Abstract
Orai (Orai1, Orai2, and Orai3) proteins form a family of highly Ca2+-selective plasma membrane channels that are regulated by stromal-interacting molecules (STIM1 and STIM2); STIM proteins are Ca2+ sensors located in the membrane of the endoplasmic reticulum. STIM and Orai proteins are expressed in vascular and airway smooth muscle and constitute the molecular components of the ubiquitous store-operated Ca2+ entry pathway that mediate the Ca2+ release-activated Ca2+ current. STIM/Orai proteins also encode store-independent Ca2+ entry pathways in smooth muscle. Altered expression and function of STIM/Orai proteins have been linked to vascular and airway pathologies, including restenosis, hypertension, and atopic asthma. In this review we discuss our current understanding of Orai proteins and the store-dependent and -independent signaling pathways mediated by these proteins in vascular and airway smooth muscle. We also discuss the current studies linking altered expression and function of Orai proteins with smooth muscle-related pathologies.
Keywords: store-operated calcium entry, Orai, stromal-interacting molecule, calcium signaling, vascular and airway disease, smooth muscle remodeling
smooth muscle remodeling is a feature of many diseases, including restenosis, atherosclerosis, hypertension, and atopic asthma (10, 63). This remodeling is initiated by various insults in the form of mechanical injury or inflammatory mediators. In response to these insults, smooth muscle cells (SMCs) undergo phenotypic modulation, altering their transcriptional profile from a quiescent contractile phenotype to a more synthetic phenotype, whereby SMCs proliferate, migrate, and deposit extracellular matrix (9, 60). This phenotypic modulation is characterized by a remodeling of the repertoire of ion channels in smooth muscle (36). Recently, SMCs have also been shown to act as immune effector cells that actively participate in the inflammatory response through production and secretion of various cytokines and inflammatory factors [e.g., transforming growth factor (TGF)-β, interleukin (IL)-11, IL-6, and leukemia inhibitory factor], further exacerbating the disease (21). These pathologically related phenotypic changes enhance and sustain vascular and airway remodeling and are the basis of various cardiovascular and respiratory diseases.
Alterations in Ca2+ homeostasis directly regulate smooth muscle phenotypic modulation, and changes in protein expression of various Ca2+ channel proteins have been shown to contribute to smooth muscle remodeling in vascular and respiratory diseases. Stromal-interacting molecule (STIM)/Orai family members are the molecular components of the store-operated Ca2+ entry (SOCE) pathway, as well as the store-independent Ca2+ entry pathway, in SMCs (6, 30, 67, 107, 108). Deficiency in STIM1 or Orai1 expression is associated with immune deficiency, autoimmune disease, skeletal muscle hypotonia, and ectodermal dysplasia associated with sweat gland dysfunction and defects in development of tooth enamel (40). Gain-of-function mutations in STIM1 and Orai1, resulting in constitutive unregulated Ca2+ entry, which is associated with a number of diseases, including forms of myopathy, have been reported (40). In smooth muscle, enhanced expression of STIM/Orai proteins is a feature of vascular and airway remodeling in animal models of mechanical balloon injury in vessels (30, 104, 106) and atopic asthma (81). Upregulation of STIM/Orai expression and enhanced Ca2+ signals contribute to phenotypic modulation of vascular and airway SMCs (2, 6, 30, 31, 46, 81, 92, 104). STIM/Orai-mediated Ca2+ signaling pathways activate downstream effector proteins and transcription factors that promote SMC proliferation and migration (see below). In this review we discuss our current understanding of Ca2+ entry pathways mediated by STIM/Orai proteins in SMCs and discuss the role of these Ca2+-selective channels in vascular and airway disease.
Orai Proteins in Smooth Muscle
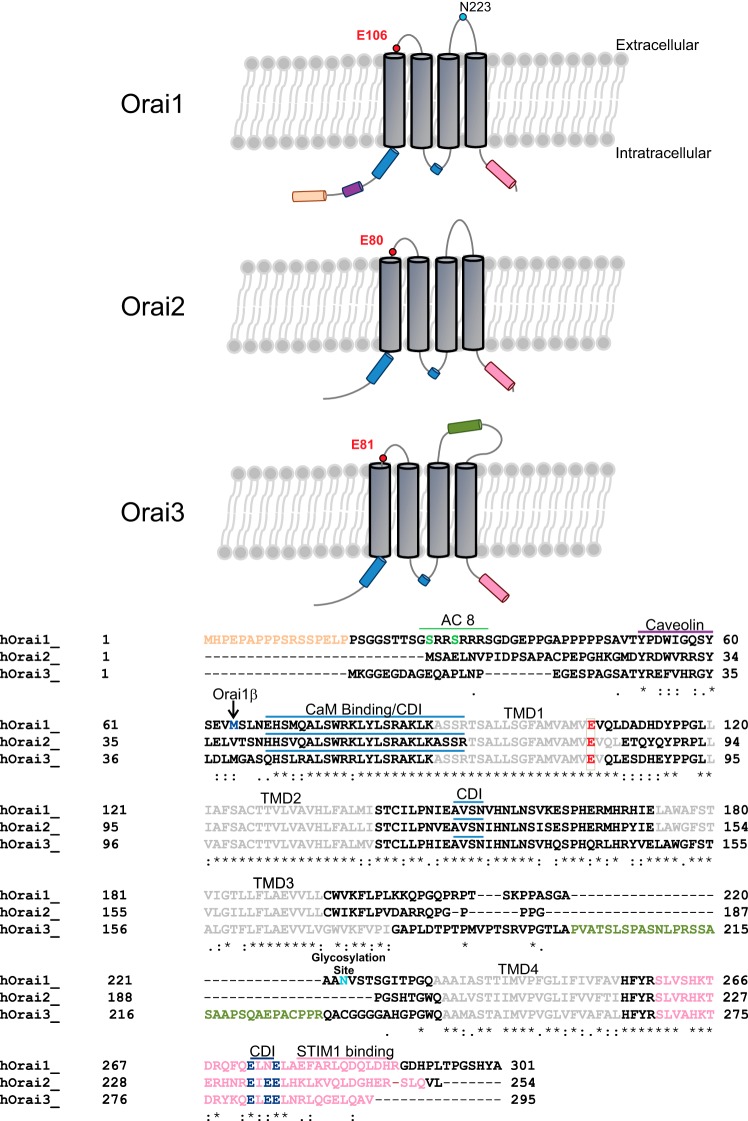
Mammalian Orai proteins are a class of Ca2+-selective plasma membrane channel proteins made up of three homologs, Orai1, Orai2, and Orai3, encoded by three independent genes (77). Invertebrates have one single Orai gene; all vertebrates possess two Orai genes, Orai1 and Orai2; the Orai3 gene is exclusive to mammals. Orai1 is expressed in human embryonic kidney (HEK-293) cells as two different isoforms: a long form, Orai1α, containing an additional NH2-terminal 63 amino acids, and a short form, Orai1β (Fig. 1). These Orai1 isoforms are the result of alternative translation-initiation sites within the Orai1 message; the long-form, Orai1α, is exclusive to mammals (26).
Fig. 1.
Orai homolog structure and domain composition. A: protein domains for each Orai homolog. B: amino acid alignments. Conserved domains within the Orai family of proteins consist of an intracellular NH2 terminus containing a calmodulin-binding region, which plays a role in Ca2+-dependent inhibition (CDI) of Orai channels (blue lines) (56): 4 transmembrane-spanning domains, TMD1, TMD2, TMD3, and TMD4 (gray) with extracellular loops between TMD1 and TND2 and between TMD3 and TMD4. TMD1 and the first extracellular loop contain a highly conserved pore region with a conserved “E” residue (red) representing the selectivity filter. The intracellular loop contains residues 153–157, proposed to mediate fast Ca2+-dependent inhibition (blue line) (82). The COOH-terminal region contains a coiled-coil domain, required for stromal-interacting molecule 1 (STIM1) binding and gating of Orai channels (pink). Orai1 contains a distinct proline-rich NH2-terminal domain (peach) from M1 to P17. The conserved CaM-binding domain (blue line) spans E68–R91. Orai1 contains a conserved CaM-binding domain (blue) spanning E68–R91. Orai1 also has 4 conserved transmembrane domains (TMD1, TMD2, TMD3, and TMD4), a conserved pore residue at E106 (red), and a distinct glycosylation site within the second extracellular loop at N223 (blue). The shorter form of Orai1 (Orai1β) originates from an alternative initiation-translation of the Orai1 mRNA at methionine 63 (M63; blue) (17, 26). Orai1 contains a region shown to mediate interaction with the Ca2+-sensitive adenylate cylase 8 (AC8; green line) (98), which contains 2 serine residues (S27 and S30; green), which are targets for phosphorylation by protein kinase C (34), and a putative caveolin-binding domain (purple line). Orai2, the smallest of the 3 isoforms, contains all conserved functional domains, with its CaM-binding domain spanning H42–R65, its pore-selectivity filter residue at E80, and its COOH-terminal coiled-coil domain spanning residues S221–Q252. The Orai3 CaM-binding domain spans residues Q43–R66; the homologous residue to E106 in Orai1 is E81. Orai3 contains a distinctly long extracellular loop (olive) between TMD3 and TMD4 and its COOH-terminal coiled-coil domain spanning residues S269–V295. Conserved COOH-terminal glutamate residues (3 in Orai2/Orai3 and 2 in Orai1; dark blue) determine more enhanced fast Ca2+-dependent inactivation of Orai2/Orai3 than Orai1 (41). ∗, Conserved residues among all 3 Orai homologs; conserved residues between 2 Orai homologs.
