Abstract
Background/Aims
NELL-1 is a novel osteochondral differentiation factor protein with increasing usage in tissue engineering. Previously, we reported the expression patterns of NELL-1 in bone-forming skeletal tumors. With increasing interest in the use of NELL-1 protein, we sought to examine the expression of NELL-1 in cartilage-forming tumors.
Methods
Immunohistochemical expression was examined in human pathologic specimens.
Results
Consistent NELL-1 overexpression across all cartilage-forming tumors was observed. Similar degrees of expression were observed in enchondroma, chondrosarcoma, and chondroblastic osteosarcoma. NELL-1 expression did not significantly vary by tumor grade.
Conclusion
In summary, NELL-1 demonstrates reliable and consistent expression across cartilage-forming skeletal tumors.
Keywords: Nell-1, Enchondroma, Chondrosarcoma, Osteosarcoma, Chondroblastic osteosarcoma
1. Introduction
NELL-1 (NEL-like Protein 1) is a novel osteochondral differentiation factor that has been increasingly used for tissue engineering applications.1, 2, 3, 4, 5 NELL-1 is a secreted protein of 810-amino acids with a molecular weight of about 90 kDa before N-glycosylation and oligomerization.6 NELL-1 was first identified to have bone-forming properties by its overexpression in fusing cranial sutures.7 Since that time, NELL-1 has also been shown to be pro-chondrogenic in what appears to be a cell- and context-dependent manner. In mature adult chondrocytes, NELL-1 induces cellular proliferation, as well as the chondrogenic markers, Aggrecan and Type II Collagen, and increases cartilage specific matrix deposition in 3-D culture.8 Mechanistically, NELL-1 has been recently shown to bind the cell surface receptor integrin β1,9 resulting in FAK,9 MAPK,10 and Canonical Wnt signaling activation.11
The importance of avoiding tumorigenic effects cannot be overemphasized in the field of tissue engineering and regeneration. This issue has growing importance with cytokine-based skeletal repair. For example, the main FDA approved recombinant protein for bone formation is BMP2 (Bone Morphogenetic Protein 2).12, 13 BMP ligands and BMP receptors are expressed in most osteosarcoma14, 15 and chondrosarcoma subtypes.16 Moreover, although disagreement in the literature exists, the presence of BMP signaling in osteosarcoma may impart a worse prognosis.15, 17, 18 On the cellular level, BMP signaling appears to mediate pro-migratory effects in both chondrosarcoma and osteosarcoma cell types.19 Likewise, parathyroid hormone (PTH) is the main FDA approved anabolic agent in the treatment of osteoporosis.20, 21, 22, 23 Unfortunately, the clinical duration of use for PTH is limited to 24 months, owing to the risk of osteosarcomagenesis.24 Thus, currently approved agents for bone formation are not without potential risks for sarcomagenesis.
Recently, we reported the expression patterns of NELL-1 in benign and malignant bone tumors.25 Briefly, we found that among benign bone tumors (osteoid osteoma and osteoblastoma), strong and diffuse NELL-1 expression was observed, which spatially correlated with markers of osteogenic differentiation. In contrast, a relative reduction in NELL-1 staining was observed in osteosarcoma, accompanied by increased variation between tumors. Furthermore, among osteosarcoma specimens, NELL-1 expression did not correlate well with markers of osteogenic differentiation. These results suggested alternative bioactive effects of NELL-1 in malignant bone tumors. In the present manuscript, we sought to expand these findings to human tumors of cartilage.
2. Materials and methods
2.1. Antibodies and reagents
Primary antibodies used in this study were anti-NEL like protein 1 (NELL-1) (GTX111493, GeneTex, Inc., Irvine, CA). All other reagents were purchased from Dako unless otherwise specified.
