Abstract
The swallowing muscles that influence upper esophageal sphincter (UES) opening are centrally controlled and modulated by sensory information. Activation and deactivation of neural inputs to these muscles, including the intrinsic cricopharyngeus (CP) and extrinsic submental (SM) muscles, results in their mechanical activation or deactivation, which changes the diameter of the lumen, alters the intraluminal pressure, and ultimately reduces or promotes flow of content. By measuring the changes in diameter, using intraluminal impedance, and the concurrent changes in intraluminal pressure, it is possible to determine when the muscles are passively or actively relaxing or contracting. From these “mechanical states” of the muscle, the neural inputs driving the specific motor behaviors of the UES can be inferred. In this study we compared predictions of UES mechanical states directly with the activity measured by electromyography (EMG). In eight subjects, pharyngeal pressure and impedance were recorded in parallel with CP- and SM-EMG activity. UES pressure and impedance swallow profiles correlated with the CP-EMG and SM-EMG recordings, respectively. Eight UES muscle states were determined by using the gradient of pressure and impedance with respect to time. Guided by the level and gradient change of EMG activity, mechanical states successfully predicted the activity of the CP muscle and SM muscle independently. Mechanical state predictions revealed patterns consistent with the known neural inputs activating the different muscles during swallowing. Derivation of “activation state” maps may allow better physiological and pathophysiological interpretations of UES function.
Keywords: deglutition, dysphagia, pressure, impedance, diameter, electromyography, neural pathways, upper esophageal sphincter, cricopharyngeus muscle, submental muscles
the upper esophageal sphincter (UES) is a region of high pressure located at the juncture between the pharynx and esophagus. The UES is a gatekeeper, which is tonically contracted to prevent air from entering the esophagus during inspiration and to guard against aspiration of gastric refluxate. The UES high-pressure zone must also relax, resulting in an open lumen to allow unimpeded passage of swallowed food (26). The neural inputs to the swallowing muscles that effect UES relaxation and luminal opening are controlled by central circuits, and these are modulated by sensory information. Activation of these neural inputs results in a complex sequence of changes in the striated muscles of the UES high-pressure zone (7). These changes could see these muscles contract or relax, which in turn influences the intraluminal pressures and diameters ultimately determining the initiation of swallowed food propulsion.
The pathophysiology of UES dysfunction needs to be accurately diagnosed to guide appropriate therapeutic interventions to ameliorate symptoms of dysphagia. We have previously described a novel, nonradiological, in vivo technique in which we have used intraluminal impedance recorded in parallel with manometry during swallowing to determine changes in the diameter of the UES lumen and the corresponding changes in UES pressure (22). By defining this relationship between changes in diameter and pressure, we have determined the “mechanical states” of the UES muscles. By applying the methodology we can theoretically determine in real time when the muscle is actively contracting or relaxing, consistent with patterns of activity of central motor neurons of the nucleus ambiguus.
The muscles most relevant to UES opening include the intrinsic cricopharyngeus muscle (CP) and the extrinsic submental muscles (SM), which are mechanically coupled to the UES (5). During swallowing, the CP relaxes in coordination with activation of the SM complex, which serves to lift the hyolaryngeal complex and pull open the UES. Both of these actions are necessary for distension of the esophageal lumen (3). To assess neural inputs to these muscles, simultaneous electromyography (EMG) recordings are usually required (1, 12, 13, 19, 21, 23). However, intramuscular EMG recordings are time intensive, require extensive training for the procedure and analysis, and are not in the scope of practice of all clinicians who take part in the diagnosis of dysphagia. Luminal manometry of high spatial resolution provides information over several pressure sensors straddling a broad region of interest and is simpler and easier to apply than CP-EMG. Therefore, manometry potentially offers an advantage over EMG, provided that the information gathered on motility of the UES can give similar insights regarding the state of activation of the CP muscle.
We hypothesized that the neural activation state of CP and SM muscles can each be deduced by analysis of UES mechanical states using combined manometry and impedance recordings (22). To test this hypothesis, we performed a study recording, simultaneously, pharyngoesophageal pressure-impedance and EMG activity of the CP and SM muscles. The mechanical states of the UES muscles were determined from pressure and impedance recordings and then compared with time-linked recordings of muscle activation/deactivation.
METHODS
The UES Mechanical States Concept
By examining the relationships that exist between changes in diameter and the corresponding changes in pressure, recorded at the same point in space and time, the mechanical states of the muscle can be determined (4). These mechanical states predict when the muscle is actively contracting or relaxing during periods of luminal occlusion or distension.
Pressure changes occurring within the UES high-pressure zone during swallowing can be measured with an indwelling manometry catheter, and diameter changes of the UES lumen can be simultaneously inferred by recording intraluminal impedance (22). We have previously shown a correlation between changes in diameter and changes in impedance in the human UES (22).
