Abstract
Urocortins (Ucns) 1, 2, and 3 and corticotropin-releasing factor receptor 2 (CRF2) mRNA are prominently expressed in various layers of the upper gut. We tested whether Ucns and CRF2 variants are also expressed in the different layers of the rat colon, regulated by LPS (100 μg/kg ip) and play a modulatory role in the colonic immune response to LPS. Transcripts of Ucns and CRF2b, the most common isoform in the periphery, were detected in all laser microdissected layers, including myenteric neurons. LPS increased the mRNA level of Ucn 1, Ucn 2, and Ucn 3 and decreased that of CRF2b in both the colonic mucosa and submucosa + muscle (S+M) layers at 2, 6, and 9 h after injection with a return to basal at 24 h. In addition, CRF2a, another variant more prominent in the brain, and a novel truncated splice variant CRF2a-3 mRNA were detected in all segments of the large intestine. LPS reciprocally regulated the colonic expression of these CRF2 variants by decreasing both CRF2a and CRF2b, while increasing CRF2a-3 in the mucosa and S+M. The CRF2 antagonist astressin2-B further enhanced LPS-induced increase of mRNA level of interleukin (IL)-1β, TNF-α, and inducible nitric oxide synthase in S+M layers and IL-1β in the mucosa and evoked TNF-α expression in the mucosa. These data indicate that Ucns/CRF2 variants are widely expressed in all colonic layers and reciprocally regulated by LPS. CRF2 signaling dampens the CD14/TLR4-mediated acute inflammatory response to Gram-negative bacteria in the colon.
Keywords: astressin2-B, colon, corticotropin-releasing factor receptor type 2 variants, cytokines, lipopolysaccharide, urocortins
the corticotropin-releasing factor (CRF) peptide family encompasses, in addition to the 41-amino acid peptide CRF, three mammalian CRF-like paralogs, urocortins (Ucns), namely Ucn 1, Ucn 2, and Ucn 3 (16, 59). These peptides bind to two distinct receptor subtypes, CRF1 and CRF2, which belong to the class B1 G protein-coupled receptor superfamily (16, 18). Although these two CRF receptors share 69% amino acid identity, they exhibit distinct ligand specificity (16, 19). CRF and Ucn 1 bind with high affinity to the CRF1 receptor, while Ucn 1 displays 40 times higher binding affinity to CRF2 than CRF (16, 18). In contrast, Ucn 2 and Ucn 3 bind selectively to CRF2 and, thus, are considered to be the endogenous ligands of this receptor subtype (16, 19). In rodents, the CRF2 exists in two protein-coding splice variants, CRF2a and CRF2b (previously denoted as CRF2α and CRF2β, respectively), which are also highly conserved among mammalian species, including humans (16, 19). Although these two isoforms are structurally distinct in their NH2-terminal extracellular domains, they share almost identical binding affinity for Ucns leading to the activation of adenylate cyclase in cells expressing rodent CRF2a and CRF2b (16, 35). In rats, the distribution of CRF2a variant was originally reported to be confined to brain neurons, while CRF2b was expressed in nonneuronal cells of the brain and primarily in peripheral tissues, including the skeletal muscle and viscera (12, 36, 61, 63). However, recent studies provided evidence that CRF2a variant and more prominently, the additional splice variant CRF2a-3 were also expressed in the esophagus and stomach (61, 63). So far, the detailed knowledge on Ucn ligands and CRF2 receptor transcript variants in various layers of the large intestine is still limited compared with the upper gut (61, 63) or other visceral organ, such as the heart (54).
Convergent reports support the existence of a peripheral CRF/CRF1 signaling system that has physiological relevance in the acute stress response, particularly pertaining to the stimulation of colonic secretory motor function, the development of visceral pain and the promotion of intestinal inflammation and angiogenesis (24, 34, 53). By contrast, the activation of CRF2 receptor induced by peripheral injection of Ucn 2 induces opposite biological effects on the colon, namely, the inhibition of intraperitoneal CRF-induced CRF1-mediated stimulation of colonic secretory motor function and visceral hyperalgesia, while not influencing basal colonic propulsive motility in rodents (14, 38, 40, 41, 46). Activation of Ucn 2/CRF2 receptor signaling pathway also dampened colonic inflammation assessed by histological damage and cytokine production and promoted mucosal repair in colitis models (5, 23, 24, 29).
Bacterial lipopolysaccharide (LPS) is widely used to study the innate defense response to Gram-negative bacteria infection inducing acute immunological stress (32). LPS injected intraperitoneally at 50 μg/kg alters the gene expression of CRF2b and Ucn 1 in peripheral organs, such as the pituitary, heart, or thymus (25, 26, 28). We recently reported that LPS injected intraperitoneally at 100 μg/kg increased Ucns' mRNA levels and differentially regulated the expression of CRF2 receptor variants in the gastric corpus, which was relevant to the modulation of gastric motor inhibition associated with LPS injection (63). Whether LPS also regulates the gene expression of Ucns and CRF2 splice variants in the rat colon is still unknown.
In the present study, we first assessed the profile of Ucns and CRF2b gene expression in the different layers of the proximal colon, including myenteric neurons in naïve rats using laser microdissection system (LMD) combined with RT-PCR. Then, we tested the hypothesis that LPS regulates Ucns/CRF2b gene expression in the colonic mucosa and submucosa plus muscle layers (S+M). As we have previously shown that in addition to the CRF2b variant, the splice variants CRF2a and, more prominently, CRF2a-3, which contains the entire intron 3 (61), were expressed in the rat stomach (63), we tested whether they were also expressed in the large intestine and regulated by LPS. Lastly, on the basis of the important roles of peripheral Ucns in regulating the inflamed intestine (5, 29, 64), we examined whether alterations in Ucns/CRF2 gene expression induced by LPS modulated the colonic expression of cytokines using peripheral injection of the CRF2 selective antagonist, astressin2-B (49).
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Harlan Laboratory, San Diego, CA) weighing 260–310 g were housed under controlled conditions of temperature and lighting (22–24°C, lights on from 6:00 AM to 6:00 PM). Animals had free access to a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water. Studies were conducted under the approved protocol of the Department of Veterans Affairs Animal Research Committee (no. 05-058-02). All experiments were performed between 7:00 and 10:00 AM except in the 6- and 9-h time course study, which ended at 1:00 PM and 4:00 PM, respectively.
Urocortins and CRF2b Gene Expression in the Colon
Tissue collection.
Four naïve rats were euthanized by decapitation, and the proximal colon was harvested and separated into the mucosa and S+M. All tissue samples were immediately frozen on dry ice and stored at −80°C until processed for RNA extraction and RT-PCR determinations.
RNA extraction and RT-PCR.
Tissue samples were homogenized in RNA-Bee (TEL-TEST, Friendswood, TX) using a polytron homogenizer (Brinkmann Instruments, Westbury, NY), following the manufacturer's recommended protocol. RNA pellets were resuspended in diethylpyrocarbonate (DEPC)-treated water and further digested with DNase I for 60 min at 37°C (Promega, Madison, WI). The quality and amount of RNA samples were estimated on the basis of the ratio of absorbance at 260/280 nm by UV spectrophotometer (ND-1000, NanoDrop, Wilmington, DE). Then, 5 μg of total RNA was reverse-transcribed to cDNA using ThermoScript RT (Invitrogen, Carlsbad, CA) in a total volume of 20 μl reaction buffer. The reaction was terminated by incubation at 85°C for 5 min. One microliter of RNase H was added into the reaction mixture to remove the RNA template. Subsequently, 1 μl of first-strand cDNA was amplified directly by the PCR, as described previously (61). The sequences of primers specific for rat Ucn 1, Ucn 2, Ucn 3, and CRF2b are listed in Table 1. Rat CRF2 receptor primers were designed to amplify the NH2-terminal regions specific to CRF2b. RT-PCR for acidic ribosomal protein (ARP), a housekeeping gene, served as an internal control to assess RNA quality and cDNA normalization (61). PCR products were separated by 1% agarose gel electrophoresis, visualized with ethidium bromide. Gel images were acquired by Kodak EDAS 290 system and quantitative densitometry was performed with NIH image software (Scion, Frederick, MD).
Table 1.
