Abstract
The purpose of this paper is to review the clinical indications for acetabular reconstruction in patients with underlying peri-prosthetic segmental and cavitary defects, evaluate steps in pre-operative planning, and present the American Academy of Orthopaedic Surgeons (AAOS) and Paprosky classification systems to categorize acetabular defects. We also present a review of the current surgical techniques to reconstruct the acetabular socket which includes a cementless acetabular component with morselized bone, structural allograft, jumbo and oblong cups, reinforcement rings, bone cages, custom triflange acetabular constructs, and trabecular metal components.
Keywords: Acetabular reconstruction, Paprosky classification, Reinforcement rings, Triflange constructs, Revision hip arthroplasty
1. Introduction
Total hip arthroplasty (THA) is perhaps the most recognized operation in the field of orthopedic surgery and regarded as a benchmark treatment of end-stage hip joint disease. The aging population and growing incidence in obesity will continue to increase the number of hip replacements. Despite excellent clinical results, many patients outlive the typical lifespan of implants with approximately 17% of all primary hips eventually failing and requiring revision.1 Acetabular revision in the context of poor bone stock is a technically challenging procedure; therefore, it is imperative for the arthroplasty surgeon to understand the advantages and disadvantages of the available acetabular component systems. In this paper, we review clinical indications for acetabular revision, radiographic classification systems, and pre-operative planning. We also include a summary of available acetabular component systems and highlight unique features.
2. Clinical evaluation
Clinical presentation depends on the fundamental etiology for acetabular implant failure, which include aseptic loosening, infection, instability, wear, trauma, and osteolysis.2 Groin or buttock pain is a characteristic patient complaint associated with acetabular implant failure while thigh pain is often associated with femoral implant failure.2, 3 A comprehensive medical history and focused physical exam should be performed on all patients regardless of clinical presentation. Laboratory studies including complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), nuclear scans, and aspiration arthrogram with culture and sensitivity are recommended when underlying infection is suspected.4 Pre-operative templating requires an upright weight-bearing anteroposterior (AP) pelvis, femoral lateral, and full-length views to evaluate the extent of disease. Judet views can help detect acetabular column disease. Furthermore, 3D pelvis CT scans provide ancillary evidence of osteolytic lesions as plain films often underestimate the degree of bone loss. Multiple studies report that CT scan is better than plain film at identifying peri-acetabular osteolytic lesions.5, 6, 7, 8 Frail cortical bone is often difficult to distinguish from bone cement in poor quality films. Finally, CT angiogram of pelvis vasculature and possible vascular surgery consultation may be necessary when Kohler's (ilio-ischial) line is interrupted and the acetabular component is markedly displaced.
3. Classification of acetabular defects
An ideal radiological classification system provides accurate and standardized algorithm to evaluate the extent of bone loss, assist in pre-operative planning and clinical management. Acetabular defects are routinely described using the American Academy of Orthopaedic Surgeons (AAOS) and Paprosky classification system.
The AAOS classification system categorizes lesions into cavitary, segmental, combined cavitary and segmental, pelvic discontinuities, and arthrodesis.9 Cavitary defects are localized within the acetabular cavity and do not involve the anterior, superior, and posterior rim. Cavitary defects include any kind of dome lesion. Segmental defects occur along the acetabular rim and include the medial wall. Segmental defects are subdivided into anterior or posterior. Pelvic discontinuity occurs when anterior and posterior columns separate and disrupt the rostral hemipelvis from the distal aspect.10 It constitutes less than 5% of all acetabular revisions and requires careful planning.10
The Paprosky classification system uses pre-operative imaging and intraoperative assessment to describe acetabular defects.11, 12 Using this classification system, acetabular defects are graded from Type I to Type III based on location and extent of bone loss (Fig. 1).
Fig. 1.
