Abstract
Membrane contact sites (MCS) are critical junctions that form between the endoplasmic reticulum (ER) and membranes of various organelles, including the plasma membrane (PM). Signaling complexes, including mediators of Ca2+ signaling, are assembled within MCS, such as the ER/PM junction. This is most evident in polarized epithelial cells, such as pancreatic cells. Core Ca2+ signaling proteins cluster at the apical pole, the site of inositol 1,4,5-trisphosphate-mediated Ca2+ release and Orai1/transient receptor potential canonical-mediated store-dependent Ca2+ entry. Recent advances have characterized the proteins that tether the membranes at MCS and the role of these proteins in modulating physiological and pathological intracellular signaling. This review discusses recent advances in the characterization of Ca2+ signaling at ER/PM junctions and the relation of these junctions to physiological and pathological Ca2+ signaling in pancreatic acini.
Keywords: signaling, PM, ER, junctions, pancreas
intracellular signaling pathways are mediated by signaling complexes that interact with each other, often synergistically, to enhance downstream physiological responses. These signal transduction proteins must be in close physical proximity to allow these interactions. Each pathway is composed of several proteins with defined functions that regulate both the spatial and temporal fidelity of the signals and convey them to targets within the cell interior. The proteins that make the pathways are assembled into signaling complexes that interact with each other in molecular distances. To promote organized signaling cascades, the various steps are compartmentalized within specific cellular sites. Compartmentalized signaling complexes are present in the plasma membrane (PM), endosomes, lysosomes, mitochondria, and the endoplasmic reticulum (ER). Within these compartments, signaling complexes are further restricted to defined microdomains.
In recent years, an improved molecular understanding of these microdomains has emerged, especially of the membrane contact sites (MCS) that form between the ER and other cellular membranes. MCS are formed by tethering proteins at the ER/PM junctions (7, 31), ER/mitochondria (113), ER/endosomes/lysosomes (81) [vacuoles in yeast (85)], and ER/nuclear membrane (85). Many MCS serve to communicate and transfer material between membranes, particularly lipids in a nonvesicular form of transport (50). This review focuses on one particular MCS, the ER/PM junction, due to its role in Orai1/stromal interacting molecule 1 (STIM1)-mediated Ca2+ signaling. Discussion of other MCS can be found in several excellent recent reviews (31, 50, 81, 85, 88, 113).
The Pancreatic ER/PM Junctions
Secretory epithelial cells, including those in the pancreas, lung, salivary glands, and other gastrointestinal organs are all polarized structurally with basal and apical poles. This structure allows their polarized functions of exocytosis and transcellular fluid and electrolyte secretion (54). Accordingly, signaling machinery in these cell types, including the Ca2+ signaling apparatus, are organized into complexes in ER/PM junctions. The ER/PM junctions in secretory cells are most prominent at the apical pole. All G protein-coupled receptors (GPCRs) examined, including the cholecystokinin and muscarinic receptors that signal through changes in cytoplasmic Ca2+ {intracellular Ca2+ concentration ([Ca2+]i)} and the vasoactive intestinal peptide receptors that signal by increasing cellular cAMP, are localized at the tight junctions (32, 99). Immunolocalization and functional studies show that all inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) are expressed at high levels at the apical pole (56, 126), with IP3R2 and IP3R3 being the dominant IP3Rs in exocrine secretory cells (125). The PM Ca2+-ATPase pump (PMCA) isoform 4 is confined to the apical pole (99), while the housekeeping PMCA1 is localized at the lateral and basal membranes (55). Exocrine cells also express high level of transient receptor potential canonical 1 (TRPC1), TRPC3, and TRPC6 at their apical pole (34, 43). The store-operated Orai1 channel is markedly enriched at the apical pole, and, on depletion of ER Ca2+, the ER Ca2+ sensor STIM1 clusters at the apical pole (34). Finally, the scaffolding Homer proteins that bind IP3Rs, TRPC channels, GPCRs (117, 122), and PMCA (121) are confined to the apical pole.
The placement of Ca2+ signaling components at ER/PM junctions in exocrine cells accounts for polarized Ca2+ signaling in these cells. Polarized cell signaling is designed to meet the specific functions of the cells. This is reflected very well in the function of pancreatic and salivary acinar cells. The cardinal function of these cells is exocytosis of digestive enzymes and secretion of isotonic, NaCl-rich fluid (54). Both secretory functions depend on critical apical pole proteins and transporters. Pancreatic exocytosis is mediated by increases in free cytoplasmic Ca2+ ([Ca2+]i) (66), while exocytosis by salivary gland acinar cells is triggered by increases in cytoplasmic cAMP (86). Fluid secretion by both acinar cell types follows Ca2+ oscillations at the apical pole. The receptor-evoked Ca2+ signal starts with Ca2+ release from the ER, followed by activation of Ca2+ influx across the PM through the Orai and TRPC store-operated Ca2+ influx channels (SOCs). ER Ca2+ release is limited, and Ca2+ influx sustains the Ca2+ signal and reloads intracellular/ER stores with Ca2+ at the end of the stimulation period. The increase in [Ca2+]i activates the PMCA and ER/sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA) pump, which remove Ca2+ from the cytoplasm to restore basal cytoplasmic Ca2+. At physiological stimulus intensity, the channels periodically inactivate, and the cycle of Ca2+ release and influx is repeated, resulting in Ca2+ oscillations. Overactivation of Ca2+ influx causes a sustained, pathological increase in [Ca2+]i. Hence, Ca2+ influx mediates both physiological Ca2+ oscillations and pathological sustained increases in [Ca2+]i.