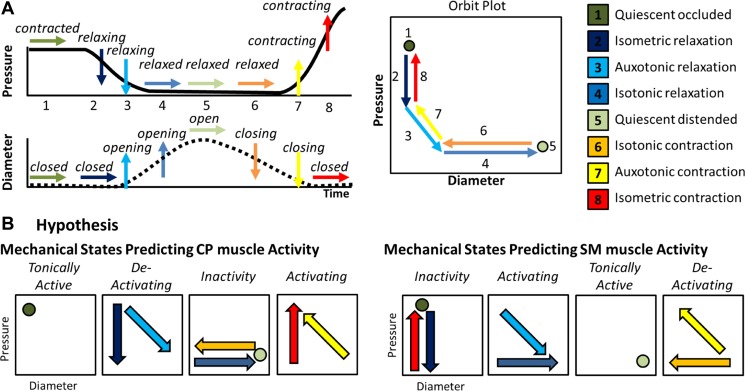
During a healthy human swallow, these changes in diameter and pressure can be seen to follow a typical pattern sequence of pressure change in relation to diameter change (Fig. 1A). The relationship between diameter and pressure over time can also be visualized by way of an “orbit” plot (Fig. 1A). Previous studies examining ex vivo peristalsis in the lower gut have defined 12 possible mechanical states (4, 6, 27) of which eight are common for the UES for healthy swallows (22) (Fig. 1A).
Fig. 1.
Explanations and hypothesis in relation to upper esophageal sphincter (UES) mechanical states. A: typical pattern of pressure and diameter change that the UES undergoes during normal swallowing. A typical UES pressure profile is shown with arrows indicating periods when pressure is static (→), decreasing (↓), or increasing (↑). A typical diameter profile is shown with arrows indicating periods when the diameter is static (→), increasing (↑), or decreasing (↓). When the pressure and diameter data are interpreted together, different mechanical states can be defined based on the direction of pressure change and in relation to whether the lumen is open, closed, or changing in diameter. The relationship of diameter vs. pressure over time can also be visualized by way of an “orbit” plot. The mechanical states numbered 1–8 typify the normal sequence of UES contractility and UES opening during swallowing. B: we hypothesized that the 8 states can be consolidated into 4 groups defining when the muscle is tonically active, activating, deactivating, or inactive. Since the cricopharyngeus (CP) and extrinsic submental (SM) muscles perform mechanically reciprocal functions when activated, the association of states to muscle activity needs to be done separately for each muscle group type, depicted here as orbit plots.
The UES is comprised of intrinsic striated muscles and is affected by striated muscles extrinsic to the UES. The physiology governing the smooth muscle gut peristalsis is not directly comparable, and therefore a different mechanistic interpretation for the UES needs to be garnered (Fig. 1B). Resting UES pressure is predominantly generated by neurally mediated tonic active contraction of the CP muscle as well as passive elastic forces generated by the UES wall. During normal swallowing, the measured UES pressure fluctuates in concert with neural activation; CP active contraction, neural deactivation, and neural reactivation contribute to UES opening and closing, respectively. The hyoid bone is an anatomical structure mechanically coupled to the larynx and UES. The UES opens due to superior-anterior movement of the hyolaryngeal complex, affected by the action of the submental muscle groups. Based on the current understanding of UES relaxation and opening mechanisms, the many different muscle states can be consolidated into four groups: 1) tonically active contractions, 2) transient active contractions, 3) deactivating relaxations, and 4) inactivity. Since the activation of CP and SM muscles performs a reciprocal function (i.e., CP muscle activation keeps the lumen closed while SM activation opens the lumen), the muscle states must be interpreted differently with respect to each muscle group. As a starting point, we proposed a hypothesis defining which mechanical states are most likely to be associated with different states of activation of the CP and SM muscles (Fig. 1B).
Study Procedures
The muscle activity, diameter and pressure relationships were determined for the UES by use of intramuscular EMG electrodes in conjunction with intraluminal impedance and pressure measurement using an indwelling catheter placed across the UES. Combined recordings of pharyngeal swallows were analyzed. Our study included eight healthy participants (5 men) between 20 and 43 yr old (mean 27 ± 7 yr) without a history of swallowing, respiratory, or neurological deficits. Each participant provided written, informed consent, and the protocol was approved by the Institutional Review Board of the University of Wisconsin-Madison. The participants were instructed not to eat for 4 h and not to drink for 2 h before testing to avoid any potential confounding effect of satiety. Each subject underwent placement of CP and SM electrodes and was then intubated with a manometry catheter and performed a standardized swallow protocol.
Electromyography.
Procedures for CP-EMG have been described previously and are reviewed here (7, 8, 10, 12, 13). The CP-EMG signals were recorded with a 50-μm-diameter, bipolar hook-wire intramuscular electrode (MicroProbes, Gaithersburg, MD) and a surface ground electrode (A10058-SRT; Vermed, Bellows Falls, VT) placed on the forehead.
Before CP electrode insertion, 1 ml of 1% lidocaine hydrochloride with epinephrine (1:100,000) was subcutaneously injected into the neck through a 30-gauge needle. The intramuscular electrode was then inserted with a 27-gauge needle. The characteristic CP muscle pattern of quiescence during a swallow followed by a burst of activity after the swallow was consistent with accurate placement. Bilateral surface EMG electrodes were placed in the submental region between the mandible and the hyoid bone, each at 1 cm from midline.
The EMG signals were amplified, band-pass filtered from 100 Hz to 6 kHz (model 15LT; Grass Technologies, Warwick, RI), and digitized at 20 kHz (LabChart version 6.1.3; ADInstruments, Colorado Springs, CO).
High-resolution manometry with impedance.
Following successful placement of CP-EMG electrodes, pharyngeal motor patterns were measured with a 4.2-mm-diameter solid state pressure and impedance catheter incorporating 36 1-cm-spaced pressure sensors and 18 adjoining impedance segments, each of 2-cm length (Given Imaging). Data were recorded at a sampling rate of 50 Hz (ManoScan Data Acquisition; Given Imaging). The catheter was calibrated before use with each participant according to manufacturer specifications.