mRNA of all three Orai homologs is ubiquitously expressed, including expression in vascular and airway SMCs from different species, including humans. Protein expression levels in contractile vascular SMCs obtained from human or rat aorta are negligible (5, 67). However, in cultured aortic SMCs, which are reminiscent of synthetic dedifferentiated SMCs found in disease states, expression levels of STIM1, Orai1, Orai2, and Orai3 are dramatically increased (5, 6, 30, 67). Expression levels of Orai1α and Orai1β have not been characterized in SMCs, but such studies are warranted, as these isoforms have been shown to have distinct signaling and regulatory properties (17).
Orai1 contains four transmembrane domains (23), with its NH2- and COOH-terminal domains residing in the cytosol (69) (Fig. 1). The use of myc-tagged Orai1 at the NH2- or COOH-terminal end confirmed Orai1 plasma membrane (PM) localization. In vascular SMCs, Orai1 colocalizes with the well-known PM marker wheat germ agglutinin (6). Similarly, when Orai2 and Orai3 proteins are ectopically expressed, they locate at the PM (32) through their four highly conserved transmembrane domains, which are highly conserved among all Orai homologs. Between these transmembrane domains are two extracellular loops: the first is shorter, with a large number of acidic residues (69) made up of nine conserved amino acids, including a glutamate residue [E106 (Orai1), E80 (Orai2), and E81 (Orai3)], a glutamine residue [Q108 (Orai1), Q81 (Orai2), and Q83 (Orai3)], and within Orai1 three aspartate residues (D110, D112, and D114) (96). Orai1:E106, in particular, and its homologs in Orai2 and Orai3 are critical for Ca2+ selectivity of Orai channels (69, 94, 101). Orai1 constructs where E106 was substituted for glutamine (E106Q) or alanine (E106A) yield a dead channel, and when E106 was mutated to a similar amino acid, aspartate (E106D), Orai1 channels lose their Ca2+ selectivity and mediate monovalent and divalent cation currents equally (69, 101), highlighting the importance of the size and charge of pore-forming domain structures in selectivity of this ion channel. The second extracellular loop, located between transmembrane domains 3 and 4, is much longer in Orai3 than in Orai1 or Orai2 (Fig. 1).
The cytosolic domains are also conserved among all Orai homologs. Residues within the NH2 terminus contain a Ca2+/calmodulin (CaM)-binding domain first shown in Orai1 to mediate Ca2+-dependent inactivation (CDI) of the channel (45, 56). Srikanth et al. (82) showed that five residues spanning 153–157 (NVHNL) within the intracellular loop of Orai1 also participate in the fast Ca2+-dependent inactivation. Within STIM1, a string of acidic residues (475–483) that are COOH-terminal to the STIM-Orai-activating region (SOAR; residues 344–442) are required for mediation of CDI of Orai1 channels (16, 41, 56). Furthermore, recent data from our laboratory showed much more pronounced CDI in Orai1α than Orai1β channels (17); however, the molecular basis of this difference and the 63 NH2-terminal residues within Orai1α that contribute to enhanced CDI remain unknown. Orai1 contains a distinct proline- and arginine-rich region in its NH2 terminus (8) that binds much more weakly to STIM1 than does the COOH-terminal region but was, nonetheless, proposed to regulate channel gating by STIM1 (102). The NH2 terminus contains a region involved in interaction with the Ca2+-sensitive adenylate cyclase 8 (98) and contains two protein kinase C phosphorylation sites (34). A putative caveolin-binding domain is also located in the NH2 terminus (Fig. 1). The COOH-terminal domain of all three Orai homologs contains a conserved STIM-binding coiled-coil domain. The importance of this coiled-coil domain in gating Orai channels was first identified in Orai1 (25). This COOH-terminal coiled-coil domain is essential for Orai1 channel activation by virtue of its role in mediating binding to STIM1. Upon store depletion, a 100-amino acid region in STIM1, SOAR, interacts with the COOH-terminal coiled-coil domain of Orai1 to activate the Orai1 channel (54, 55, 62, 102). Within this COOH-terminal coiled-coil domain of Orai channels, Lee et al. (41) showed that three conserved glutamates in the COOH terminus of Orai2 and Orai3 (E233, E235, and E236 in Orai2; Fig. 1) mediate fast Ca2+-dependent inactivation, which is more prominent in Orai2 and Orai3 than in Orai1. Interestingly, Frischauf et al. (25) reported that COOH-terminal coiled-coil domain probability varies between Orai homologs, with the lowest probability observed for Orai1 and a five- to six-times-greater probability for Orai2 and Orai3 COOH-terminal coiled-coil regions; this suggests a correlation between this coiled-coil probability and the interactions between different Orai isoforms and STIM1. A single-residue mutation in the Orai1 coiled-coil region (L273S) disrupted the interaction of Orai1 with the STIM1 COOH-terminal domain, while equivalent mutations in Orai2 or Orai3 supported moderate interaction with STIM1 and channel activation. Reciprocally, decreasing coiled-coil probability of the second coiled-coil region in the COOH terminus of STIM1 by mutation of one residue (L373S) abrogated activation of Orai1 while allowing partial activation of Orai2 and Orai3 (25).
SOCE in Smooth Muscle
Vascular smooth muscle.
SOCE, a widespread means of regulated Ca2+ entry into cells from the extracellular space, was first introduced by Putney (71). PM receptors that couple to isoforms of phospholipase C (PLC) induce production of inositol 1,4,5-triphosphate (IP3) (83). IP3 causes Ca2+ release from the endoplasmic reticulum (ER) through the IP3 receptor, and this ER Ca2+ store depletion is the signal that triggers activation of PM store-operated Ca2+ channels. SOCE can be activated pharmacologically by specific inhibitors of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), such as thapsigargin and cyclopiazonic acid (CPA), which passively deplete internal Ca2+ stores without producing second messengers (86). The Ca2+ current mediating SOCE, Ca2+ release-activated Ca2+ current (ICRAC), was first identified in mast cells by whole cell patch-clamp recordings. ICRAC is activated when store depletion is achieved by inclusion of Ca2+ chelators (alone or with IP3) in the patch pipette; ICRAC is an inwardly rectifying Ca2+-selective current that has a reversal potential around +60 mV and is voltage-insensitive (35). After nearly two decades, STIM and Orai molecules were finally identified as the genuine molecular components of SOCE and ICRAC (70). STIM and Orai proteins are also expressed in cells of the vasculature and mediate SOCE in endothelial cells and SMCs (1, 67, 75). STIM proteins are single-pass transmembrane proteins located in the ER membrane with an NH2-terminal luminal low-affinity EF-hand. STIM proteins sense Ca2+ store depletion within the lumen of the ER, while Orai proteins are the store-operated Ca2+ channels at the PM. ER Ca2+ store depletion causes STIM molecules to accumulate at junctional ER-PM spaces, where they trap and gate Orai channels through physical interactions between the cytosolic COOH-terminal SOAR domain of STIM and the COOH-terminal coiled-coil domain of Orai; to interact with STIM, Orai channels diffuse to these regions of the PM closely associated with the ER.
The molecular identity of the PM SOCE channels in SMCs and in other cell types was a matter of intense debate over the past decades (for review see Ref. 68). With the discovery of STIM and Orai proteins as the bona fide molecular components of SOCE in Drosophila Schneider 2 cells and in mammalian lymphocytes (70), Peel et al. (64, 65) used siRNA knockdown to document roles for STIM1 and Orai1 in mediating SOCE in cultured human airway SMCs. Our laboratory provided evidence for the existence of the highly Ca2+-selective store depletion-activated current ICRAC in cultured rat vascular cells as well as airway SMCs. We established a role for STIM1 and Orai1 in mediating SOCE and ICRAC in these cells in response to passive store depletion or agonist stimulation, namely, by the SMC proproliferative promigratory agonist platelet-derived growth factor (PDGF) (6, 67, 81).