An increase in [Ca2+]i activates the Ca2+-activated Cl− channel anoctamin 1 (ANO1) [transmembrane protein (TMEM) 16A] at the apical membrane (9, 79) and K+ channels at both the apical and basolateral membranes (2, 89, 90). This results in K+ and Cl− efflux and cell shrinkage. Electrical neutrality is achieved by transcellular Na+ flux through the tight junctions. NaCl secretion dives fluid secretion through the water channel aquaporin 5 (25). Cell shrinkage inhibits Ca2+ signaling via unknown mechanisms; this results in [Ca2+]i reduction back to baseline, inhibition of the Cl− and K+ channels, and inhibition of fluid secretion. Simultaneously, cell shrinkage activates the basolateral Na+-K+-2Cl− cotransporter 1 (NKCC1) (54, 65), which is regulated by the cell volume and osmolality sensitive kinases WNK (with no lysine) and SPAK (Ste20-related proline-alanine-rich kinase) (25, 39). The Na+ entering the cells through NKCC1 is exchanged with K+ by the Na+ pump. Restoration of cytoplasmic K+ and Cl− by NKCC1 is followed first by restoration of cell volume and then by a second Ca2+ spike, initiating another secretory event. In this manner, acinar cells function as fluid and electrolyte secretory pumps.
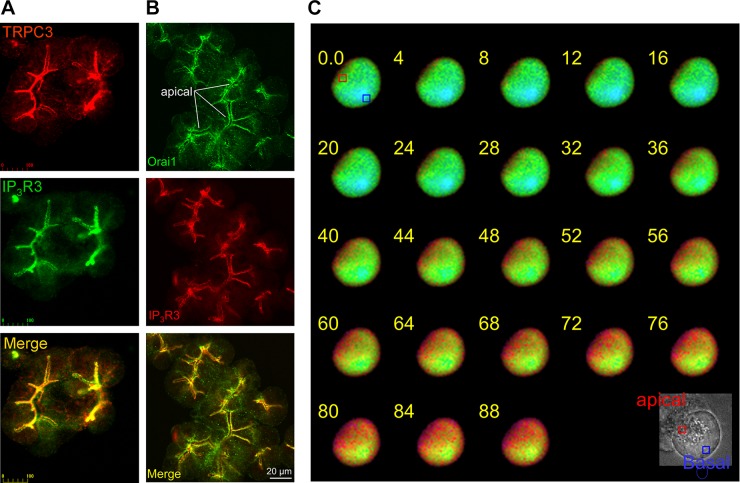
The discussion above highlights the reason for generation of signaling mediators at the apical pole of epithelial cells. Indeed, the Ca2+ signal in acinar (40, 106) and duct cells (56, 99) always initiates at the apical pole and propagates to the basal pole. Moreover, each receptor has a Ca2+ signaling signature with a defined initiation site and propagation pattern (99), which is regulated by specific RGS (regulators of G protein signaling) proteins (118, 127). Mapping receptor-stimulated Ca2+ signaling by localized stimulation at cellular microdomains revealed much higher sensitivity of the apical than the basal pole to agonist stimulation (57), likely due to the high level of GPCRs at this pole, as described above. Physiological stimulus intensity evokes Ca2+ oscillations that can remain confined to the apical pole (82), where they stimulate the Ca2+-activated Cl− channels (79). A mitochondrial belt at the apical pole around the secretory granules helps confine the Ca2+ signal to the apical pole (5, 107). Similarly, localized release of IP3 at low concentrations readily releases Ca2+ from the ER at the apical pole, while a much higher concentration of IP3 is needed to release Ca2+ from the basal pole (24). Although Ca2+ can enter the cells across the basolateral membrane when presented only to this membrane (68), Ca2+ mainly enters the cells when Ca2+ release from the ER activates the SOC Orai1 and the TRPC channels at the apical pole (13, 34). Localization of the TRPC3 and Orai1 channels allowing Ca2+ entry at the ER/PM junctions of acinar cells is illustrated in Fig. 1.
Fig. 1.
Clustering of Ca2+ influx channels and Ca2+ influx at the apical pole of pancreatic acini. A and B: colocalization of inositol 1,4,5-trisphosphate receptor 3 (IP3R3) and of transient receptor potential canonical 3 (A) and Orai1 (B) at the apical pole of pancreatic acini. C: polarized pancreatic acinar cells (note the apical granules at the differential interference contrast image, bottom right) was treated with 0.5 mM carbachol and 25 μM of the endoplasmic reticulum (ER)/sarcoplasmic reticulum Ca2+-ATPase inhibitor cyclopiazonic acid in Ca2+-free solution to deplete the stores and maximally activate Ca2+ influx. The cell was then rapidly perfused with a solution containing 5 mM Ca2+, and images were collected every 0.3 s to identify the site on Ca2+ influx. It is clear that Ca2+ enters the cell primarily from the apical region, where the Ca2+ influx channels are clustered. A is reproduced from Ref. 43, and B and C are from Ref. 34.
Components of the cAMP-dependent signaling pathway are also localized at the apical pole ER/PM junctions of acinar cells. Several Ca2+-dependent adenylyl cyclases (ACs) are localized at the apical pole (73). Localization of ACs is determined by A-kinase anchoring proteins that interact with apical cytoskeletal proteins like Ezrin (95, 128). Clear compartmentalization of all components of the cAMP pathway has been extensively demonstrated in muscles, where they are expressed at the SR/PM junctions (23, 29). Moreover, receptor stimulation causes large elevations in cAMP, leading to PKA activation at the junctions (23, 92). In muscles and secretory cells, the Ca2+ and cAMP signaling pathways are in close proximity, allowing their functional interaction. Indeed, cross-activation (15) and synergism (1, 80) between these two pathways are well established. Of particular interest is the regulation of AC8 and perhaps other Ca2+-dependent ACs by Ca2+ entering the cells through Orai1 (114) and TRPC1 (115). This regulation depends on the presence of all components in caveolae (74) and requires the direct interaction of AC8 with Orai1 (114) and perhaps with TRPC channels. The ER/PM junctions are enriched in caveolae (74).
Several of the key questions in understanding signaling at the ER/PM junctions are how the junctions are formed, how they are regulated, and how they affect cell function. There is very little information on the proteins that form and maintain the junctions in vivo or in any secretory cells. However, studies of nonvesicular lipid transport in yeast (50, 85) and in model mammalian cell systems (for reviews, see Refs. 7, 31, 85) are providing information that is relevant to all cells and is discussed below.