Primers used for RT-PCR experiments
| Genes | Direction | Primer (5′-3′) | Position | Product Size, bp |
|---|---|---|---|---|
| Ucn 1 | forward | GCTACGCTCCTGGTAGCGTTGCTGCTTCTG | 133–162 | 356 |
| reverse | GCCGATCACTTGCCCACCGAGTCGAATATG | 459–488 | ||
| Ucn 2 | forward | ATGATGACCAGGTGGGCACTG | 85–105 | 367 |
| reverse | AGGTCACCCCATCTTTATGAC | 431–451 | ||
| Ucn 3 | forward | AGCGATGCTGATGCCCACTTAC | 505–526 | 516 |
| reverse | CCTGCCTGGTCTTTGCTTTATTTC | 997–1020 | ||
| CRF2a and CRF2a-3 | forward | GCGGCCCCTCATCTCCGTGAG | 1–22 | 353, 483* |
| reverse | CTGGTCCAAGGTCGTGTTGCA | 333–353 | ||
| CRF2b | forward | ATGGGGACCCCAGGCTCT | 215–232 | 198 |
| reverse | CTGGTCCAAGGTCGTGTTGCA | 392–412 | ||
| PGP 9.5 | forward | ATGAATCAGACCATCGGGAAC | 227–297 | 505 |
| reverse | GCTAAAGCTGCAAACCAAGGG | 761–781 | ||
| ARP | forward | GTTGAACATCTCCCCCTTCTC | 604–624 | 402 |
| reverse | ATGTCCTCATCGGATTCCTCC | 985–1005 |
Variant CRF2a-3 (including intron 3).
Laser microdissection.
Four naïve rats were euthanized by decapitation, and whole-thickness proximal colon tissues were embedded in OCT (Tissue-Tek, Sakura Finete, Torrance, CA) and snap-frozen in isopentane (Sigma-Aldrich, St. Louis, MO) cooled with dry ice. The proximal colons from two rats were sectioned vertically or flatly in a cryostat, and different layers, including the epithelia, lamina propria, crypts, submucosa, circular muscle, and individual myenteric neurons, were dissected using Pixcell II LCM System (Arcturus Engineering, Mountain View, CA), and the procedures were essentially as described previously (61). The vertical or flat cryostat sections (8 μm) of the proximal colons from another two rats were mounted onto polyethylene naphthalate (PEN)-membrane glass slides (Leica Microsystems, Bannockburn, IL), air dried, fixed in 70% ethanol, and then rehydrated in DEPC-treated water. Vertical sections were stained with hematoxylin and eosin. Flat sections crossing the myenteric plexus were stained with cuprolinic blue, an established histochemical staining specific for enteric neurons (21). After mounting face down, the sections on the Leica LMD 7000 (Leica Microsystems) were examined using a Leica binocular dissecting microscope with zoom magnification (Leica Microsystems). The boundaries of different colonic layers, including the epithelia, lamina propria, crypts, submucosa, circular muscle, and individual myenteric neurons, were outlined onto the monitor using an ultra-fine point marking pen (Fig. 2, top). The pulsed UV laser beam was carefully directed along the borders of each layer with the following setting: power 45, aperture 11, speed 10, and offset 39 for cutting colonic tissue layers under a 10× objective and power 8, aperture 5, speed 4, and offset 39 for dissecting myenteric neurons under a 40× objective. Each different layer and 300–500 neurons were cut from 4–5 adjacent slides per animal and then were transferred by gravity alone into a 0.5 ml microcentrifuge tube cap placed directly underneath the section, thereby providing a contact-free and contamination-free transfer. The tube cap was filled with 30 μl of RNA extraction buffer (PicoPure RNA isolation kit; Arcturus, Mountain View, CA) to ensure isolation of intact RNA. Tissue and neuron collections were verified by inspecting the tube cap.
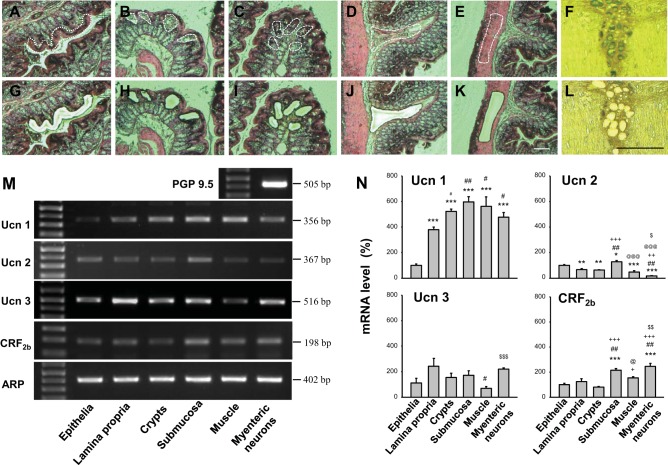
Fig. 2.
Laser microdissection (LMD) of freshly frozen vertical and flat sections crossing the myenteric plexus (8 μm) of rat proximal colon in naïve rats. The surface epithelia, lamina propria, crypts, submucosa, and circle muscle layers were dissected separately from the whole-thickness tissue sections stained with hematoxylin and eosin (A–E, G–K). The individual myenteric neurons were clearly defined with the cuprolinic blue staining and dissected by LMD (F, L); untargeted tissues remained attached to the glass slide. Representative images before dissection (A–F) and after dissection (G–L). Scale bar = 100 μm. M: representative gel images of RT-PCR products showing the mRNA expression of Ucn 1, Ucn 2, Ucn 3, CRF2b, and ARP in LMD dissected layers and myenteric neurons identified by the neuronal marker protein gene product 9.5 (PGP 9.5). N: semiquantitative analysis of mRNA levels obtained with NIH image software (Scion, Frederick, MD). Values are expressed as percent mRNA levels of the epithelia taken as 100%. Each column represents the means ± SE of four rats. *P < 0.05; **P < 0.01, and ***P < 0.001 compared with the epithelia. #P < 0.05 and ##P < 0.01 compared with lamina propria. ++P < 0.01 and +++P < 0.001 compared with the crypts. @P < 0.05 and @@@P < 0.001 compared with the submucosa. $P < 0.05, $$P < 0.01, and $$$P < 0.001 compared with the muscle layer.
RNA isolation and RT-PCR for LMD samples.
The tissue samples isolated with LCM and adhered on the LCM caps were processed for RT-PCR, as detailed previously (61). The tissue layers and neurons collected with LMD 7000 were processed for total RNA isolation with PicoPure RNA isolation kit (Arcturus), according to the manufacturer's recommendations with a DNase treatment by RNase-Free DNase Set (Qiagen, Valencia, CA). Total RNA extracted from each LMD sample (25 ng) was denatured at 65°C for 5 min and then was reverse transcribed to cDNA using ThermoScript reverse transcriptase (Invitrogen) in a total volume of a 20-μl reaction buffer. Two microliters of cDNA was used for PCR with 45 cycles at 92°C for 40 s, 57°C for 40 s, 72°C for 2 min, and a final extension step at 72°C for 5 min using specific primers, as described above. PCR for protein gene product 9.5 (PGP 9.5), a pan-neuronal marker (30) was carried out to ascertain the neuronal identity of dissected myenteric cells. Negative control contained all reagents, except that 1 μl H2O was substituted for reverse transcriptase in the RT reaction to exclude the possibility of genomic or other DNA contamination. All PCR products corresponding to the predicted rat Ucn 1, Ucn 2, Ucn 3, and CRF2b were separated by 1% agarose gel electrophoresis, visualized with ethidium bromide and extracted with QIAquick gel extraction kit (Qiagen) and sequenced to confirm their identities, as previously described (61). Gel images acquired by Kodak EDAS 290 system were processed for densitometric analysis with NIH image software (Scion).
Regulation of urocortins and CRF2b receptor gene expression in the colon by LPS: time course.