Studies from our laboratory used cultured proliferative migratory vascular SMCs (also referred to as synthetic cells), which represent a good in vitro model for dedifferentiated SMCs under pathophysiological conditions such as restenosis and atherosclerosis (60). Passive store depletion in synthetic vascular SMCs activated Ca2+ entry, which bears pharmacological features of the classical SOCE pathway, characterized in lymphocytes and HEK-293 cells (61), including inhibition by 30–50 μM 2-aminoethoxydiphenyl borate (2-APB), 50 μM ML-9, and relatively low concentrations (5 μM) of lanthanides (93). In the same study we showed that store depletion activated ICRAC in cultured synthetic vascular SMCs (67). Knockdown of STIM1 or Orai1 inhibited SOCE and ICRAC in these cells, while knockdown of Orai2, Orai3, and the transient receptor potential (TRP)-canonical (TRPC) channels TRPC1, TRPC4, and TRPC6 had no effect on SOCE or ICRAC. These findings are supported by other studies in a number of mammalian cells where native SOCE is mediated by STIM1 and Orai1, despite ubiquitous protein expression of Orai2 and Orai3 in all these cells (90). The only exception is a subset of estrogen receptor-positive breast cancer cells, where Orai3, along with STIM1, mediates SOCE (52, 53). TRP channels, especially isoforms belonging to the TRPC subfamily, have long been proposed to mediate SOCE in many cell types, including those of the vasculature (SMCs and endothelial cells) (29, 91). However, while there is evidence that certain isoforms of TRPC channels work closely and in concert with Orai channels, are regulated by STIM1 proteins, and fulfill nonoverlapping physiological roles (11, 13), the general consensus is that TRPC channels are activated secondarily to store depletion by receptor-mediated mechanisms, including Orai1-generated Ca2+ signals and lipid second messengers generated downstream from the PLC pathway (19).
SOCE measured by fura 2 imaging is upregulated in synthetic vascular SMCs compared with quiescent contractile SMCs, which were acutely isolated from vessels and studied within 6 h of isolation (67). This enhanced SOCE in synthetic SMCs correlated with increased protein expression of STIM1 and Orai1 compared with levels of these proteins in freshly isolated quiescent vascular SMCs. Knockdown of STIM1 and Orai1 using siRNA attenuated proliferation and migration in synthetic vascular SMCs (67). Inhibition of Orai1 was associated with a compensatory downregulation of Na+/Ca2+ exchanger type 1 and PM Ca2+ pump isoform 1 that would, presumably, reduce Ca2+ extrusion. In this same study, immunohistochemistry revealed co-clustering of Orai1 and Na+/Ca2+ exchanger type 1 in regions of the PM (4).
The pathophysiological SMC agonist PDGF activates Ca2+ entry in synthetic vascular SMCs through STIM1/Orai1-mediated SOCE, as well as proliferation and migration of these cells. Knockdown of STIM1 or Orai1 in synthetic vascular SMCs attenuated PDGF-mediated Ca2+ entry and SMC migration, while silencing of Orai2, Orai3, TRPC1, TRPC4, and TRPC6 did not affect the magnitude of PDGF-mediated Ca2+ entry; knockdown of Orai2, Orai3, and STIM2 had a marginal effect on PDGF-induced vascular SMC migration (6). The PDGF receptor is upregulated in vascular occlusive disease and promotes vascular remodeling through initiation of SMC migration, proliferation, and extracellular matrix deposition, subsequently contributing to remodeling and neointimal hyperplasia (72). Ogawa et al. (59) showed that the PDGF-activated Akt/mammalian target of rapamycin pathway enhances SOCE and pulmonary artery SMC proliferation through upregulation of STIM1 and Orai1 expression. Inhibition of Akt reduced SOCE, proliferation, and STIM/Orai expression (59). Munoz et al. (57) showed that nonsteroidal anti-inflammatory drugs indirectly inhibit SOCE in A10 vascular SMCs by causing mitochondrial depolarization, thus inhibiting mitochondrial uptake of cytosolic Ca2+ and causing cytosolic Ca2+-dependent inhibition of SOCE. This provides a potential mechanism by which nonsteroidal anti-inflammatory drugs inhibit vascular SMC proliferation, including proliferation of A10 SMCs as described by Munoz et al.
The vasoactive agonist urotensin II, which also promotes vascular SMC proliferation, enhanced Ca2+ influx through a pathway pharmacologically reminiscent of SOCE in vascular SMCs (18, 73). Under conditions where cytosolic Ca2+ was buffered to 150 nM by high concentrations of a Ca2+ chelator (10 mM BAPTA-5 mM CaCl2), urotensin II activated whole cell currents in cultured aortic SMCs that were biophysically and pharmacologically reminiscent of ICRAC, including inward rectification and inhibition by 5 μM Gd3+ (73). Interestingly, urotensin II-mediated Ca2+ entry measured with fura 2 in these cultured aortic SMCs was dependent on TRPC1, in addition to STIM1 and Orai1 (73). This likely reflects close interactions between STIM1/Orai1-mediated ICRAC and nonselective TRPC channels. The latter are likely activated secondarily to store depletion and Ca2+ entry through Orai1 channels (e.g., direct activation by Ca2+ or Ca2+-dependent delivery to the PM of TRPC-containing vesicles) (11, 12, 17, 91). Consistent with this notion, coimmunoprecipitation experiments showed that urotensin II enhanced the STIM1-Orai1 and Orai1-TRPC1 interactions in cultured aortic SMCs (73). Rodriguez-Moyano et al. (73) also showed that pharmacological inhibition of SOCE (by 50 μM 2-APB or 10 μM ML-9) significantly inhibited epidermal growth factor receptor and ERK phosphorylation in response to urotensin II. Reciprocally, pharmacological inhibition of epidermal growth factor receptor and ERK inhibited the Ca2+ increase and proliferation of cultured aortic SMCs in response to urotensin II. Pharmacological inhibition of SOCE or knockdown of STIM1, Orai1, or TRPC1 inhibited urotensin II-mediated activation of the transcription factor cAMP response element-binding protein (CREB), suggesting a role for vascular SMC Ca2+ signaling in CREB activation and cell proliferation (73).
Despite the involvement of STIM1 and Orai1 in vascular SMC SOCE, the SOCE pathway has distinct pharmacological characteristics in vascular SMCs compared with other cell types that, potentially, could be exploited for selective drug targeting of vascular SOCE for the purpose of therapy. Earlier work from our laboratory showed that 2-APB fully inhibits rat aortic vascular SMC SOCE at concentrations 10-fold lower than those required to block SOCE in other cell types (67). A subsequent study showed that the SOCE blocker S66 inhibits human saphenous vein SMC SOCE and cell migration at concentrations roughly two orders of magnitude lower than those required to block SOCE in other cell types (42). While the molecular basis for this enhanced SOCE sensitivity to channel blockers in vascular SMCs remains unknown, these data suggest that Orai1 in vascular SMCs has high sensitivity for pharmacological compounds and could be selectively targeted without affecting other cell types, including leukocytes, where SOCE plays a prominent role in immune function and competence.
The characteristic sustained Ca2+ signal that is mediated by SOCE regulates various Ca2+/CaM-dependent enzymes, including the serine/threonine phosphatase calcineurin (24). In an agonist-dependent manner, calcineurin regulates transcriptional programs in vascular SMCs through activation of transcription factors, most notably isoforms of nuclear factor of activated T cells (NFAT). NFAT resides in the cytosol under basal conditions in a phosphorylated state; upon SOCE activation, NFAT is dephosphorylated by calcineurin, promoting its nuclear translocation, DNA binding, and transcriptional regulation of genes. NFAT-regulated genes are involved in various processes, including inflammation, proliferation, and migration (33). In synthetic vascular SMCs, passive store depletion by thapsigargin or receptor stimulation by PDGF leads to the nuclear translocation of NFAT (47, 104, 106). Knockdown of STIM1 or Orai1 expression prevents nuclear translocation of NFAT in vascular SMCs, with a corresponding decrease in promoter activity, as assessed by luciferase reporter assays (104). Synthetic vascular SMCs downregulate expression of SERCA2a, which is abundantly expressed in contractile vascular SMCs (44). Bobe et al. (7) reported that restoration of SERCA2a expression in synthetic human coronary artery SMCs by gene transfer altered the nature of agonist-induced Ca2+ signaling from sustained to oscillatory, abrogated SOCE by inhibiting STIM1-Orai1 interactions, and inhibited NFAT nuclear translocation and SMC proliferation and migration, providing further evidence that sustained, robust Ca2+ entry mediated by SOCE couples to NFAT and vascular SMC growth and remodeling.