Topical 2% viscous lidocaine hydrochloride was applied to the nasal passages and to the manometric catheter as a topical anesthetic and lubricant to ease passage of the catheter through the nasal cavity and pharynx. Once the catheter was inserted, the participants rested for ∼5 min to adjust to the catheter and electrodes before performing the swallow protocol.
Swallow protocol.
With the subject sitting upright and in the head neutral position, five saliva swallows and five bolus swallows each of 2, 5, 10, and 20 ml saline solution (0.9% NaCl) were administered. These were simultaneously recorded by the EMG system and the pressure-impedance acquisition system. The boluses were administered at >20-s intervals to the mouth via a syringe and subjects were asked to swallow on command (i.e., cued volitional swallowing).
Data Analysis
Measurement of UES pressure and admittance profiles.
The UES pressure, impedance, CP-EMG, and SM-EMG data were time linked by means of a transistor-to-transistor logic signal. The corresponding pressure data and impedance data for each swallow were exported from the acquisition systems in text-file (.txt) format. The pressure, impedance, and EMG data were analyzed with a customized MATLAB program (MathWorks, Natick, MA). The EMG signals were rectified and low-pass filtered by using the MATLAB resample function to match the 50-Hz sampling rate of the pressure-impedance signals. The segment of time-series data for UES pressure and the CP muscle voltage were time aligned for each trial.
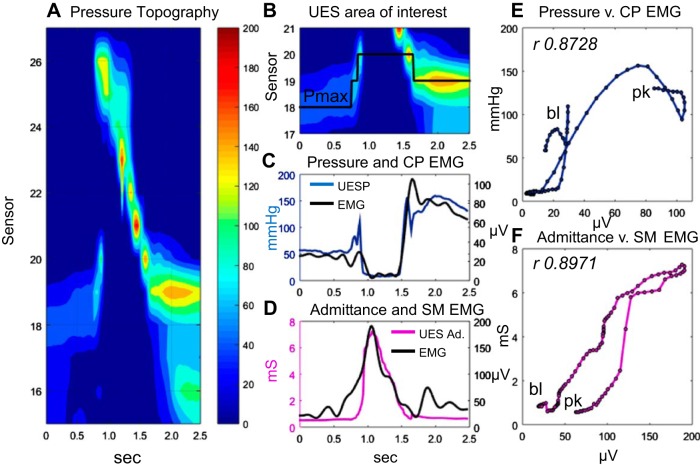
Impedance data were spatially interpolated to increase the spatial dataset to match the pressure dataset (1 sample per 1 cm). To account for known nonlinearity of the impedance-area relationship, the impedance values were converted to the inverse product of impedance (1/impedance) henceforth called “admittance” expressed in millisiemens (mS). The UES undergoes a 2-cm or more elevation before complete UES relaxation (14). The manometry catheter itself elevates ∼1 cm during swallowing as well, asynchronous to UES elevation (13). UES pressure and impedance data were therefore analyzed within an area of interest corresponding to the region from the distal margin of the UES high-pressure zone to the estimated apogee position of the UES during swallow, and for the time period from 1 s before the onset to 1 s after the offset of CP pause (on CP-EMG) (Fig. 2, A and B).
Fig. 2.
An example of a 10-ml liquid swallow showing the relationship of CP-electromyography (EMG) to UES pressure and SM-EMG to UES intraluminal admittance. A: pressure topography plot of the entire pharyngoesophageal segment. B: area of interest defined for the UES high-pressure zone. Pmax, location of maximum axial UES pressure during the swallow. C: plot of UES pressure (defined by Pmax) and simultaneously recorded CP-EMG. D: plot of UES admittance (Ad.) (at Pmax) and simultaneously recorded SM-EMG. E: time correlation (Pearson rho) of CP-EMG vs. pressure data is shown for the period preswallow baseline (bl) to postrelaxation peak (pk) when diameter and pressure changes predominantly occur. F: time correlation of SM-EMG vs. admittance for the same baseline-to-peak period.
The maximum axial UES pressure during the swallow was measured within the limits of UES area of interest over time. The location of maximum axial pressure was used to track the superior and inferior movement of the UES based on the method of Ghosh and colleagues (9) (Fig. 2B). Consecutive pressure and admittance values mapped to the corresponding position of the UES over time were used to derive an optimal profile of pressure (Fig. 2C) and admittance (Fig. 2D) during the swallow, which could be correlated with the single CP-EMG (Fig. 2, C and E) and SM-EMG (Fig. 2, D and F) recordings.
Definition of UES mechanical states using UES admittance and UES pressure profiles.
