Abstract
In the mid-20th century, Hans Ussing developed a chamber that allowed for the simultaneous measurement of current and labeled probe flux across epithelia. Using frog skin as a model, Ussing used his results to propose mechanisms of transcellular Na+ and K+ transport across apical (exterior/luminal) and basolateral (interior) membranes that is essentially unchanged today. Others took advantage of Ussing's chambers to study mucosal tissues, including bladder and intestines. It quickly became clear that, in some tissues, passive paracellular flux, i.e., across the tight junction, was an important component of overall transepithelial transport. Subsequent work demonstrated that activation of the apical Na+-glucose cotransporter SGLT1 regulated paracellular permeability such that intestinal paracellular transport could coordinate with and amplify transcellular transport. Intermediates in this process include activation of p38 MAPK, the apical Na+/H+ exchanger NHE3, and myosin light chain kinase (MLCK). Investigators then focused on these processes in disease. They found that TNF induces barrier dysfunction via MLCK activation and downstream caveolin-1-dependent endocytosis of the tight junction protein occludin. TNF also inhibited NHE3, and both barrier loss and PKCα-dependent NHE3 inhibition were required for TNF-induced acute diarrhea, emphasizing the interplay between transcellular and paracellular transport. Finally, studies using immune-mediated inflammatory bowel disease models showed that mice lacking epithelial MLCK were initially protected, but became ill as epithelial damage progressed and provided a tight junction-independent means of barrier loss. None of these advances would have been possible without the insights provided by Ussing and others using Ussing's ingenious, and still useful, chambers.
Keywords: cytokine, myosin, myosin light chain kinase, tight junction, TNF, intestine, permeability
for the past century, investigators have studied ion flux across membranes. In the first half of the 20th century, it was shown that cells handle Na+ and K+ ions differently, and that Na+ was actively extruded by many cells. Measurement of ion transport across epithelial barriers was, however, a more challenging problem (51). In 1951, Hans Ussing made a giant leap in overcoming this challenge (95). He developed an apparatus with paired compartments between which a tissue, e.g., frog skin, was mounted (96) (Fig. 1A). By putting the same solution on both sides and short-circuiting the transepithelial electrical potential, Ussing was able to simultaneously measure current and 24Na+ flux across frog skin. This allowed him to calculate the electrical resistance to Na+ diffusion and the electromotive force of the active Na+ transport system. Through a series of manipulations to alter potential difference across the skin without affecting Na transport in non-short-circuited skin, Ussing found that Na flux and current were closely correlated under all conditions. Ussing also applied defined chemical or electrical potentials across the skin to determine, in relative terms, the electromotive force of active Na transport (96). While important, the results were not entirely unexpected, as others (28, 82) had measured current across short-circuited frog skin and Ussing himself had previously assessed 24Na+ flux across frog skin (51). The advance here was developing a device that allowed simultaneous measurement of transport using electrical and chemical methods. This allowed Ussing to show that active Na+ transport was entirely responsible for the measured current (96). He was also able to conclude that “the active sodium transport mechanism is located at the inner border (i.e., the interior tissue interface) and should possibly be considered a forced exchange of Na+ with K+ from the inner solution” (48) (Fig. 1B). This was followed by studies using the Ussing chamber to show that active transport of Na+ and K+ are linked (10) and that, under most conditions, Cl− transport across frog skin is passive (43, 49). Thus, by combining electrical and chemical measurements and applying a great deal of precision and creativity, Ussing and others were able to rapidly advance our understanding of both active and passive transport across epithelia. It did not take long before other investigators began using the chambers and methods of analyses developed by Ussing to study other transporting epithelia, including those within the gastrointestinal tract (6, 29, 70). Space prohibits a complete discussion of the important discoveries made during this time. Because there has been so much important work done in these areas, the discussion is necessarily incomplete, and many paradigm-shifting and other significant studies are not presented. A number of outstanding reviews on the topic have, however, been published (5, 8, 20, 31, 41, 42, 44, 46, 50, 71, 76, 98–101).
Fig. 1.
A: Ussing's original chamber. By short-circuiting the transepithelial electrical potential, Ussing could simultaneously measure Na24 flux and current across a given sample tissue (96). This allowed him to accurately assess active transport across epithelia. I, microammeter; V, voltmeter; A, apical side of tissue; B, basolateral side of tissue. B: Koefoed-Johnsen and Ussing (48) proposed passive Na+ across the outer membrane and active exchange of Na+ for K+ at the inner membrane.
While Ussing discovered processes of ion transport, others concentrated on the structures relevant to transport and barrier functions. With the development of the electron microscope and a variety of ways to prepare tissues for analysis came an explosion of morphologic analyses of never-before-seen structures. Farquhar and Palade (21) described intercellular junctions of simple epithelia and found shared features across diverse tissues. They took advantage of experimental hemoglobinuria and the electron-dense nature of iron to show that hemoglobin within the nephron lumen was unable to cross the zonula occludens (ZO), or tight junction (21). This and the detailed morphological studies that followed (32, 81) supported the conclusion that “the tight junction is impervious to concentrated protein solutions and appears to function as a diffusion barrier or ‘seal’” (21). It did not take long, however, for Machen and others (53) to combine ultrastructural analyses with transport physiology. These studies rapidly led to the inescapable conclusion that “salt-transporting epithelia [should be divided] into two main groups, those with ”leaky“ junctional complexes and those with tight junctional complexes…” (53). Examples of the latter include frog skin and toad urinary bladder, while gallbladder and ileum are examples of leaky epithelia.