Technical goals in acetabular revision are to reconstitute bone stock, restore anatomic hip center of rotation, limb-length, offset, and secure the prosthesis to the native acetabular socket.13 These steps are necessary to reduce risk of post-operative dislocations, increase wear time, and avoid particle-induced osteolysis that may permanently alter hip biomechanics.13 A number of options are available to help the arthroplasty surgeon achieve sufficient acetabular bony contact and return hip center to normal anatomic position, including the use of bone cages, allografts, jumbo and oblong cups, triflange implants, and porous acetabular metal augments. The decision to proceed depends on the localization and extent of disease, patient anatomy, and experience of the arthroplasty surgeon.
4. Cavitary defects
Cementless hemispheric acetabular components are generally used for patients with cavitary defects. Small cavitary defects can be reamed with a larger size reamer to increase contact area between native bone and implant. The acetabular shell is then impacted into the socket and transacetabular screws are placed in the posterior quadrants to provide ancillary fixation to the ileum and ischium. Anterosuperior and anteroinferior placements of screws increase the risk of injury to external iliac and obturator vessels respectively and should be done with care.
4.1. Morselized bone grafts with a cementless acetabular cup
Over-reaming large cavitary defects may cause further damage to pre-existing bone along the acetabular rim and should be supplemented with morselized bone grafts.
Cementless hemispheric acetabular components with morselized cancellous bone allografts are generally used in the setting of type 1 Paprosky contained defects with an intact rim, columns, and dome.14, 15 The literature recommends that at least 50% host bone contact is needed to prevent mechanical loosening between the prosthesis and native bone.1, 2, 14 Femoral head, distal femur, and acetabular allografts can be used to fill in the gaps. The operating surgeon may consider autogenic graft as they are less immunogenic, but difficult to harvest in some patients with pathological bone disease.14, 15 Intraoperatively, the arthroplasty surgeon uses a bone mill or rangeur to generate small chunks of bone that are impacted with a smooth acetabular impaction domes. Reverse reaming technique can be used alternatively to impact bone into the acetabular socket.15 Subsequently, the cup is pressfit and secured to the pelvis with multiple screws for ancillary fixation.
5. Segmental defects
Under circumstances of substantial superior segmental defects, the arthroplasty surgeon may consider placing the joint in a superiorly elevated position using a cementless hemispherical cup as described previously or restore hip center with supplementation of bulk allograft or using a larger size cup.
5.1. Jumbo and oblong cups
Cup parameters may be altered to augment fixation when standard hemispheric cups are incapable of providing necessary fixation in patients presenting with segmental and combined defects.
A jumbo cup increases contact area to provide appropriate biologic fixation and can be combined with a large femoral head to increase stability in the post-operative setting. Gustke et al. reported low rates of aseptic loosening (1.5%), 98% implant survival at 4 years and 96% at 16 years using a jumbo cup in a series of 196 cases.16 Paprosky Type III hips with moderate to severe teardrop, superolateral acetabulum, and posterior column deficiencies should be reconstructed using a device that provides better fixation than a jumbo cup, like a acetabular augments or cup-cage construct.
An oblong cup is designed to have a longer diameter in the longitudinal plane relative to the transverse plane.