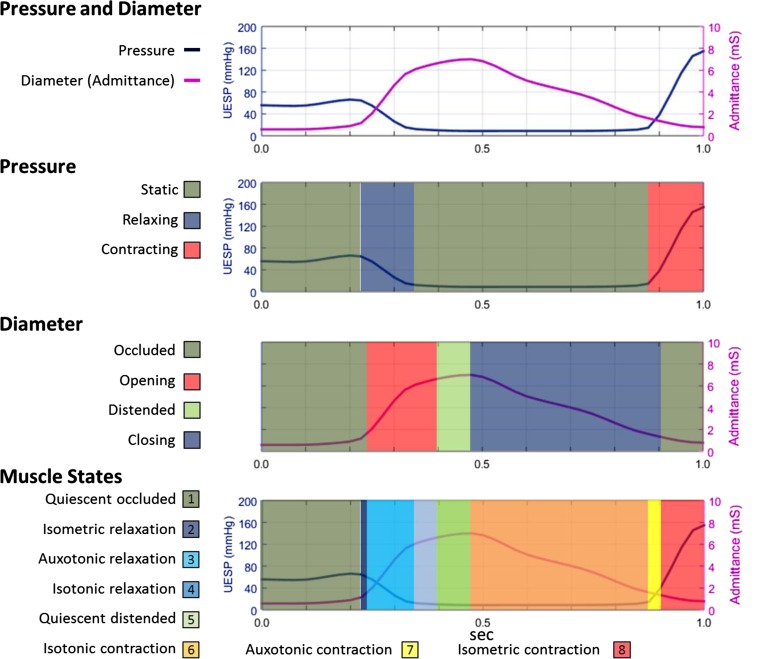
Mechanical state analysis was performed by using the optimal UES pressure and UES admittance profiles measured from preswallow baseline to postrelaxation peak (Fig. 3). As stated above, the mechanical states are calculated from changes in intraluminal pressure in relation to the changes in diameter at the same location. For this analysis, admittance change was used to estimate UES diameter change. This negated the need for radiology, which was not technically possible for us to perform simultaneously with EMG at this time. Previous in vivo recordings have validated UES admittance change as an estimate of UES diameter change based on sequential measurement of the width of a barium bolus visualized radiologically at the level of the tracheal air column (22).
Fig. 3.
UES mechanical states determined from the UES pressure (UESP) and UES admittance profile from preswallow baseline to postrelaxation peak. Using the UES admittance and pressure data array for each of the swallows, we determined muscle states based on the direction of contraction or relaxation and in relation to the occluded or distended state of the lumen. Example is from the same swallow shown in Fig. 2.
To match the sampling rate utilized in our previous validation study (22), the pressure and admittance data were interpolated to achieve a temporal resolution of 40 samples/s. Using the UES admittance and pressure data array for each of the swallows, muscle states were determined based on the direction of contraction or relaxation and in relation to whether the lumen was in an occluded or distended state (Fig. 3). The eight predominant muscle states (Fig. 1) were determined by using a decision tree applying the gradient of admittance and pressure with respect to time (i.e., the slopes of sections of any orbit). Optimal admittance criteria were applied as defined previously (22), summarized as follows: Admittance of ≤1.5 mS defined an occluded state. A rate of admittance change above +10 mS/s or below −1.5 mS/s defined whether the diameter was increasing or decreasing. A pressure change threshold of >250 mmHg/s was used to define pressure increasing above baseline tone and a threshold of < −150 mmHg/s used to define pressure decreasing below basal tone.
Statistical analysis.
Statistical analysis was performed with SPSS Statistics 22 (IBM). Pearson correlation rho (r) was used to assess strength of temporal correlation between the UES pressure profile and CP-EMG and the UES admittance profile and SM-EMG. These were simultaneous data acquired for the time period from preswallow baseline to postrelaxation peak. An r ranging between 0.60 and 0.79 was considered to indicate a substantial correlation, and r > 0.80 was considered to indicate an excellent correlation.
The validity of the different muscles states, as predictors of EMG activity, was also determined over time. This was assessed by accessing the simultaneously recorded EMG, whereby the mean EMG level (μV) and EMG gradient (μV/s) were determined in relation to when each of the muscle state predictions was occurring during each swallow. EMG level and gradient data were normalized to each participant and the average during the eight predicted muscle states was separately determined for each bolus volume consumed. Comparisons were performed by repeated-measures ANOVA among volumes and for volume effects within specific muscle states (general linear model with repeated volume measures and post hoc analysis with Bonferroni correction for multiple comparisons). A P value <0.05 was considered to represent statistical significance; however, P values 0.05–0.099 were also reported representing a nonsignificant trend. Partial eta squared (ηp2) was used as a measure of effect size (ηp2 of 0.1 = small effect, 0.3 = medium effect, 0.5 = large effect).
RESULTS
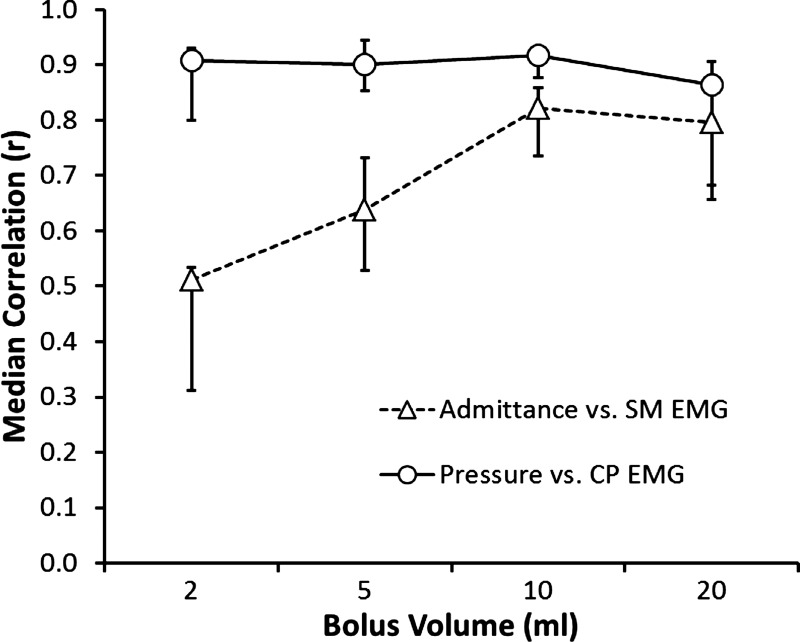
The UES pressure profile showed a substantial to excellent correlation with CP-EMG activity across bolus volumes (Fig. 4). The UES admittance profile showed a good correlation with SM-EMG activity, but only when bolus volumes of 5–20 ml were swallowed (Fig. 4). At all volumes, UES pressure did not correlate with SM-EMG activity and UES admittance did not correlate with CP-EMG activity (data not shown). Because of the discordance of admittance and SM-EMG during 2-ml volume swallows, muscle state predictions were only compared with EMG recordings for 5- to 20-ml boluses.