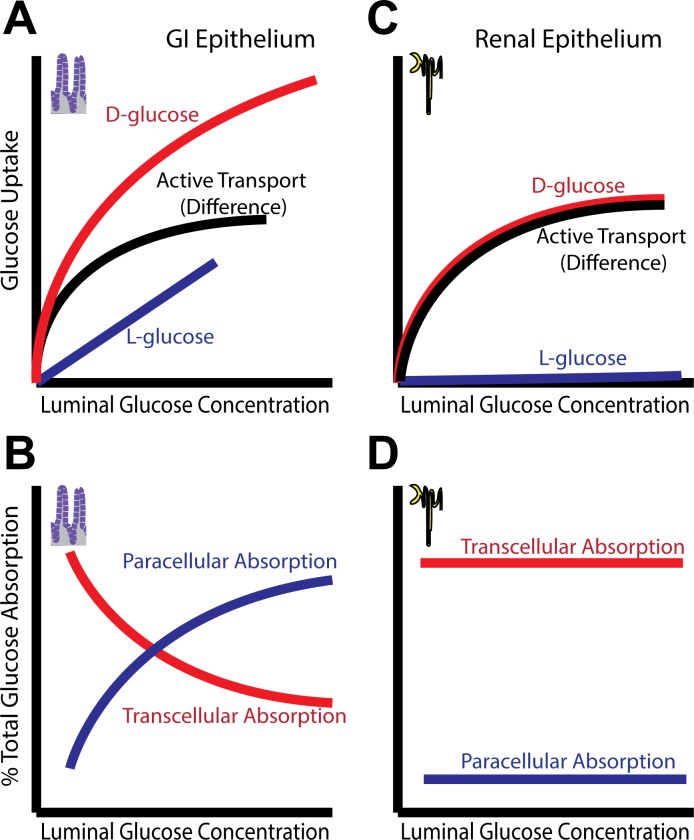
Despite recognizing two classes of tight junctions, the physiological importance of “leaky” tight junctions had yet to be defined. Nevertheless, a logarithmic relationship between the number of strands seen in freeze-fracture replicas and measured electrical resistance was demonstrated in diverse epithelia (14, 15). Such a relationship could be explained by pores within the strands. While he did not suspect that pores would be found within tight junctions, and the topic of tight junctions remains controversial (47), Ussing did theorize, in 1957, that water transport across the skin was passive and occurred via pores (2). He also showed that osmotically driven water movement could result in flux of solutes across the same pores via solvent drag (2). Three decades later, Pappenheimer, Madara, and Reiss demonstrated the presence of regulated solvent drag, triggered by Na+-nutrient cotransport and accompanied by increased tight junction permeability to small molecules, in rodent small intestine (59, 66, 67). These data also provide a structural basis for Meddings' observation that, despite saturation of specific d-glucose update at 50–60 mM, uptake of l-glucose, which is not recognized by the Na+-glucose cotransporter, continued to increase at concentrations exceeding 100 mM in rat jejunum (64) (Fig. 2A). In other words, these data indicate the existence of a passive transport process that lacks stereospecific recognition of d-glucose. This passive process is explained by solvent drag as a result of osmotically driven water absorption and resulting paracellular absorption, e.g., of l-glucose, across tight junctions with enhanced permeability to small molecules. While the best available data suggest that solvent drag is an important mechanism of intestinal nutrient absorption, support for this is not universal. With rare exception, however, careful review of studies that failed to detect solvent drag-mediated absorption of water and nutrients shows that there was little transport of any sort measured (19, 24–27, 30, 57, 80, 90, 92–94).
Fig. 2.
Comparison of interactions between active and passive transport in two different epithelia. A: in gastrointestinal (GI) epithelium, SGLT1-mediated Na+-glucose cotransport activates Na+/H+ exchanger (NHE3)-mediated Na+ absorption and also increases paracellular permeability to small molecules. Together with the osmotic gradient generated by transcellular transport, the increased paracellular permeability allows enhanced passive glucose uptake, as measured by l-glucose (which is not transported by SGLT1) by solvent drag, thereby increasing overall nutrient absorption. B: at high luminal glucose concentrations, paracellular flux via solvent drag can represent a significant fraction of total glucose absorption. C and D: Na+-glucose cotransport in renal tubular epithelium does regulate tight junction permeability. Solvent drag does not significantly amplify transcellular glucose absorption, resulting in excess glucose in the urine at high blood glucose concentrations.
Solvent drag allows the intestine to absorb all luminal glucose, even when concentrations exceed the capacity of the transcellular transport system; at low concentrations, the transcellular pathway absorbs luminal glucose, but as concentrations increase, a growing fraction of glucose absorption occurs via the paracellular pathway (Fig. 2B). Similar processes likely contribute to amino acid absorption (3, 58). It should be noted that an alternative explanation for passive transepithelial glucose absorption has been suggested based on the observation that the facilitated glucose transporter GLUT2 is recruited to the brush border membrane following Na+-glucose cotransporter SGLT1 activation (45). While this could establish a passive, transcellular route for d-glucose absorption, it fails to account for the increased transepithelial flux of mannitol and l-glucose following SGLT1 activation and, therefore cannot explain the increased paracellular absorption observed during active Na+-glucose cotransport. Solvent drag in the intestine contrasts sharply with the situation in the kidney, where excessively high levels of blood glucose are partially corrected by urinary excretion due to the inability of the nephron to reabsorb glucose that exceeds the capacity of the transcellular pathway (Fig. 2, C and D). The evolutionary advantage of intestinal solvent drag at times when meals were irregularly spaced may therefore have been enormous.
This new understanding of tight junction permeability as a dynamic, rather than static, entity and the profound impact of paracellular flux on overall transepithelial transport led to interest in the mechanisms responsible for these regulatory processes. As a reductionist model, the well-differentiated Caco-2BBe intestinal epithelial cell line was transfected to express the intestinal Na+-glucose cotransporter, SGLT1 (89). Activation of apical Na+-glucose cotransport triggered a reversible increase in paracellular conductance of ions and small, glucose-sized molecules, e.g., mannitol, but not the larger paracellular probe inulin (91). Like glucose-elicited tight junction regulation in rodent mucosae (3), the SGLT1 inhibitor phlorizin prevented paracellular permeability increases in SGLT1-expressing Caco-2BBe monolayers (91). This validated the Caco-2BBe model as a tool with which to define the molecular mechanisms underlying physiological tight junction regulation.