5.2. Bone cages with allografts
Bone cages are generally indicated in Type III defects with moderate superior hip center displacement, disruption of the teardrop, and Kohler's line. The cage is constructed from titanium alloy and thought to transfer load off the acetabular socket and onto the peri-acetabular bone through adjustable illeal and ischial flanges that provide intimate contact with native bone when secured to the pelvis using cancellous screws.10, 17 Cages protect underlying bone graft from lysis and permit remodeling with native bone. True survivorship of cages is unclear but has ranged from less than 70% to 100% according to Sembrano et al. who report that the end-points of each study varied depending on time of cage removal, evidence of radiographic loosening, and reason for revision.17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Regis et al. reported an 87% (49/56) survival rate at a mean follow-up of 11.7 years in type IIIA (32%) and IIIB (68%) hips using cages and structural allografts using radiographic evidence of bone ingrowth and remodeling as the primary endpoint.28 The major disadvantage of cages includes high complication rates previously reported by multiple authors.14, 29, 30, 31, 32 There is a higher risk of neurovascular injury since large exposure of the ileum and ischium is needed to anchor the cage.29, 30, 31 In addition, cages generally fail to incorporate native bone in the long run because the non-porous surface makes ingrowth problematic.17 There is a stronger association with fatigue failure when cages are used in patients with pelvic discontinuity.14, 32
5.3. Reinforcement rings with allografts
Reinforcement rings are designed to manage contained cavitary and minor segmental defects with more than 50% of native bone intact. Like cages, rings protect allograft from biomechanical lysis. Rings contain pliable flanges that can be adjusted intraoperatively to increase contact area with native bone Flanges are secured to the ileum and ischium with cancellous screws to relieve stress forces placed on incompetent native bone.10 Still, biological fixation is a concern since rings are made from a biologically incompatible material.10, 33 Failure rates as high as 25% have been reported in the literature.33
5.4. Trabecular metal cups and augments
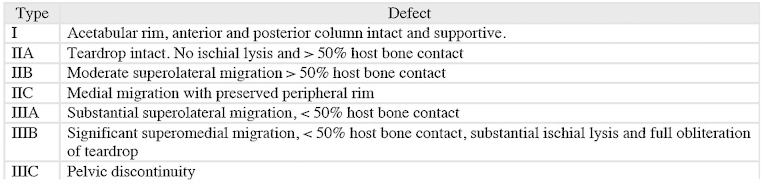
Porous trabecular metal (Zimmer, Warsaw, IN) components made from tantalum provide excellent biological fixation in Type II and III acetabular defects with moderate to severe superior hip center migration and teardrop lysis.29 Trabecular metal (TM) components are approximately 70–80% porous and high shear strength profile reduces micromotion between native bone and prosthesis. Tantalum is like cancellous bone with a previously reported modulus of elasticity between 2.5 and 3.9 MPa to prevent stress shielding.29, 34, 35, 36 Mid-term studies have so far yielded good results using tantalum augments in acetabular reconstruction. Weeden and Schmidt reported 98% success rate using radiograph failure as the primary endpoint with trabecular metal augments and notably only 1 case (4%) of aseptic loosening in 26 hips at the minimum follow-up of 2 years (range, 2–4 years).36 Del Gaizo et al. reported 97.3% survival rate with aseptic loosening as the endpoint in 37 Paprosky Type IIIA hips with a mean follow-up of 60 months (range, 26–106 months).37 Longer follow-up studies are needed to capture long-term survival and loosening rates. The potential to produce metal debris may warrant further studies to analyze the effect of tantalum on surrounding tissues (Fig. 2, Fig. 3).
Fig. 2.
Pre and postoperative imaging showing acetabular reconstruction using porous metal augmentation in a Paprosky Type IIIA hip. The patient is 72-year-old male with history of avascular necrosis of the right hip.
Fig. 3.
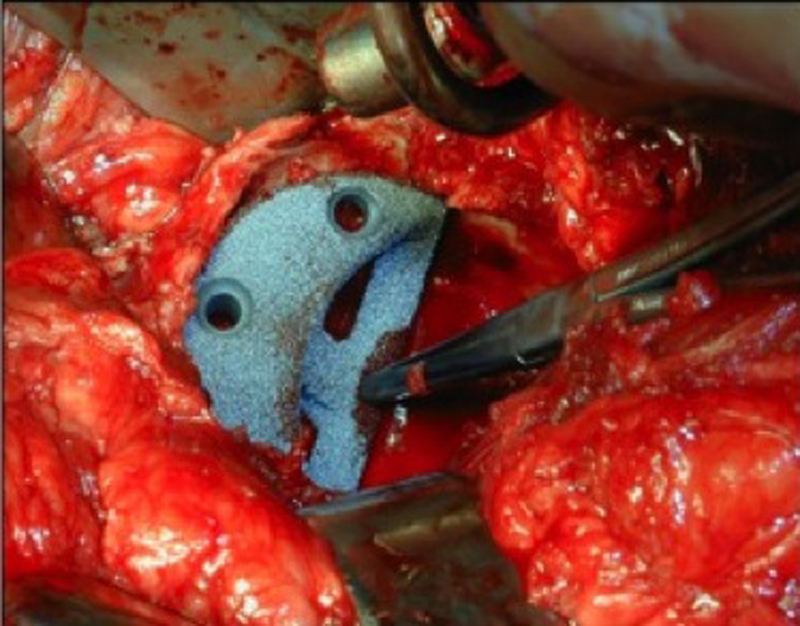
Intraoperative view of a porous trabecular augment used in acetabular reconstruction.