Damage endured by the vessel in response to removal of the atherosclerotic plaque using a procedure called balloon angioplasty and stent insertion often leads to restenosis. Restenosis involves vessel remodeling, whereby vascular SMCs proliferate and migrate to the luminal side of the injured vessel, depositing excess matrix proteins and forming the neointimal layer, leading to recidivous occlusion of the vessel (60, 105). The use of this mechanical vascular injury procedure in rat carotid arteries provides an in vivo model to study vascular SMC dedifferentiation during disease. STIM1 and Orai1 protein expression were upregulated in the medial and neointimal layers of balloon-injured rat carotid arteries (74, 104). These injured vessels also showed an increase in vascular muscle proliferation compared with controls as assessed by increased expression of the proliferative markers Ki-67 and proliferating cell nuclear antigen in the SMCs. Infection of injured vessels with a lentivirus containing shRNA against STIM1 or Orai1 prevented Orai1 and STIM1 upregulation in the media and neointima, inhibited cell proliferation, and attenuated neointima formation (2, 104). Subsequent studies confirming these earlier findings showed that knockdown of STIM1 and Orai1 inhibited angiotensin II-activated Ca2+ entry and proliferation of synthetic vascular SMCs. Knockdown of STIM1 and Orai1 using siRNA inhibited neointimal growth induced by angiotensin II after vascular injury, suggesting a potential role for SOCE in angiotensin II-induced SMC proliferation during vascular disease (31).
STIM1 and Orai1 were implicated in the pathogenesis of arterial hypertension, whereby protein expression of Orai1 and STIM1 was increased in aortas derived from stroke-prone spontaneously hypertensive rats compared with wild-type Wistar-Kyoto rats. Store depletion caused a greater STIM1- and Orai1-dependent SOCE in arteries from hypertensive than normotensive rats. Giachini et al. (28) stimulated vessels with thapsigargin to activate SOCE and relied on nonspecific inhibitors such as 2-APB and Gd3+ at high concentrations (100 μM) to link SOCE function to enhanced vessel contraction in hypertensive rats. Nevertheless, enhanced SOCE in hypertensive rats might indeed account for enhanced receptor signaling through contractile mediators (e.g., phenylephrine and angiotensin II) (38, 47). Notwithstanding an effect on contractility, STIM1/Orai1 and SOCE upregulation in vessels from hypertensive animals might suggest the establishment of SMC remodeling, which is a major contributor to the chronic phase of hypertension.
Mancarella et al. (47) generated SMC-specific STIM1 knockout (sm-STIM1-KO), STIM2 knockout (sm-STIM2-KO), and STIM1/STIM2 double-knockout mice. The double-knockout mice were perinatally lethal, while sm-STIM1-KO mice exhibited high mortality and reduced body weight; sm-STIM2-KO mice did not show an obvious phenotype. The use of external potassium chloride to assess depolarization-induced contraction on aortas of sm-STIM1-KO mice showed normal contractility in these mice. However, α1-adrenergic-mediated contraction of sm-STIM1-KO mice was inhibited by ∼26%, suggesting a defect in contractile α1-adrenergic signaling (47). Consistent with previous results with rat balloon injury, neointima formation caused by carotid artery ligation was reduced by 54% in sm-STIM1-KO mice compared with littermate controls. Furthermore, in vascular SMCs isolated from sm-STIM1-KO mice and stimulated with PDGF, in vitro proliferation was strongly inhibited, while Ca2+ entry and NFAT nuclear translocation were essentially abrogated (47). A subsequent study from our laboratory generated sm-STIM1-KO mice, as well as endothelial-specific STIM1 knockout (ec-STIM1-KO) mice, and showed that body weight was reduced in sm-STIM1-KO and ec-STIM1-KO mice. Consistent with the results of Mancarella et al., we also showed that artery vessel contraction to α1-adrenergic stimulation with phenylephrine was significantly reduced only in sm-STIM1-KO mice, while contraction to thromboxane A2 agonist and potassium chloride was normal. Mancarella et al. showed that sm-STIM1-KO mice show abnormally developed vascular and intestinal smooth muscle tissues with distended and thinned morphology. Therefore, using siRNA, we acutely downregulated STIM1 in arteries ex vivo and showed that the contractile response to phenylephrine was inhibited, with no effect on contractility in response to thromboxane, suggesting that defects in contractile α1-adrenergic signaling are not developmentally related (38). An Ossabaw miniature swine model was used in an animal study to address the issue of accelerated coronary artery disease in areas adjacent to stenting (peri-stent) in metabolic disease. Animals fed an excess-calorie atherogenic diet (metabolic syndrome) show increased SOCE and coronary artery disease in non- and peri-stent segments of coronary arteries. Exercise reverses coronary artery disease and increases in SOCE, as well as STIM1, Orai1, and TRPC1 upregulation (20).
In an early study that predates the discovery of STIM and Orai proteins, Lin et al. (43) were the first to show upregulation of SOCE and diacylglycerol (DAG)-activated Ca2+ entry in pulmonary artery SMCs during hypoxic pulmonary hypertension and correlated these enhanced Ca2+ entry routes to increased expression of the TRPC isoforms TRPC1, TRPC3, and TRPC6 (TRPC3 and TRPC6 are receptor-activated channels gated by DAG). Ng et al. (58) subsequently proposed that interactions between TRPC1, Orai1, and STIM1 underlie SOCE induced by acute hypoxia in pulmonary artery SMCs. Wang et al. (97) proposed that peroxisome proliferator-activated receptor-γ (PPARγ) plays a protective role in pulmonary hypertension induced by chronic hypoxia. They proposed that PPARγ decreases proliferation and migration of pulmonary artery SMCs through inhibition of SOCE, which is upregulated by exposure to hypoxia. In the same study, they correlated their findings with expression of TRPC1 and TRPC6 proteins; however, STIM/Orai isoform expression was not addressed (97). In a subsequent study, Yang et al. (100) suggested that enhanced SOCE in hypoxia-exposed pulmonary artery SMCs is mediated through the upregulation of caveolin-1, arguing that SOCE channels are organized within caveolin-rich regions. Molecular knockdown of caveolin-1 inhibited hypoxia-mediated increases in SOCE, and the PPARγ agonist GW1929 reduced SOCE and caveolin-1 expression under hypoxic conditions (100).
Zhang et al. (103) reported that SOCE was increased in pulmonary artery SMCs from patients with idiopathic pulmonary hypertension (IPAH) compared with control normotensive patients. In a subsequent study, Song et al. (80) showed that STIM2 expression level was increased in pulmonary artery SMCs from IPAH patients, while STIM1 levels were decreased and knockdown of STIM2 in these cells inhibited SOCE and proliferation, while STIM2 knockdown in pulmonary artery SMCs from controls had no effect on SOCE or cell proliferation. Overexpression of STIM2 in control cells did not cause an increase in SOCE or proliferation, leading the authors to conclude that STIM2 plays a necessary, but not sufficient, role in upregulation of SOCE and enhanced pulmonary artery SMC proliferation during IPAH (80). The failure of STIM2 overexpression to cause an increase in SOCE and proliferation in control cells is likely due to the fact that native Orai channel expression is limiting in control cells and that concurrent upregulation of different isoforms of Orai channels in IPAH is required for enhanced SOCE. Indeed, follow-up studies showed that STIM2 and Orai2 are upregulated in proliferating pulmonary artery SMCs compared with quiescent contractile cells, suggesting a role for STIM2/Orai2-mediated SOCE in phenotypic modulation and proliferation of pulmonary artery SMCs (22).
A recent study by Daskoulidou et al. (15) described the upregulation of all STIM and Orai proteins by chronic treatment with high glucose (25 mM), which correlated with enhanced SOCE in response to store depletion in vascular endothelial cells. This upregulation could be prevented by cyclosporin A and knockdown of NFATc3, leading the authors to conclude that upregulation of STIM/Orai proteins by hyperglycemia is mediated by the calcineurin/NFAT pathway, which enhances SOCE and causes endothelial dysfunction (15). The enhanced expression of STIM/Orai isoforms was also observed in aortas from diabetic patients and from two different animal models of diabetes (15). However, whether STIM/Orai upregulation in response to high glucose or during diabetes also occurs in vascular SMCs and whether upregulation of STIM/Orai isoforms causes enhancement of modes of regulated Ca2+ entry other than SOCE have not been studied.
Airway smooth muscle.
Initial studies characterizing SOCE in acutely dispersed porcine airway SMC showed store depletion by the use of the SERCA inhibitor CPA or caffeine-activated Ca2+ entry from the extracellular space. This Ca2+ entry pathway was unaffected by the L-type Ca2+ channel blocker nifidipine but was inhibited by 1 μM La3+ and 10 μM SKF-96365, a nonspecific SOCE inhibitor (3). SOCE has also been characterized in cultured human airway SMCs (65), in which all three Orai homologs are expressed. Molecular knockdown of Orai1 in cultured human airway SMCs inhibited thapsigargin- and CPA-induced Ca2+ entry. Knockdown of Orai3 also inhibited SOCE in these cells, although to a smaller degree, while knockdown of Orai2 expression had no inhibitory effects on SOCE. The same study showed that knockdown of Orai1 in cultured human airway SMCs inhibited CPA-activated ICRAC-like currents. Interestingly, Orai3 knockdown also attenuated Ca2+ release, leading Peel et al. (65) to propose an additional role for Orai3 in regulating basal levels of ER Ca2+ or in mediating Ca2+ release from internal stores.
