertensive state.
To further differentiate the factors contributing to the renal microvascular autoregulatory dysfunction in DOCA-salt hypertension, we conducted additional experiments to determine whether the impairment arose due to DOCA-salt treatment or to the elevation of arterial pressure. We treated DOCA-salt rats with TTx to prevent development of hypertension while maintaining the elevated mineralocorticoid environment. Although these rats received the same DOCA-salt treatment, the afferent arterioles from DOCA-salt+TTx rats showed normal autoregulatory behavior. The profile of pressure-dependent vasoconstriction was similar to the response in UNx rats but was distinguished from DOCA-salt hypertensive rats, suggesting that elevated arterial pressure, rather than a direct effect of the mineralocorticoid-salt treatment per se, leads to impaired renal autoregulation. Our results are in agreement with the findings of Klanke et al. (27), who treated DOCA-salt hypertensive rats with TTx or spironolactone. They found that only the TTx prevented the enhanced gene expression of chemokines, adhesion molecules, and profibrotic cytokines in DOCA-salt kidneys despite the fact that both treatments ameliorated glomerulosclerosis and interstitial fibrosis, suggesting that high blood pressure triggers an inflammatory cascade in DOCA-salt hypertensive kidneys.
ATP is an endogenous ligand for P2X and P2Y receptors. ATP is postulated to act as a mediator in the juxtaglomerular transmission in tubuloglomerular feedback signals (37). Activation of renal microvascular P2X1 signaling is essential for renal autoregulation, as P2X1 receptor blockade or deletion blunts autoregulation (22, 23, 39). In keeping with this hypothesis, we found that UNx rats exhibited normal autoregulatory behavior and concentration-dependent vasoconstriction to either ATP or the P2X1 receptor agonist β,γ-methylene ATP. In contrast, both ATP- or β,γ-methylene ATP-mediated arteriolar vasoconstriction were blunted in DOCA-salt rats whereas the vasoconstrictor response to norepinephrine remained unaltered. When DOCA-salt rats were treated with PPS, ATP-mediated vasoconstriction was significantly improved compared with the response in DOCA-salt hypertensive rats. Importantly, PPS treatment preserved afferent arteriolar vasoconstriction to β,γ-methylene ATP, a more selective P2X1 receptor agonist. Consistent with the normal autoregulatory behavior of afferent arterioles in DOCA-salt+TTx rats, we also found that suppressing hypertension with TTx preserved afferent arteriolar vasoconstrictor responses to both ATP and β,γ-methylene ATP in DOCA-salt rats. Altogether, these findings indicate that the inflammation and high blood pressure per se contribute to impaired renal microvascular reactivity to P2X1 receptor activation. These data are consistent with our previous findings in an ANG II-hypertensive model that renal autoregulatory impairment is accompanied by impaired P2X1 receptor signaling in afferent arterioles (14, 15, 21).
Both P1 and P2Y2 purinoceptors are expressed in afferent arterioles. Adenosine, the endogenous P1 ligand, is believed to mediate tubuloglomerular feedback responses via A1 receptor activation at afferent arterioles (5, 47). Activation of P2Y2 receptors with UTP also causes vasoconstriction of preglomerular microvessels (20, 53). In the current study, the vasoconstriction of afferent arterioles by adenosine or UTP in DOCA-salt rats was not different from the response in UNx rats, suggesting that afferent arteriolar reactivity to A1 and P2Y2 receptor activation is intact in DOCA-salt rats and cannot be implicated in the impaired autoregulatory behavior in DOCA-salt hypertensive rats.