Morphological studies in rodent mucosae had identified two principal changes induced by activation of Na+-glucose cotransport; development of intrajunctional dilatations and perijunctional cytoskeletal condensation (3). The latter suggested that cytoskeletal, i.e., actomyosin, contraction, and evidence of this occurring in SGLT1-expressing Caco-2BBe monolayers was therefore sought. Indeed, Na+-glucose cotransport resulted in a greater than twofold increase in phosphorylation of the myosin regulatory light chain (MLC), a biochemical marker of actomyosin contraction (91). Both MLC phosphorylation and increased tight junction permeability after activation of Na+-glucose cotransport could be blocked by the relatively nonspecific myosin light chain kinase (MLCK) inhibitors ML-7 and ML-9 (91) as well as the highly specific peptide inhibitor of MLCK, PIK (105). ML-9 also blocked paracellular permeability increases in rodent small intestine (91) but did not prevent development of intrajunctional dilatations (Turner JR and Madara JL, unpublished observations). The latter observation suggests that the dilatations formed as a consequence of the massive water absorption that occurs in response to transcellular Na+ and glucose absorption and were not a specific morphological feature of tight junction regulation. Finally, analysis of human jejunal mucosae showed that Na+-glucose cotransport increased paracellular permeability and that this was associated with enhanced MLC phosphorylation within the perijunctional actomyosin ring (7). Overall, these studies marked the start of an integrated understanding of the structural and functional mechanisms of tight junction remodeling.
Coordinated Regulation of Transcellular and Paracellular Transport
After MLCK was identified as a critical intermediate in Na+-glucose cotransport-induced tight junction regulation, efforts focused on elucidating the events that preceded and followed MLCK activation. One potential link could be the cellular volume increase, and subsequent regulated volume decrease, that follow initiation of Na+-glucose cotransport. One study showed that extracellular Na+ in general, and basolateral Na+/H+ exchange mediated by NHE1 in particular, was necessary for the regulated volume decrease activated by treatment of rodent jejunal villus epithelia with 5% hypotonic media, which induced cell swelling of a magnitude similar to Na+-glucose cotransport (54, 55). Subsequent analysis of Na+/H+ exchange in SGLT1-expressing Caco-2BBe monolayers failed to demonstrate NHE1 regulation, but instead showed that the apical Na+/H+ exchanger NHE3 was activated following initiation of Na+-glucose cotransport (87). This difference is not particularly surprising since, despite similar volume changes, signaling events differ in regulated volume decreases following hypotonic or Na+-nutrient cotransport-induced cell swelling (56).
Increased NHE3-mediated transport was due to rapid trafficking of the transporter from subapical cytoplasmic vesicles to the brush border (104). This was triggered by a signaling cascade in which Na+-glucose cotransport resulted in phosphorylation and activation of p38 mitogen-associated protein kinase (MAPK) that, in turn, phosphorylated and activated MAPK-activated protein kinase-2 (MAPKAPK2), Akt2, and ezrin (39, 79, 104). This pathway is also directly linked to tight junction regulation, as specific NHE3 inhibition reduced paracellular permeability of SGLT1-expressing Caco-2BBe monolayers during active Na+-glucose cotransport but not when SGLT1 was inhibited by phlorizin (88).
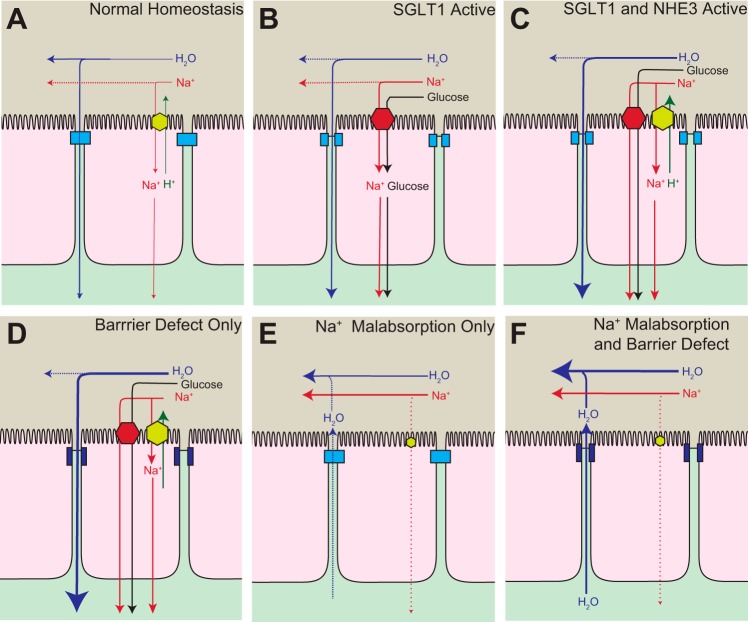
At a superficial level, one might puzzle at the benefit of Na+-glucose cotransport increasing NHE3-mediated Na+ absorption. The most likely explanation is that, in addition to acting as a transporter, SGLT1 serves as a nutrient sensor that activates villus enterocyte absorptive processes more globally (Fig. 3, A–C). This may have significant benefits under conditions of stress. For example, SGLT1-mediated transport of a nonmetabolizable glucose analog reverses cholera toxin-induced NHE3 inhibition (52). It is therefore likely that tight junction regulation and restoration of NHE3 activity synergize to account for some of the benefit afforded by oral rehydration therapy.
Fig. 3.
States of malabsorption and diarrhea in the small intestine. A: under normal conditions, transcellular Na+ absorption occurs primarily via NHE3 and water is absorbed paracellularly. B: SGLT1 serves as a nutrient sensor and triggers rearrangement of the tight junction to increase paracellular fluid and nutrient absorption. C: SGLT1 also increases NHE3 activity that mediates greater Na+ absorption and further increases the osmotic gradient for water absorption across the tight junction. D: pathologic barrier dysfunction, i.e., paracellular permeability increases, can enhance fluid absorption when the transepithelial osmotic gradient is maintained. This occurs experimentally when mice are treated with LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for herpesvirus entry mediator on T cells) (17). E: inhibition of Na+ malabsorption leads to a diminished osmotic gradient and less water absorption, ultimately causing mild diarrhea. This occurs experimentally in NHE3 knockout mice (74). F: in cases where there is both a barrier defect and reduced Na+ malabsorption, as in mice treated with TNF (16, 17), luminal Na+ is retained and the osmotic gradient favors movement of fluid from the tissue into the lumen. The barrier dysfunction allows greater fluid efflux than would occur with Na+ malabsorption alone.