The allergen-induced mucosal injury characteristic of asthmatic airways is believed to initiate a repair cascade that involves secretion of growth factors, including PDGF, which stimulate airway SMCs (95). In whole cell patch-clamp recordings, PDGF activated a small inwardly rectifying Ca2+ current characteristic of ICRAC that could be further amplified by the use of divalent free bath solutions in rat airway SMCs (81). Knockdown of Orai1 expression in synthetic rat airway SMCs inhibited PDGF-mediated activation of SOCE and ICRAC and attenuated airway SMC proliferation and migration (81). Using chemotaxis chamber assays, Suganuma et al. (84) investigated the role of STIM1 and Orai1 in PDGF-mediated human airway SMC migration. They showed that PDGF-activated Ca2+ entry and migration in these cells were significantly reduced by siRNA against STIM1 or Orai1, confirming the potential role for SOCE in human airway SMC remodeling; in this study, knockdown of STIM2 with siRNA had no effect on SOCE or cell migration (84). Zou et al. (109) showed that, similar to synthetic vascular SMCs, SOCE and STIM1/Orai1 expression were enhanced during airway SMC proliferation. Pharmacological blockade of SOCE using the inhibitors SKF-96365 (10 μM) and BTP2 (100 nM) or molecular knockdown of STIM1 or Orai1 inhibited SOCE and reduced airway SMC proliferation in response to serum (109). Gao et al. (27) suggested that TGFβ1 (10 ng/ml) induced an increase in expression of STIM1 and Orai1 in airway SMCs with enhancement of basal cytosolic Ca2+ levels and SOCE in response to thapsigargin. They reported that rat airway SMC proliferation in response to serum and TGFβ1 was partly reduced by 10 μM SKF-96365 (27). Jia et al. (37) showed that IL-13 and TNFα enhance puncta formation of ectopically expressed fluorescently tagged STIM1 and increased SOCE in airway SMCs, suggesting that inflammatory cytokines might contribute to airway hyperresponsiveness through increased SOCE in human airway SMCs during disease.
While airway SMC hyperresponsiveness to contractile agonists is a hallmark of asthmatic airways, airway SMC phenotypic modulation involving proliferation and hypertrophy is also a major contributor to the pathophysiology of asthma and other airway obstructive diseases (14, 66). Patients with severe asthma tend to have an increase in airway smooth muscle mass and enhanced distance between airway smooth muscle and luminal epithelium, suggesting increased airway SMC proliferation, hypertrophy, and rate of migration (66). STIM1 and Orai1 proteins are upregulated in airway SMCs from asthmatic mice, suggesting a potential role for SOCE in the pathogenesis of atopic asthma (81). However, the specific role of STIM and Orai proteins in asthma development and airway SMC remodeling has not been examined. In addition to this role in promoting the airway SMC synthetic proliferative phenotype, SOCE signaling has been proposed to play a role in regulating Ca2+ signals that mediate airway SMC contraction. Based on the use of 3-fluoropyridine-4-carboxylic acid, a nonspecific pharmacological blocker of SOCE in guinea pig airway SMCs, Sutovska et al. (85) proposed that G protein-coupled receptor agonists such as histamine regulate airway SMC contractility through activation of the SOCE pathway. However, extensive studies are required to clarify the role of SOCE in airway SMC contractility. Wylam et al. (99) incubated human airway SMCs with cigarette smoke extract and showed enhanced SOCE and increased expression of Orai1 and STIM1. Knockdown of Orai1 inhibited the effects of cigarette smoke extract on SOCE. These authors also reported that cigarette smoke extract enhanced proliferation of airway SMCs and, thus, concluded that enhanced expression of Ca2+ signaling proteins such as Orai1 and STIM1 and subsequent increase in SOCE activity could play a role in the pathogenesis of airway disease in smokers (99).
Store-Independent Ca2+ Entry in Smooth Muscle
STIM and Orai proteins also encode a different receptor-mediated Ca2+ entry pathway that is store-independent (74, 79). This store-independent pathway is activated by receptor-mediated production of arachidonic acid (AA) or its metabolite leukotriene C4 (LTC4) (30, 49, 76), and the conductance it mediates was originally characterized in HEK-293 cells and termed AA-regulated Ca2+ current (IARC) (48). IARC and ICRAC are not additive in the same cell, suggesting that these two conductances are mediated by physically distinct channels (48). IARC is biophysically similar to ICRAC, and both pathways often co-exist in the same cells, suggesting that they control distinct cellular and physiological functions. IARC has some unique features: it has a less pronounced Ca2+-dependent fast inactivation under a voltage-step protocol, and it is not inhibited by high concentrations (50 μM) of 2-APB or by reductions in external pH (78).
Production of AA or its downstream metabolite LTC4 after receptor ligation can occur through a range of enzymatic pathways, including 1) direct generation of AA from membrane glycerophospholipids by the action of the phospholipase A2 family of enzymes or 2) from sequential catalytic activities of PLC on phosphatidylinositol 4,5-bisphosphate (which generates DAG) and DAG lipase action on DAG, which will yield AA, and 3) through the activity of phosphatidic acid phosphohydrolase on phosphatidic acid produced through phospholipase D (78). Nevertheless, IARC was predominantly studied by addition of relatively low concentrations (8 μM) of exogenous AA to the bath solution, which causes activation of Ca2+ entry and membrane currents without measurable depletion of internal Ca2+ stores. Using HEK-293 cells, Shuttleworth and colleagues (50, 51, 87) showed that IARC required STIM1, Orai1, and Orai3 and proposed that the minor pool of STIM1 expressed at the plasma membrane is involved in the activation of IARC while the NH2-terminal domain of Orai3 determines the selectivity for activation by AA over store depletion.
Vascular smooth muscle.
Studies from our laboratory showed that the mitogenic and inflammatory agonist thrombin activates store-independent Ca2+ entry and membrane currents in cultured rat aortic SMCs that were additive to SOCE and ICRAC activated by PDGF (30). Maximal concentrations of thrombin do not cause sustained store depletion, and blockade of IP3 receptor-mediated Ca2+ release through dialysis of heparin in the patch pipette failed to inhibit thrombin-activated currents (30). Thrombin-activated currents were highly Ca2+-selective currents, similar to ICRAC. However, they displayed some unique features that were distinct from ICRAC, as they did not show the typical phenomenon of depotentiation in divalent free solutions (whereby large Na+ currents through ICRAC are activated but immediately inactivate) and were not inhibited by 50 μM 2-APB. Using molecular knockdown, we showed that this store-independent thrombin-activated Ca2+ entry pathway requires Orai1, Orai3, and STIM1, with the pool of STIM1 that is restricted to the ER being necessary and sufficient (107). Activation of this Ca2+ entry route requires receptor-mediated production of AA through the actions of PLC and DAG lipase and AA metabolism into LTC4 by the action of LTC4 synthase (LTC4S) (30, 106). Interestingly, exogenous AA added to the bath solution is metabolized by cells into LTC4 for optimal channel activation; dialysis of LTC4 through the patch pipette activated similar currents that were not additive to thrombin- or AA-activated currents (30). We showed that the STIM1 COOH-terminal coiled-coil domain is constitutively interacting with store-independent Orai1/Orai3 heteromultimeric channels through the Orai3 COOH terminus and that this interaction is required for channel activation by LTC4 in vascular SMCs (107). We thus named this conductance LTC4-regulated Ca2+ current (ILRC) (30). In a follow-up study, we set out to establish whether IARC in HEK-293 cells and ILRC in vascular SMCs are encoded by the same or different pools of STIM1, Orai1, and Orai3 proteins. We undertook comparative whole cell patch-clamp recordings of IARC (activated by exogenous application of 8 μM AA) and ILRC (activated by dialysis of 100 nM LTC4 through the patch pipette) in HEK-293 cells and vascular SMCs and discovered that, regardless of cell type, IARC and ILRC are not additive and require STIM1, Orai1, and Orai3, strongly arguing that these two currents are mediated by the same channel. Regardless of cell type (HEK-293 cells or vascular SMCs), a nonmetabolizable form of AA, 5,8,11,14-eicosatetraenoic acid, activated ∼50% of the current activated by AA. Similarly, when exogenous AA was added to cells treated with a 5-lipoxygenase inhibitor to prevent AA downstream metabolism, only ∼50% of the current densities were obtained compared with currents activated by dialysis of LTC4 into the cytosol of cells treated with the 5-lipoxygenase inhibitor (108). These data strongly argue that while AA is capable of activating these store-independent channels, AA metabolism into LTC4 is required for full or optimal activation. We also addressed the discrepancy between data on HEK-293 cells from Thompson and Shuttleworth (88) and our own data on vascular SMCs (30) regarding the requirement of PM-STIM1 for IARC vs. the requirement of ER-STIM1 for ILRC (108). We found that when whole cell patch-clamp recordings were used in both cell types, a construct expressing STIM1 at the ER and PM was required for current activation by LTC4 or AA. However, exclusive expression of STIM1 at the ER was sufficient to mediate current activation by LTC4 or AA when the perforated patch-clamp technique or fura 2 Ca2+ imaging was used. We concluded in this study that a soluble factor might be involved in interacting with the STIM1-Orai1-Orai3 complex and required for store-independent Orai1/Orai3 channel activation by LTC4; this factor would be mostly dialyzed out when whole cell patch-clamp recordings are considered, but PM-STIM1 would help maintain its interaction with the channel complex. However, in the case of an intact cytosol, the soluble factor would be retained in the cytosol and the PM-STIM1 requirement would be bypassed (89).