6. Pelvic discontinuity
6.1. Custom triflange implants
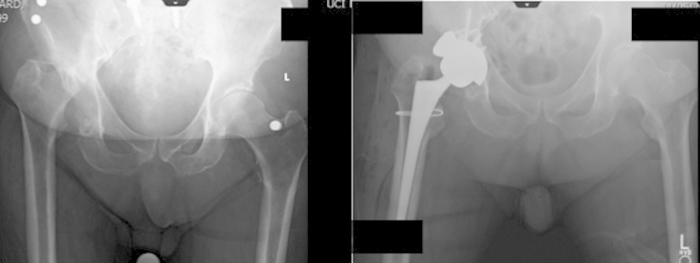
Triflange implants are custom-made, porous coated titanium alloy components considered a final therapeutic salvage option in patients with pelvic discontinuity and/or prior radiation to pelvis. A triflange construct is designed from pelvis CT scans with metal subtraction software converted into a three-dimensional (3D) representation of the patient's hemipelvis. The implant manufacture generates individualized implants from the respective imaging (Fig. 4). Some authors have reported variable results with triflange components. DeBoer et al. reported no cases (0/20) requiring revision and average Harris Hip Score (HHS) of 80 at 10 years post-op.38 Taunton et al. reported a revision rate of 30% (20/57) at 5.4 years and 21% dislocation rate most likely attributable to instability generated from pre-operative trochanteric escape performed in 51% of patients and possible traction injury to the superior gluteal nerve during exposure.31 When comparing manufacture costs, triflange components are priced similar to other constructs used to treat pelvic discontinuity, including the “cup-cage” construct, a trabecular metal cup with an antiprotrusio cage (Zimmer, Inc, Warsaw, IN, USA). The typical cost of the triflange construct (cup, screws, and polyethylene liner) is $12,500 and cup-cage construct is $11,250.31 The major drawback of the triflange construct is it may take several months to prepare the implant for surgery.31, 38
Fig. 4.
Postoperative radiograph demonstrating the use of a patient specific custom-made triflanged acetabular component in a 74-year-old patient with previous history of multiple hip arthroplasties.
6.2. Cup-cage construct
The cup-cage construct is an alternative option to correct large acetabular defects and pelvic discontinuity. Using this technique, a porous metal cup is secured to host bone and allograft if used; acetabular distraction technique can be used in cases of pelvic discontinuity (see Section 6.3). A bone cage is subsequently anchored to the porous cup (Zimmer, Inc., Warsaw, IN, USA). The rational behind is the cage removes loading forces on the cup and allows time to optimize ingrowth of new bone into the cup.39 Cup-cage reconstruction has yielded encouraging short-term outcomes, including one study that demonstrated no clinical or radiological evidence of loosening in 23 out of 26 (88.5%) hips with an average follow-up 44.6 months (range 24–68).40 Despite the main concern that cages having a tendency to undergo fatigue failure, combining a biologically compatible porous cup with the cage may counteract the high failure rates associated with using just cages, longer-term data is needed to validate the longevity of this construct39, 40 (Fig. 5).
Fig. 5.
