obstructive sleep apnea (OSA) is one of the common sleep disorders that affects the health and quality of life for millions of people in the US, particularly obese or overweight middle-aged men. OSA is characterized by brief periodic recesses in breathing or instances of shallow breathing during sleep due to partial or complete collapse of the upper airway, which consequently leads to a decrease in oxygen (O2) saturation (6). Symptoms of OSA include unexplained daytime sleepiness, snoring, nocturnal gasping/choking, and insomnia. OSA has been strongly implicated in the pathogenesis of cardiovascular disease. In fact, individuals with OSA have a higher incidence of cardiovascular morbidities including hypertension, ischemic heart disease, chronic heart failure, arrhythmias, and stroke compared with the general population (8). Acute sympathetic activation is considered an important mechanism of cardiovascular morbidities and mortality in patients with OSA (7). Additionally, sympathetic activation is associated with the generation of cardiac arrhythmias in congenital long QT syndrome (LQTS) patients (1). LQTS is a familial cardiac condition characterized by prolonged ventricular repolarization and increased risk of arrhythmogenic episodes that may result in sudden cardiac death due to ventricular fibrillation (6). A recent study reported that OSA patients with congenital LQTS exhibit increased QT prolongation and sudden cardiac death (6).
KCNH2, also known as the human ether-a-go-go-related gene (hERG), encodes the pore-forming subunit of the delayed rectifier voltage-gated K+ channel (9). These channels are expressed in numerous cell types and are implicated in a variety of cellular functions, but they are most notable for their role in cardiac myocytes. hERG K+ channels are essential for maintaining normal cardiac electrical activity as they conduct K+ during the terminal repolarization phase of the action potential. Indeed, Curran and colleagues (2) determined that inherited loss-of-function mutations in hERG K+ channels are associated with LQTS. Most missense mutations in KCNH2 have been linked to defective trafficking of KCNH2 channels; however, approximately 25% of KCNH2 mutations result in premature stop codons which may undergo nonsense-mediated decay (3).
In a recent issue of AJP Cell Physiology, Wang et al. (10) provide evidence for hypoxia-mediated hERG K+ channel protein degradation via reactive oxygen species (ROS)-dependent activation of Ca2+-sensitive calpain proteases. The authors have previously shown that ROS generated by hypoxia were capable of downregulating hERG translation and attenuating hERG-mediated K+ current in HEK293 (4). As recurrent apneas result in chronic intermittent hypoxia (IH), a hallmark of OSA, the authors wanted to determine whether downregulation of hERG proteins by IH contributes to heart rate abnormalities associated with OSA. The authors found that expression of hERG proteins is attenuated in SH-SY5Y neuroblastoma cells and neonatal rat adrenal medullary chromaffin cells by IH in a stimulus-dependent manner. Furthermore, this IH-induced decrease in hERG protein expression in SH-SY5Y cells was also associated with attenuated hERG K+ current in a stimulus-dependent manner. Using RT-PCR, the authors discovered that hERG mRNA levels are increased compared with control cells, which suggests that transcriptional changes are unlikely the reason for the IH-mediated downregulation of hERG channel expression. Treatment with proteosomal or lysosomal inhibitors failed to prevent degradation of hERG proteins by IH, suggesting that proteosomal/lysosomal activity did not account for the IH-mediated degradation of hERG proteins in SH-SY5Y cells. Previously, the authors discovered that IH-mediated ROS production increases [Ca2+]i levels in rat PC12 cells (5); therefore they sought to determine whether ROS-dependent Ca2+ signaling contributes to hERG protein degradation. The authors reported that neuroblastoma cells exposed to IH had increased ROS levels and hERG protein degradation, which was prevented via pretreatment with a membrane-permeable ROS scavenger. The authors found that basal [Ca2+]i was increased in IH-stimulated cells; furthermore this effect could be inhibited by application of an ROS scavenger. In addition, Ca2+ chelation prevented hERG degradation by IH whereas Ca2+ ionophores decreased hERG protein levels under normoxic conditions. Calpain, a Ca2+-dependent protease, was shown to display increased enzyme activity in IH-exposed cells, which was blocked by ALLM, a potent inhibitor of calpains. Furthermore, the author indicated that ALLM prevented the degradation of hERG protein and restored hERG-mediated currents. Finally, the authors found that hERG proteins are one of the substrates for calpain as revealed by immunoblot experiments. Therefore these results presented in this study suggest that activation of calpains by ROS-dependent elevation of [Ca2+]i mediates hERG protein degradation by IH.