Ten groups of conscious rats (4 or 5/group) were injected intraperitoneally (0.3 ml) with either vehicle (sterile saline) or LPS (100 μg/kg, Escherichia coli serotype O26: B6; code 3755, lot no. 37H4095; Sigma Chemical), and then euthanized by decapitation at 1, 2, 6, 9, and 24 h after the intraperitoneal injection. The LPS dose chosen was based on our previous studies showing that Ucns' gene expressions and hormones were altered at this dose in the stomach (60, 61, 63). Another group of nontreated rats (n = 4) was used as a naive control and euthanized at the beginning of the experiment. The proximal colon was collected as whole thickness and separated into mucosa and S+M layers, frozen on dry ice, and stored at −80°C for 1–4 days until RNA extraction. RT-PCR for Ucn 1, Ucn 2, Ucn 3, and CRF2b mRNA levels and quantitative analysis were performed as described above.
CRF2a and variant: gene expression and regulation by LPS in the lower gut.
Four naïve rats were euthanized by decapitation and the cecum, proximal colon, distal colon, and rectum were harvested and kept as whole thickness. In another two groups (5 rats/group), rats were injected intraperitoneally (0.3 ml) with either saline or LPS (100 μg/kg). The proximal colon was harvested 6 h later (corresponding to sustained changes based on previous time course study) and separated into mucosa and S+M layers, frozen on dry ice, and stored at −80°C for 1–4 days until RNA extraction. Tissue samples were processed to assess the transcript variant of CRF2a with total RNA extraction performed, as described above, using the primer designed to amplify the NH2-terminal-specific region of CRF2a (Table 1).
LPS upregulates inflammatory mediators in colon: effect of CRF2 antagonist.
Four groups of rats (6 rats/group) were pretreated subcutaneously (0.3 ml/rat sc) with astressin2-B (100 μg/kg dissolved in distilled water; Clayton Foundation Laboratories, Salk Institute, La Jolla, CA) or vehicle (distilled water) 15 min before the injection (0.3 ml) of LPS (100 μg/kg ip) or vehicle (saline). The regimen of astressin2-B was based on our previous report showing full reversal of peripheral Ucn 2-induced inhibition of gastric emptying (39). Two hours after intraperitoneal injection of LPS or vehicle, the proximal colon was collected as whole thickness and separated in mucosa and S+M layers, processed for RNA extraction and cDNA synthesis, as described above. Real-time quantitative PCR for IL-1β, TNF-α, and inducible nitric oxide synthase (iNOS) was performed in duplicates using StepOnePlus real-time PCR system (Applied Biosystems) in a 25-μl reaction volume. The optimized reaction contained 12.5 μl of SYBR Premix Ex Taq II (Tli RNaseH Plus) (Takara Mirus Bio, Madison, WI), 0.5 μl each of oligonucleotide primers (10 μM), 1 μl of the cDNA synthesis reaction, and 10.5 μl of H2O. A housekeeping gene, rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was also analyzed as internal controls. Selected forward and reverse primers for real-time PCR are listed in Table. 2. Thermal conditions were as follows: 95°C for 4 min, followed by 40 cycles of 95°C for 5 s, and 60°C for 60 s. The specificity of the amplification reaction was determined by performing a melting curve analysis of the PCR fragments. Each target gene was normalized by GAPDH and calculated using the comparative ΔΔCt method using the equation 2 −ΔΔCt. Results are expressed as fold change in reference to the control group as previously (61).
Table 2.
Primers used for real time PCR
| Gene | Forward | Reverse | Size, bp |
|---|---|---|---|
| IL-1β | CAGGAAGGCAGTGTCACTCA | AGACAGCACGAGGCATTTTT | 232 |
| TNFα | CCTCACACTCAGATCATCTTCTCA | GTGGGTGAGGAGCACATAG | 237 |
| iNos | CACCTTGGAGTTCACCCAGT | ACCACTCGTACTTGGGATGC | 170 |
| GAPDH | AGACAGCCGCATCTTCTTGT | TGATGGCAACAATGTCCACT | 142 |
Statistical analysis.
In all RT-PCR studies, the band intensity of each targeted PCR product was normalized to its corresponding ARP, the reference gene. Levels of Ucns and CRF2b receptor mRNA in the colonic mucosa and S+M layers were expressed as a mean for four animals in corrected arbitrary units and compared by Student's t-test. mRNA levels in laser microdissected samples were averaged from values obtained in 4 or 5 adjacent slides in each layer for each animal, and data from four animals were used to calculate the mean, and results were expressed as percent of the epithelia, which was defined as 100%. Changes in CRF2a and CRF2a-3 mRNA levels induced by LPS at 6 h postinjection compared with intraperitoneal vehicle were expressed as a percentage of CRF2a-3 in the saline group, which was defined as 100%. All of these data and those of inflammation markers in the colon were analyzed by one-way ANOVA followed by Dunnett's multiple-comparison post hoc test. In the 24-h time-course study, the normalized values were expressed as a percentage of the naïve control group, which was defined as 100%, and data were analyzed by two-way ANOVA with LPS treatment and time as fixed factors followed by post hoc comparisons using Bonferroni's method. A P value of <0.05 was considered statistically significant.
RESULTS
Urocortins and CRF2b Gene Expression in the Proximal Colon of Naïve Rats
Mucosa vs. submucosa + muscle layers.
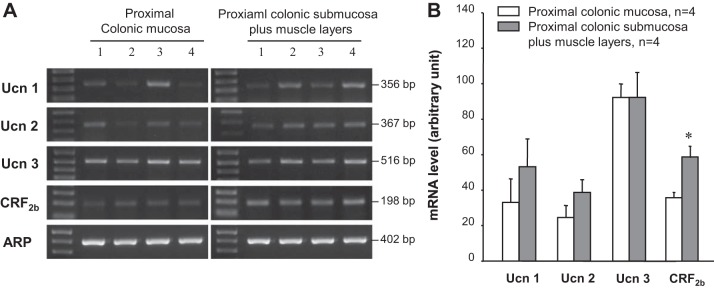
RT-PCR experiments performed on tissues of the proximal colon separated into mucosa and S+M layers showed bands for Ucn 1, Ucn 2, or Ucn 3 mRNA that occurred at the predicted sizes with similar intensity in the two layers (Fig. 1A). No significant difference was seen between Ucns mRNA levels in the mucosa vs. S+M (Fig. 1B). By contrast, the clear band for CRF2b detected in both layers had strong intensity in the S+M (Fig. 1A). The semiquantitative analysis showed that CRF2b receptor mRNA levels were significantly higher by 1.5-fold in the S+M than in the mucosa (Fig. 1B).
Fig. 1.
Ucn1, Ucn 2, Ucn 3, and CRF2b gene expression in the mucosa and submucosa plus muscle layer of the proximal colon in naïve rats. A: gel images of RT-PCR products showing predicted Ucn 1 (356 bp), Ucn 2 (367 bp), Ucn 3 (516 bp), CRF2b (198 bp), and housekeeping gene acidic ribosomal protein (ARP) (402 bp). The numbers represent samples collected from four individual rats. B: semiquantitative analysis of mRNA levels. Values are expressed as the normalized mRNA levels in arbitrary units, and each column represents the mean ± SE of four rats. *P < 0.05 vs. mucosa.
Various layers and myenteric neurons obtained by LMD.
Using LMD 7000, the surface epithelia (Fig. 2, A and G), lamina propria (Fig. 2, B and H), crypts (Fig. 2, C and I), submucosa (Fig. 2, D and J), and muscle layer (circular muscle) (Fig. 2, E and K) were separately cut from the vertical sections of the whole-thickness proximal colon leaving the untargeted tissues attached to the PEN-membrane glass slide (Fig. 2, G–L). In the flat sections across the colonic myenteric plexus, individual myenteric neurons stained with cuprolinic blue were clearly defined, while the dendrites and processes of neurons were devoid of staining (Fig. 2F). The cuprolinic blue-stained cells were precisely dissected (Fig. 2L). The bands of PCR products for housekeeping gene ARP were detected with the predicted size and similar intensity in all layers and neurons (Fig. 2M). This indicates the consistency of the cDNA in LMD-dissected colonic samples and reliability of normalized mRNA values by ARP. The PCR product for PGP 9.5 was detected from the myenteric cells, confirming their neuronal identities (Fig. 2M). The negative control showed no visible band (data not shown), suggesting no genomic or other DNA contaminations. Ucns and CRF2b mRNAs were detected in all layers and myenteric neurons (Fig. 2M). The similar patterns of PCR products were also detected in the colonic samples dissected with LCM (data not shown). The PCR products were semiquantified and expressed as a percentage of the epithelia, which was defined as 100% (Fig. 2N). Significantly higher levels of Ucn 1 mRNA were observed in the submucosa, muscle, crypt, myenteric neurons, and lamina propria reaching 596%, 562%, 522%, 477%, and 393%, respectively, compared with the epithelia (Fig. 2N). Ucn 2 mRNA level in the submucosa was significantly higher than that of all other layers, while that in the myenteric neurons was significantly lower compared with all other layers (Fig. 2N). For Ucn 3, similar mRNA levels were observed in the various layers with the muscle layer being significantly lower than those in myenteric neurons and the lamina propria (Fig. 2N). CRF2b was detected with the highest mRNA levels in the myenteric neurons and submucosa with a significant difference from those in the epithelia, lamina propria crypts, and muscle (Fig. 2N). These results indicate that both Ucns and CRF2b are widely expressed in the rat colonic layers.