Orai channel heteromultimerization and store-independent Orai channel activation likely represent means to enhance the diversity of receptor-activated Ca2+ entry routes, whereby different PLC-coupled agonists activate specific Ca2+ entry pathways in the same cell type. In fact, the downstream Ca2+-responsive pathways activated by ILRC in vascular SMCs are distinct from those activated by ICRAC (106). Unlike PDGF, stimulation of vascular SMCs with thrombin failed to induce NFAT nuclear translocation (106). On the other hand, knockdown of Orai3 or LTC4S caused a more robust and sustained phosphorylation of Akt1 and Akt2 on Ser473/Ser474 after serum stimulation, and this was accompanied by a decrease in SMC migration in vitro, suggesting an inhibitory effect of Akt signaling on vascular SMC migration. In vivo, Orai3 and LTC4S proteins, as well as ILRC densities, are increased in medial and neointimal SMCs acutely isolated from rat carotid arteries 14 days after balloon injury (30, 106). In vivo lentiviral transduction of injured carotid arteries with shRNA against Orai3 or LTC4S to prevent Orai3 and LTC4S upregulation inhibited ILRC in acutely isolated SMCs and reduced neointimal hyperplasia 14 days postinjury compared with injured vessels infected with control nontargeting shRNA (30, 106). Interestingly, Akt activity in acutely isolated SMCs, as assessed by phosphorylation on Ser473/Ser474, was abrogated in neointimal and medial SMCs at 14 days after carotid injury and was rescued when vessels were infected with lentiviral particles encoding shRNA against LTC4S or Orai3 (106). In a recent work, we used mouse embryonic fibroblasts isolated from Orai1 knockout mice and HEK-293 cells where endogenous Orai1 expression was silenced by siRNA (17). In each case, Orai1 expression was restored with the use of cDNA plasmids that exclusively express Orai1α or Orai1β driven by a weak promoter to recapitulate the low physiologically relevant levels of Orai1 expression and prevent the dominant-negative effects associated with Orai1 overexpression (17). We discovered that ICRAC could be recapitulated with Orai1α or Orai1β, while IARC/ILRC requires the long exclusively mammalian isoform Orai1α (17). Although it is expected that IARC/ILRC in vascular SMCs would also require Orai1α, this specific experiment remains to be performed.
Airway smooth muscle.
Recently, Thompson et al. (89) used fura 2 imaging to describe AA-mediated Ca2+ oscillations in human airway SMCs when 1–10 μM AA was used. These AA-activated Ca2+ oscillations depended on Ca2+ entry from the extracellular space, while other fatty acids, including the DAG analog 1-oleolyl-2-acetylglycerol, oleic acid, and palmitic acid, at 10 μM were unable to produce Ca2+ signals. Surprisingly, pharmacological inhibition of ryanodine receptor (with 10 μM ryanodine) or IP3 receptor (with 1 μM xestospongin C) to inhibit Ca2+ release from sarcoplasmic reticulum reduced the frequency and amplitude of AA-activated Ca2+ oscillations, while the SOCE inhibitor SKF-96365 did not, leading Thompson et al. to suggest that AA-activated Ca2+ influx could trigger sarcoplasmic reticulum Ca2+ release through Ca2+-induced Ca2+ release to regulate Ca2+ oscillations (89).
Thompson et al. (89) showed that siRNA knockdown of STIM1 and Orai3 inhibited the amplitude and frequency of AA-activated Ca2+ oscillations in human airway SMCs. Interestingly, similar results were obtained when caveolin-1 was knocked down with siRNA, suggesting that these channels are located within caveolin-rich regions of the PM (89); it is noteworthy that the long isoform of Orai1, Orai1α, uniquely involved in store-independent Orai1/Orai3 channels, contains a caveolin-binding region in the 63 NH2-terminal amino acids that are lacking in Orai1β (17, 26) (Fig. 1). Thompson et al. analyzed airway SMCs obtained from asthmatic patients and showed enhanced amplitude of AA-activated Ca2+ oscillations compared with controls (89), suggesting a role for this pathway in asthma pathology. Clearly, extensive whole cell electrophysiological recordings are warranted to define this AA-activated Ca2+ entry in airway SMCs. Furthermore, the specific downstream signaling mechanisms controlled by AA-activated Ca2+ oscillations in asthmatic airway SMCs and their contributions to manifestations of asthma are important issues that warrant additional investigations.
Concluding Remarks
In general, Orai-mediated store-dependent and -independent Ca2+ entry pathways (Fig. 2), which are activated by a variety of growth, migratory, and inflammatory agonists, are upregulated during phenotypic modulation of vascular and airway smooth muscle associated with vascular and airway pathophysiologies. In animal models of disease, when in vivo upregulation of these molecules is prevented, SMC dedifferentiation and remodeling are inhibited. This causal link between Orai channel function and disease is of major interest in the development of agents that target Orai-mediated pathways in prevention and treatment of vascular occlusive disease and airway obstructive disease. For example, the effective antiproliferative agent sirolimus (rapamycin), which is clinically used in drug-eluting stents to prevent postoperative vascular remodeling, has been shown to prevent proliferation of human coronary artery SMCs, at least in part through inhibition of the SOCE pathway and downstream CREB-mediated proliferative signaling (39). Novel drugs targeting specific isoforms of Orai, especially the exclusively mammalian Orai3 proteins or the heteromultimeric store-independent Orai1α/Orai3 channels, hold the promise of specific targeting of smooth muscle remodeling with limited side effects.
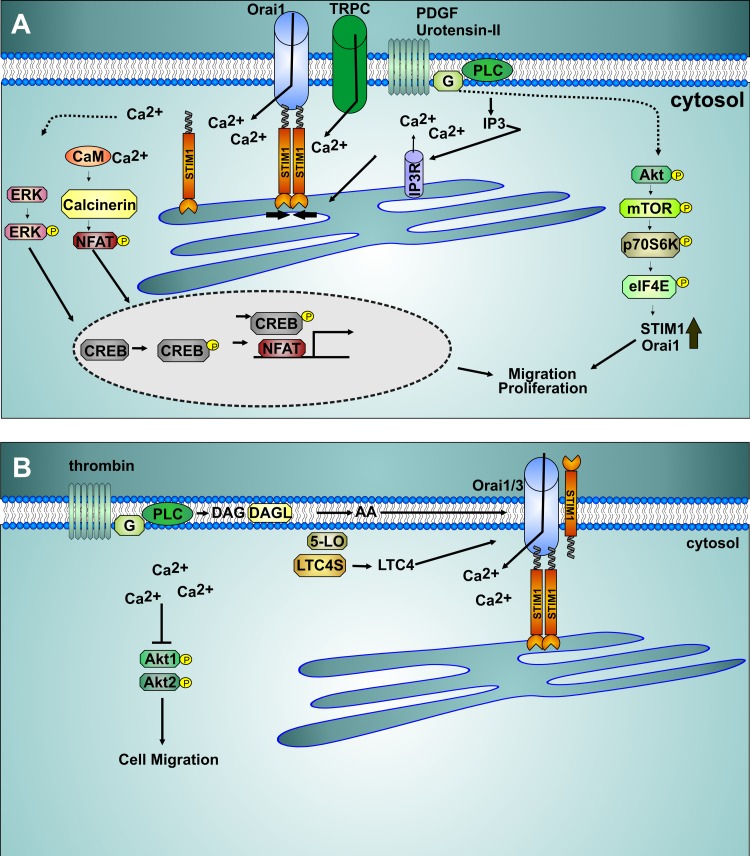
Fig. 2.