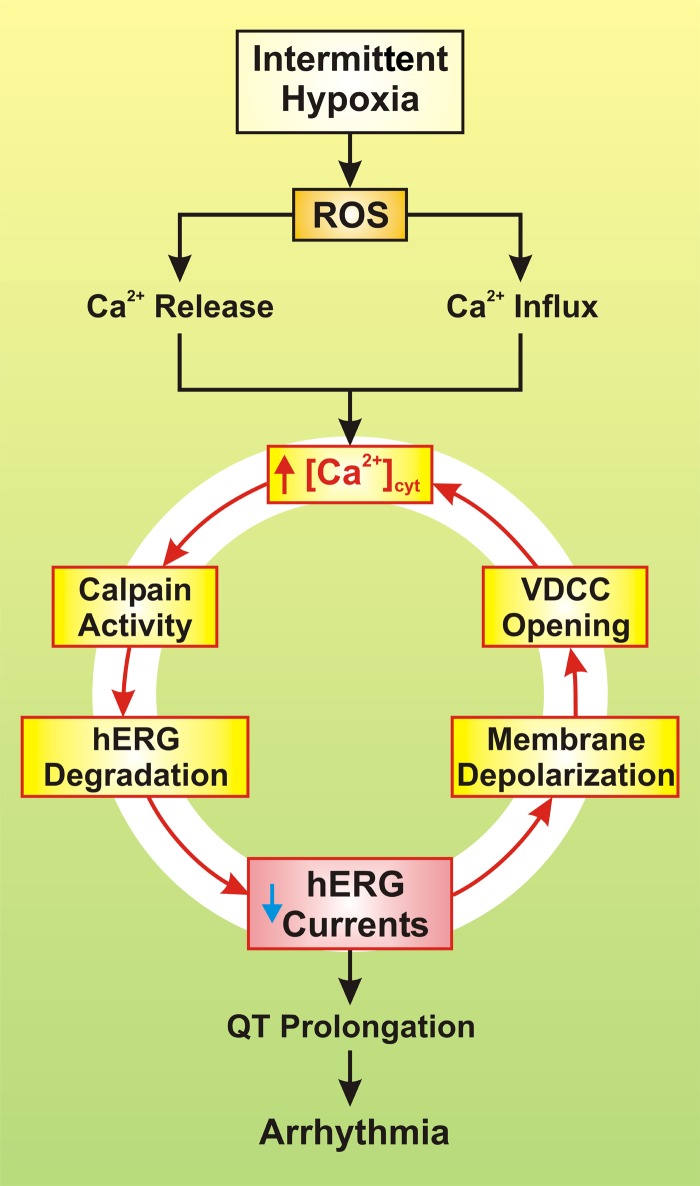
This novel finding in concert with previous studies suggests that IH serves as a trigger for hERG protein degradation and dysfunction that consequently alters cardiac QT (Fig. 1). IH stimulates ROS production, which leads to elevation of cytosolic free Ca2+ concentration ([Ca2+]cyt) via depletion of Ca2+ stores and Ca2+ influx. This leads to the activation of calpain, which appears to use hERG proteins as a substrate for degradation. Degradation or deficient trafficking of hERG proteins results in dysfunction of hERG channels, attenuates hERG-mediated K+ currents, and prolongs QT interval, which ultimately leads to development of arrhythmias.
Fig. 1.