LPS Upregulates Urocortins and Downregulates CRF2b mRNA Expression in the Colon
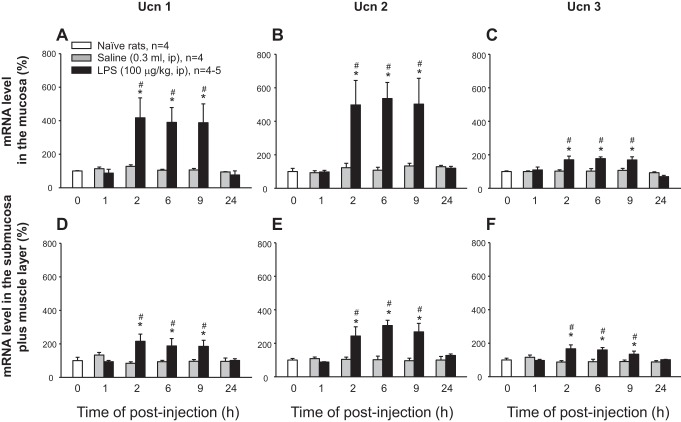
Ucn 1, Ucn 2, and Ucn 3 mRNA levels remained stable at 1, 2, 6, 9, and 24 h after intraperitoneal injection of vehicle in the mucosa and S+M layers of the rat proximal colon and did not show significant changes compared with naïve rats (Fig. 3). LPS (100 μg/kg, ip) elicited significant time-dependent increases in mRNA levels of Ucn 1, Ucn 2, and Ucn 3 in both the mucosa (Fig. 3, A–C) and S+M (Fig. 3, D–F) of the proximal colon. No change was observed after the 1 h postinjection, while a maximal upregulation occurred at 2 h, which was maintained up to 9 h postinjection, with a return to basal levels of naive rats at 24 h after LPS injection. The increased levels of Ucn 1 and Ucn 2 induced by LPS were significantly higher in the mucosa than in S+M layers with a pattern of Ucn 2 ≥ Ucn 1 > Ucn 3 among both layers (Fig. 3) A two-way ANOVA indicated a main effect of intraperitoneal injection of LPS over vehicle on the expression of Ucn 1 in the mucosa [F(1, 48) = 9.5, P < 0.01] and S+ M [F(1, 48) = 9.2, P < 0.01], Ucn 2 in the mucosa [F(1, 48) = 16.8, P < 0.001] and S+M [F(1, 48) = 25.0, P < 0.001], and Ucn 3 in the mucosa [F(1, 48) = 16.6, P < 0.001] and S+M [F(1, 48) = 15.9, P < 0.001]. There was also a main effect of time on the expression of Ucn 2 [F(5, 48) = 4.0, P < 0.001], and Ucn 3 [F(5, 48) = 6.6, P < 0.001] in the mucosa and Ucn 2 [F(5, 48) = 4.8, P < 0.05] in the S+M. The two-way interactions involving intraperitoneal injection of LPS × time was also significant for gene expression of Ucn 1 [F(5, 48) = 3.3, P < 0.05], Ucn 2 [F(5, 48) = 3.4, P < 0.05], and Ucn 3 [F(5, 48) = 4.6, P < 0.01] in the mucosa, and Ucn 1 [F(5, 48) = 3.2, P < 0.05], and Ucn 2 [F(5, 48) = 5.1, P < 0.001], Ucn 3 [F(5, 48) = 4.2, P < 0.01] in the S+M.
Fig. 3.
Time course of LPS-induced upregulation of Ucn 1, Ucn 2, and Ucn 3 gene expression in the rat proximal colonic mucosa and submucosa plus muscle layers. Groups of rats were injected intraperitoneally with vehicle (saline, 0.3 ml) or LPS (100 μg/kg) and euthanized at various time intervals postinjection. Naïve rats (n = 4) were used as a control at zero time. The gel images of RT-PCR products were semiquantified by densitometry. Relative mRNA levels were normalized to the housekeeping gene ARP and expressed in percent of zero-time control, which was defined as 100%. Each column represents the mean ± SE of 4–5 rats. *P < 0.05 compared with the control group. #P < 0.05 compared with respective saline groups. Upper panels A–C refer to mucosa, while lower panels D–F refer to submucosal plus muscle layer.
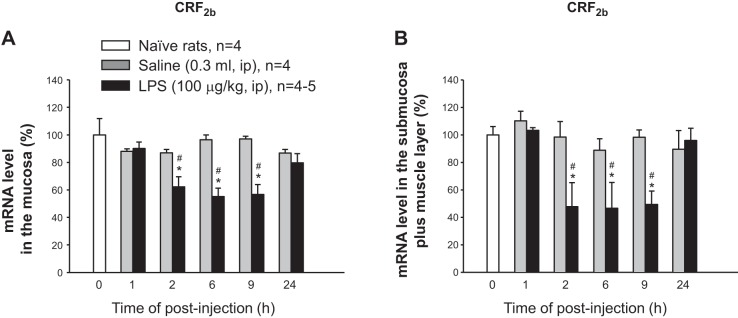
By contrast, CRF2b mRNA levels dropped in the mucosa (Fig. 4A) and M+S (Fig. 4B) at 2, 6, and 9 h, respectively, after LPS injection compared with respective vehicle groups, which did not show significant changes post intraperitoneal saline injection (Fig. 4). CRF2b mRNA levels returned to control levels at 24 h post-LPS injection in both the mucosa and the S+M layers (Fig. 4, A and B). A two-way ANOVA indicated a main effect of LPS over vehicle for CRF2b in the mucosa [F(1, 48) = 29.5, P < 0.001] and S+M [F(1, 48) = 15.3, P < 0.001] and also a main effect of time in the mucosa [F(5, 48) = 5.3, P < 0.001] and the S+M [F(5, 48) = 4.8, P <0.001]. The interaction between LPS × time was also significant for CRF2b gene expression in the mucosa [F(5, 48) = 5.5, P < 0.001] and in the S+M [F(5, 48) = 3.0, P < 0.05]. Together, these data demonstrate that Ucns/CRF2b are reciprocally regulated by LPS in the rat colon.
Fig. 4.
Time course of LPS-induced downregulation of CRF2b in the mucosa (A) and submucosa plus muscle layers (B). Data were collected from the same experiment as described in Fig. 4. Each column represents the means ± SE of 4–5 rats. *P < 0.05 compared with the zero-time control group. #P < 0.05 compared with each respective saline group.