Tethering the ER/PM Junctions
In yeast, ∼40% of the PM is tethered to the ER and is thus a robust system to study the ER/PM junctions. The ER is tethered to the PM by specialized proteins that form and stabilize the ER/PM junctions and regulate their functions. Early work in yeast identified the tricalbins as proteins that localize to the ER/PM junctions and are required for lipid transfer between the membranes (109). Localization of the tricalbins to the junctions requires their interaction with the PM lipids phosphatidylinositol-4-phosphate (PI4P) and phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2] (64, 109). Another essential protein for formation of the yeast junctions is Ist2, which has a polybasic domain that interacts with PI(4,5)P2 (60, 116). Ssy1 is a protein with a similar general domain structure to Ist2 that is targeted to the ER/PM junctions. Ssy1 also has an ER transmembrane domain, a PM lipid-binding domain, and a disordered linker that bridges the ER/PM distance (49). Although the exact function of Ssy1 is not known at present, its localization suggests that it is likely to have a specific role in the ER/PM junction, such as lipid transfer, endocytosis, or signaling. The yeast ER/PM junctions include the vesicle-associated membrane protein-associated proteins (VAPs) Scs2 and Scs22 and their partners, the oxysterol-binding homology (Osh) proteins (103). The exact role of each protein in junction formation is not clear, but deletion of all of them is required to disrupt the yeast ER/PM junction (64).
In the special case of muscle, ER(SR)/PM junction tether proteins have been studied in some details and include the junctophilins (105) and the type 1 ryanodine receptor, which interacts directly with L-type Ca2+ channels (3). Junctophilins 3 and 4 may also participate in the ER/PM junctions in nonmyocytes (105), and yeast homologs with similar functions were identified in mammalian cells. The three extended synaptotagmins (E-Syts) (28, 67) are homologs of the yeast tricalbins with multiple Ca2+ binding C2 domains and PI(4,5)P2 binding capability (28). When expressed in mammalian cells, the three E-Syts tether the ER to the PM to form ER/PM junctions (28). However, the E-Syts appear to have distinct functions. E-Syt1, but not E-Syt2 and E-Syt3, affect STIM1-Orai1 function at the ER/PM junctions (7, 62), whereas E-Syt2 and E-Syt3, but not E-Syt1, interact with activated FGF receptors (110) to mediate receptor endocytosis (36). Based on sequence similarity and predicted topology, it is possible that one or more of the 10 ANOs (also known as TMEM16A-J) (8, 94, 120) are homologs of yeast Ist2. VAP-A and VAP-B are homologs of the yeast Scs proteins. In mammalian cells, the VAPs interact with Nir proteins. Nir2 is a lipid transfer and exchange protein that translocates to the PM and maintains the level of PI(4,5)P2 by exchanging phosphatidic acid with PI(4,5)P2 (46, 47) during cell stimulation (10, 47). Nir3 maintains PM PI(4,5)P2 at the resting state (11). The cytoskeletal proteins septin 4 and septin 5 also localize at the junctions and maintain high levels of PI(4,5)P2 around Orai1 in response to cell stimulation (97). They may also control the size of the ER/PM junctions (12).
Phosphatidylserine (PS) is an important PM lipid that is enriched in the inner leaflet of the PM and functions as a signaling lipid. PS is first synthesized in the ER and is then transferred to the PM. PS can be translocated from the ER to the PM by ANO proteins. ANO6 (TMEM16F) was shown to have a lipid scramblase function that mediates transfer of PS between membrane leaflets (104). The crystal structure of the fungus Nectria haematococca ANO6 homolog suggests a fascinating potential mechanism of PS flipping (6). The transmembrane domains of the Nectria haematococca ANO6 are arranged to form a hydrophilic cavity within the lipid bilayer that can guide the charged group of the phospholipids between the membrane leaflets (6). Whether the mammalian ANO6 handles phospholipids through a similar mechanism remains to be determined.
Proteins that function in controlling PM PS belong to the oxysterol-binding protein-related protein (ORP) family, including Osh3 (108) and Osh6/7 in yeast (69) and ORP5 and ORP8 in mammalian cells (14). In yeast, Osh3 is recruited to the ER/PM junctions, where it interacts with Scs/VAP through its phenylalanines in an acidic tract (FFAT) motif, and with PM PI4P through its pH domain to mediate lipid transfer (103). The structure of Osh4 shows that it can bind PI4P or sterols, suggesting that the Osh/ORP proteins may function to exchange lipids between membranes (16). Such a mechanism was recently shown to be mediated by the ORP5/ORP8 and their yeast homologs (14, 69). Early studies in mammalian cells showed that ORP5 has a cholesterol-binding domain and localizes at the ER/endolysosomal junction by interacting with Niemann Pick type c1 protein to mediate the transfer of cholesterol from late endosomes to the ER (21). Further studies showed that the ORPs, including ORP5, can mediate the transfer of PS from the ER to the PM (61). The mechanism of PS transfer by ORP5 was described recently. When expressed in mammalian cells, ORP5 and ORP8 form ER/PM junctions that require their PI4P binding pH domain (14). ORP5 and ORP8 bind PI4P or PS and exchange lipids between bilayers (14, 69). The coupled countertransport of PI4P from the PM to the ER and of PS from the ER to the PM determines the level of the respective lipids at the respective membranes (14, 69). The significance of this form of lipid transport to Ca2+ signaling is not known at present, although it is likely to affect it by determining the PM PS level.