Fig. 4.
Pearson rho time-correlations of SM-EMG vs. admittance and CP-EMG vs. pressure in relation to different volumes. The median of all subjects and interquartile ranges for each bolus volume are shown.
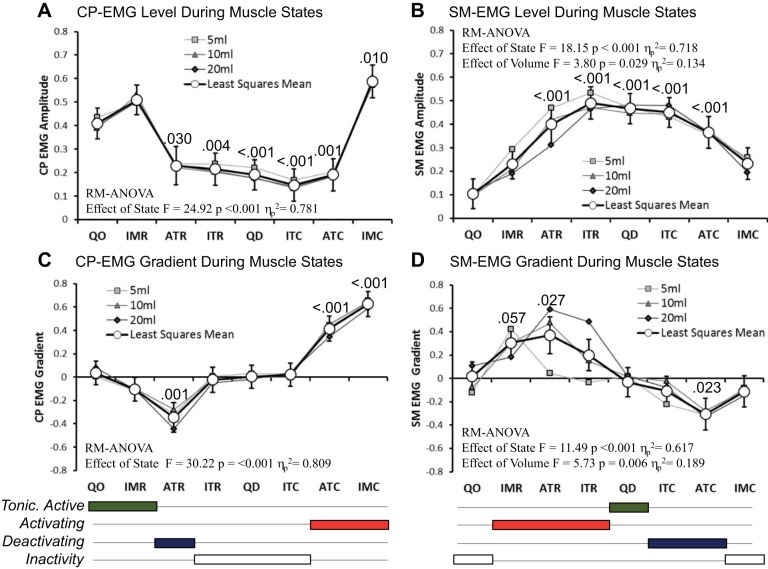
Simultaneously recorded CP-EMG and SM-EMG activity differed among the eight predicted UES muscle states (Fig. 5). Recorded EMG activity changed in relation to the predicted mechanical state. During the period from preswallow baseline to postrelaxation peak, most of the muscle state predictions were associated with a level of activity (Fig. 5, A and B) and/or direction of activity change (Fig. 5, C and D) that was consistent with our original hypothesis (described in Fig. 1B). There was one notable exception: In relation to muscle states and CP-EMG activity, we hypothesized that isometric relaxation at the start of the swallow would be associated with a decreasing CP-EMG gradient. However, tonic activity, rather than significant deactivation, was evident (see “IMR” in Fig. 5, A and C). Similarly, in relation to muscle states and SM-EMG activity, we hypothesized that isometric relaxation at the start of the swallow would occur passively and thus be associated with no change in SM-EMG activity. However, activation, rather than inactivity, was evident (see IMR in Fig. 5, B and D). Further investigation of these observations in relation the isometric relaxations suggested that this phenomenon was only related to the relaxation at the onset of swallow. Isometric relaxations observed after the postrelaxation contractile peak tended to show the anticipated pattern of decreasing CP-EMG activity with SM-EMG inactivity. Finally, we observed some volume effects of small to medium effect size in relation to SM-EMG level (Volume*State F = 2.52, P = 0.004, ηp2 = 0.261) and EMG gradient (Volume*State F = 4.04, P < 0.001, ηp2 = 0.361). These were most apparent during auxotonic and isotonic relaxations (Fig. 5D). The SM-EMG gradient during these relaxations increased, suggesting greater SM-EMG activation, with larger volume swallows.
Fig. 5.
Normalized CP-EMG and SM-EMG activity recorded when different UES muscle states were predicted. A and B: EMG level, where higher values represent greater activity. C and D: EMG gradient, where positive/negative values indicate that EMG activity is increasing/decreasing with respect to time. Based on the EMG changes in these data with respect to mechanical state, conclusions were drawn with respect to the overall state of muscle activity present when the different muscle states were predicted. Below the graphs, the 8 states are consolidated into 4 groups defining when the muscle is tonically active (green), activating (red), deactivating (blue), or inactive (white). Graphs show the individual means for each state based on 5-, 10-, and 20-ml volumes and the overall estimated marginal mean for each state for all volumes combined (white circles with 95% confidence intervals). Repeated-measures (RM) ANOVA descriptive parameters are shown for each overall estimated marginal mean comparison. P values indicate pairwise significance vs. quiescent occluded (QO) state (post hoc test following Bonferroni correction). IMR, isometric relaxation; ATR, auxotonic relaxation; ITR, isotonic relaxation; QD, quiescent distended; ITC, isotonic contraction; ATC, auxotonic contraction; IMC, isometric contraction.