2.2. Tissue procurement
Tumors were retrospectively collected from biopsy and resection specimens at the University of California, Los Angeles with IRB approval under UCLA IRB# 13-897. Tumor samples were de-identified with the use of a numeric labeling system so as to protect the identity of the patients, in full compliance with the UCLA IRB and ethics committee. Each tumor was re-examined by two blinded bone tissue pathologists to ensure accuracy of original diagnosis. Radiographic and clinical history was also consulted to ensure accuracy of diagnosis. Demographic features were recorded, including patient age, gender, anatomic location, tumor size, and clinical course including regional recurrence and distant metastasis (Supplementary Table 1).
Supplementary Table 1 related to this article can be found, in the online version, at doi:10.1016/j.jor.2015.10.001.
Patient demographics.
2.3. Histological and immunohistochemical analyses
Five-micron-thick paraffin sections of bone tumors were stained with hematoxylin and eosin (H&E). Using H&E sections, histomorphologic assessments were made to confirm tumor type and to determine characteristics of different regions within each section. Additional sections were analyzed by indirect immunohistochemistry. Briefly, unstained sections were deparaffinized in xylene and a series of graded ethanol solutions, and rehydrated using phosphate buffered solution. The slides were incubated in 3% hydrogen peroxide for 20 min at room temperature to block endogenous peroxidase activity. 0.125% trypsin induced epitope retrieval was performed for 20 min at room temperature, using the “Digest-All 2” system (Cat 00-3008, Invitrogen, Grand Island, NY). Slides were then incubated with the primary antibody for 1 h at 37 °C and 4 °C overnight. The anti-NELL-1 primary antibody was used at a dilution of 1:400. After incubation with the primary antibody, slides were incubated with the appropriate biotinylated secondary antibodies (Dako) for 1 hr at room temperature at a 1:200 dilution.
Positive immunoreactivity was detected following ABC complex (PK-6100, Vectastain Elite ABC Kit, Vector Laboratories Inc., Burlingame, CA) incubation and development with AEC chromagen (K346911-2, Dako). Negative controls for each antibody consisted of incubation with secondary antibody in the absence of primary antibody. Sections of neonatal rat spines were used in each instance as a positive staining control.11 Sections were counterstained in Modified Mayers Hematoxylin (Thermo Scientific, Waltham, MA) for 30 s, and placed under running water for 5 min. Slides were mounted using aqueous mounting medium (Dako). Photomicrographs were acquired using Olympus BX51 (100× and 200× magnification lens, UPLanFL, Olympus).
Intensity and distribution of immunohistochemical staining were determined by three blinded observers, as previously performed.25 The intensity of staining was estimated using a 3 point scale, with ‘0′ indicating no staining, ‘1+’ indicating predominantly faint/barely perceptible cytoplasmic staining within any percentage of tumor cells, ‘2+’ indicating predominantly weak/moderate cytoplasmic staining within any percentage of tumor cells, and ‘3+’ indicating strong/intense cytoplasmic staining within any percentage of tumor cells. Discrepancies in semi-quantification of intensity of staining between observers were found in <10% of samples. In this case, the intensity of stain was determined by consensus re-review of the slides by all three observers. Distribution of staining was determined on a continuous 0–100% scale, estimating the percentage of tumor cells with NELL-1 immunoreactivity. Distribution of staining for each tumor was determined as a mean value between each blinded observer.
2.4. Statistical analysis
Statistical analysis was performed using an appropriate Student's t-test when two groups of numerical values were being compared, as in the case of staining distribution. A Fisher's exact test was performed to determine statistical significance of contingency tables, as in the case of staining intensity. In general, a p-value <0.05 was considered statistically significant.