STIM1 and Orai1 at the ER/PM Junctions
The ER Ca2+ sensor STIM1 is an ER/PM junctional protein that has a key role in Ca2+ signaling and in formation of the ER/PM junctions. STIM1 is a multidomain protein, but only one of the STIM1 domains is requited to activate the SOCs: the Orais and several TRPC channels (13, 51). The NH2-terminus of STIM1 resides in the ER lumen and has a low Ca2+ affinity EF hand and sterile α motif (SAM) domain. The SAM domain aids in STIM1 clustering on depletion of ER Ca2+ (100, 102). The EF hand binds Ca2+ when the ER is filled to keep STIM1 in an unclustered, close conformation (70, 101). The single transmembrane span is followed by three coiled-coil domains that regulate unfolding of STIM1 (48, 70) to release the STIM1 Orai1 activating region (SOAR) domain (123), also known as CAD (78) and CCb9 (41). SOAR, a 98-residue domain, is the minimal STIM1 domain (123) that dimerizes (119, 123) and interacts with the COOH- and NH2-terminus of Orai1 (78, 123), leading to full Orai1 activation. SOAR is followed by the COOH-terminal inhibitory domain (CTID) (37), serine/proline rich and polybasic domains (58, 91). Activation of the Orai channels by STIM1 requires Ca2+ release from the ER and co-clustering with STIM1 at the ER/PM junctions. At the ER/PM junctions, the STIM1 SOAR domain interacts with coiled-coil domains at the COOH- and NH2-termini of Orai1, and likely of other Orai channels. In the case of TRPC channels, the SOAR domain interacts with the coiled-coil domain in the COOH-terminus of the channels, either independently (TRPC1) or after dissociation between the channels COOH- and NH2-termini coiled-coil domains (TRPC3) (51). As will be discussed in the next section, both Orai1 and the TRPC channels participate in mediating physiological and pathological store-dependent Ca2+ influx in pancreatic acinar cells.
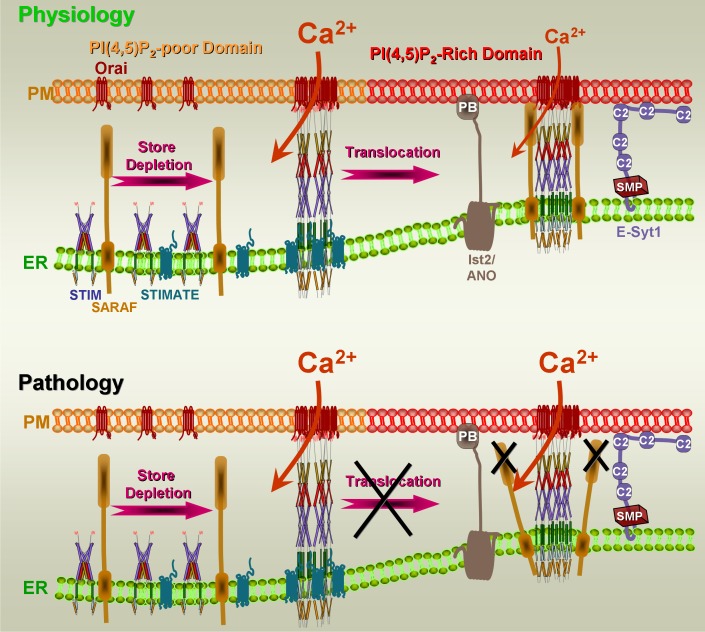
STIM1 has the canonical domains needed for localization at the ER/PM junctions: an ER localized transmembrane domain, a PI(4,5)P2 polybasic binding domain, and a coupling domain that spans the ER/PM space. Accordingly, on store depletion, STIM1 clusters and expands the ER/PM junctions (33). Clustering of STIM1 is regulated by the newly discovered STIM1 interacting protein named STIMATE/TMEM110 (38, 87). STIMATE/TMEM110 has ER membrane resident multiple transmembrane domains and a sort cytoplasmic domain that includes a polybasic sequence. STIMATE/TMEM110 interacts with STIM1 to promote its active conformation and stabilizes the STIM1-formed ER/PM junctions (38, 87). Deletion of STIMATE/TMEM110 prevents STIM1 clustering and STIM1-Orai1 interaction to inhibit the Orai1 current and Ca2+ influx (38, 87). Another protein that regulates STIM1 function is SARAF (75). SARAF is an ER resident, single transmembrane span protein with a long cytoplasmic domain that mediates the slow Ca2+-dependent inactivation of Orai1. Orai1 undergoes two types of Ca2+-dependent inactivation, fast inactivation with a time constant of ∼10 ms and slow inactivation with a time constant of 1–2 min (77). A negatively charged STIM1 sequence within CTID (37) is required for Orai1 inactivation (17, 53, 71). CTID regulates the access of SARAF to the STIM1 SOAR domain (37). Notably, SARAF interacts with STIM1 only when STIM1 is at the PI(4,5)P2-rich ER/PM junctions (62). Interestingly, the interaction between STIMATE/TMEM110 and STIM1 is Orai1 independent and is concurrent with or precedes STIM1-Orai1 interaction (38, 87). Conversely, interaction of SARAF with STIM1 takes place after and indeed requires formation of the STIM1-Orai1 complex (37). Hence, it is possible that, at the resting state, interaction of STIMATE/TMEM110 and SARAF with STIM1 is minimal. Upon store depletion, STIMATE/TMEM110 facilitates STIM1 clustering and stabilizes the active STIM1 conformation at the PI(4,5)P2-poor domain to fully activate Ca2+ influx. When the STIM1-Orai1 complex moves to the PI(4,5)P2-rich domain, SARAF is recruited to the complex to partially inhibit Ca2+ influx. This scenario of early interaction of STIMATE with STIM1 to facilitate STIM1 clustering, and delayed interaction of STIM1 with SARAF to inhibit the channel at the ER/PM junctions PI(4,5)P2-poor and PI(4,5)P2-rich domains, is illustrated in Fig. 2, top. Excessive pathological Ca2+ influx can occur when the STIMATE or SARAF do not function properly, or when translocation of Orai1-STIM1 to the PI(4,5)P2-rich domain fails. The Orai1-STIM1 complex may fail to translocate to the PI(4,5)P2-rich domain, either because the PI(4,5)P2 at the ER/PM junctions is completely hydrolyzed by strong receptor stimulation, or because STIMATE fails to facilitate STIM1 clustering and translocation, preventing SARAF from interacting with STIM1. Mutation or posttranslational modification of SARAF may also prevent SARAF interaction with STIM1. Loss of SARAF-STIM1 interaction will maintain the Ca2+ influx channels fully active, resulting in a sustained pathological increase in [Ca2+]i, as illustrated in Fig. 2, bottom.
Fig. 2.