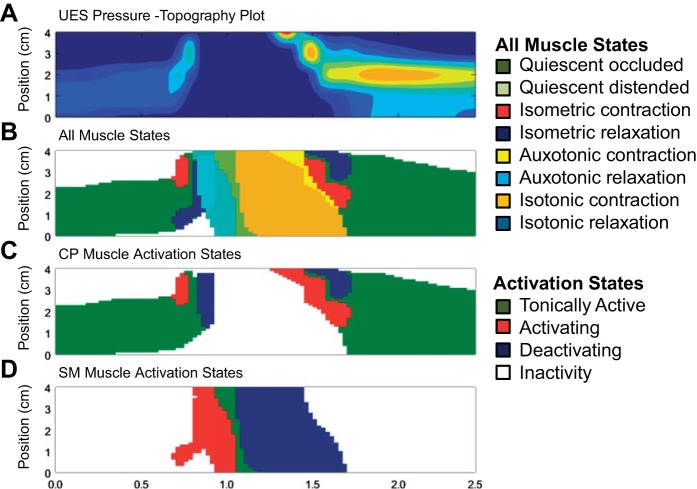
Altogether, the muscle states method allowed standard pressure topography-based assessments (Fig. 6A) to be enhanced through creation of a seamless spatiotemporal “muscle state map” of the entire UES high-pressure zone region. The states map can be configured to show all eight predominant muscle states (Fig. 6B). Furthermore, guided by the level (Fig. 5, A and B) and gradient (Fig. 5, C and D) of EMG activity, mechanical states can be consolidated into four “activation state” subgroups. These are defined when the muscle is 1) tonically active, 2) activating, i.e., transiently increasing in activity, 3) deactivating, i.e., transiently decreasing in activity, or 4) showing inactivity (refer to Fig. 5, C and D, bottom). “Activation state” spatiotemporal maps can then be generated. These define separately the CP muscle activity (Fig. 6C) and SM muscle activity (Fig. 6D).
Fig. 6.
Generation of seamless maps of the spatiotemporal distribution of mechanical states (10-ml liquid swallow, previously shown in Figs. 2 and 3). A: pressure topography plot of the UES area of interest. B: muscle state map showing the appearance of 8 main muscle states over time. C: simplified muscle state map showing predicted CP muscle activity with states consolidated into 4 groups. D: simplified muscle state map showing predicted SM muscle activity with states consolidated into 4 groups.
DISCUSSION
We conducted a validation study in healthy human subjects that combined pressure, impedance, and EMG recordings to assess UES function in vivo. The main findings of this study were 1) pressure-inferred contraction was, at all volumes, a good correlate of CP-EMG activity; 2) admittance-inferred diameter of the UES lumen was, at volumes of 5 ml and over, a good correlate of SM-EMG activity; and 3) “muscle state”-based inferences of neural inputs to the swallowing muscles were associated with appropriate EMG recorded changes in the activation state of the CP and SM muscles. Hence, the pattern of UES muscle states was consistent with known neurally dependent phasic discharge patterns of CP and SM muscle activity during swallowing.
The physiology governing UES relaxation and then UES opening follows the same fundamental mechanical principles governing propulsion of contents throughout the gastrointestinal tract. That is, the lumen ahead of the moving bolus must relax and open to allow unimpeded passage and the lumen behind the bolus must contract and close to generate propulsive force and prevent retrograde bolus escape. The mechanical states method allows prediction of these mechanical changes within the UES to be recorded in vivo during bolus swallowing. For a sphincter muscle, neural inputs and mechanical factors lead to tonic muscle contraction and luminal occlusion. In the case of the upper sphincter, relaxation represents neural deactivation, rather than activation of inhibitory neurons. As long as these caveats are understood, the mechanical states can be derived for the upper sphincter muscles and likely neural inputs can be deduced in the same way as has been proposed for the remainder of the gastrointestinal tract (4, 6, 27).
Past studies have shown that the CP muscle is the major contractile component of the UES that “relaxes” prior to being opened by traction forces applied by the anterior movement of the hyoid and larynx (1–3). UES relaxation is then followed by a pause in activity while the UES is open (1, 8, 12, 19). A central pattern generator-controlled transient burst of EMG activity then follows luminal closure, leading to a postrelaxation pressure peak (7). Our previous observations of different muscle states occurring within the UES region (22) were highly consistent with EMG studies that directly measured activation and deactivation of the CP muscle during swallowing (1, 12, 19, 21, 23). These observations suggested that pressure-impedance-based determination of mechanical states may potentially predict the level of CP muscle activation. However, to confirm this, a direct correlation of CP-EMG activity and pressure-diameter based predictions of the muscle states was needed.
Of 12 theoretically possible UES mechanical states, eight are nearly always seen during healthy swallowing (22). Different states, and the switching between states, allow specific inferences with respect to neural inputs to the different muscles. The eight states were consolidated into four general pattern groups that define when either the CP muscle or SM muscles are tonically active, activating, deactivating, and inactive. These groups were validated by simultaneous EMG recordings. We hypothesized that isometric relaxation/contraction states indicate deactivation/activation of CP muscle and inactivity of the SM muscle. Auxotonic relaxation/contraction states indicate deactivation/activation of CP muscle juxtaposed with activation/deactivation of the SM muscles. Isotonic relaxation/contraction states indicate inactivity of the CP muscle and activation/deactivation of the SM muscles. Quiescent occluded and distended states indicate tonic activation of the CP and SM muscles, respectively.