The mechanisms leading to impaired renal microvascular reactivity to P2X1 receptor activation in DOCA-salt hypertensive rats are unclear. Earlier studies show that increases in renal perfusion pressure increase the ATP concentration in renal interstitial fluid (38). Chronic ANG II infusion leads to a dramatic increase in ATP concentration in renal interstitial fluid and afferent arteriolar hypertrophy, which can be prevented by treatment with a nonspecific P2 receptor blocker, PPADS or a P2Y12 receptor blocker, clopidogrel (12). We speculate that inflammatory factors might lead to P2X1 receptor internalization or disruption of membrane lipid rafts, which may cause redistribution of P2X1 receptors and lead to the blunted responses to P2X1 receptor activation (48). A recent study by Gordienko et al. (11) in spontaneously hypertensive rats (SHR) reveals a reduction of P2X1 receptor expression and attenuation of the β,γ-methylene ATP-mediated increase in intracellular Ca2+ concentration in myocytes isolated from preglomerular microvessels of SHR compared with control WKY rats, suggesting that hypertension could compromise P2X1 receptor signaling in renal microvascular myocytes and contribute to microvascular dysfunction. Additional studies are needed to determine whether alteration of P2X1 receptor expression in renal microvessels or the alterations in P2X1 receptor-mediated Ca2+ signaling account for the blunted P2X1 receptor-mediated vasoconstriction in this hypertensive model.
MCP-1 is a chemokine that contributes to inflammation-induced renal injury. Chronic DOCA-salt treatment increased renal MCP-1/CCl2 gene expression and urinary MCP-1 excretion in rats and mice (6, 27, 33, 50). Blockade of the MCP-1/chemokine receptor 2b preserved normal autoregulatory behavior of afferent arterioles in ANG II-infused hypertensive rats fed 8% salt (7), suggesting that MCP-1 contributes to impaired renal autoregulation. However, the role of MCP-1 in renal microvascular function in DOCA-salt hypertensive rats is unknown. In the current study, urinary MCP-1 excretion was elevated in DOCA-salt rats compared with UNx rats but was significantly reduced by PPS or TTx treatment. Interestingly, plasma MCP-1 concentration was not significantly increased in DOCA-salt hypertensive rats. This finding is consistent with reports in other hypertensive rat models, including double transgenic hypertensive rats harboring the human renin and angiotensinogen genes or SHR rats (28, 31, 35), and also hypertensive human subjects (25), suggesting that local renal production of MCP-1, rather than circulating MCP-1, plays a critical role in mediating hypertension-associated renal injury. Blasi and coworkers (2) revealed that MCP-1 mRNA is rarely detectable in normal rat kidneys but was detected in damaged and regenerative tubules, interstitial macrophages, vascular smooth muscle cells, arteriolar endothelial cells, and glomerular endothelial and epithelial cells in aldosterone/salt-induced hypertensive rats. This suggests that stimulation of mineralocorticoid receptor activation can lead to increased MCP-1 in many renal cell types. PPS treatment significantly decreased both MCP-1 excretion and proteinuria in DOCA-salt rats. The level of proteinuria is positively correlated with MCP-1 excretion, suggesting that PPS treatment provides renal protection via reduction of inflammation in DOCA-salt+PPS rats, consistent with improved renal vascular function.
PPS has been widely used for treatment of interstitial cystitis (41). Recent studies show protective effects of PPS in animals with hypertension-associated kidney injury, diabetic nephropathy, and atherosclerotic lesions (3, 14, 32, 52). PPS is a highly sulfated semisynthetic polysaccharide and has similar structural and chemical properties as glycosaminoglycan. Glycosaminoglycan is considered an important constituent, forming an endothelial surface glycocalyx layer to protect endothelial cells from cytokine and leukocyte adhesion under inflammatory conditions (29, 43, 44). Therefore, an intact glycocalyx in the glomerular endothelium acts as an important microvascular permeability barrier that prevents albumin filtration (43, 45). Disruption of the endothelial glycocalyx layer has been linked to increased albuminuria, atherosclerosis, and systemic inflammation (29, 43, 44). Wu et al. (52) reported that the development/progression of nephropathy in aging diabetic mice could be prevented by PPS treatment by reduction of MCP-1 production and suppression of TNF-α and NF-κB activation, suggesting the anti-inflammatory properties of PPS. Additionally, the endothelial glycocalyx layer is thought to play a crucial role in mechanotransduction (51), a process that initiates the vascular myogenic response. However, it is unclear whether alteration of the endothelial glycocalyx layer is responsible for impaired afferent arteriole autoregulatory behavior in DOCA-salt hypertensive rats. Here, we demonstrate that PPS treatment not only protects renal microvascular function by preserving renal autoregulation but also reduces proteinuria despite persistent hypertension in DOCA-salt rats. We also showed that PPS significantly protects against renal hypertrophy in DOCA-salt rats, but the mechanisms underlying this protection need further investigation. Furthermore, if PPS provides renal protection in an established hypertension-induced kidney injury setting, it would create a unique therapeutic opportunity for translation to clinical application.