Ca2+ signaling at the ER/plasma membrane (PM) junctions. The ER/PM junctions are tethered by the tethering proteins extended synaptotagmin 1 and an anoctamin isoform (homologs of yeast Ist2) to form a phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2]-rich domain at the PM. Physiology: In the resting state, stromal interacting molecule 1 (STIM1) is in an inactive, not clustered, state and does not interact with either SARAF or STIMATE. Orai1 is at the PM PI(4,5)P2-poor domain and is not clustered. Store depletion recruits STIMATE to STIM1 to facilitate STIM1 clustering and interaction with Orai1 at the PI(4,5)P2-poor domain to fully activate Orai1 and Ca2+ influx. The STIM1-Orai1 complex then translocates to the PI(4,5)P2-rich ER/PM junctions, where SARAF interacts with STIM1 to mediate Ca2+-dependent inactivation of Orai1 and limit Ca2+ influx to that required to sustain the physiological Ca2+ response. Pathology: When the Orai1-STIM1 complex fails to translocate to the PI(4,5)P2-rich ER/PM junctions and/or SARAF fails to interact with STIM1 at the PI(4,5)P2-rich ER/PM junctions to inhibit Ca2+ influx, Ca2+ influx remains fully activated, resulting in a sustained increase in cytoplasmic Ca2+ concentration and cell toxicity, as occurs in acute pancreatitis. SMP, synaptotagmin-like-mitochondrial-lipid binding protein; PB, polybasic.
The Pancreatic ER/PM Junctions in Pathology
In all cell types, excessive and sustained increase in cytoplasmic Ca2+ ([Ca2+]i) is highly toxic, leading to cell death by disrupting key cellular functions, including mitochondrial function (20, 84), apoptosis (19), gene regulation (76) and membrane transport (54, 63). The major mechanism underlying excessive increase in [Ca2+]i is overactivation of the various Ca2+ influx channels. In nonexcitable cells, the main Ca2+ influx channels are the TRPC channels and Orai1 (7, 13). It is well established that the major cause of various pathologies in secretory epithelial cells, including the pancreas, is excessive Ca2+ influx (22, 26, 30, 35). Acute pancreatitis is a life-threatening disease with multiple potential etiologies and no cure. Uncontrolled activation of Ca2+ influx was shown as the nodal point in triggering the common forms of acute pancreatitis that are initiated by intense receptor stimulation, bile acids, and excessive alcohol consumption (26, 63). Moreover, in vitro experiments showed that buffering [Ca2+]i to prevent the sustained increase in [Ca2+]i protects against all forms of acute pancreatitis (42, 83, 111).
Due to the central role of excessive Ca2+ influx in pancreatitis, inhibition of Ca2+ influx channels has been considered as a potential treatment for the disease. In principal, inhibitors of TRPC channels, of Orai1, or STIM1 should be beneficial in the treatment of pancreatitis, each of which has advantages and drawbacks. The main advantage of TRPC channel inhibition is that inhibitors should have modest side effects: knockout of TRPC1 (59), TRPC3 (44), or TRPC6 (18) and combinations of these channels (72, 96) all have minor phenotypes. Another advantage is that, when multiple TRPC channels are expressed in the same cells, they heteromultimerize (51, 52, 124), and inhibition of one isoform is sufficient to inhibit all TRPC channel-mediated Ca2+ influx, as was found in pancreatic acinar cells (51). Accordingly, knockout of TRPC3 in mice ameliorates the damage observed in acute pancreatitis (44). Similar protection has been observed by inhibition of TRPC3 with pyrazol 3 (45). The disadvantage of inhibiting TRPC3, and possibly other TRPC channels, is that experimental inhibition protects only against mild forms of pancreatitis (44, 45), and the currently available drug is not able to inhibit the fully activated channel (45). Hence more potent TRPC channel inhibitors would be desirable for pancreatitis therapy.
Inhibitors of Orai1 have been tested recently as potential treatments for pancreatitis (27, 112). In vitro studies showed that inhibition of Orai1 prevents the sustained Ca2+ increase caused by intense receptor stimulation, by fatty acids ethyl esters (products of alcohol metabolism), and by activation of intracellular trypsin (27). The same and an additional Orai1 inhibitor were used in mice before induction of acute pancreatitis by bile acid, by stimulation with cholecystokinin, or by exposure to alcohol + fatty acids ethyl esters (112). Orai1 blockers partially but significantly protected against all models of acute pancreatitis (112). One significant advantage of Orai1 blockade is that all forms of acute pancreatitis are associated with severe inflammation (93), and Orai1-mediated Ca2+ influx is critical for activation of various inflammatory cells (98). Inhibition of Orai1 can, therefore, protect the pancreas by two mechanisms: reduction in cell damage from the sustained [Ca2+]i and reduction of the inflammatory response. However, Orai1 has many roles outside the pancreas; thus inhibitors will likely have adverse effects limiting their therapeutic efficacy. Deletion of Orai1 in mice is embryonically lethal, and Orai1 has diverse roles in cardiovascular, neuronal, and muscular physiology, as well as platelet aggregation and the immune response. Loss-of-function mutations in ORAI1 cause severe immunodeficiency, tubular aggregate myopathy, and autoimmunity (4). Hence, any potential use of Orai1 blockers will require much caution. One potential solution would be a combination of TRPC channel blockers and low-dose Orai1 inhibitors to treat acute pancreatitis while minimizing the side effects due to inhibition of Orai1.
Small-molecule inhibitors have revolutionized the fields of oncology and immunology. Simultaneous blockade of multiple noxious stimuli is achieved by targeting intracellular signaling pathways, leading to improved disease outcomes. While the development of selective Ca2+ channel inhibitors is in its infancy, recent advances in the field have opened new possibilities that could similarly shift the treatment paradigm exocrine diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S., S.P., D.M.S., and S.M. edited and revised manuscript; A.S., S.P., D.M.S., and S.M. approved final version of manuscript; S.M. prepared figures; S.M. drafted manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research intramural grant DE-000735 and by the National Foundation of Korea Grant funded by the Korean Government (NRF-2013S1A2A2035370, 2015R1A2A1A15054157).