Paracellular Transport: From Physiology to Pathophysiology
Researchers' understanding of physiologic changes in tight junctions prompted them to examine the tight junction in pathologic barrier dysfunction. Tumor necrosis factor-α (TNF) had been identified as an autocrine and paracrine regulator of intestinal epithelial tight junction permeability in vitro (86). Shortly thereafter, it was reported that TNF- neutralizing antibodies were remarkably effective as Crohn's disease treatment (4, 18) and also restored intestinal barrier function in Crohn's disease patients (85). This placed TNF at center stage in Crohn's disease pathogenesis and prompted investigation of the mechanisms of TNF-induced barrier loss. TNF treatment of Caco-2BBe monolayers resulted in doubling of MLC phosphorylation (105). While the increase in MLC phosphorylation and associated loss of barrier function developed over days, acute MLCK inhibition with the specific peptide PIK triggered complete recovery of barrier function and nearly complete normalization of MLC phosphorylation in less than 1 h (105). Thus, TNF uses, in part, a physiological mechanism of tight junction regulation. However, there are also significant differences between Na+-glucose cotransport-induced and TNF-induced barrier loss. While the former is size-selective and not associated with morphologically evident redistribution of tight junction proteins, TNF-induced barrier loss is characterized by tight junction protein endocytosis and relatively non-size-selective increases in paracellular permeability (102). Thus, while MLCK is required for TNF-induced barrier loss and is sufficient to increase paracellular permeability in vitro and in vivo, even absent of other stimuli (77, 84), other signaling events must also contribute to the impact of TNF on intestinal transport.
To better understand the in vivo contributions of tight junction regulation to immune-mediated diarrhea, a relevant model was required. Disseminated T cell activation following injection of an anti-CD3 antibody induces acute, T-cell-mediated diarrhea in mice and humans (13, 22, 23) that is abrogated by corticosteroids or TNF neutralization (22, 23, 65). In this model, serum TNF levels peak by 1.5 h and return to baseline within 4 h of anti-CD3 treatment (23), which closely matched the kinetics of diarrhea development and resolution (65). Notably, histologic changes in the first 4 h after T cell activation are limited (16); severe changes develop only 20 h or more after anti-CD3 treatment (23, 68). Nevertheless, electron microscopic examination demonstrated that, within 3 h, anti-CD3 treatment induced perijunctional actomyosin contraction that was nearly identical to that previously associated with Na+-glucose cotransport (3, 16). Further, anti-CD3 treatment was associated with increased intestinal epithelial MLC phosphorylation within the perijunctional actomyosin ring. In vivo analyses of transmucosal transport showed that anti-CD3 treatment increased albumin leak from the bloodstream into the intestinal lumen, indicative of barrier loss, as well as net fluid secretion, i.e., diarrhea (16). Both barrier loss and diarrhea were dose-dependently inhibited by luminal administration of the specific MLCK inhibitor PIK (16). Further, mice lacking the intestinal epithelial MLCK isoform long MLCK were protected from anti-CD3-induced barrier loss and diarrhea. These data firmly established epithelial MLCK as a critical mediator of physiological and pathophysiological regulation of intestinal epithelial barrier function.
Integration of Paracellular and Transcellular Transport: Back to the Chambers
PIK was able to completely normalize barrier function following anti-CD3 treatment. However, despite inducing a return to net fluid absorption, PIK did not fully restore absorption in quantitative terms (17). To understand this discordance between fluid transport and barrier function, the effects of individual cytokines elevated following anti-CD3 treatment were assessed. Although serum interferon-γ (IFN-γ) increased more than 20-fold by anti-CD3 treatment, administration of recombinant IFN-γ did not acutely affect barrier function or fluid transport. In contrast, administration of either TNF or the TNF-related molecule lymphotoxin-like inducible protein that competes with glycoprotein D for herpesvirus entry mediator on T cells (LIGHT) significantly increased paracellular albumin flux. Notably, unlike the in vitro studies where IFN-γ-dependent TNF receptor 2 (TNFR2) expression was required for TNF effects on barrier function, exogenous IFN-γ was not required for TNF-induced barrier loss.
While the effects of TNF and LIGHT on barrier function and MLC phosphorylation were quantitatively similar, each resulted in only ∼50% of the barrier loss induced by anti-CD3 treatment (17). The barrier loss induced by TNF and LIGHT was additive, and combined treatment resulted in paracellular albumin flux that was quantitatively similar to that following anti-CD3 treatment. TNF alone was sufficient to cause net fluid secretion that was quantitatively indistinguishable from that induced by anti-CD3 treatment. In contrast, LIGHT caused a small, but significant increase in fluid absorption (Fig. 3D). When TNF and LIGHT were combined, the TNF effect dominated, and net fluid secretion was identical to that induced by TNF alone or anti-CD3 (17).
To explain the discordance between barrier function and fluid flux following TNF or LIGHT treatment, the underlying mechanisms of fluid secretion were considered. Previous analyses showed that neither the cystic fibrosis transmembrane rectifier (CFTR), the dominant chloride channel responsible for fluid secretion in cholera, nor calcium-activated chloride channels were involved in anti-CD3-induced barrier loss or fluid secretion (16). Conversely, while inhibition of Na+/H+ exchangers, including NHE3, either pharmacologically or by elimination of luminal Na+, did not affect anti-CD3-induced barrier loss or fluid secretion, these treatments did reduce the magnitude of net fluid absorption in the absence of T cell activation (16). Further, pharmacological or genetic NHE3 inhibition or elimination of luminal Na+ completely abolished fluid absorption in LIGHT-treated mice (17). These data suggested that inhibition of Na+/H+ exchange might contribute to TNF-induced fluid secretion. That hypothesis was explored in Ussing chambers using Na22 as a tracer of Na+ transport, much as Ussing himself had done half a century earlier. Na+ absorption was similar in jejunal mucosae from control or LIGHT-treated mice and was reduced by ∼80% following treatment with a specific NHE3 inhibitor or in mucosae from NHE3-deficient mice (Fig. 3E). Net Na+ absorption in jejunal mucosae from TNF-treated mice was reduced by ∼80% relative to mucosae from control or LIGHT-treated mice and was not significantly affected by the NHE3 inhibitor (17). Thus, in addition to triggering MLCK-dependent barrier loss, TNF inhibited NHE3 function.