Expression of CRF2a and Its Variant in the Various Segments of the Large Intestine and Differential Regulation by LPS in the Colon
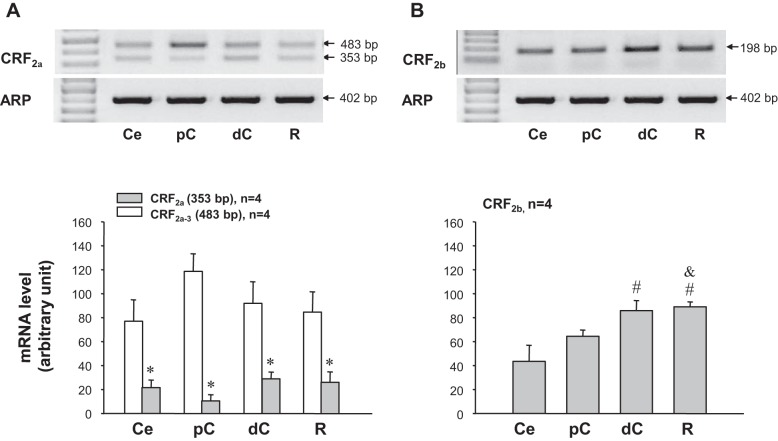
Using primers specific for the rat CRF2a, the band at the expected size 353 bp for CRF2a was detected within all segments of the large intestine (cecum, proximal and distal colon, and rectum) (Fig. 5A). In addition, another band with 483 bp in size was also found on the gel (Fig. 5A). This product was identified and confirmed by sequencing as one of the novel splicing variant of CRF2a found in the rat esophagus (61). Because of an unspliced intron 3 retained in the CRF2a mRNA sequence, this new variant was named CRF2a-3 (61). The gene expression level of CRF2a was similarly low in all colonic segments, while CRF2a-3 was significantly higher than that of CRF2a by 3.6-fold in the cecum, 11.4-fold in the proximal colon, and 3.2-fold in the distal colon and rectum (Fig. 5A). The expression of CRF2b was the highest in the distal colon and rectum compared with the cecum and proximal colon, which displayed similar levels (Fig. 5B).
Fig. 5.
Expression patterns of CRF2 receptor mRNA splice variants along the large intestine of naïve rats. The upper panels show gel images of RT-PCR amplified CRF2 slide variants 2a and 2b. The lower panels show semiquantitative analysis with the values expressed as the corrected arbitrary unit and each column represents the mean ± SE of four rats. A: CRF2a. The band for CRF2a at the expected size 353 bp was detected in the cecum (Ce), proximal colon (pC), distal colon (dC), and rectum (R). In addition, a larger band of 483 bp in size was found in all segments of the large intestine with stronger intensity compared with that at 353 bp. This product was confirmed as a splice variant CRF2a-3 previously identified in the esophagus (61). *P < 0.05 compared with CRF2a-3 values in Ce, pC, dC, and R, respectively. #P <0.05 compared with values in Ce. &P < 0.05 compared with values in pC.
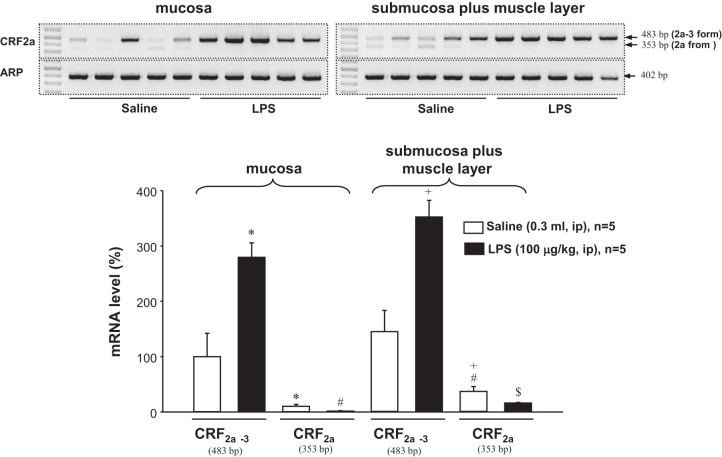
We next assessed the expression of the CRF2a variants in the mucosa vs. S+M and their regulation by intraperitoneal LPS at 6 h postinjection. In control animals treated with intraperitoneal saline, CRF2a mRNA level was significantly higher in S+M than in mucosa (32.6 ± 8.0 vs. 10.5 ± 3.3, P < 0.05) while CRF2a-3 mRNA level is expressed equally in both layers (100.0 ± 42.1 in mucosa vs. 144.2 ± 38.4 in S+M, P = 0.215) (Fig. 6). LPS injection decreased significantly CRF2a mRNA by 52% in mucosa and 82% in S+M, while the endotoxin increased significantly CRF2a-3 by 175% in the mucosa and 143% in M+S of the proximal colon (Fig. 6).
Fig. 6.
Expression of CRF2a and CRF2a-3 in both proximal colonic mucosa and submucosa plus muscle layer (S+M) and regulation by LPS at 6 h postinjection. The results were normalized to the housekeeping gene ARP and expressed as a percentage of CRF2a-3 in the vehicle group (100%). Each column represents the mean ± SE of five rats. *P < 0.05 compared with CRF2a-3. #P < 0.05 compared with CRF2a in the in the mucosa of the saline group. +P < 0.05 compared with CRF2a-3. $P < 0.05 compared with CRF2a in S+M of the vehicle group.
CRF2 Receptor Antagonist Enhanced LPS-Induced Gene Upregulation of Inflammatory Mediators in the Colon
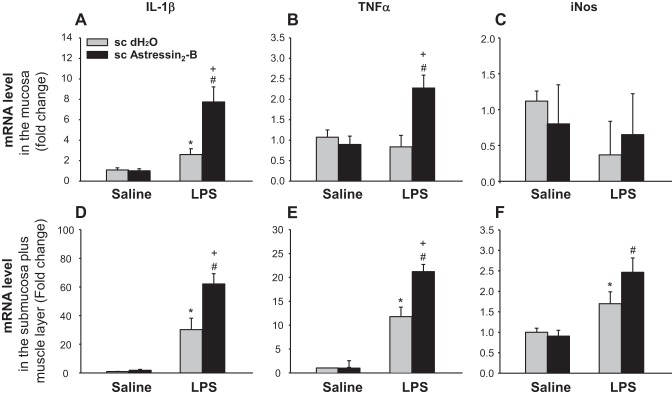
Lastly, we investigated whether the upregulation of Ucn ligands by LPS had any effect on the expression of proinflammatory mediators in the proximal colon using CRF2 antagonist. In the colonic mucosa of subcutaneous vehicle-pretreated rats, LPS (100 μg/kg, ip) induced a significant 2.6-fold increase of IL-1β mRNA level, while TNF-α and iNOS mRNA levels were not altered at 2 h after LPS injection (Fig. 7, A–C). By contrast in the S+M, LPS induced a 35.3-, 13.3-, and 2.2-fold-fold increase of IL-1β, TNF-α, and iNOS mRNA levels, respectively (Fig. 7, D–F). In the mucosa, astressin2-B (100 μg/kg sc) pretreatment 15 min before LPS induced a further three-fold increase of the elevated IL-1β gene expression (Fig. 7A), a significant 2.9-fold increase in TNF-α (Fig. 7B), while having no effect on iNOS mRNA levels (Fig. 7C). In the S+M, the pretreatment with astressin2-B further enhanced significantly LPS-induced IL-1β and TNF-α expression by 1.8-, and 1.6-fold, respectively, compared with sc vehicle + LPS (Fig. 7, D and E), while only displaying a tendency to further enhance the increased iNOS mRNA level induced by LPS (Fig. 7F). Astressin2-B in intraperitoneal saline-treated group did not modify gene expression of IL-1β, TNFα, and iNOS (Fig. 7). These data indicate that the blockade of CRF2 signaling potentiates the inflammatory response to Gram negative bacteria in the colon.
Fig. 7.
The CRF2 antagonist, astressin2-B enhanced LPS-induced upregulation of proinflammatory mediators in the proximal colon. Overnight-fasted rats were injected subcutaneously (0.3 ml) with astressin2-B (100 μg/kg) or vehicle (distilled water) 15 min prior to the intraperitoneal injection of LPS (100 μg/kg ip) or saline. Two hours later, the proximal colon was harvested and separated in mucosal and submucosa plus muscle layers for mRNA determination. Each bar represents the mean ± SE of six rats per group. *P < 0.05 vs. subcutaneous vehicle/intraperitoneal saline. #P < 0.05 vs. subcutaneous astressin2-B/intraperitoneal saline. +P < 0.05 vs. subcutaneous vehicle/intraperitoneal LPS.
DISCUSSION
The present study provides new evidence that the transcripts for three Ucns and several CRF2 variants are broadly expressed in various layers of the rat proximal colon. The gene expression of Ucns/CRF2 variants are regulated by LPS with a sustained and prominent upregulation of Ucn 1 and Ucn 2 and downregulation of both functional isoforms CRF2b and CRF2a, while the truncated splice variant CRF2a-3 is upregulated. In addition, we showed that peripheral injection of a CRF2 receptor antagonist enhanced LPS-induced upregulation of colonic cytokines and iNOS gene expression. These data indicate that Ucns in the colon exert a local CRF2-mediated anti-inflammatory effect in response to a Gram-negative bacterial endotoxin.