Our original hypothesis (Fig. 1) was for the most part supported by CP- and SM-EMG recordings. However, the association of isometric relaxation with tonic CP muscle activity and SM muscle activation was an unexpected finding. If correct, this suggests that the CP muscle undergoes neural deactivation after it begins to manometrically relax. Hence, at swallow onset, isometric relaxation may represent a passive pressure drop due to extrinsic traction counteracting the neurogenic and passive elastic factors responsible for generating the UES basal pressure. This concept of traction from superior-anterior hyoid movement, potentially overcoming a tonically active CP contraction, has been previously described (26).
For CP muscle states, pressure change is the critical measure defining muscle deactivation/activation. Intraluminal impedance is important for differentiating whether the muscle is tonically active or inactive since this inference depends on whether the lumen is occluded or open. Muscle state predictions for the CP muscle appeared to reliably predict muscle activity over the range of bolus volumes tested. This was most likely due to the fact that UES pressure and CP-EMG showed substantial to excellent time correlation across the bolus volume range. We therefore conclude that the muscle states measurements can be used to infer effectively neural inputs to the CP muscle.
For SM muscle states, diameter change (impedance measured) is critical for defining muscle activation/deactivation. Muscle state predictions of SM muscle activity were found most reliable at the highest 10- to 20-ml volumes, where the time correlations of admittance and SM-EMG were excellent to substantial. We conclude, therefore, that it is also possible for the muscle states approach to be used to infer, successfully, neural inputs to the SM muscles. However, the ability to swallow volumes of 10–20 ml is an important caveat, problematic for patients who are unable to swallow these volumes, or who demonstrate piecemeal swallowing of orally administered boluses. Surface electrode SM-EMG, unlike CP-EMG, is not technically difficult to record and therefore the ability to predict the activity of the SM muscles in vivo may only represent limited added value.
In our study we applied a previously developed admittance-based model to define mechanical states of the muscle (22). While interpolation functions are used to spatially align pressure and impedance measurements, we recognize that the resolution and mode of sensing used to achieve pressure and impedance recordings are not the same. Namely pressure is discretely measured by strain gauges at 1-cm intervals, whereas impedance is measured over a 2-cm electrode segment. Simultaneous fluoroscopic imaging and approximation of UES opening and closure in the lateral view would have further added to the validation information available and improved the predictive model applied. However, this was not technically available in our laboratory at this time. Nevertheless, as applied and despite these limitations, the methodology we used to derive UES mechanical states in vivo produced results that were consistent with our study hypothesis that the neural activation state of CP and SM muscles can each be deduced.
Methods to assess CP muscle activity are needed to clinically differentiate motor dysfunctions that cause dysphagia. The present study shows that mechanical state analysis alone can predict CP muscle neural activation states. Given that CP-EMG is difficult to clinically apply, while high-resolution impedance-manometry is already in widespread use, our method may have broad clinical use. Objective determination of the mechanical states of the muscle may differentiate specific CP muscle dysfunctions (e.g., neurogenic UES relaxation failure following brain stem stroke) from structural pathology (e.g., CP fibrosis following radiotherapy) and submental muscle weakness (e.g., weak UES opening due to motor neuron disease). This understanding may guide appropriate treatments such as Botox injection, to diminish tonic activation of a nonrelaxing CP (16); surgical myotomy or pneumatic dilatation, to disrupt a nonopening fibrotic CP muscle (2, 11, 17, 18, 20); and/or suprahyoid muscle strengthening exercises or swallow maneuvers, to augment upper esophageal sphincter opening (15, 24, 25). Further studies assessing mechanical states in different patient subgroups and following therapeutic intervention are indicated.
In conclusion, we present findings in relation to a novel method to assess UES function using the assessment of UES mechanical states based on the relationship of admittance and pressure with reliable inference of the neural activation of the relevant muscle. Mechanical state predictions were simple for us to apply using software and revealed patterns consistent with the measured neural inputs activating the different muscles during swallowing. Changes in mechanical states correlate with CP- and SM-EMG recordings of muscle activity and are consistent with the established understanding of UES opening mechanics. In this study we have consolidated the analysis into four states that allow key interpretations regarding UES function. Further studies are needed to extend these observations into patients with dysphagia symptoms due to UES pathophysiology.
GRANTS
T. I. Omari is the recipient of a National Health & Medical Research Council Senior Research Fellowship. C. Cock received grants from The Repat Foundation. T. M. McCulloch, C. A. Jones, and M. J. Hammer are supported by National Institutes of Health (NIH) Grant DC011130. M. J. Hammer is also supported by NIH Grants DC010900 and DC014519. C. A. Jones is also supported by NIH Grant T32 GM007507.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.I.O., C.C., P.G.D., L.W., M.C.C., and T.M.M. conception and design of research; T.I.O., C.A.J., M.J.H., C.C., and T.M.M. performed experiments; T.I.O. and C.A.J. analyzed data; T.I.O., C.A.J., M.J.H., C.C., and T.M.M. interpreted results of experiments; T.I.O. prepared figures; T.I.O., C.A.J., M.J.H., and C.C. drafted manuscript; T.I.O., C.A.J., M.J.H., C.C., P.G.D., L.W., M.C.C., and T.M.M. edited and revised manuscript; T.I.O., C.A.J., M.J.H., C.C., P.G.D., L.W., M.C.C., and T.M.M. approved final version of manuscript.