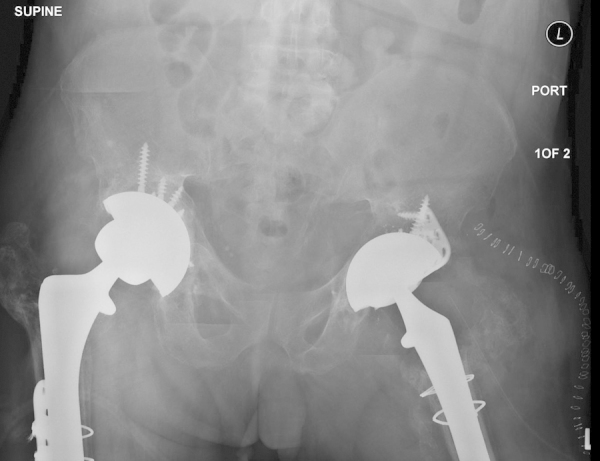
Postoperative radiograph showing a well-seated cup-cage construct.
6.3. Acetabular distraction with porous cup
The acetabular distraction technique is another novel approach for managing pelvic discontinuities. While the techniques described above utilize screws and plates to compress nonunion and achieve mechanical fixation, the underlying basis of acetabular distraction is the address nonunion of fracture lines using distraction to expand the defect and create elastic recoil forces to compress the porous metal construct.41 Intraoperatively, a Cobb elevator is used to delineate the fracture line and debridement of the granulation tissue and reaming is performed to define bone suitable for fixation using augmentation.41 Sporer et al. reported good mid-term results using acetabular distraction including one case (1/20) requiring revision for aseptic loosening at 9 months.42 At 4 years follow-up, four hips demonstrated migration of the acetabular component but were clinically stable. The acetabular distraction technique is a reasonable option for many patients but the long-term data is limited in the regard.
7. Conclusion
In summary, many constructs are available to achieve sufficient acetabular bony contact and return hip center to normal anatomic position; including the use of bone cages, allografts, jumbo and oblong cups, triflange implants, and porous acetabular metal augments. It is important to understand the advantages and disadvantages of the available acetabular component systems to deal with poor bone stock.
Conflicts of interest
The authors have none to declare.
References
- 1.Choplin R.H., Henley C.N., Edds E.M., Capello W., Rankin J.L., Buckwalter K.A. Total hip arthroplasty in patients with bone deficiency of the acetabulum. Radiographics. 2008;28:771–786. doi: 10.1148/rg.283075085. [DOI] [PubMed] [Google Scholar]
- 2.Taylor E.D., Browne J.A. Reconstruction options for acetabular revision. World J Orthop. 2012;3:95–100. doi: 10.5312/wjo.v3.i7.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid C., Grobler G.P., Dower B.J., Nortje M.B., Walters J. Revision total hip arthroplasty: addressing acetabular bone loss. SA Orthop J. 2012;11:34–46. [Google Scholar]
- 4.Leiberman J.R. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2009. American Academy of Orthopaedic Surgeons Comprehensive Orthopaedic Review. [Google Scholar]
- 5.Claus A.M., Totterman S.M., Sychterz C.J. Computed tomography to assess pelvic lysis after total hip replacement. Clin Orthop Relat Res. 2004:167. doi: 10.1097/01.blo.0000129345.22322.8a. [DOI] [PubMed] [Google Scholar]
- 6.Johanson N.A., Driftmier K.R., Cerynik D.L., Stehman C.C. Grading acetabular defects: the need for a universal and valid system. J Arthroplasty. 2010;25:425–431. doi: 10.1016/j.arth.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Leung S., Naudie D., Kitamura N. Computed tomography in the assessment of periacetabular osteolysis. J Bone Joint Surg Am. 2005;87:592–597. doi: 10.2106/JBJS.D.02116. [DOI] [PubMed] [Google Scholar]
- 8.Sandgren B., Crafoord J., Garellick G., Carlsson L., Weidenheilm Olivecrona H. Computed tomography vs digital radiography assessment for detection of osteolysis in asymptomatic patients with uncemented cups: a proposal of a new classification system based on computed tomography. J Arthroplasty. 2013;28:1608–1613. doi: 10.1016/j.arth.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 9.D’Antonio J.A., Capello W.N., Borden L.S. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1989;243:126–137. [PubMed] [Google Scholar]