Proposed mechanisms involved in intermittent hypoxia-mediated arrhythmia. Reactive oxygen species (ROS), increased by intermittent hypoxia, trigger Ca2+ release from the sarcoplasmic reticulum (SR) and mitochondria and causes Ca2+ influx through voltage-dependent Ca2+ channels (VDCC) and transient receptor potential vanilloid (TRPV) channels. The resulting increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) then activates calpain, a Ca2+-dependent, non-lysosomal cysteine protease, which causes degradation of human ERG (hERG) channels and decrease in whole cell K+ currents through hERG channels. Reduced hERG K+ current then leads to prolongation of the Q-T interval and ultimately to arrhythmia and sudden cardiac death. The reduced hERG K+ current also causes membrane depolarization that opens VDCC, enhances Ca2+ influx through VDCC, and further increases [Ca2+]cyt in the cardiomyocytes. The loop formed by the initial ROS-mediated increase in [Ca2+]cyt and the calpain-mediated decrease in whole cell hERG K+ currents may serve as a pathogenic sequence of events for ROS-mediated arrhythmia in patients with OSA or individuals exposed to intermittent hypoxia.
Patients with OSA and those with LQTS in particular may experience increased risk of developing life-threatening arrhythmias. However, some studies have indicated no direct connection between OSA and increased risk of arrhythmias (1). Therefore the controversial relationship of OSA and LQTS should be resolved before any assumptions are made. Reduced hERG K+ current efflux also causes membrane depolarization that enhances Ca2+ influx through voltage-dependent Ca2+ channels (VDCC) and further increases [Ca2+]cyt. Thus further studies should provide insight into this positive feedback loop formed by the initial ROS-mediated increase in [Ca2+]cyt and the calpain-mediated decrease in whole cell hERG K+ currents that may serve as a pathogenic sequence of events for ROS-mediated arrhythmia in patients with OSA or individuals exposed to intermittent hypoxia. Additionally, this study employs the use of a cell line to explore IH-induced hERG degradation which may not reflect actual pathogenic mechanisms; therefore it is important to characterize the IH-mediated effects in human cardiomyocytes. Overall, this study provides valuable insight into the mechanisms of IH-mediated hERG degradation and dysfunction that may provide the future impetus for developing therapeutic targets of calpain, potentially reducing the incidence of arrhythmias and sudden cardiac death in patients with OSA and LQTS.
GRANTS
This work was supported in part by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL-11504, HL-055012, and HL-125208).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.J.A. and J.X.-J.Y. prepared figures; R.J.A. drafted manuscript; R.J.A., H.T., and J.X.-J.Y. edited and revised manuscript; R.J.A., H.T., and J.X.-J.Y. approved final version of manuscript.
REFERENCES
- 1.Barta K, Szabó Z, Kun C, Munkácsy C, Bene O, Tünde Magyar M, Csiba L, Lörincz I. The effect of sleep apnea on QT interval, QT dispersion, and arrhythmias. Clin Cardiol 33: E35–E39, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curran ME, Splawski I, Timothy KW, Vincen GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80: 795–803, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Gong Q, Zhang L, Vincent GM, Horne BD, Zhou Z. Nonsense mutations in hERG cause a decrease in mutant mRNA transcripts by nonsense-mediated mRNA decay in human long-QT syndrome. Circulation 116: 17–24, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanduri J, Wang N, Bergson P, Yuan G, Ficker E, Prabhakar NR. Mitochondrial reactive oxygen species mediate hypoxic down-regulation of hERG channel protein. Biochem Biophys Res Commun 373: 309–314, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA 106: 1199–1204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shamsuzzaman AS, Somers VK, Knilans TK, Ackerman MJ, Wang Y, Amin RS. Obstructive sleep apnea in patients with congenital long QT syndrome: implications for increased risk of sudden cardiac death. Sleep 38: 1113–1119, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement From the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing In Collaboration With the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118: 1080–1111, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K+ channels: structure, function, and clinical significance. Physiol Rev 92: 1393–1478, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Wang N, Kang HS, Ahmmed G, Khan SA, Makarenko VV, Prabhakar NR, Nanduri J. Calpain activation by ROS mediates human ether-a-go-go-related gene protein degradation by intermittent hypoxia. Am J Physiol Cell Physiol 310: C329–C336, 2016. doi: 10.1152/ajpcell.00231.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]