3. Results
3.1.1. NELL-1 expression in enchondroma
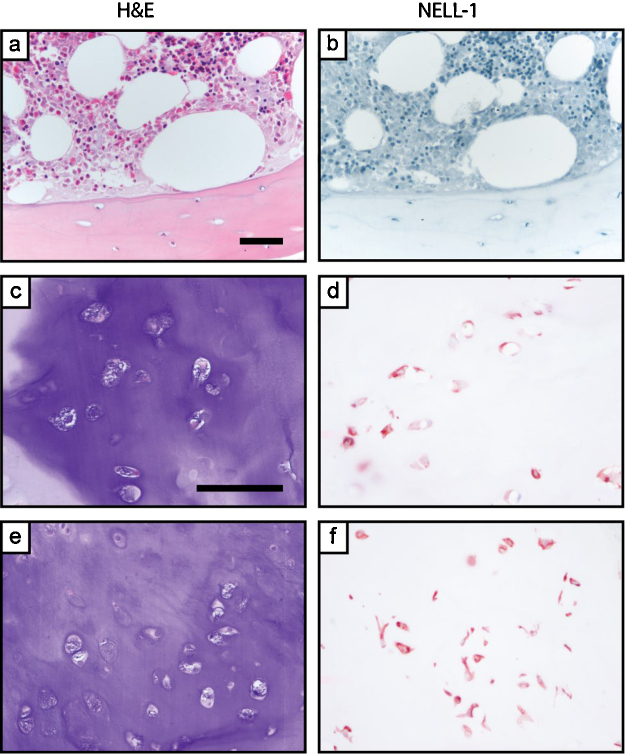
All cases of enchondroma demonstrated characteristic histologic findings, including a lobular proliferation of low-grade cartilage without significant cytologic atypia, with inconspicuous mitotic activity, and without any observed bone permeation or soft tissue extension (Fig. 1). Peri-lesional adult cortical bone and adjacent bone marrow had negligible NELL-1 immunoreactivity. In comparison, enchondromas showed staining in chondrocytes in a multifocal distribution. Immunoreactivity was generally in a cytosolic or combined cytosolic and nuclear distribution. Moderate to strong NELL-1 immunoreactivity was observed across all tumors (2–3+ staining intensity), and was on average present in 47% of tumor cells (±10%) (Table 1).
Fig. 1.
NELL-1 expression in peri-lesional, uninvolved human trabecular bone and enchondroma. (a, b) Representative appearance of routine H&E staining and NELL-1 immunohistochemical staining in cortical bone and bone marrow. (c–f) Representative H&E staining and NELL-1 immunohistochemical staining in enchondroma. Images shown use the GeneTex NELL-1 antibody. Scale bar: 100 μm.
Table 1.
Semi-quantitative assessment of NELL-1 immunohistochemistry, by tumor type.
| Tumor type | Staining intensity (% of cases stained) |
Staining distribution | |||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | Mean % of cells stained (±SD) | |
| Enchondroma | – | – | 2/5 (40%) | 3/5 (60%) | 47.0% (±10%) |
| Chondrosarcoma | – | 1/12 (8.3%) | 5/12 (42%) | 6/12 (50%) | 39.6% (±30%) |
| Grade 1 | – | – | 3/5 (60%) | 2/5 (40%) | 38.0% (±33%) |
| Grade 2 | – | 1/5 (20%) | 2/5 (40%) | 2/5 (40%) | 33.0% (±31%) |
| Grade 3 | – | – | – | 2/2 (100%) | 60.0% (±28%) |
| Chondroblastic OS | – | 3/12 (25%) | 8/12 (67%) | 1/12 (8.3%) | 49.0% (±40%) |
3.1.2. NELL-1 expression in chondrosarcoma
All cases of chondrosarcoma were intramedullary with a radiographic impression of a malignant cartilage neoplasm. Bone permeation and/or soft tissue extension was present in most cases. Results showed that among chondrosarcoma specimens, a similar pattern of NELL-1 expression was observed with intertumor more variability. As with enchondroma, a multifocal pattern of staining was apparent in lesional chondrocytes (Fig. 2). Some degree of NELL-1 immunoreactivity was observed in all cases, although this varied from weak to strong immunoreactivity (1–3+ intensity) and with a wider variation in percentage of cells stained (mean 39.6% ± 30%). No statistically significant difference was observed between NELL-1 expression in enchondroma and chondrosarcoma in terms of staining intensity (p = 1.0) or distribution (p = 0.61).