- 10.Villanueva M., Rious-Luna A., Pereiro De Lamo J., Fahandez-Saddi H., Bostrom M.P.G. A review of the treatment of pelvic discontinuity. HSS J. 2008;4:128–137. doi: 10.1007/s11420-008-9075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paprosky W.G., Perona P.G., Lawrence J.M. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty. 1994:33. doi: 10.1016/0883-5403(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 12.Bradford M.S., Paprosky W.G. Acetabular defect classification: a detailed radiographic approach. Semin Arthroplasty. 1995;6:76. [PubMed] [Google Scholar]
- 13.Haddad F.S., Shergill N., Muirhead-Altwood S.K. Acetabular reconstruction with morcelized allograft and ring support: a medium-term review. J Arthroplasty. 1999;14:788–795. doi: 10.1016/s0883-5403(99)90026-8. [DOI] [PubMed] [Google Scholar]
- 14.Burns A.W.R., McCalden R.W. Current techniques and new developments in acetabular revision surgery. Curr Orthop. 2006;20:162–170. [Google Scholar]
- 15.Peng K.T., Hsu W.H., Shih H.N., Chen C.C., Yeh J.H. Revision total hip arthroplasty for large medial defects with witch's hat-shaped structural allografts-minimum 10-year follow-up. J Arthroplasty. 2014;29:428–431. doi: 10.1016/j.arth.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Gustke K.A., Levering M.F., Miranda M.A. Use of jumbo cups for revision of acetabulae with large bony defects. J Arthroplasty. 2014;29:199–203. doi: 10.1016/j.arth.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Sembrano J.M., Cheng E.Y. Acetabular cage survival and analysis of factors related to failure. Clin Orthop Relat Res. 2008;466:1657–1665. doi: 10.1007/s11999-008-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry D.J., Müller M.E. Revision arthroplasty using an anti-protrusio cage for massive acetabular bone deficiency. J Bone Joint Surg Br. 1992;74:711–715. doi: 10.1302/0301-620X.74B5.1527119. [DOI] [PubMed] [Google Scholar]
- 19.Böhm P., Banzhaf S. Acetabular revision with allograft bone: 103 revisions with 3 reconstruction alternatives, followed for 0.3–13 years. Acta Orthop Scand. 1999;70:240–249. doi: 10.3109/17453679908997800. [DOI] [PubMed] [Google Scholar]
- 20.Goodman S., Saastamoinen H., Shasha N., Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19:436–446. doi: 10.1016/j.arth.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Paprosky W., Sporer S., O’Rourke M.R. The treatment of pelvic discontinuity with acetabular cages. Clin Orthop Relat Res. 2006;453:183–187. doi: 10.1097/01.blo.0000246530.52253.7b. [DOI] [PubMed] [Google Scholar]
- 22.Perka C., Ludwig R. Reconstruction of segmental defects during revision procedures of the acetabulum with the Burch-Schneider anti-protrusio cage. J Arthroplasty. 2001;16:568–574. doi: 10.1054/arth.2001.23919. [DOI] [PubMed] [Google Scholar]
- 23.Pieringer H., Auersperg V., Bohler N. Reconstruction of severe acetabular bone-deficiency: the Burch-Schneider antiprotrusio cage in primary and revision total hip arthroplasty. J Arthroplasty. 2006;21:489–496. doi: 10.1016/j.arth.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Rosson J., Schatzker J. The use of reinforcement rings to reconstruct deficient acetabula. J Bone Joint Surg Br. 1992;74:716–720. doi: 10.1302/0301-620X.74B5.1527120. [DOI] [PubMed] [Google Scholar]
- 25.Van Koeveringe A.J., Ochsner P.E. Revision cup arthroplasty using Burch-Schneider anti-protrusio cage. Int Orthop. 2002;26:291–295. doi: 10.1007/s00264-002-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wachtl S.W., Jung M., Jakob R.P., Gautier E. The Burch-Schneider antiprotrusio cage in acetabular revision surgery: a mean follow-up of 12 years. J Arthroplasty. 2000;15:959–963. doi: 10.1054/arth.2000.17942. [DOI] [PubMed] [Google Scholar]