REFERENCES
- 1.Asoh R, Goyal RK. Manometry and electromyography of the upper esophageal sphincter in the opossum. Gastroenterology 74: 514–520, 1978. [PubMed] [Google Scholar]
- 2.Clary MS, Daniero JJ, Keith SW, Boon MS, Spiegel JR. Efficacy of large-diameter dilatation in cricopharyngeal dysfunction. Laryngoscope 121: 2521–2525, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brasseur JG, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 257: G748–G759, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Costa M, Wiklendt L, Arkwright JW, Spencer NJ, Omari T, Brookes SJH, Dinning PG. An experimental method to identify neurogenic and myogenic active mechanical states of intestinal motility. Front Syst Neurosci 7: 7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crary MA, Carnaby GD, Groher ME. Biomechanical correlates of surface electromyography signals obtained during swallowing by healthy adults. J Speech Lang Hear Res 49: 186–193, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Dinning PG, Wiklendt L, Omari T, Arkwright JW, Spencer NJ, Brookes SJH, Costa M. Neural mechanisms of peristalsis in the isolated rabbit distal colon: a neuromechanical loop hypothesis. Front Neurosci 8: 75, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ertekin C, Aydogdu I. Electromyography of human cricopharyngeal muscle of the upper esophageal sphincter. Muscle Nerve 26: 729–739, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Ertekin C, Pehlivan M, Aydoğdu I, Erta̦s M, Uludağ B, Celebi G, Colakoğlu Z, Sağduyu A, Yüceyar N. An electrophysiological investigation of deglutition in man. Muscle Nerve 18: 1177–1186, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol 291: G525–G531, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Hammer MJ, Jones CA, Mielens JD, Kim CH, McCulloch TM. Evaluating the tongue-hold maneuver using high-resolution manometry and electromyography. Dysphagia 29: 564–570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatlebakk J, Castell J, Spiegel J, Paoletti V, Katz P, Castell D. Dilatation therapy for dysphagia in patients with upper esophageal dysfunction — manometric and symptomatic response. Dis Esophagus 11: 254–259, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Jones CA, Hammer MJ, Hoffman MR, McCulloch TM. Quantifying contributions of the cricopharyngeus to upper esophageal sphincter pressure changes by means of intramuscular electromyography and high-resolution manometry. Ann Otol Rhinol Laryngol 123: 174–182, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CA, Ciucci MR, Hammer MJ, McCulloch TM. A multisensor approach to improve manometric analysis of the upper esophageal sphincter. Laryngoscope 2015 Aug 22. doi: 10.1002/lary.25506 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahrilas P, Dodds W, Dent J, Logemann J, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterology 95: 52–62, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Kahrilas PJ, Logemann J, Kruger C, Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol Gastrointest Liver Physiol 260: G450–G456, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Kelly EA, Koszewski IJ, Jaradeh SS, Merati AL, Blumin JH, Bock JM. Botulinum toxin injection for the treatment of upper esophageal sphincter dysfunction. Ann Otol Rhinol Laryngol 122: 100–108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly JH. Management of upper esophageal sphincter disorders: indications and complications of myotomy. Am J Med 108 Suppl: 43S–46S, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Lan Y, Xu G, Dou Z, Wan G, Yu F, Lin T. Biomechanical changes in the pharynx and upper esophageal sphincter after modified balloon dilatation in brainstem stroke patients with dysphagia. Neurogastroenterol Motil 25: e821–e829, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Lang IM, Dantas R, Cook I, Dodds J. Analysis of canine upper esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 260: G911–G919, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Mason RJ, Bremner CG, DeMeester TR, Crookes PF, Peters JH, Hagen JA, DeMeester SR. Pharyngeal swallowing disorders: selection for and outcome after myotomy. Ann Surg 228: 598–608, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medda BK, Lang I, Dodds W, Christl M, Kern M, Hogan W, Shaker R. Correlation of electrical of the cricopharyngeus and contractile activities muscle in the cat. Am J Physiol Gastrointest Liver Physiol 273: G470–G479, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Omari TI, Wiklendt L, Dinning P, Costa M, Rommel N, Cock C. Upper esophageal sphincter mechanical states analysis: a novel methodology to describe UES relaxation and opening. Front Syst Neurosci 8: 1–18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Overbeek J, Wit H, Paping R, Segenhout H. Simultaneous manometry and electromyography in the pharyngoesophageal segment. Laryngoscope 95: 582–584, 1985. [DOI] [PubMed] [Google Scholar]
- 24.Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, Grande B, Kazandjian M, Dikeman K. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology 122: 1314–1321, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Shaker R, Kern M, Eytan B, Taylor A, Stewart E, Raymend G, Arndorfer R, Hofmann C, Bonnevier J. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by excercise. Am J Physiol Gastrointest Liver Physiol 272: G1518–G1522, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am J Med 108 Suppl: 27S–37S, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Wiklendt L, Costa M, Dinning PG. Inference of mechanical states of intestinal motor activity using hidden Markov models. BMC Physiol 13: 14, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]