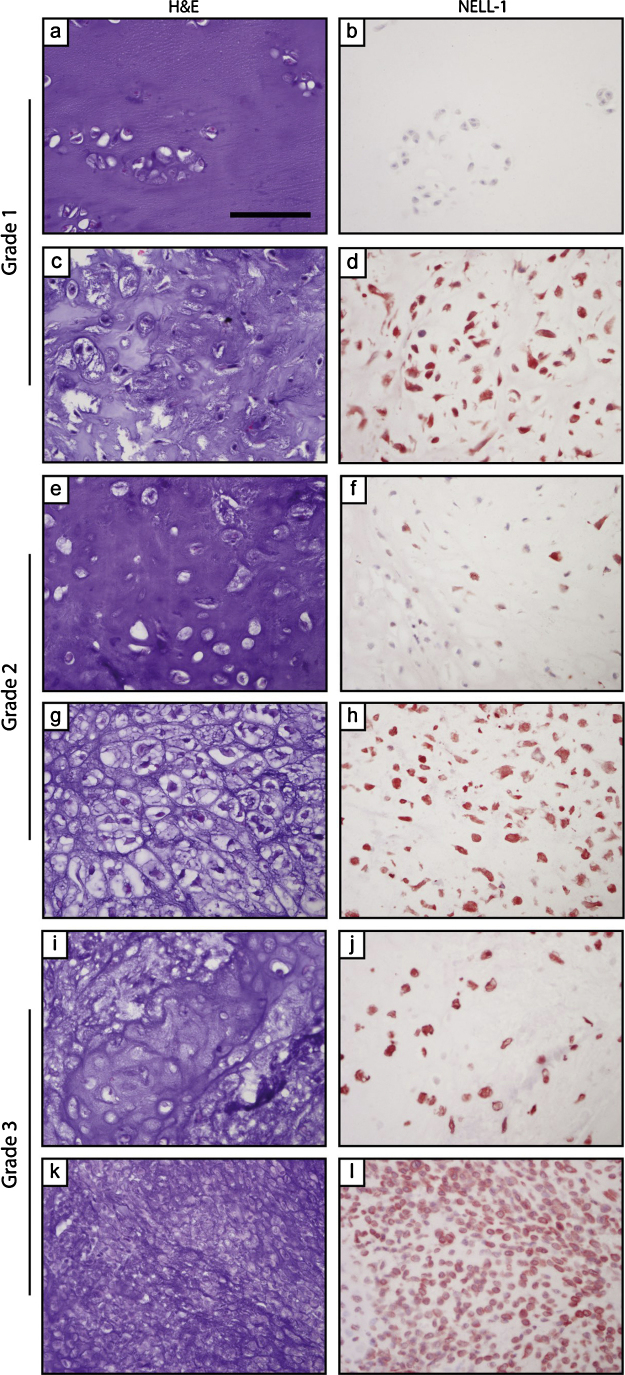
Fig. 2.
NELL-1 expression in chondrosarcoma. (a–d) Appearance of H&E staining and NELL-1 immunohistochemical staining in chondrosarcoma, grade I. (e–h) Appearance of H&E staining and NELL-1 immunohistochemical staining in chondrosarcoma, grade II. (i–l) Appearance of H&E staining and NELL-1 immunohistochemical staining in chondrosarcoma, grade III. Scale bar: 100 μm.
Qualitative impression showed that NELL-1 seemed to have more immunoreactivity within areas of increasing hypercellularity among chondrosarcoma specimens (Fig. 2). To further investigate, chondrosarcoma specimens were next stratified by tumor grade (Table 1).