TNF-induced NHE3 inhibition was associated with movement of NHE3 from the apical brush border into a subapical cytoplasmic compartment. Such NHE3 inhibition had been previously reported following PKCα activation. Consistent with PKCα-mediated NHE3 endocytosis, PKCα inhibition prevented both NHE3 removal from the apical membrane and reduced Na+ absorption following TNF treatment (17). Finally, either pharmacological or genetic PKCα inhibition prevented diarrhea following TNF treatment, while the PKCα activator synergized with LIGHT to cause net fluid secretion (17). Thus, the difference in fluid movement following TNF or LIGHT treatment can be explained by the observations that, while both LIGHT and TNF activate MLCK to effect barrier loss, only TNF triggers PKCα-dependent NHE3 inhibition (Fig. 3F). These data reinforce our understanding of the paracellular pathway as a passive transport process driven by electrochemical gradients and demonstrate that NHE3-dependent Na+ absorption significantly contributes to the gradients that drive paracellular fluid absorption.
TNF-Induced Tight Junction Reorganization
Despite extensive examination, tight junction protein distribution was only modestly affected by TNF or anti-CD3 treatment (16, 61). The most notable change was endocytosis of the tight junction protein occludin, which was blocked by either pharmacological or genetic MLCK inhibition (16, 61). Occludin internalization began in a synchronized fashion 1.5 h after TNF administration and preceded luminal fluid accumulation by 0.75 h (61). Morphometric analysis of electron micrographs demonstrated the appearance of new vesicles with diameters of ∼80 nm that coincided with occludin internalization. These matured progressively into larger vesicles with diameters of ∼125 nm, ∼170 nm, and ∼240 nm over the next 0.5 h. Correlative immunofluorescent analyses showed the occludin-containing vesicles that appeared at 1.5 h were positive for caveolin-1 and became progressively positive for the early endosomal marker EEA1 over the next 0.5 h. In contrast, clathrin heavy chain did not colocalize significantly with occludin at any time before or after TNF treatment. In vitro studies showed similar internalization of occludin into EEA1-positive vesicles after IFN-γ treatment of T84 intestinal epithelial monolayers (11) or latrunculin-A-mediated actin depolymerization in MDCK cell monolayers (78). In vivo, pharmacological inhibition of caveolar, but not clathrin-mediated endocytosis or micropinocytosis, prevented TNF-induced occludin endocytosis, barrier loss, and net fluid secretion (61). This correlated with in vitro studies of IFN-γ- and TNF-induced occludin internalization in Caco-2 monolayers as well as latrunculin-A-induced occludin internalization in MDCK monolayers, but were not consistent with studies concluding that macropinocytosis was responsible for occludin endocytosis in IFN-γ-treated T84 cells (11). The difference may well reflect nonspecific effects of pharmacological inhibitors under the conditions of those studies. The ultimate evidence is that caveolin-1 knockout prevented in vivo TNF-induced occludin endocytosis, barrier loss, and net fluid secretion (61). Consistent with this, expression of dominant negative dynamin, which blocks clathrin- and caveolin-1-mediated endocytosis, but not micropinocytosis, prevented latrunculin-A-induced occludin internalization in MDCK monolayers (78). Internalization of occludin and other proteins via clathrin-mediated endocytosis has also been reported. This is unlikely to be physiologically relevant, as the conclusion is largely based on studies using pharmacological inhibitors and has not been replicated in vivo (40). Nevertheless, these studies do underscore the importance of occludin, which is further emphasized by in vivo the observation that occludin overexpression limits TNF-induced barrier loss and diarrhea as well as reports that occludin knockdown reduces barrier function in cultured intestinal epithelial cells and mouse intestine (1, 12). While not proven, it is likely that occludin is degraded following internalization, thereby explaining the reduction in occludin expression that is seen in chronic disease (37).
The data above and other work link MLCK-dependent, caveolin-1-mediated occludin endocytosis to TNF- and LIGHT-induced, MLCK-dependent barrier loss (60, 61, 75). This, however, seemed at odds with reports that intestinal barrier function was unaffected by occludin knockout (73). Nevertheless, intestinal epithelial overexpression of occludin partially protected against TNF-induced barrier loss and restored net fluid absorption (61). Potential explanations to resolve the paradox presented by these conflicting results include the possibility that knockout mice are able to compensate for intestinal epithelial occludin loss, perhaps by upregulating another tight junction-associated MARVEL protein (TAMP) (69), or, alternatively, that the contributions of occludin to tight regulation are more important than those to basal barrier function. Studies of the responses of occludin-deficient mice to stress, which have not been reported to date, may provide better understanding. Nevertheless, the in vitro observations that barrier function is reduced in a manner similar to that induced by TNF in occludin-deficient Caco-2BBe monolayers (12), that TNF does not cause further barrier loss in either Caco-2BBe or MDCKII monolayers lacking occludin (12, 97), and that disruption of occludin-ZO-1 binding prevents both TNF-induced barrier loss and occludin endocytosis (12) provide strong evidence that occludin is a critical component of the circuitry responsible for TNF-induced barrier loss and diarrhea.
Towards an Understanding of Barrier Loss in Chronic Disease
Barrier loss alone does not trigger human or experimental inflammatory bowel disease in otherwise normal subjects, as demonstrated by the observation that neither mice nor humans with chronically increased intestinal permeability develop disease (38, 63, 84). However, epithelial injury can contribute to a susceptible host developing inflammatory bowel disease, as demonstrated by acceleration of disease pathogenesis by either NSAIDs or H. hepaticus infection in IL-10 knockout mice (35, 62) and, potentially, the small increases in inflammatory bowel disease incidence following an episode of infectious gastroenteritis (33). To better define the contributions of isolated tight junction barrier loss to chronic colitis, transgenic mice expressing a constitutively active myosin light chain kinase (CA-MLCK) within the intestinal epithelium were developed (84). Despite increased paracellular permeability, these mice are clinically normal (84), much like some fist degree relatives of Crohn's disease patients (38). Thus, the CA-MLCK transgenic mice may be a model of healthy subjects who are at risk of developing inflammatory bowel disease. Consistent with this, CA-MLCK transgenic mice display subclinical mucosal immune activation characterized by TH1 immune polarization. Nevertheless, CA-MLCK transgenic mice develop disease more rapidly and suffer greater morbidity and mortality when studied in the context of chronic, immune-mediated, adoptive transfer colitis (84). Thus, while tight junction barrier loss is insufficient to cause disease in an immunologically intact individual, it can accelerate development and augment disease progression (84). Further, these data demonstrate that MLCK-dependent barrier regulation, which is both physiologically and pathophysiologically relevant, is sufficient to promote disease.