Gene Expression of Ucns and CRF2 Variants in the Colonic Layers of Naïve Rats
In this study, transcripts of Ucn 1, Ucn 2, and Ucn 3 were detected by RT-PCR in the colon with equal mRNA levels in the mucosa and S+M under basal conditions. RT-PCR with samples obtained by two different laser microdissection systems demonstrated the ubiquitous expression and similar distribution patterns of Ucn 1, Ucn 2, and Ucn 3 mRNA in each layer of the mucosa, including the epithelium, lamina propria, and crypts, as well as submucosa and muscle layers. We also identified the mRNA expression of Ucn 1, Ucn 2, and Ucn 3 in laser microdissected myenteric neurons identified by the neuronal marker PGP 9.5 (30). These data extend previous reports in whole-thickness tissue of rat colon (proximal and/or distal) showing the gene expression of Ucn 1 (6, 15), Ucn 2 (7, 62), or Ucn 3 (37) detected by RT-PCR and the expression of Ucn 1 in colonic myenteric neurons by in situ hybridization (15). Likewise, in human colon, Ucn 1 mRNA has been identified in the epithelium and lamina propria of resected tissues (43), Ucn 2 mRNA in the mucosa (42), and Ucn 3 mRNA in myenteric and submucosal nervous plexus and smooth muscle by in situ hybridization (51). Collectively these data demonstrate that the colon harbors prominent gene expression of Ucns indicative of local endogenous synthesis of these peptides.
Urocortins are well established to have nonselective (Ucn 1) or selective (Ucn 2 and Ucn 3) high affinity to CRF2 (16, 19). We found that in addition to Ucns, CRF2b was expressed in all LMD layers of the proximal colon. CRF2b mRNA levels were 1.5-fold higher in the S+M than M, which is consistent with the differential expression obtained from laser microdissected layers showing dense bands in the submucosa, muscle layers, and myenteric neurons and lesser intensity bands in other mucosal layers. In rodents, the expression of CRF2b is thought to be the primary variant expressed in nonneuronal cells of the brain and peripheral tissues, while CRF2a is exclusively expressed in brain neurons (9, 36). However, our study showed the expression of CRF2a with 3.1-fold higher mRNA levels in the S+M than mucosal layers. The difference may be indicative of the prominent expression of CRF2a in myenteric neurons contained in S+M layers in keeping with the expression of this variant in neuronal cells (36). These data expand the presence of CRF2a variant to the lower gut, initially found in the esophagus and stomach (61, 63). In addition, we also confirmed by gene sequencing, the expression of the CRF2a-3 splice variant, which contains the unspliced intron 3 (GenBank accessing no. EF078963) encoding a 144-amino acid truncated receptor lacking all seven transmembrane domains as a result of a frame shift (61). CRF2a-3 expression occurred at higher levels than CRF2a in different segments of the large intestine. These findings, combined with the previous identification of CRF2a-3 in the stomach (corpus, antrum, pylorus) at higher mRNA levels than CRF2a (63), suggest a preponderant expression of this transcript throughout the gut. Whether the occurrence of this splice variant is specific to the gastrointestinal tract needs to be assessed, as CRF2a, in general, is not expressed in rat heart (45), and another mouse isoform, iv-mCRF2b, was found to be restricted to the mouse heart but not yet found in rat tissues (52). Overall, the detailed expression pattern of CRF2 mirrored that observed for Ucn ligands in the various layers of the colon and provided anatomical support for a role in local autocrine/paracrine action of Ucns/CRF2. Functional studies in rats showed that peripheral administration of Ucns act through CRF2 receptor to influence physiological processes occurring within the different components of the colonic layers. In particular, the activation of CRF2 stimulated colonic blood flow (2) and reduced noxious colorectal distension-induced visceral pain in vivo and activation of inferior sensatory afferent fibers in vitro (41). Urocortins acting through CRF2 receptor also inhibit exogenous and/or endogenous peripheral CRF1-mediated activation of colonic myenteric neurons, propulsive motility, and visceral hyperalgesia to colorectal distension in vivo (14, 40, 46) and colonic muscle strip contractions and angiogenesis in vitro (24, 46). Recent studies also provided evidence that activation of CRF2 exerts a negative regulation of colorectal cancer epithelial growth (50).
Regulation of Colonic Ucns/CRF2 Systems by LPS
We have previously established that LPS injected intraperitoneally at a single dose of 100 μg/kg mimics clinical features of an acute Gram-negative bacterial infection (reduction of food intake, gastric emptying, and circulating ghrelin) (4, 33, 60) without apparent signs of severe sickness found in endotoxin shock occurring at LPS doses ranging from 25 to 45 mg/kg (47, 56). Here, we found that LPS upregulates Ucn 1, Ucn 2, and Ucn 3 gene expression in both the mucosa and S+M layers after 1 h, to reach a maximal response at 2 h that was maintained up to 9 h with a return to normal level by 24 h postinjection. Still little has been known about the regulation of Ucn gene expression by LPS so far. Under similar conditions of LPS administration, Ucn mRNA levels in the gastric corpus are upregulated with a similar time course (63). Other studies indicate that Ucn 1 mRNA levels are increased in the thymus and the heart, but decreased in the spleen, indicative of distinct patterns of regulation by LPS depending upon the tissues examined (22, 25, 26, 48). However, our present and previous findings (63) support that Ucns within the gastrointestinal tract are upregulated under conditions of innate defense response to Gram-negative bacteria infection. We also found that the upregulation process differs among the Ucn family members in terms of magnitude and localization making Ucn 1 and Ucn 2 in the mucosa more responsive to the upregulation by LPS compared with Ucn 3. This is in keeping with the recently demonstrated prominent role of Ucn 2 in colonic epithelial cells to promote cell proliferation in vivo and in vitro (20).
With regard to the CRF2 receptor, LPS decreased mRNA levels of CRF2b and CRF2a variants in the colon from 2 to 9 h postinjection, as observed in the gastric corpus under similar conditions (63). Downregulation of CRF2 mRNA levels induced by LPS has been reported in the rodent heart (17, 26, 48), although in other peripheral tissues, such as spleen or skeletal muscle, CRF2 mRNA levels were increased (17). The parallel kinetic of upregulation of three Ucns and downregulation of two functional CRF2 isoforms in the colon over the 9-h post-LPS injection may be indicative of ligand-induced downregulation of G protein receptors, as documented for Ucn 1-CRF2 regulations in other visceral tissues (3, 11, 26, 48). Indeed, previous reports indicated that while peripheral injection of LPS, glucocorticoids, Ucn 1, or cytokines like IL-1α and TNF-α downregulated CRF2 gene expression in the rodent heart, only Ucn 1 was able to exert a suppressive effect in vitro (11, 17, 26, 27). Further studies will be required to establish the direct and/or indirect role of Ucn ligands, endocrine and/or immune factors recruited by LPS (26, 28), contributing to the downregulation of CRF2a and CRF2b in the colon and whether it relates to alterations of gene transcription and/or mRNA stability.
By contrast to the functional CRF2 isoforms, LPS increased CRF2a-3 variant by 175% in the mucosa and by 143% in the M+S of the proximal colon. Similarly, in the mouse heart, a report showed an opposite downregulation of the CRF2b and upregulation of the noncoding isoform iv-mCRF2b under conditions of stress (52). The differential gene expression of CRF2a and CRF2a-3 under basal and LPS treatment may reflect a state of alternative splicing through the control of posttranscriptional processing, whereby the dominant expression of the aberrant transcript occurred at the expense of the CRF2a transcripts. These findings highlight the importance of selecting primer pairs in PCR analysis distinguishing functional transcripts from other CRF2 splice variants, particularly when the alternate variants are more highly expressed and differentially regulated, as found here for CRF2a-3 compared with CRF2a. This will be important in the context of the human colonic biopsies in which CRF2 gene expression has been detected by RT-PCR, while only using primers that did not discriminate between the splice variants (8, 42, 50). Also, we note the fact that CRF2a is the predominant isoform expressed in human viscera (44). Additionally, increasing evidence indicates that truncated CRF2 receptors, while devoid of signaling and/or binding properties, may alter the function of endogenous ligands or modulate the signaling of the CRF2a/b receptors (10, 52).