REFERENCES
- 1.Ahuja M, Jha A, Maleth J, Park S, Muallem S. cAMP and Ca2+ signaling in secretory epithelia: crosstalk and synergism. Cell calcium 55: 385–393, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almassy J, Won JH, Begenisich TB, Yule DI. Apical Ca2+-activated potassium channels in mouse parotid acinar cells. J Gen Physiol 139: 121–133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannister RA. Bridging the myoplasmic gap: recent developments in skeletal muscle excitation-contraction coupling. J Muscle Res Cell Motil 28: 275–283, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bergmeier W, Weidinger C, Zee I, Feske S. Emerging roles of store-operated Ca2+ entry through STIM and ORAI proteins in immunity, hemostasis and cancer. Channels (Austin) 7: 379–391, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce JI, Giovannucci DR, Blinder G, Shuttleworth TJ, Yule DI. Modulation of [Ca2+]i signaling dynamics and metabolism by perinuclear mitochondria in mouse parotid acinar cells. J Biol Chem 279: 12909–12917, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516: 207–212, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Choi S, Maleth JJ, Park S, Ahuja M, Muallem S. The ER/PM microdomain, PI(4,5)P and the regulation of STIM1-Orai1 channel function. Cell calcium 58: 342–348, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Catalan MA, Kondo Y, Pena-Munzenmayer G, Jaramillo Y, Liu F, Choi S, Crandall E, Borok Z, Flodby P, Shull GE, Melvin JE. A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc Natl Acad Sci U S A 112: 2263–2268, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CL, Hsieh TS, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao JC, Liou J. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep 5: 813–825, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Chang CL, Liou J. Phosphatidylinositol 4,5-bisphosphate homeostasis regulated by Nir2 and Nir3 proteins at endoplasmic reticulum-plasma membrane junctions. J Biol Chem 290: 14289–14301, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao JT, Wong AK, Tavassoli S, Young BP, Chruscicki A, Fang NN, Howe LJ, Mayor T, Foster LJ, Loewen CJ. Polarization of the endoplasmic reticulum by ER-septin tethering. Cell 158: 620–632, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Choi S, Maleth J, Jha A, Lee KP, Kim MS, So I, Ahuja M, Muallem S. The TRPCs-Orai interaction. Hand Exp Pharmacol 223: 1035–1054, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P. Intracellular transport. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349: 428–432, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper DM. Store-operated Ca2+-entry and adenylyl cyclase. Cell calcium 58: 368–375, 2015. [DOI] [PubMed] [Google Scholar]
- 16.de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, Antonny B, Drin G. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol 195: 965–978, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derler I, Fahrner M, Muik M, Lackner B, Schindl R, Groschner K, and Romanin C. A Ca2+ release-activated Ca2+ (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2+-dependent inactivation of ORAI1 channels. J Biol Chem 284: 24933–24938, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25: 6980–6989, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol 1: 405–434, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Dorn GW 2nd, Kitsis RN. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res 116: 167–182, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du X, Kumar J, Ferguson C, Schulz TA, Ong YS, Hong W, Prinz WA, Parton RG, Brown AJ, Yang H. A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J Cell Biol 192: 121–135, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhut M, Wallace H. Ion channels in inflammation. Pflügers Arch 461: 401–421, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Filadi R, Pozzan T. Generation and functions of second messengers microdomains. Cell calcium 58: 405–414, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Fogarty KE, Kidd JF, Tuft RA, Thorn P. A bimodal pattern of InsP(3)-evoked elementary Ca2+ signals in pancreatic acinar cells. Biophys J 78: 2298–2306, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagnon KB, Delpire E. Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport. Physiol Rev 92: 1577–1617, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerasimenko JV, Gerasimenko OV, Petersen OH. The role of Ca2+ in the pathophysiology of pancreatitis. J Physiol 592: 269–280, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerasimenko JV, Gryshchenko O, Ferdek PE, Stapleton E, Hebert TO, Bychkova S, Peng S, Begg M, Gerasimenko OV, Petersen OH. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc Natl Acad Sci U S A 110: 13186–13191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P(2)-dependent and Ca2+-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell 153: 1494–1509, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorelik J, Wright PT, Lyon AR, Harding SE. Spatial control of the betaAR system in heart failure: the transverse tubule and beyond. Cardiovasc Res 98: 216–224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hecquet CM, Malik AB. Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb Haemost 101: 619–625, 2009. [PMC free article] [PubMed] [Google Scholar]
- 31.Henne WM, Liou J, Emr SD. Molecular mechanisms of inter-organelle ER-PM contact sites. Curr Opin Cell Biol 35: 123–130, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Hodges RR, Zoukhri D, Lightman JP, Dartt DA. Identification and cellular localization of the components of the VIP signaling pathway in the lacrimal gland. Adv Exp Med Biol 438: 169–176, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Hogan PG. The-ORAI1 microdomain. Cell calcium 58: 357–367, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic 12: 232–245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilatovskaya DV, Staruschenko A. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am J Physiol Renal Physiol 309: F393–F397, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jean S, Mikryukov A, Tremblay MG, Baril J, Guillou F, Bellenfant S, Moss T. Extended-synaptotagmin-2 mediates FGF receptor endocytosis and ERK activation in vivo. Dev Cell 19: 426–439, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Jha A, Ahuja M, Maleth J, Moreno CM, Yuan JP, Kim MS, Muallem S. The STIM1 CTID domain determines access of SARAF to SOAR to regulate Orai1 channel function. J Cell Biol 202: 71–79, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing J, He L, Sun A, Quintana A, Ding Y, Ma G, Tan P, Liang X, Zheng X, Chen L, Shi X, Zhang SL, Zhong L, Huang Y, Dong MQ, Walker CL, Hogan PG, Wang Y, Zhou Y. Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat Cell Biol 17: 1339–1347, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahle KT, Khanna AR, Alper SL, Adragna NC, Lauf PK, Sun D, Delpire E. K-Cl cotransporters, cell volume homeostasis, and neurological disease. Trends Mol Med 21: 513–523, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasai H, Augustine GJ. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature 348: 735–738, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun 385: 49–54, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JY, Kim KH, Lee JA, Namkung W, Sun AQ, Ananthanarayanan M, Suchy FJ, Shin DM, Muallem S, Lee MG. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology 122: 1941–1953, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem 281: 32540–32549, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology 137: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology 140: 2107–2115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S, Kedan A, Marom M, Gavert N, Keinan O, Selitrennik M, Laufman O, Lev S. The phosphatidylinositol-transfer protein Nir2 binds phosphatidic acid and positively regulates phosphoinositide signalling. EMBO Rep 14: 891–899, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim YJ, Guzman-Hernandez ML, Wisniewski E, Balla T. Phosphatidylinositol-phosphatidic acid exchange by Nir2 at ER-PM contact sites maintains phosphoinositide signaling competence. Dev Cell 33: 549–561, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korzeniowski MK, Manjarres IM, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal 3: ra82, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kralt A, Carretta M, Mari M, Reggiori F, Steen A, Poolman B, Veenhoff LM. Intrinsically disordered linker and plasma membrane-binding motif sort Ist2 and Ssy1 to junctions. Traffic 16: 135–147, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Lahiri S, Toulmay A, Prinz WA. Membrane contact sites, gateways for lipid homeostasis. Curr Opin Cell Biol 33: 82–87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KP, Choi S, Hong JH, Ahuja M, Graham S, Ma R, So I, Shin DM, Muallem S, Yuan JP. Molecular determinants mediating gating of transient receptor potential canonical (TRPC) channels by stromal interaction molecule 1 (STIM1). J Biol Chem 289: 6372–6382, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent function of transient receptor potential canonical (TRPC) channels tunes their store-operated mode. J Biol Chem 285: 38666–38673, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc Natl Acad Sci U S A 106: 14687–14692, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev 92: 39–74, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J Biol Chem 272: 15771–15776, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem 272: 15765–15770, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Li Q, Luo X, Muallem S. Functional mapping of Ca2+ signaling complexes in plasma membrane microdomains of polarized cells. J Biol Chem 279: 27837–27840, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci U S A 104: 17542–17547, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maass K, Fischer MA, Seiler M, Temmerman K, Nickel W, Seedorf M. A signal comprising a basic cluster and an amphipathic alpha-helix interacts with lipids and is required for the transport of Ist2 to the yeast cortical ER. J Cell Sci 122: 625–635, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, Gavin AC. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature 501: 257–261, 2013. [DOI] [PubMed] [Google Scholar]
- 62.Maleth J, Choi S, Muallem S, Ahuja M. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat Commun 5: 5843, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maleth J, Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell calcium 55: 337–345, 2014. [DOI] [PubMed] [Google Scholar]
- 64.Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell 23: 1129–1140, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Messenger SW, Falkowski MA, Groblewski GE. Ca2+-regulated secretory granule exocytosis in pancreatic and parotid acinar cells. Cell calcium 55: 369–375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Min SW, Chang WP, Sudhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc Natl Acad Sci U S A 104: 3823–3828, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mogami H, Nakano K, Tepikin AV, Petersen OH. Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell 88: 49–55, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Moser von Filseck J, Copic A, Delfosse V, Vanni S, Jackson CL, Bourguet W, and Drin G. Intracellular transport. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science 349: 432–436, 2015. [DOI] [PubMed] [Google Scholar]
- 70.Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, Romanin C. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J 30: 1678–1689, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci U S A 106: 15495–15500, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nilius B, Flockerzi V. What do we really know and what do we need to know: some controversies, perspectives, and surprises. Hand Exp Pharmacol 223: 1239–1280, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Nlend MC, Schmid A, Sutto Z, Ransford GA, Conner GE, Fregien N, Salathe M. Calcium-mediated, purinergic stimulation and polarized localization of calcium-sensitive adenylyl cyclase isoforms in human airway epithelia. FEBS Lett 581: 3241–3246, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ong HL, Ambudkar IS. Molecular determinants of TRPC1 regulation within ER-PM junctions. Cell calcium 58: 376–386, 2015. [DOI] [PubMed] [Google Scholar]
- 75.Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell 149: 425–438, 2012. [DOI] [PubMed] [Google Scholar]
- 76.Parekh AB. Store-operated CRAC channels: function in health and disease. Nat Rev Drug Discov 9: 399–410, 2010. [DOI] [PubMed] [Google Scholar]
- 77.Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park MK, Lomax RB, Tepikin AV, Petersen OH. Local uncaging of caged Ca2+ reveals distribution of Ca2+-activated Cl− channels in pancreatic acinar cells. Proc Natl Acad Sci U S A 98: 10948–10953, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park S, Shcheynikov N, Hong JH, Zheng C, Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, Seki G, Yule D, Mikoshiba K, Muallem S. Irbit mediates synergy between Ca2+ and cAMP signaling pathways during epithelial transport in mice. Gastroenterology 145: 232–241, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Penny CJ, Kilpatrick BS, Eden ER, Patel S. Coupling acidic organelles with the ER through Ca2+ microdomains at membrane contact sites. Cell calcium 58: 387–396, 2015. [DOI] [PubMed] [Google Scholar]
- 82.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol 70: 273–299, 2008. [DOI] [PubMed] [Google Scholar]
- 83.Petersen OH, Tepikin AV, Gerasimenko JV, Gerasimenko OV, Sutton R, Criddle DN. Fatty acids, alcohol and fatty acid ethyl esters: toxic Ca2+ signal generation and pancreatitis. Cell calcium 45: 634–642, 2009. [DOI] [PubMed] [Google Scholar]
- 84.Pizzo P, Drago I, Filadi R, Pozzan T. Mitochondrial Ca2+ homeostasis: mechanism, role, and tissue specificities. Pflügers Arch 464: 3–17, 2012. [DOI] [PubMed] [Google Scholar]
- 85.Prinz WA. Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. J Cell Biol 205: 759–769, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Proctor GB, Carpenter GH. Salivary secretion: mechanism and neural regulation. Monogr Oral Sci 24: 14–29, 2014. [DOI] [PubMed] [Google Scholar]