Additionally, the current study also reveals that suppressing hypertension development with TTx not only preserves normal autoregulatory behavior of afferent arterioles but also alleviates urinary protein excretion in DOCA-salt hypertensive rats, supporting the concept that maintaining normal autoregulation is important for preventing glomerular injury in hypertension. Loss of renal autoregulatory efficiency leads to glomerular stress and subsequent glomerular injury, as shown by elevation of urinary protein excretion. Better control of blood pressure is critical for preventing loss of renal autoregulation and hence protects against hypertensive glomerular injury.
In conclusion, the current studies provide evidence that hypertension activates inflammatory cascades which contribute to impaired renal autoregulation and blunted P2X1 receptor-mediated renal microvascular vasoconstriction in DOCA-salt hypertensive rats. Simultaneous initiation of anti-inflammatory treatment with PPS or suppression of hypertension development with TTx, at the start of DOCA-salt, prevents impairment of renal autoregulation despite persistent hypertension. This is accompanied by normalizing the renal microvascular response to P2X1 receptor activation and reducing MCP-1 excretion and protein excretion. We also demonstrated that P2Y2, A1, and adrenergic receptor reactivity remains intact in DOCA-salt afferent arterioles, suggesting that impairment of renal autoregulation in DOCA-salt hypertension cannot be attributed to alteration of P2Y2 and A1 receptor signaling or to a general decline in afferent arteriolar reactivity to vasoconstrictor stimuli. Overall, anti-inflammatory intervention with PPS and suppression of hypertension with TTx confer significant renal protection against the decline of renal autoregulation, and this is associated with preservation of P2X1 receptor reactivity.
GRANTS
This study was supported by a postdoctoral fellowship (0825465E) from the Greater Southeast Affiliate and by a National Scientist Development Grant (10SDG3770010) from the American Heart Association to Z. Guan and by National Institutes of Health Grants DK044628, HL074167, HL098135, and HL095499 to E. W. Inscho and HL69999 and HL095499 to J. S. Pollock and D. M. Pollock.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.G., J.S.P., D.M.P., and E.W.I. provided conception and design of research; Z.G., S.T.S., H.C., J.P.V.B., and A.K.C. performed experiments; Z.G., S.T.S., H.C., J.P.V.B., and A.K.C. analyzed data; Z.G., S.T.S., J.S.P., D.M.P., and E.W.I. interpreted results of experiments; Z.G. prepared figures; Z.G. drafted manuscript; Z.G., J.S.P., D.M.P., and E.W.I. edited and revised manuscript; Z.G., S.T.S., H.C., J.P.V.B., A.K.C., J.S.P., D.M.P., and E.W.I. approved final version of manuscript.