3.1.3. NELL-1 expression in chondroblastic osteosarcoma
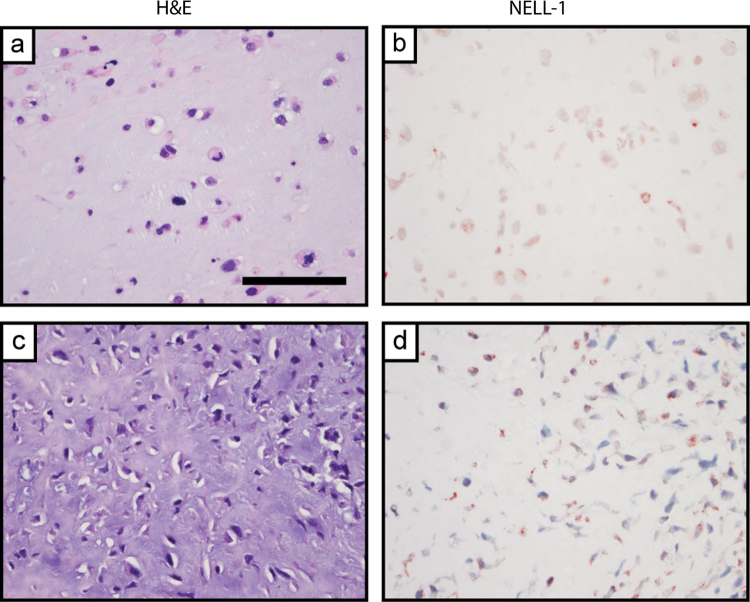
Chondroblastic osteosarcoma specimens were defined as osteosarcoma specimens with a majority of chondroblastic phenotype (Fig. 3). A wide range of NELL-1 staining intensity was observed (1–3+), with a non-significant trend toward reduced staining intensity in comparison to chondrosarcoma tumors (Table 1). Overall a qualitative increase in staining among chondroblastic areas with increasing cellularity was observed, consistent with our prior observations.25 However, some element of NELL-1 staining was observed in each tumor sample.
Fig. 3.
NELL-1 expression in chondroblastic osteosarcoma. (a–d) Appearance of H&E staining and NELL-1 immunohistochemical staining in representative chondroblastic osteosarcoma (OS). Scale bar: 100 μm.
4. Discussion
In brief, the present study has identified several unique features of NELL-1 in skeletal tumor biology. First, NELL-1 expression is increased across all cartilage-forming skeletal tumors when compared to non-neoplastic adult bone. Second, both benign and malignant chondrocytic tumors’ cells demonstrate consistent NELL-1 staining with some heterogeneity between samples and tumor type. Third, NELL-1 immunoreactivity is a consistent features among both chondrosarcoma and chondroblastic osteosarcoma specimens.
The study of osteochondrogenic proteins in skeletal sarcomas is essentially to understand the roles and risks of exogenous protein application in skeletal tissue regeneration efforts. There is clear precedent that currently used proteins have real or potential risks for sarcomagenesis or influencing sarcoma progression. For example, the clinical duration of use for PTH is limited to 24 months, owing to the risk of osteosarcomagenesis as seen in murine studies.24 To date, it is unknown if these PTH risks in animal models translate to human biology. The main local differentiation factor for use in bone tissue engineering, BMP2, also has theoretical risks.12, 13 BMP ligands and BMP receptors are expressed in most osteosarcoma14, 15 and chondrosarcoma subtypes.16 Moreover, although disagreement in the literature exists, the presence of BMP signaling in osteosarcoma may impart a worse prognosis.15, 17, 18 On the cellular level, BMP signaling appears to mediate pro-migratory effects in both chondrosarcoma and osteosarcoma cell types.19 Thus, in a search for alternative osteochondral differentiation factors, it is important to consider their importance in skeletal tumor biology.