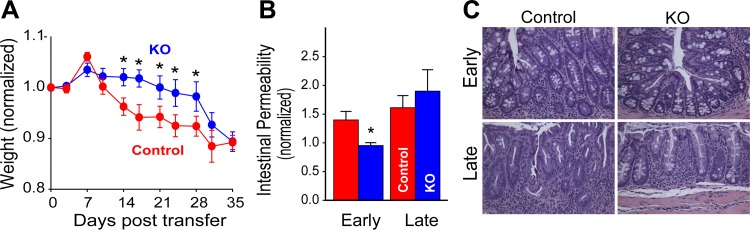
While increased MLCK activity can enhance disease, whether MLCK inhibition can prevent or limit disease remained unknown. The question is relevant, however, as MLCK expression is transcriptionally upregulated by TNF in experimental models (34, 102) and in patients with active inflammatory bowel disease (9). This was initially explored by taking advantage of the observation that intestinal epithelial TNFR2 expression is required for activation of MLCK (103). By using the adoptive transfer colitis model, it was possible to provide lymphocytes with normal TNFR1 and TNFR2 expression to recipient mice lacking TNFR1 or TNFR2 on other cell types (83). TNFR2, but not TNFR1, knockout recipients were markedly protected from disease in terms of weight loss, mucosal TNF production, and histopathology (Fig. 4). This could be explained by the absence of increased intestinal epithelial MLCK expression and MLC phosphorylation in TNFR2, but not TNFR1, knockout recipients (83). Further study using wild-type donor lymphocytes and recipient mice lacking the epithelial long MLCK isoform showed decreased MLC phosphorylation and reduced histopathology relative to MLCK-expressing mice (Fig. 4). The long MLCK knockout mice also displayed preservation of barrier function and body weight, and reduced mucosal TNF production and clinical scores at early, but not late times during disease development (83). This apparent failure of MLCK knockout to prevent disease was explained by progressively greater immune-mediated epithelial damage as disease advanced. One way to explain these data is that epithelial damage sufficient to cause tight junction independent barrier loss made inhibition of MLCK-dependent barrier loss irrelevant late in the disease course. Consistent with this, MLCK knockout mice were protected from epithelial apoptosis at early, but not late stages of disease (83). This protection was not a direct effect of MLCK deficiency, as acute, high-dose TNF administration caused similar degrees of epithelial apoptosis in wild-type and long MLCK knockout mice. Further, when subjected to the epithelial injury-dependent DSS model of colitis, disease was actually more severe in long MLCK knockout, relative to wild-type, mice. Thus, while epithelial MLCK inhibition has promise in immune-mediated diseases, such as inflammatory bowel disease, it may actually be harmful in disorders dominated by epithelial injury. This can be understood when one recalls that MLCK is important to both epithelial migration and wound closure (72).
Fig. 4.
Adoptive transfer colitis is less severe in mice lacking epithelial myosin light chain kinase (MLCK). A: Rag1−/−MLCK−/− and Rag1−/− received naïve T cells. MLCK knockout (KO) mice were protected from weight loss at early, but not late, stages of disease. B: intestinal permeability is increased in Rag1−/−, but not Rag1−/−MLCK−/−, at day 15 after adoptive transfer. This difference is abrogated by day 35 and coincides with increased epithelial apoptosis. C: despite weight loss, epithelial MLCK knockout mice demonstrate protection from histopathological evidence of disease at early and late times. [From Su et al. (83) with permission from Elsevier.]
Future Opportunities
Despite these and many other significant advances in our understanding of epithelial transport, much remains to be explored. This is true in terms of fundamental discovery, and particularly pressing if this knowledge is to be used for therapeutic benefit. As one example, although the data above suggest that clinical use of MLCK inhibitors may have promise, the identity of smooth muscle and epithelial MLCK catalytic domains presents a potential obstacle to developing therapies that target MLCK, as smooth muscle MLCK inhibition is likely to result in hypotension, intestinal dysmotility, and dysfunction of other organs, e.g., the bladder (36). In addition, the complex physiologic interactions between occludin, ZO-1, and the claudin family have yet to be fully elucidated, although these do appear to be critical tight junction regulation. Other gaps in understanding of transcellular transport and its integration with paracellular transport also remain to be filled. Finally, while the gastrointestinal microbiome has become a frequent topic of study, little is known about how interactions with luminal bacteria, viruses, and other materials impact epithelial transport and barrier functions. Nevertheless, epidemiological studies linking acute infectious gastroenteritis to subsequent development of inflammatory bowel disease suggest that this may be a particularly fruitful future avenue for investigation. Thus, much remains to be done. The great strides forward in our understanding of epithelial transport in the relatively short time since Ussing created his chamber are, nevertheless, remarkable. And, as is often the case, this knowledge has led to even more exciting frontiers for exploration.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01 DK-68271, R01 DK-61931, and R24 DK-099803.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.R.H. and J.R.T. prepared figures; J.R.H. and J.R.T. drafted manuscript; J.R.H. and J.R.T. edited and revised manuscript; J.R.H. and J.R.T. approved final version of manuscript.