REFERENCES
- 1.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 22: 1–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 63: 1791–1800, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bobadilla NA, Tack I, Tapia E, Sanchez-Lozada LG, Santamaria J, Jimenez F, Striker LJ, Striker GE, Herrera-Acosta J. Pentosan polysulfate prevents glomerular hypertension and structural injury despite persisting hypertension in 5/6 nephrectomy rats. J Am Soc Nephrol 12: 2080–2087, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Boesen EI, Williams DL, Pollock JS, Pollock DM. Immunosuppression with mycophenolate mofetil attenuates the development of hypertension and albuminuria in deoxycorticosterone acetate-salt hypertensive rats. Clin Exp Pharmacol Physiol 37: 1016–1022, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castrop H. Mediators of tubuloglomerular feedback regulation of glomerular filtration: ATP and adenosine. Acta Physiol (Oxf) 189: 3–14, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II - salt hypertension. Hypertension 50: 1069–1076, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco M, Bautista-Perez R, Perez-Mendez O. Purinergic receptors in tubulointerstitial inflammatory cells: a pathophysiological mechanism of salt-sensitive hypertension. Acta Physiol (Oxf) 214: 75–87, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Franco M, Bautista R, Tapia E, Soto V, Santamaria J, Osorio H, Pacheco U, Sanchez-Lozada LG, Kobori H, Navar LG. Contribution of renal purinergic receptors to renal vasoconstriction in angiotensin II-induced hypertensive rats. Am J Physiol Renal Physiol 300: F1301–F1309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Sanchez EP, Zhou M, Gomez-Sanchez CE. Mineralocorticoids, salt and high blood pressure. Steroids 61: 184–188, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Gordienko D, Povstyan O, Sukhanova K, Raphael M, Harhun M, Dyskina Y, Lehen'kyi V, Jama A, Lu ZL, Skryma R, Prevarskaya N. Impaired P2X signalling pathways in renal microvascular myocytes in genetic hypertension. Cardiovasc Res 105: 131–142, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol 294: F161–F169, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan Z, Fellner RC, Van Beusecum J, Inscho EW. P2 receptors in renal autoregulation. Curr Vasc Pharmacol 12: 818–828, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in ANG II-infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Renal Physiol 298: F1276–F1284, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan Z, Giddens MI, Osmond DA, Cook AK, Hobbs JL, Zhang S, Yamamoto T, Pollock JS, Pollock DM, Inscho EW. Immunosuppression preserves renal autoregulatory function and microvascular P2X1 receptor reactivity in ANG II-hypertensive rats. Am J Physiol Renal Physiol 304: F801–F807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haberle DA, Konigbauer B, Davis JM, Kawata T, Mast C, Metz C, Dahlheim H. Autoregulation of the glomerular filtration rate and the single-nephron glomerular filtration rate despite inhibition of tubuloglomerular feedback in rats chronically volume-expanded by deoxycorticosterone acetate. Pflügers Arch 416: 548–553, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Harhun MI, Povstyan OV, Gordienko DV. Purinoreceptor-mediated current in myocytes from renal resistance arteries. Br J Pharmacol 160: 987–997, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, hypertension. Hypertension 57: 132–140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imig JD, Passmore JC, Anderson GL, Jimenez AE. Chloride alters renal blood flow autoregulation in deoxycorticosterone-treated rats. J Lab Clin Med 121: 608–613, 1993. [PubMed] [Google Scholar]
- 20.Inscho EW. Mysteries of renal autoregulation. Hypertension 53: 299–306, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inscho EW, Cook AK, Andrea Clarke N, Zhang S, Guan Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in Ang II-infused hypertensive rats fed a high salt diet. Hypertension 57: 780–787, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand 181: 445–453, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol 10, Suppl 11: S178–S183, 1999. [PubMed] [Google Scholar]
- 25.Jilma B, Li-Saw-Hee FL, Wagner OF, Beevers DG, Lip GY. Effects of enalapril and losartan on circulating adhesion molecules and monocyte chemotactic protein-1. Clin Sci (Lond) 103: 131–136, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kaloyanides GJ, Bastron RD, DiBona GF. Impaired autoregulation of blood flow and glomerular filtration rate in the isolated dog kidney depleted of renin. Circ Res 35: 400–412, 1974. [DOI] [PubMed] [Google Scholar]