The expression of NELL-1 in skeletal malignancy raises intriguing questions regarding its role in the basic function in tumor biology. Several pieces of data suggest that NELL-1 expression is downregulated in epithelial malignancies (carcinomas). For example, NELL-1 has been found to be epigenetically silenced via methylation in several carcinomas, including colon adenocarcinoma,26 esophageal adenocarcinoma,27 as well as renal cell carcinoma.28 Our study, in combination with our previous observations,25 suggests that the opposite is true in skeletal sarcomas, including consistent NELL-1 overexpression in both osteosarcoma and chondrosarcoma. The bioactive effects of NELL-1 in skeletal sarcomas remain a matter of speculation; however, recent understanding of NELL-1 as an integrin β1 ligand may reveal new insights.9
Several limitations exist for broader extrapolation of the results from the present study. First, we rely on immunohistochemical-based detection of NELL-1. Clinical samples vary in their processing, with variable lengths of ischemic and fixation times. How these factors influence the NELL-1 antigen is not yet known. Second, the present study is a survey of NELL-1 expression, and as such has a modest sample size of any given tumor type. Nevertheless, the present study highlights the presence of NELL-1 across benign and malignant cartilage tumors.
Conflicts of interest
Drs. XZ, KT, and CS are inventors of NELL-1 related patents. Drs. XZ, KT, and CS are founders and or board members of Bone Biologics Inc. which sublicenses NELL-1 patents from the UC Regents, which also hold equity in the company.
Acknowledgments
The present work was supported by the UCLA Department of Pathology and Laboratory Medicine, the Translational Research Fund, the UCLA Daljit S. and Elaine Sarkaria Fellowship award, and the Orthopaedic Research and Education Foundation with funding provided by the Musculoskeletal Transplant Foundation. The authors thank the staff of UCLA Translational Pathology Core Laboratory, and A.S. James for their excellent technical assistance.
References
- 1.Aghaloo T., Cowan C.M., Chou Y.F. Nell-1-induced bone regeneration in calvarial defects. Am J Pathol. 2006;169:903–915. doi: 10.2353/ajpath.2006.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W., Lee M., Whang J. Delivery of lyophilized Nell-1 in a rat spinal fusion model. Tissue Eng Part A. 2010;16:2861–2870. doi: 10.1089/ten.tea.2009.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W., Zara J.N., Siu R.K. Nell-1 enhances bone regeneration in a rat critical-sized femoral segmental defect model. Plast Reconstr Surg. 2011;127:580–587. doi: 10.1097/PRS.0b013e3181fed5ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siu R.K., Lu S.S., Li W. Nell-1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng Part A. 2011;17:1123–1135. doi: 10.1089/ten.tea.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan W., James A.W., Asatrian G. NELL-1 based demineralized bone graft promotes rat spine fusion as compared to commercially available BMP-2 product. J Orthop Sci. 2013;18:646–657. doi: 10.1007/s00776-013-0390-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X., Zara J., Siu R.K., Ting K., Soo C. The role of NELL-1: a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 2010;89:865–878. doi: 10.1177/0022034510376401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting K., Vastardis H., Mulliken J.B. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14:80–89. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Lee M., Siu R.K., Ting K., Wu B.M. Effect of Nell-1 delivery on chondrocyte proliferation and cartilaginous extracellular matrix deposition. Tissue Eng Part A. 2010;16:1791–1800. doi: 10.1089/ten.TEA.2009.0384. [DOI] [PubMed] [Google Scholar]
- 9.Shen J., James A.W., Chung J. NELL-1 promotes cell adhesion and differentiation via Integrinβ1. J Cell Biochem. 2012;113:3620–3628. doi: 10.1002/jcb.24253. [DOI] [PubMed] [Google Scholar]