REFERENCES
- 1.Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 300: G1054–G1064, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen B, Ussing HH. Solvent drag on non-electrolytes during osmotic flow through isolated toad skin and its response to antidiuretic hormone. Acta Physiol Scand 39: 228–239, 1957. [DOI] [PubMed] [Google Scholar]
- 3.Atisook K, Carlson S, Madara JL. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am J Physiol Cell Physiol 258: C77–C85, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Baert FJ, D'Haens GR, Peeters M, Hiele MI, Schaible TF, Shealy D, Geboes K, Rutgeerts PJ. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology 116: 22–28, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Barry RJ, Smyth DH, Wright EM. Short-circuit current and solute transfer by rat jejunum. J Physiol 181: 410–431, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am J Physiol Gastrointest Liver Physiol 281: G1487–G1493, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Binder HJ, Rajendran V, Sadasivan V, Geibel JP. Bicarbonate secretion: a neglected aspect of colonic ion transport. J Clin Gastroenterol 39: S53–S58, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 86: 191–201, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Bricker NS, Biber T, Ussing HH. Exposure of the isolated from skin to high potassium concentrations at the internal surface. I. Bioelectric phenomena and sodium transport. J Clin Invest 42: 88–99, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 19: 923–933, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, Breskin LA, Wu L, Anderson K, Turner JR, Weber CR. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell 24: 3056–3068, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatenoud L. Therapeutic use of the OKT3 anti-T cell monoclonal antibody: mode of action and side effects. Transplant Proc 20: 79–83, 1988. [PubMed] [Google Scholar]
- 14.Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol 39: 219–232, 1978. [DOI] [PubMed] [Google Scholar]
- 15.Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol 58: 390–400, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest 115: 2702–2715, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116: 2682–2694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: a European multicenter trial. Gastroenterology 116: 1029–1034, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Diamond JM, Bossert WH. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol 50: 2061–2083, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farquhar M, Palade G. Junctional complexes in various epithelia. J Cell Biol 17: 375–412, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferran C, Dy M, Merite S, Sheehan K, Schreiber R, Leboulenger F, Landais P, Bluestone J, Bach JF, Chatenoud L. Reduction of morbidity and cytokine release in anti-CD3 MoAb-treated mice by corticosteroids. Transplantation 50: 642–648, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Ferran C, Sheehan K, Dy M, Schreiber R, Merite S, Landais P, Noel LH, Grau G, Bluestone J, Bach JF, Chatenoud L. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol 20: 509–515, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Ferraris RP, Diamond J. Crypt-villus site of glucose transporter induction by dietary carbohydrate in mouse intestine. Am J Physiol Gastrointest Liver Physiol 262: G1069–G1073, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Fine KD, Ana CAS, Porter JL, Fordtran JS. Mechanism by which glucose stimulates the passive absorption of small solutes by the human jejunum in-vivo. Gastroenterology 107: 389–395, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of changing intestinal flow rate on a measurement of intestinal permeability. Gastroenterology 108: 983–989, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of d-glucose on intestinal permeability and its passive absorption in human small intestine in vivo. Gastroenterology 105: 1117–1125, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Francis W, Pumphrey R. The electrical properties of frog skin. Part I. Introductory. J Exp Biol 10: 379–385, 1933. [Google Scholar]
- 29.Fromter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol 8: 259–301, 1972. [DOI] [PubMed] [Google Scholar]
- 30.Fromter E, Diamond J. Route of passive ion permeation in epithelia. Nat New Biol 235: 9–13, 1972. [DOI] [PubMed] [Google Scholar]
- 31.Ghishan FK, Kiela PR. Epithelial transport in inflammatory bowel diseases. Inflamm Bowel Dis 20: 1099–1109, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol 45: 272–290, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterology 137: 495–501, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem 281: 26205–26215, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Hale LP, Gottfried MR, Swidsinski A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm Bowel Dis 11: 1060–1069, 2005. [DOI] [PubMed] [Google Scholar]
- 36.He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, Feil R, Kamm KE, Stull JT, Gao X, Zhu MS. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology 135: 610–620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129: 550–564, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 105: 883–885, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Hu Z, Wang Y, Graham WV, Su L, Musch MW, Turner JR. MAPKAPK-2 is a critical signaling intermediate in NHE3 activation following Na+-glucose cotransport. J Biol Chem 281: 24247–24253, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell 15: 176–188, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeck N, Schlingmann KP, Reinalter SC, Komhoff M, Peters M, Waldegger S, Seyberth HW. Salt handling in the distal nephron: lessons learned from inherited human disorders. Am J Physiol Regul Integr Comp Physiol 288: R782–R795, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Jentsch TJ, Maritzen T, Zdebik AA. Chloride channel diseases resulting from impaired transepithelial transport or vesicular function. J Clin Invest 115: 2039–2046, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnsen VK, Levi H, Ussing HH. The mode of passage of chloride ions through the isolated frog skin. Acta Physiol Scand 25: 150–163, 1952. [DOI] [PubMed] [Google Scholar]
- 44.Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol 73: 261–281, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J 350: 155–162, 2000. [PMC free article] [PubMed] [Google Scholar]
- 46.Khanal RC, Nemere I. Regulation of intestinal calcium transport. Annu Rev Nutr 28: 179–196, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Kirschner N, Brandner JM. Barriers and more: functions of tight junction proteins in the skin. Ann NY Acad Sci 1257: 158–166, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Koefoed-Johnsen V, Ussing HH. The nature of the frog skin potential. Acta Physiol Scand 42: 298–308, 1958. [DOI] [PubMed] [Google Scholar]
- 49.Koefoed-Johnsen V, Ussing HH, Zerahn K. The origin of the short-circuit current in the adrenaline stimulated frog skin. Acta Physiol Scand 27: 38–48, 1952. [DOI] [PubMed] [Google Scholar]
- 50.Larsen EH, Mobjerg N. Na+ recirculation and isosmotic transport. J Membr Biol 212: 1–15, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Levi H, Ussing HH. Resting potential and ion movements in the frog skin. Nature 164: 928, 1949. [DOI] [PubMed] [Google Scholar]
- 52.Lin R, Murtazina R, Cha B, Chakraborty M, Sarker R, Chen TE, Lin Z, Hogema BM, de Jonge HR, Seidler U, Turner JR, Li X, Kovbasnjuk O, Donowitz M. d-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology 140: 560–571, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machen TE, Erlij D, Wooding FB. Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J Cell Biol 54: 302–312, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacLeod RJ, Hamilton JR. Activation of Na+/H+ exchange is required for regulatory volume decrease after modest “physiological” volume increases in jejunal villus epithelial cells. J Biol Chem 271: 23138–23145, 1996. [DOI] [PubMed] [Google Scholar]