Anti-Inflammatory Effect of Ucns/CRF2 in Colon in Response to LPS
Lastly, we assessed whether the local alterations in expression of colonic Ucns/CRF2 by LPS influence the inflammatory response in the colon through the use of a peripheral injection of the selective and long-acting CRF2 antagonist, astressin2-B (49). Earlier studies showed that peripherally injected Ucn 1 inhibited the elevated serum levels of TNF-α, and IL-1β induced 90–180 min after systemic injection of LPS at 4 μg/kg in mice (1). In the present study, LPS at 100 μg/kg increased gene expression of IL-1β in the mucosa and S+M and that of TNF-α in the S+M layers of the colon at 2 h postinjection. Importantly, under conditions of CRF2 blockade, LPS enhanced the colonic inflammatory response as shown by the heightened levels of IL-1β and TNF-α in both the mucosa and S+M. We also found that LPS increased iNOS mRNA levels selectively in the S+M, which were further elevated in the presence of CRF2 blockade. This may reflect the prominent expression of iNOS in various cells of the S+M, including smooth muscle, macrophage, and myenteric neurons, as well as the synergistic regulation of iNOS by IL-1β and TNF-α, reported in rat colonic cultured circular smooth muscle cells (31). The lack of an effect of the CRF2 antagonist by itself indicates that the Ucns-CRF2 system does not regulate the low levels of cytokines or iNOS in the colon under basal conditions. Collectively, these data support an immunomodulatory role played by the Ucns-CRF2 system in the colon to reduce the early colonic inflammatory response to Gram-negative bacteria. In another model of colonic inflammation induced by dextran sodium sulfate, we previously reported that mice treated with astressin2-B or with genetic deletion of CRF2 had increased levels of TNF-α, IL-6, and chemokine ligand 1 in whole-thickness colonic tissue compared with those in vehicle control (20, 24). The underlying mechanisms involved in the CRF2-mediated modulation of colonic inflammatory response to LPS are still to be established. Existing evidence indicates that CRF2 ligands interacting with CRF2 exert a direct early anti-inflammatory effect by inhibiting LPS-induced TNF-α and IL-1β release in rodent macrophages and accelerate macrophage apoptosis (13, 57, 58).
In summary, we have provided evidence that there is gene expression of CRF2b receptor and Ucn ligands in the different layers of the rat colon, including the epithelium, lamina propria, crypts, submucosa, myenteric neurons, and muscles, and this finding provides molecular support for their local paracrine/autocrine actions. In addition, CRF2a and, more prominently, the related novel truncated isoform CRF2a-3, which contains the entire exon 3, are also expressed from the cecum to the rectum. LPS administered at 100 μg/kg ip induces within 2 h the upregulation of Ucns and differential downregulation/upregulation of CRF2a,b/CRF2a-3 in the colon. CRF2 blockade with selective receptor antagonist heightened the colonic inflammatory response to LPS, as shown by enhanced expression of IL-1β, TNF-α, and iNOS. These findings show the Ucns/CRF2 pathways in the colon to be a relevant adaptive system that modulates the inflammatory response to Gram-negative bacteria, as recently observed under conditions of murine colitis (20, 24). Future investigations should focus on clarifying the precise mechanisms by which endocrine/cytokine cues are triggered by LPS in the regulation of Ucns/CRF2 systems, as well as the role that noncoding CRF2 isoforms upregulated by LPS have in modulating the CRF2 signaling in the colon, as has been established in the heart (52).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.-Q.Y. and Y.T. conception and design of research; P.-Q.Y. and S.V.W. performed experiments; P.-Q.Y. and S.V.W. analyzed data; P.-Q.Y., S.V.W., and Y.T. interpreted results of experiments; P.-Q.Y. prepared figures; P.-Q.Y. and Y.T. drafted manuscript; P.-Q.Y., S.V.W., C.P., and Y.T. edited and revised manuscript; P.-Q.Y., C.P., and Y.T. approved final version of manuscript.
ACKNOWLEDGMENTS
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases R01 grant DK-33061 (to Y. Taché), Center grant DK-41301 (Animal Core) (to Y. Taché and C. Pothoulakis), and DK-101691(to C. Pothoulakis) and Veteran Administration Research Career Scientist Award (Y. Taché). We thank the Advanced Light Microscopy/Spectroscopy Core of the California Nanosystems Institute, University of California at Los Angeles for providing the LMD 7000 facility and helpful advices and Dr. Jean Rivier (Clayton Foundation Laboratories, Salk Institute, La Jolla, CA) for the generous supply of astressin2-B, as well as Honghui Liang for excellent technical assistance.
REFERENCES
- 1.Agnello D, Bertini R, Sacco S, Meazza C, Villa P, Ghezzi P. Corticosteroid-independent inhibition of tumor necrosis factor production by the neuropeptide urocortin. Am J Physiol Endocrinol Metab 275: E757–E762, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Akiba Y, Kaunitz JD, Million M. Peripheral corticotropin-releasing factor receptor type 2 activation increases colonic blood flow through nitric oxide pathway in rats. Dig Dis Sci 60: 858–867, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asaba K, Makino S, Nishiyama M, Hashimoto K. Regulation of type-2 corticotropin-releasing hormone receptor mRNA in rat heart by glucocorticoids and urocortin. J Cardiovasc Pharmacol 36: 493–497, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Basa NR, Wang L, Arteaga JR, Heber D, Livingston EH, Tache Y. Bacterial lipopolysaccharide shifts fasted plasma ghrelin to postprandial levels in rats. Neurosci Lett 343: 25–28, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Buckinx R, Adriaensen D, Nassauw LV, Timmermans JP. Corticotrophin-releasing factor, related peptides, and receptors in the normal and inflamed gastrointestinal tract. Front Neurosci 5: 54, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang J, Adams MR, Clifton MS, Liao M, Brooks JH, Hasdemir B, Bhargava A. Urocortin 1 modulates immunosignaling in a rat model of colitis via corticotropin-releasing factor receptor 2. Am J Physiol Gastrointest Liver Physiol 300: G884–G894, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang J, Hoy JJ, Idumalla PS, Clifton MS, Pecoraro NC, Bhargava A. Urocortin 2 expression in the rat gastrointestinal tract under basal conditions and in chemical colitis. Peptides 28: 1453–1460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatzaki E, Anton PA, Million M, Lambropoulou M, Constantinidis T, Kolios G, Taché Y, Grigoriadis DE. Corticotropin-releasing factor receptor subtype 2 in human colonic mucosa: down-regulation in ulcerative colitis. World J Gastroenterol 19: 1416–1423, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen A, Perrin M, Brar B, Li C, Jamieson P, DiGruccio M, Lewis K, Vale W. Mouse corticotropin-releasing factor receptor type 2α gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol 19: 441–458, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. A soluble mouse brain splice variant of type 2α corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci USA 102: 2620–2625, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coste SC, Heldwein KA, Stevens SL, Tobar-Dupres E, Stenzel-Poore MP. IL-1α and TNFα down-regulate CRH receptor-2 mRNA expression in the mouse heart. Endocrinology 142: 3537–3545, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Dautzenberg FM, Higelin J, Wille S, Brauns O. Molecular cloning and functional expression of the mouse CRF2(a) receptor splice variant. Regul Pept 121: 89–97, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Rey E, Chorny A, Varela N, Robledo G, Delgado M. Urocortin and adrenomedullin prevent lethal endotoxemia by down-regulating the inflammatory response. Am J Pathol 168: 1921–1930, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gourcerol G, Wu SV, Yuan PQ, Pham H, Miampamba M, Larauche M, Sanders P, Amano T, Mulak A, Im E, Pothoulakis C, Rivier J, Taché Y, Million M. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology 140: 1586–1596, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada S, Imaki T, Naruse M, Chikada N, Nakajima K, Demura H. Urocortin mRNA is expressed in the enteric nervous system of the rat. Neurosci Lett 267: 125–128, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 55: 21–26, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Heldwein KA, Duncan JE, Stenzel P, Rittenberg MB, andStenzel-Poore MP. Endotoxin regulates corticotropin-releasing hormone receptor 2 in heart and skeletal muscle. Mol Cell Endocrinol 131: 167–172, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 27: 260–286, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hoare SR, Sullivan SK, Fan J, Khongsaly K, Grigoriadis DE. Peptide ligand binding properties of the corticotropin-releasing factor (CRF) type 2 receptor: pharmacology of endogenously expressed receptors, G protein-coupling sensitivity and determinants of CRF2 receptor selectivity. Peptides 26: 457–470, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman JM, Baritaki S, Ruiz JJ, Sideri A, Pothoulakis C. Corticotropin-releasing hormone receptor 2 signaling promotes mucosal repair response following colitis. Am J Pathol 186: 134–144, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holst MC, Powley TL. Cuprolinic blue (quinolinic phthalocyanine) counterstaining of enteric neurons for peroxidase immunocytochemistry. J Neurosci Methods 62: 121–127, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K, Tojo K, Inada Y, Takada Y, Sakamoto M, Lam M, Claycomb WC, Tajima N. Regulation of urocortin I and its related peptide urocortin II by inflammatory and oxidative stresses in HL-1 cardiomyocytes. J Mol Endocrinol 42: 479–489, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Im E. Multi-facets of corticotropin-releasing factor in modulating inflammation and angiogenesis. J Neurogastroenterol Motil 21: 25–32, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im E, Rhee SH, Park YS, Fiocchi C, Taché Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology 138: 2457–2467, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kageyama K, Bradbury MJ, Zhao L, Blount AL, Vale WW. Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology 140: 5651–5658, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Kageyama K, Gaudriault GE, Bradbury MJ, Vale WW. Regulation of corticotropin-releasing factor receptor type 2β messenger ribonucleic acid in the rat cardiovascular system by urocortin, glucocorticoids, and cytokines. Endocrinology 141: 2285–2293, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kageyama K, Gaudriault GE, Suda T, Vale WW. Regulation of corticotropin-releasing factor receptor type 2β mRNA via cyclic AMP pathway in A7r5 aortic smooth muscle cells. Cell Signal 15: 17–25, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kageyama K, Li C, Vale WW. Corticotropin-releasing factor receptor type 2 messenger ribonucleic acid in rat pituitary: localization and regulation by immune challenge, restraint stress, and glucocorticoids. Endocrinology 144: 1524–1532, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun 24: 41–48, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krammer HJ, Karahan ST, Rumpel E, Klinger M, Kuhnel W. Immunohistochemical visualization of the enteric nervous system using antibodies against protein gene product (PGP) 9.5. Ann Anat 175: 321–325, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Kuemmerle JF. Synergistic regulation of NOS II expression by IL-1β and TNF-α in cultured rat colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 274: G178–G185, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Langhans W. Bacterial products and the control of ingestive behavior: clinical implications. Nutrition 12: 303–315, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Langhans W. Anorexia of infection: current prospects. Nutrition 16: 996–1005, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Larauche M, Kiank C, Taché Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol 60 Suppl 7: 33–46, 2009. [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2α and CRF2β receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology 136: 4139–4142, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Mahajan S, Liao M, Barkan P, Takahashi K, Bhargava A. Urocortin 3 expression at baseline and during inflammation in the colon: corticotropin releasing factor receptors cross-talk. Peptides 54: 58–66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther 301: 611–617, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Million M, Maillot C, Saunders PR, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF-related peptide, displays selective CRF2-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol 282: G34–G40, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Taché Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol 292: R1429–R1438, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu VS, Wei JY, Rivier J, Vale W, Mayer EA, Taché Y. CRF2 receptor activation prevents colorectal distension-induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut 55: 172–181, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moss AC, Anton P, Savidge T, Newman P, Cheifetz AS, Gay J, Paraschos S, Winter MW, Moyer MP, Karalis K, Kokkotou E, Pothoulakis C. Urocortin II mediates pro-inflammatory effects in human colonocytes via corticotropin-releasing hormone receptor 2α. Gut 56: 1210–1217, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides 21: 1799–1809, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Nazarloo HP, Buttrick PM, Saadat H, Dunn AJ. The roles of corticotropin-releasing factor-related peptides and their receptors in the cardiovascular system. Curr Protein Pept Sci 7: 229–239, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Nishikimi T, Miyata A, Horio T, Yoshihara F, Nagaya N, Takishita S, Yutani C, Matsuo H, Matsuoka H, Kangawa K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am J Physiol Heart Circ Physiol 279: H3031–H3039, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Nozu T, Takakusaki K, Okumura T. A balance theory of peripheral corticotropin-releasing factor receptor type 1 and type 2 signaling to induce colonic contractions and visceral hyperalgesia in rats. Endocrinology 155: 4655–4664, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Otterbein L, Sylvester SL, Choi AM. Hemoglobin provides protection against lethal endotoxemia in rats: the role of heme oxygenase-1. Am J Respir Cell Mol Biol 13: 595–601, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Pournajafi NH, Tanaka Y, Dorobantu M, Hashimoto K. Modulation of corticotropin-releasing hormone receptor type 2 mRNA expression by CRH deficiency or stress in the mouse heart. Regul Pept 115: 131–138, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem 45: 4737–4747, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez JA, Huerta-Yepez S, Law IKM, Baay-Guzman GJ, Tirado-Rodriguez B, Hoffman JM, Illiiopoulos D, Hommes DW, Verspage HW, Chang L, Pothoulakis C, Barita KS. Diminished expression of corticotropin-releasing hormone receptor 2 in human colon cancer promotes tumor growth and epithelial-to-mesenchymal transition via persistent interleukin-6/STAT3 signaling. Cell Mol Gastro Hepat 1: 610–630, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saruta M, Takahashi K, Suzuki T, Fukuda T, Torii A, Sasano H. Urocortin 3/stresscopin in human colon: possible modulators of gastrointestinal function during stressful conditions. Peptides 26: 1196–1206, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Sztainberg Y, Kuperman Y, Issler O, Gil S, Vaughan J, Rivier J, Vale W, Chen A. A novel corticotropin-releasing factor receptor splice variant exhibits dominant negative activity: a putative link to stress-induced heart disease. FASEB J 23: 2186–2196, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taché Y, Million M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil 21: 8–24, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi K. Distribution of urocortins and corticotropin-releasing factor receptors in the cardiovascular system. Int J Endocrinol 2012: 395284, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi K, Totsune K, Murakami O, Shibahara S. Urocortins as cardiovascular peptides. Peptides 25: 1723–1731, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Thomas RC, Bath MF, Stover CM, Lambert DG, Thompson JP. Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system. Peptides 61: 56–60, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, Gravanis A, Margioris AN. Urocortin 1 and Urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett 579: 4259–4264, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Tsatsanis C, Androulidaki A, Dermitzaki E, Gravanis A, Margioris AN. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-α release from macrophages via induction of COX-2 and PGE2. J Cell Physiol 210: 774–783, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Vaughan JM, Donaldson CJ, Fischer WH, Perrin MH, Rivier JE, Sawchenko PE, Vale WW. Posttranslational processing of human and mouse urocortin 2: characterization and bioactivity of gene products. Endocrinology 154: 1553–1564, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St Pierre DH, Taché Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol 291: G611–G620, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Wu SV, Yuan PQ, Wang L, Peng YL, Chen CY, Taché Y. Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology 148: 1675–1687, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamauchi N, Otagiri A, Nemoto T, Sekino A, Oono H, Kato I, Yanaihara C, Shibasaki T. Distribution of urocortin 2 in various tissues of the rat. J Neuroendocrinol 17: 656–663, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Yuan PQ, Wu SV, Taché Y. Urocortins and CRF type 2 receptor isoforms expression in the rat stomach are regulated by endotoxin: role in the modulation of delayed gastric emptying. Am J Physiol Gastrointest Liver Physiol 303: G20–G31, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu H, Wang J, Li J, Li S. Corticotropin-releasing factor family and its receptors: pro-inflammatory or anti-inflammatory targets in the periphery? Inflamm Res 60: 715–721, 2011. [DOI] [PubMed] [Google Scholar]