- 10.Chen F., Walder B., James A.W. NELL-1-dependent mineralisation of Saos-2 human osteosarcoma cells is mediated via c-Jun N-terminal kinase pathway activation. Int Orthop. 2012;36:2181–2187. doi: 10.1007/s00264-012-1590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James A.W., Zhang S.J., Asatrian X. NELL-1 in the treatment of osteoporotic bone loss. Nat Commun. 2015;6:7362. doi: 10.1038/ncomms8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freire M.O., You H.K., Kook J.K., Choi J.H., Zadeh H.H. Antibody-mediated osseous regeneration: a novel strategy for bioengineering bone by immobilized anti-bone morphogenetic protein-2 antibodies. Tissue Eng Part A. 2011;17:2911–2918. doi: 10.1089/ten.tea.2010.0584. [DOI] [PubMed] [Google Scholar]
- 13.Friedlaender G.E., Perry C.R., Dean Cole J. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(suppl 1 (Pt 2)):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 14.Gobbi G., Sangiorgi L., Lenzi L. Seven BMPs and all their receptors are simultaneously expressed in osteosarcoma cells. Int J Oncol. 2002;20:143–147. [PubMed] [Google Scholar]
- 15.Nguyen A., Scott M.A., Dry S.M., James A.W. Roles of bone morphogenetic protein signaling in osteosarcoma. Int Orthop. 2014;38:2313–2322. doi: 10.1007/s00264-014-2512-x. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa H., Rettig W.J., Lane J.M. Immunohistochemical detection of bone morphogenetic proteins in bone and soft-tissue sarcomas. Cancer. 1994;74:842–847. doi: 10.1002/1097-0142(19940801)74:3<842::aid-cncr2820740309>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa H., Takaoka K., Hamada H., Ono K. Clinical significance of bone morphogenetic activity in osteosarcoma. A study of 20 cases. Cancer. 1985;56:1682–1687. doi: 10.1002/1097-0142(19851001)56:7<1682::aid-cncr2820560735>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa H., Takaoka K., Masuhara K., Ono K., Sakamoto Y. Prognostic significance of bone morphogenetic activity in osteosarcoma tissue. Cancer. 1988;61:569–573. doi: 10.1002/1097-0142(19880201)61:3<569::aid-cncr2820610324>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Sotobori T., Ueda T., Myoui A. Bone morphogenetic protein-2 promotes the haptotactic migration of murine osteoblastic and osteosarcoma cells by enhancing incorporation of integrin beta1 into lipid rafts. Exp Cell Res. 2006;312:3927–3938. doi: 10.1016/j.yexcr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Jilka R.L. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neer R.M., Arnaud C.D., Zanchetta J.R. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 22.Cusano N.E., Bilezikian J.P. Combination anabolic and antiresorptive therapy for osteoporosis. Endocrinol Metab Clin North Am. 2012;41:643–654. doi: 10.1016/j.ecl.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Body J.J., Gaich G.A., Scheele W.H. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87:4528–4535. doi: 10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 24.Vahle J.L., Sato M., Long G.G. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 25.Shen J., LaChaud G., Khadarian K. NELL-1 expression in benign and malignant bone tumors. Biochem Biophys Res Commun. 2015;460:368–374. doi: 10.1016/j.bbrc.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 26.Mori Y., Cai K., Cheng Y. A genome-wide search identifies epigenetic silencing of somatostatin: tachykinin-1, and 5 other genes in colon cancer. Gastroenterology. 2006;131:797–808. doi: 10.1053/j.gastro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Jin Z., Mori Y., Yang J. Hypermethylation of the nel-like 1 gene is a common and early event and is associated with poor prognosis in early-stage esophageal adenocarcinoma. Oncogene. 2007;26:6332–6340. doi: 10.1038/sj.onc.1210461. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura R., Oyama T., Tajiri R. Expression and regulatory effects on cancer cell behavior of NELL1 and NELL2 in human renal cell carcinoma. Cancer Sci. 2015;106:656–664. doi: 10.1111/cas.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient demographics.