- 55.MacLeod RJ, Hamilton JR. Volume regulation initiated by Na+-nutrient cotransport in isolated mammalian villus enterocytes. Am J Physiol Gastrointest Liver Physiol 260: G26–G33, 1991. [DOI] [PubMed] [Google Scholar]
- 56.MacLeod RJ, Lembessis P, Hamilton JR. Differences in Ca(2+)-mediation of hypotonic and Na(+)-nutrient regulatory volume decrease in suspensions of jejunal enterocytes. J Membr Biol 130: 23–31, 1992. [DOI] [PubMed] [Google Scholar]
- 57.Madara JL. Sodium-glucose cotransport and epithelial permeability. Gastroenterology 107: 319–320, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Madara JL, Carlson S. Supraphysiologic l-tryptophan elicits cytoskeletal and macromolecular permeability alterations in hamster small intestinal epithelium in vitro. J Clin Invest 87: 454–462, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol 100: 149–164, 1987. [DOI] [PubMed] [Google Scholar]
- 60.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol 5: 119–144, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 189: 111–126, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matharu KS, Mizoguchi E, Cotoner CA, Nguyen DD, Mingle B, Iweala OI, McBee ME, Stefka AT, Prioult G, Haigis KM, Bhan AK, Snapper SB, Murakami H, Schauer DB, Reinecker HC, Mizoguchi A, Nagler CR. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology 137: 1380–1390 e1381–e1383, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn's disease? Gastroenterology 104: 1627–1632, 1993. [DOI] [PubMed] [Google Scholar]
- 64.Meddings JB, Westergaard H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin Sci (Lond) 76: 403–413, 1989. [DOI] [PubMed] [Google Scholar]
- 65.Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest 110: 1739–1747, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pappenheimer JR. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J Membr Biol 100: 137–148, 1987. [DOI] [PubMed] [Google Scholar]
- 67.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular-junctions to absorption of nutrients by the small-intestine of the Rat. J Membr Biol 100: 123–136, 1987. [DOI] [PubMed] [Google Scholar]
- 68.Radojevic N, McKay DM, Merger M, Vallance BA, Collins SM, Croitoru K. Characterization of enteric functional changes evoked by in vivo anti-CD3 T cell activation. Am J Physiol Regul Integr Comp Physiol 276: R715–R723, 1999. [DOI] [PubMed] [Google Scholar]
- 69.Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marvelD3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 21: 1200–1213, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reuss L, Finn AL. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol 25: 115–139, 1975. [DOI] [PubMed] [Google Scholar]
- 71.Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev 95: 297–340, 2015. [DOI] [PubMed] [Google Scholar]
- 72.Russo JM, Florian P, Shen L, Graham WV, Tretiakova MS, Gitter AH, Mrsny RJ, Turner JR. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology 128: 987–1001, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11: 4131–4142, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 75.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology 132: 2383–2394, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekine T, Miyazaki H, Endou H. Molecular physiology of renal organic anion transporters. Am J Physiol Renal Physiol 290: F251–F261, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 119: 2095–2106, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16: 3919–3936, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shiue H, Musch MW, Wang Y, Chang EB, Turner JR. Akt2 phosphorylates ezrin to trigger NHE3 translocation and activation. J Biol Chem 280: 1688–1695, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soergel KH. Showdown at the tight junction. Gastroenterology 105: 1247–1250, 1993. [DOI] [PubMed] [Google Scholar]
- 81.Staehelin LA, Mukherjee TM, Williams AW. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma 67: 165–184, 1969. [DOI] [PubMed] [Google Scholar]
- 82.Stapp P. Efficiency of electrical energy production by surviving frog skin, measured by iodine coulometer. Exp Biol Med (Maywood) 46: 382–384, 1941. [Google Scholar]
- 83.Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai PY, Fu YX, Abraham C, Turner JR. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 145: 407–415, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136: 551–563, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol 97: 2000–2004, 2002. [DOI] [PubMed] [Google Scholar]
- 86.Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology 114: 657–668, 1998. [DOI] [PubMed] [Google Scholar]
- 87.Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na+ -glucose cotransport in intestinal epithelia. Am J Physiol Cell Physiol 281: C1533–C1541, 2001. [DOI] [PubMed] [Google Scholar]
- 88.Turner JR, Black ED, Ward J, Tse CM, Uchwat FA, Alli HA, Donowitz M, Madara JL, Angle JM. Transepithelial resistance can be regulated by the intestinal brush border Na+-H+ exchanger NHE3. Am J Physiol Cell Physiol 279: C1918–C1924, 2000. [DOI] [PubMed] [Google Scholar]
- 89.Turner JR, Lencer WI, Carlson S, Madara JL. Carboxy-terminal vesicular stomatitis virus G protein-tagged intestinal Na+-dependent glucose cotransporter (SGLT1): maintenance of surface expression and global transport function with selective perturbation of transport kinetics and polarized expression. J Biol Chem 271: 7738–7744, 1996. [DOI] [PubMed] [Google Scholar]
- 90.Turner JR, Madara JL. Physiological regulation of intestinal epithelial tight junctions as a consequence of Na+-coupled nutrient transport. Gastroenterology 109: 1391–1396, 1995. [DOI] [PubMed] [Google Scholar]
- 91.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol Cell Physiol 273: C1378–C1385, 1997. [DOI] [PubMed] [Google Scholar]
- 92.Uhing MR, Arango V. Intestinal absorption of proline and leucine in chronically catheterized rats. Gastroenterology 113: 865–874, 1997. [DOI] [PubMed] [Google Scholar]
- 93.Uhing MR, Kimura RE. Active transport of 3-O-methyl-glucose by the small intestine in chronically catheterized rats. J Clin Invest 95: 2799–2805, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uhing MR, Kimura RE. The effect of surgical bowel manipulation and anesthesia on intestinal glucose absorption in rats. J Clin Invest 95: 2790–2798, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ussing HH. Transport through biological membranes. Annu Rev Physiol 15: 1–20, 1953. [DOI] [PubMed] [Google Scholar]
- 96.Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23: 110–127, 1951. [DOI] [PubMed] [Google Scholar]
- 97.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci 123: 2844–2852, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venkatasubramanian J, Ao M, Rao MC. Ion transport in the small intestine. Curr Opin Gastroenterol 26: 123–128, 2010. [DOI] [PubMed] [Google Scholar]
- 99.Verkman AS. Aquaporins in clinical medicine. Annu Rev Med 63: 303–316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Visentin M, Diop-Bove N, Zhao R, Goldman ID. The intestinal absorption of folates. Annu Rev Physiol 76: 251–274, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Viswanathan VK, Hodges K, Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol 7: 110–119, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 166: 409–419, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 131: 1153–1163, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao H, Shiue H, Palkon S, Wang Y, Cullinan P, Burkhardt JK, Musch MW, Chang EB, Turner JR. Ezrin regulates NHE3 translocation and activation after Na+-glucose cotransport. Proc Natl Acad Sci USA 101: 9485–9490, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123: 163–172, 2002. [DOI] [PubMed] [Google Scholar]