- 87.Quintana A, Rajanikanth V, Farber-Katz S, Gudlur A, Zhang C, Jing J, Zhou Y, Rao A, Hogan PG. TMEM110 regulates the maintenance and remodeling of mammalian ER-plasma membrane junctions competent for STIM-ORAI signaling. Proc Natl Acad Sci U S A 112: E7083–E7092, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raiborg C, Wenzel EM, Stenmark H. ER-endosome contact sites: molecular compositions and functions. Embo J 34: 1848–1858, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romanenko V, Nakamoto T, Srivastava A, Melvin JE, Begenisich T. Molecular identification and physiological roles of parotid acinar cell maxi-K channels. J Biol Chem 281: 27964–27972, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol 581: 801–817, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rudolf R, Magalhaes PJ, Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol 173: 187–193, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol 29: 523–530, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scott JD. A-kinase-anchoring proteins and cytoskeletal signalling events. Biochem Soc Trans 31: 87–89, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Sexton JE, Desmonds T, Quick K, Taylor R, Abramowitz J, Forge A, Kros CJ, Birnbaumer L, Wood JN. The contribution of TRPC1, TRPC3, TRPC5 and TRPC6 to touch and hearing. Neurosci Lett 610: 36–42, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature 499: 238–242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shaw PJ, Feske S. Regulation of lymphocyte function by ORAI and STIM proteins in infection and autoimmunity. J Physiol 590: 4157–4167, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG, Muallem S. Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. J Biol Chem 276: 44146–44156, 2001. [DOI] [PubMed] [Google Scholar]
- 100.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem 281: 35855–35862, 2006. [DOI] [PubMed] [Google Scholar]
- 101.Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi-Seabrook GM, Muik M, Romanin C, Ikura M. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat Commun 4: 2963, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stathopulos PB, Zheng L, Ikura M. Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J Biol Chem 284: 728–732, 2009. [DOI] [PubMed] [Google Scholar]
- 103.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 144: 389–401, 2011. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468: 834–838, 2010. [DOI] [PubMed] [Google Scholar]
- 105.Takeshima H, Hoshijima M, Song LS. Ca2+ microdomains organized by junctophilins. Cell calcium 58: 349–356, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell 74: 661–668, 1993. [DOI] [PubMed] [Google Scholar]
- 107.Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J 18: 4999–5008, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tong J, Yang H, Yang H, Eom SH, Im YJ. Structure of Osh3 reveals a conserved mode of phosphoinositide binding in oxysterol-binding proteins. Structure 21: 1203–1213, 2013. [DOI] [PubMed] [Google Scholar]
- 109.Toulmay A, Prinz WA. A conserved membrane-binding domain targets proteins to organelle contact sites. J Cell Sci 125: 49–58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tremblay MG, Herdman C, Guillou F, Mishra PK, Baril J, Bellenfant S, Moss T. Extended synaptotagmin interaction with the fibroblast growth factor receptor depends on receptor conformation, not catalytic activity. J Biol Chem 290: 16142–16156, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voronina SG, Sherwood MW, Gerasimenko OV, Petersen OH, Tepikin AV. Visualizing formation and dynamics of vacuoles in living cells using contrasting dextran-bound indicator: endocytic and nonendocytic vacuoles. Am J Physiol Gastrointest Liver Physiol 293: G1333–G1338, 2007. [DOI] [PubMed] [Google Scholar]
- 112.Wen L, Voronina S, Javed MA, Awais M, Szatmary P, Latawiec D, Chvanov M, Collier D, Huang W, Barrett J, Begg M, Stauderman K, Roos J, Grigoryev S, Ramos S, Rogers E, Whitten J, Velicelebi G, Dunn M, Tepikin AV, Criddle DN, Sutton R. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology 149: 481–492 e487, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Westermann B. The mitochondria-plasma membrane contact site. Curr Opin Cell Biol 35: 1–6, 2015. [DOI] [PubMed] [Google Scholar]
- 114.Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, Klussmann E, Cooper DM. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal 5: ra29, 2012. [DOI] [PubMed] [Google Scholar]
- 115.Willoughby D, Ong HL, De Souza LB, Wachten S, Ambudkar IS, Cooper DM. TRPC1 contributes to the Ca2+-dependent regulation of adenylate cyclases. Biochem J 464: 73–84, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolf W, Kilic A, Schrul B, Lorenz H, Schwappach B, Seedorf M. Yeast Ist2 recruits the endoplasmic reticulum to the plasma membrane and creates a ribosome-free membrane microcompartment. PLoS One 7: e39703, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K, Muallem S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell calcium 42: 363–371, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu X, Zeng W, Popov S, Berman DM, Davignon I, Yu K, Yowe D, Offermanns S, Muallem S, Wilkie TM. RGS proteins determine signaling specificity of Gq-coupled receptors. J Biol Chem 274: 3549–3556, 1999. [DOI] [PubMed] [Google Scholar]
- 119.Yang X, Jin H, Cai X, Li S, Shen Y. Structural and mechanistic insights into the activation of stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci U S A 109: 5657–5662, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008. [DOI] [PubMed] [Google Scholar]
- 121.Yang YM, Lee J, Jo H, Park S, Chang I, Muallem S, Shin DM. Homer2 protein regulates plasma membrane Ca2+-ATPase-mediated Ca2+ signaling in mouse parotid gland acinar cells. J Biol Chem 289: 24971–24979, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan JP, Lee KP, Hong JH, Muallem S. The closing and opening of TRPC channels by Homer1 and STIM1. Acta Physiol (Oxf) 204: 238–247, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 11: 337–343, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yule DI, Betzenhauser MJ, Joseph SK. Linking structure to function: recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis. Cell calcium 47: 469–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yule DI, Ernst SA, Ohnishi H, Wojcikiewicz RJ. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J Biol Chem 272: 9093–9098, 1997. [DOI] [PubMed] [Google Scholar]
- 127.Zeng W, Xu X, Popov S, Mukhopadhyay S, Chidiac P, Swistok J, Danho W, Yagaloff KA, Fisher SL, Ross EM, Muallem S, Wilkie TM. The N-terminal domain of RGS4 confers receptor-selective inhibition of G protein signaling. J Biol Chem 273: 34687–34690, 1998. [DOI] [PubMed] [Google Scholar]
- 128.Zinn VZ, Khatri A, Mednieks MI, Hand AR. Localization of cystic fibrosis transmembrane conductance regulator signaling complexes in human salivary gland striated duct cells. Eur J Oral Sci 123: 140–148, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]