- 27.Klanke B, Cordasic N, Hartner A, Schmieder RE, Veelken R, Hilgers KF. Blood pressure versus direct mineralocorticoid effects on kidney inflammation and fibrosis in DOCA-salt hypertension. Nephrol Dial Transplant 23: 3456–3463, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension 51: 352–359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolarova H, Ambruzova B, Svihalkova Sindlerova L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm 2014: 694312, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res 38: 332–340, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Lorkowska B, Bartus M, Franczyk M, Kostogrys RB, Jawien J, Pisulewski PM, Chlopicki S. Hypercholesterolemia does not alter endothelial function in spontaneously hypertensive rats. J Pharmacol Exp Ther 317: 1019–1026, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Lupia E, Zheng F, Grosjean F, Tack I, Doublier S, Elliot SJ, Vlassara H, Striker GE. Pentosan polysulfate inhibits atherosclerosis in Watanabe heritable hyperlipidemic rabbits: differential modulation of metalloproteinase-2 and -9. Lab Invest 92: 236–245, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol 297: F740–F748, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menzies RI, Unwin RJ, Bailey MA. Renal P2 receptors and hypertension. Acta Physiol (Oxf) 213: 232–241, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Mervaala EM, Muller DN, Park JK, Schmidt F, Lohn M, Breu V, Dragun D, Ganten D, Haller H, Luft FC. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension 33: 389–395, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Moore LC, Schnermann J, Yarimizu S. Feedback mediation of SNGFR autoregulation in hydropenic and DOCA- and salt-loaded rats. Am J Physiol Renal Fluid Electrolyte Physiol 237: F63–F74, 1979. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama A, Navar LG. ATP mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 283: R273–R275, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama A, Rahman M, Inscho EW. Role of interstitial ATP and adenosine in the regulation of renal hemodynamics and microvascular function. Hypertens Res 27: 791–804, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Osmond DA, Inscho EW. P2X1 receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol 298: F1360–F1368, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer BF. Disturbances in renal autoregulation and the susceptibility to hypertension-induced chronic kidney disease. Am J Med Sci 328: 330–343, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Parsons CL, Mulholland SG. Successful therapy of interstitial cystitis with pentosan polysulfate. J Urol 138: 513–516, 1987. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt-sensitive hypertension. Hypertens Res 33: 975–980, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol 23: 1339–1350, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol 226: 562–574, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Satchell S. The role of the glomerular endothelium in albumin handling. Nat Rev Nephrol 9: 717–725, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 126: 267–274, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol 65: 501–529, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Vial C, Evans RJ. Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J Biol Chem 280: 30705–30711, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Wang DH. Aggravated renal inflammatory responses in TRPV1 gene knockout mice subjected to DOCA-salt hypertension. Am J Physiol Renal Physiol 297: F1550–F1559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Zhu M, Xu H, Cui L, Liu W, Wang X, Shen S, Wang DH. Role of the monocyte chemoattractant protein-1/C-C chemokine receptor 2 signaling pathway in transient receptor potential vanilloid type 1 ablation-induced renal injury in salt-sensitive hypertension. Exp Biol Med (Maywood) 240: 1223–1234, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9: 121–167, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Guan TJ, Zheng S, Grosjean F, Liu W, Xiong H, Gordon R, Vlassara H, Striker GE, Zheng F. Inhibition of inflammation by pentosan polysulfate impedes the development and progression of severe diabetic nephropathy in aging C57B6 mice. Lab Invest 91: 1459–1471, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Zhao X, Cook AK, Field M, Edwards B, Zhang S, Zhang Z, Pollock JS, Imig JD, Inscho EW. Impaired Ca2+ signaling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension. Hypertension 46: 562–568, 2005. [DOI] [PubMed] [Google Scholar]