- 27.Winter E., Piert M., Volkmann R., Maurer F., Eingartner C., Weise K., Weller S. Allogeneic cancellous bone graft and a Burch-Schneider ring for acetabular reconstruction in revision hip arthroplasty. J Bone Joint Surg Am. 2001;83:862–867. doi: 10.2106/00004623-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Regis D., Magnan B., Sandri A., Bartolozzi P. Long-term results of anti-protrusio cage and massive allografts for the management of peri-prosthetic acetabular bone loss. J Arthroplasty. 2008;23:826–832. doi: 10.1016/j.arth.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Van Kleunen J.P., Lee G., Lementowsky P.W., Nelson C.L., Garino J.P. Acetabular revisions using trabecular metal cups and augments. J Arthroplasty. 2009;24:64–68. doi: 10.1016/j.arth.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Lackstein D., Backstein D., Safir O., Koshashvili Y., Gross A.E. Trabecular metal cups for acetabular defects with 50% or less host bone contact. Clin Orthop Relat Res. 2009;467:2318–2324. doi: 10.1007/s11999-009-0772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taunton M.J., Fehring T.K., Edwards P., Bernasek T., Holt G.E., Christie M.J. Pelvic discontinuity treated with custom triflange component: a reliable option. Clin Orthop Relat Res. 2012;470:428–434. doi: 10.1007/s11999-011-2126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters C.L., Miller M., Erickson J. Acetabular revision with a modular anti-protrusio acetabular component. J Arthroplasty. 2004;19:67–72. doi: 10.1016/j.arth.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 34.Bobyn J.D., Hacking S.A., Chan S.P. Characterization of new porous tantalum biomaterial for reconstructive orthopaedics. Scientific Exhibition: 66th Annual Meeting of the American Academy of Orthopaedic Surgeons; Anaheim, CA; 1999. [Google Scholar]
- 35.Hanslik J.A., Day J.S. Bone ingrowth in well-fixed retrieved porous tantalum implants. J Arthroplasty. 2013;28:922–927. doi: 10.1016/j.arth.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weeden S.H., Schmidt R.H. The use of tantalum porous metal implants for Paprosky 3A and B defects. J Arthroplasty. 2007;22:151. [Google Scholar]
- 37.Del Gaizo D.J., Kancherla V., Sporer S.M., Paprosky W.G. Tantalum augments for Paprosky type IIIa defects remain stable at midterm follow-up. Clin Orthop Relat Res. 2012;470:395–401. doi: 10.1007/s11999-011-2170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeBoer D.K., Christie M.J., Brinson M.F., Morrison J.C. Revision total hip arthroplasty for pelvic discontinuity. J Bone Joint Surg Am. 2007;89:835–840. doi: 10.2106/JBJS.F.00313. [DOI] [PubMed] [Google Scholar]
- 39.Abolghasemian M., Tangsataporn T., Kuzyk P.R.T., Safir O.A., Backstein D.J., Gross A.E. Cup-cage solution for pelvic discontinuity. Semin Arthroplasty. 2012;23:171–175. [Google Scholar]
- 40.Kosashvili Y., Backstein D., Safir O. Acetabular revision using an anti-protrusion (ilio-ischial) cage and trabecular metal acetabular component for severe acetabular bone loss associated with pelvic discontinuity. J Bone Joint Surg Br. 2009;91:870–876. doi: 10.1302/0301-620X.91B7.22181. [DOI] [PubMed] [Google Scholar]
- 41.Shah R.P., Christy J.M., Sporer S.M., Paprosky W.G. Pelvic discontinuity: where are we today? Semin Arthroplasty. 2014;25:156–158. [Google Scholar]
- 42.Sporer S.M., Bottros J.J., Hulst J.B., Kancherla V.K., Moric M., Paprosky W.G. Acetabular distraction: an alternative for severe defects with chronic pelvic discontinuity? Clin Orthop Rel Res. 2012;470:3156–3163. doi: 10.1007/s11999-012-2514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]