Store-dependent and -independent Ca2+ entry pathways in smooth muscle. A: activation of plasma membrane phospholipase C (PLC)-coupled receptors initiates production of inositol 1,4,5-triphosphate (IP3), promoting Ca2+ release and endoplasmic reticulum store depletion. Store depletion causes stromal-interacting molecule 1 (STIM1) translocation and binding to Orai1 Ca2+ channel to activate Ca2+ entry. Transient receptor potential canonical (TRPC) channel isoforms are also concomitantly activated in response to receptor stimulation in smooth muscle. Ca2+ entry activates the phosphatase calcinerin, with subsequent dephosphorylation and nuclear translocation of nuclear factor of activated T cells (NFAT), leading to transcription of genes involved in migration and proliferation. Store-operated Ca2+ entry (SOCE) was proposed to promote ERK and cAMP response element-binding protein (CREB) phosphorylation, enhancing CREB smooth muscle cell proliferation. Receptor activation of the Akt/mammalian target of rapamycin (mTOR)/P70S6 kinase (P70S6K)/eukaryotic translation initiation factor 4E (eIF4E) pathway promotes enhanced expression of STIM1 and Orai1 and enhanced SOCE. CaM, calmodulin; IP3R, IP3 receptor; G, G protein. B: receptor activation can lead to arachidonic acid (AA) generation through several routes, including metabolism of diacylglycerol (DAG) generated through PLC by DAG lipase. AA is further metabolized into downstream metabolites, including leukotrienes. Leukotriene C4 (LTC4) and AA activate Ca2+-selective channels composed of Orai1, Orai3, and STIM1, and this activation is independent of store depletion. Inhibition of these store-independent Orai1/Orai3 channels caused enhanced Akt1 and Akt2 phosphorylation and inhibited vascular smooth muscle cell migration. LTC4S, LTC4 synthase; 5-LO, 5-lipoxygenase.
GRANTS
Research in the authors' laboratory is funded by National Heart, Lung, and Blood Institute Grants R01 HL-123364 and R01 HL-097111 and American Heart Association Grant 14GRNT18880008 (to M. Trebak).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.S. and M.T. prepared the figures; A.M.S. and M.T. drafted the manuscript; A.M.S. and M.T. approved the final version of the manuscript; M.T. edited and revised the manuscript.
REFERENCES
- 1.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res 103: 1289–1299, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, Lechat P, Lompre AM, Hulot JS. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther 17: 455–462, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L909–L917, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297: C1103–C1112, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol 295: C779–C790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd'heuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol 298: C993–C1005, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I, Liang L, Limon I, Lompre AM, Hatem SN, Hajjar RJ, Lipskaia L. SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol 50: 621–633, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium 42: 133–144, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JH, Campbell GR. Smooth muscle phenotypic modulation—a personal experience. Arterioscler Thromb Vasc Biol 32: 1784–1789, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Cazzola M, Page CP, Rogliani P, Matera MG. β2-Agonist therapy in lung disease. Am J Respir Crit Care Med 187: 690–696, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLos Biol 9: e1001025, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution of TRPC1 and Orai1 to Ca2+ entry activated by store depletion. Adv Exp Med Biol 704: 435–449, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi S, Maleth J, Jha A, Lee KP, Kim MS, So I, Ahuja M, Muallem S. The TRPCs-Orai interaction. Hand Exp Pharmacol 223: 1035–1054, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, Pavord ID, McCormack D, Chaudhuri R, Miller JD, Laviolette M. Asthma control during the year after bronchial thermoplasty. N Engl J Med 356: 1327–1337, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Daskoulidou N, Zeng B, Berglund LM, Jiang H, Chen GL, Kotova O, Bhandari S, Ayoola J, Griffin S, Atkin SL, Gomez MF, Xu SZ. High glucose enhances store-operated calcium entry by upregulating ORAI/STIM via calcineurin-NFAT signalling. J Mol Med 93: 511–521, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Derler I, Fahrner M, Muik M, Lackner B, Schindl R, Groschner K, Romanin CA. A Ca2+ release-activated Ca2+ (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2+-dependent inactivation of ORAI1 channels. J Biol Chem 284: 24933–24938, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, Trebak M. Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci Signal 8: ra74, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez-Rodriguez A, Diaz I, Rodriguez-Moyano M, Calderon-Sanchez E, Rosado JA, Ordonez A, Smani T. Urotensin-II signaling mechanism in rat coronary artery: role of STIM1 and Orai1-dependent store operated calcium influx in vasoconstriction. Arterioscler Thromb Vasc Biol 32: 1325–1332, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, Byrd JP, Kumar S, Obukhov AG, Sturek M. Exercise training decreases store-operated Ca2+ entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res 85: 631–640, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias JA, Wu Y, Zheng T, Panettieri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am J Physiol Lung Cell Mol Physiol 273: L648–L655, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez RA, Wan J, Song S, Smith KA, Gu Y, Tauseef M, Tang H, Makino A, Mehta D, Yuan JX. Upregulated expression of STIM2, TRPC6, and Orai2 contributes to the transition of pulmonary arterial smooth muscle cells from a contractile to proliferative phenotype. Am J Physiol Cell Physiol 308: C581–C593, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Feske S, Okamura H, Hogan PG, Rao A. Ca2+/calcineurin signalling in cells of the immune system. Biochem Biophys Res Commun 311: 1117–1132, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Frischauf I, Muik M, Derler I, Bergsmann J, Fahrner M, Schindl R, Groschner K, Romanin C. Molecular determinants of the coupling between STIM1 and Orai channels: differential activation of Orai1-3 channels by a STIM1 coiled-coil mutant. J Biol Chem 284: 21696–21706, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukushima M, Tomita T, Janoshazi A, Putney JW. Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities. J Cell Sci 125: 4354–4361, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao YD, Zheng JW, Li P, Cheng M, Yang J. Store-operated Ca2+ entry is involved in transforming growth factor-β1 facilitated proliferation of rat airway smooth muscle cells. J Asthma 50: 439–448, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Giachini FR, Chiao CW, Carneiro FS, Lima VV, Carneiro ZN, Dorrance AM, Tostes RC, Webb RC. Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: a novel insight into vascular dysfunction. Hypertension 53: 409–416, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Cobos JC, Trebak M. TRPC channels in smooth muscle cells. Front Biosci 15: 1023–1039, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Cobos JC, Zhang X, Zhang W, Ruhle B, Motiani RK, Schindl R, Muik M, Spinelli AM, Bisaillon JM, Shinde AV, Fahrner M, Singer HA, Matrougui K, Barroso M, Romanin C, Trebak M. Store-independent Orai1/3 channels activated by intracrine leukotriene C4: role in neointimal hyperplasia. Circ Res 112: 1013–1025, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo RW, Yang LX, Li MQ, Pan XH, Liu B, Deng YL. Stim1- and Orai1-mediated store-operated calcium entry is critical for angiotensin II-induced vascular smooth muscle cell proliferation. Cardiovasc Res 93: 360–370, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem 282: 16232–16243, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Hill-Eubanks DC, Gomez MF, Stevenson AS, Nelson MT. NFAT regulation in smooth muscle. Trends Cardiovasc Med 13: 56–62, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Hooper R, Zhang X, Webster M, Go C, Kedra J, Marchbank K, Gill DL, Weeraratna AT, Trebak M, Soboloff J. Novel protein kinase C-mediated control of Orai1 function in invasive melanoma. Mol Cell Biol 35: 2790–2798, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355: 353–356, 1992. [DOI] [PubMed] [Google Scholar]
- 36.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflügers Arch 456: 769–785, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia L, Delmotte P, Aravamudan B, Pabelick CM, Prakash YS, Sieck GC. Effects of the inflammatory cytokines TNF-α and IL-13 on stromal interaction molecule-1 aggregation in human airway smooth muscle intracellular Ca2+ regulation. Am J Respir Cell Mol Biol 49: 601–608, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kassan M, Zhang W, Aissa KA, Stolwijk J, Trebak M, Matrougui K. Differential role for stromal interacting molecule 1 in the regulation of vascular function. Pflügers Arch 467: 1195–1202, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konig S, Browne S, Doleschal B, Schernthaner M, Poteser M, Machler H, Wittchow E, Braune M, Muik M, Romanin C, Groschner K. Inhibition of Orai1-mediated Ca2+ entry is a key mechanism of the antiproliferative action of sirolimus in human arterial smooth muscle. Am J Physiol Heart Circ Physiol 305: H1646–H1657, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Lacruz RS, Feske S. Diseases caused by mutations in ORAI1 and STIM1. Ann NY Acad Sci 1356: 45–79, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc Natl Acad Sci USA 106: 14687–14692, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, McKeown L, Ojelabi O, Stacey M, Foster R, O'Regan D, Porter KE, Beech DJ. Nanomolar potency and selectivity of a Ca2+ release-activated Ca2+ channel inhibitor against store-operated Ca2+ entry and migration of vascular smooth muscle cells. Br J Pharmacol 164: 382–393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res 97: 488–495, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Zheng X, Mueller GA, Sobhany M, DeRose EF, Zhang Y, London RE, Birnbaumer L. Crystal structure of calmodulin binding domain of Orai1 in complex with Ca2+ calmodulin displays a unique binding mode. J Biol Chem 287: 43030–43041, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lompre AM, Benard L, Saliba Y, Aubart F, Fauconnier J, Hulot JS. STIM1 and Orai in cardiac hypertrophy and vascular proliferative diseases. Front Biosci 5: 766–773, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Mancarella S, Potireddy S, Wang Y, Gao H, Gandhirajan RK, Autieri M, Scalia R, Cheng Z, Wang H, Madesh M, Houser SR, Gill DL. Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J 27: 893–906, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mignen O, Shuttleworth TJ. IARC, a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J Biol Chem 275: 9114–9119, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Mignen O, Shuttleworth TJ. Permeation of monovalent cations through the non-capacitative arachidonate-regulated Ca2+ channels in HEK293 cells. Comparison with endogenous store-operated channels. J Biol Chem 276: 21365–21374, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol 586: 185–195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol 579: 703–715, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem 285: 19173–19183, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M. Orai3 is an estrogen receptor-α-regulated Ca2+ channel that promotes tumorigenesis. FASEB J 27: 63–75, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J Biol Chem 284: 8421–8426, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem 283: 8014–8022, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci USA 106: 15495–15500, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munoz E, Valero RA, Quintana A, Hoth M, Nunez L, Villalobos C. Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/Orai channels normally prevented by mitochondria. J Biol Chem 286: 16186–16196, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng LC, O'Neill KG, French D, Airey JA, Singer CA, Tian H, Shen XM, Hume JR. TRPC1 and Orai1 interact with STIM1 and mediate capacitative Ca2+ entry caused by acute hypoxia in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 303: C1156–C1172, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Ogawa A, Firth AL, Smith KA, Maliakal MV, Yuan JX. PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 302: C405–C411, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pavord ID, Thomson NC, Niven RM, Corris PA, Chung KF, Cox G, Armstrong B, Shargill NS, Laviolette M. Safety of bronchial thermoplasty in patients with severe refractory asthma. Ann Allergy Asthma Immunol 111: 402–407, 2013. [DOI] [PubMed] [Google Scholar]
- 64.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res 7: 119, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol 38: 744–749, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, Ludwig MS, Martin JG, Hamid Q. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol 116: 544–549, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J 23: 2425–2437, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potier M, Trebak M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflügers Arch 457: 405–415, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev 95: 1383–1436, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium 7: 1–12, 1986. [DOI] [PubMed] [Google Scholar]
- 72.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev 15: 237–254, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Moyano M, Diaz I, Dionisio N, Zhang X, Avila-Medina J, Calderon-Sanchez E, Trebak M, Rosado JA, Ordonez A, Smani T. Urotensin-II promotes vascular smooth muscle cell proliferation through store-operated calcium entry and EGFR transactivation. Cardiovasc Res 100: 297–306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruhle B, Trebak M. Emerging roles for native Orai Ca2+ channels in cardiovascular disease. Curr Top Membr 71: 209–235, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shinde AV, Motiani RK, Zhang X, Abdullaev IF, Adam AP, Gonzalez-Cobos JC, Zhang W, Matrougui K, Vincent PA, Trebak M. STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci Signal 6: ra18, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shuttleworth TJ. Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J Biol Chem 271: 21720–21725, 1996. [DOI] [PubMed] [Google Scholar]
- 77.Shuttleworth TJ. Orai3—the “exceptional” Orai? J Physiol 590: 241–257, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shuttleworth TJ. STIM and Orai proteins and the non-capacitative ARC channels. Front Biosci 17: 847–860, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda) 19: 355–361, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Song MY, Makino A, Yuan JX. STIM2 contributes to enhanced store-operated Ca entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ 1: 84–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spinelli AM, Gonzalez-Cobos JC, Zhang X, Motiani RK, Rowan S, Zhang W, Garrett J, Vincent PA, Matrougui K, Singer HA, Trebak M. Airway smooth muscle STIM1 and Orai1 are upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC currents, proliferation, and migration. Pflügers Arch 464: 481–492, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Srikanth S, Jung HJ, Ribalet B, Gwack Y. The intracellular loop of Orai1 plays a central role in fast inactivation of Ca2+ release-activated Ca2+ channels. J Biol Chem 285: 5066–5075, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306: 67–69, 1983. [DOI] [PubMed] [Google Scholar]
- 84.Suganuma N, Ito S, Aso H, Kondo M, Sato M, Sokabe M, Hasegawa Y. STIM1 regulates platelet-derived growth factor-induced migration and Ca2+ influx in human airway smooth muscle cells. PLos One 7: e45056, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sutovska M, Adamkov M, Kocmalova M, Mesarosova L, Oravec M, Franova S. CRAC ion channels and airway defense reflexes in experimental allergic inflammation. Adv Exp Med Biol 756: 39–48, 2013. [DOI] [PubMed] [Google Scholar]
- 86.Takemura H, Hughes AR, Thastrup O, Putney JW Jr.. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem 264: 12266–12271, 1989. [PubMed] [Google Scholar]
- 87.Thompson J, Mignen O, Shuttleworth TJ. The N-terminal domain of Orai3 determines selectivity for activation of the store-independent ARC channel by arachidonic acid. Channels (Austin) 4: 398–410, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson JL, Shuttleworth TJ. A plasma membrane-targeted cytosolic domain of STIM1 selectively activates ARC channels, an arachidonate-regulated store-independent Orai channel. Channels (Austin) 6: 370–378, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson MA, Prakash YS, Pabelick CM. Arachidonate-regulated Ca2+ influx in human airway smooth muscle. Am J Respir Cell Mol Biol 51: 68–76, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trebak M. PLC: Johnny-come-lately to ORAI and the ups and downs of calcium signalling. J Physiol 589: 5337–5338, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trebak M. STIM1/Orai1, ICRAC, and endothelial SOC. Circ Res 104: e56–e57, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trebak M. STIM/Orai signalling complexes in vascular smooth muscle. J Physiol 590: 4201–4208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trebak M, Bird GS, McKay RR, Putney JW Jr.. Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem 277: 21617–21623, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol 16: 2073–2079, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest 123: 417s–422s, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Deng X, Hewavitharana T, Soboloff J, Gill DL. Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clin Exp Pharmacol Physiol 35: 1127–1133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, Lu W, Yang K, Wang Y, Zhang J, Jia J, Yun X, Tian L, Chen Y, Jiang Q, Zhang B, Chen X, Wang J. Peroxisome proliferator-activated receptor-γ inhibits pulmonary hypertension targeting store-operated calcium entry. J Mol Med 93: 327–342, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, Klussmann E, Cooper DM. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal 5: ra29, 2012. [DOI] [PubMed] [Google Scholar]
- 99.Wylam ME, Sathish V, VanOosten SK, Freeman M, Burkholder D, Thompson MA, Pabelick CM, Prakash YS. Mechanisms of cigarette smoke effects on human airway smooth muscle. PLos One 10: e0128778, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang K, Lu W, Jiang Q, Yun X, Zhao M, Jiang H, Wang J. Peroxisome proliferator-activated receptor γ-mediated inhibition on hypoxia-triggered store-operated calcium entry. A caveolin-1-dependent mechanism. Am J Respir Cell Mol Biol 53: 882–892, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443: 226–229, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 11: 337–343, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 292: L1202–L1210, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M, Singer HA, Matrougui K, Trebak M. Orai1-mediated ICRAC is essential for neointima formation after vascular injury. Circ Res 109: 534–542, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang W, Trebak M. Vascular balloon injury and intraluminal administration in rat carotid artery. J Vis Exp. In press. [DOI] [PMC free article] [PubMed]
- 106.Zhang W, Zhang X, Gonzalez-Cobos JC, Stolwijk JA, Matrougui K, Trebak M. Leukotriene-C4 synthase, a critical enzyme in the activation of store-independent Orai1/Orai3 channels, is required for neointimal hyperplasia. J Biol Chem 290: 5015–5027, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X, Gonzalez-Cobos JC, Schindl R, Muik M, Ruhle B, Motiani RK, Bisaillon JM, Zhang W, Fahrner M, Barroso M, Matrougui K, Romanin C, Trebak M. Mechanisms of STIM1 activation of store-independent leukotriene C4-regulated Ca2+ channels. Mol Cell Biol 33: 3715–3723, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X, Zhang W, Gonzalez-Cobos JC, Jardin I, Romanin C, Matrougui K, Trebak M. Complex role of STIM1 in the activation of store-independent Orai1/3 channels. J Gen Physiol 143: 345–359, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zou JJ, Gao YD, Geng S, Yang J. Role of STIM1/Orai1-mediated store-operated Ca2+ entry in airway smooth muscle cell proliferation. J Appl Physiol 110: 1256–1263, 2011. [DOI] [PubMed] [Google Scholar]