Data from the present study reveal that preventing the reduction of shear stress during prolonged sitting with local heating abolishes the impairment in popliteal artery endothelial function. Therefore, this study provides evidence that a reduction in shear stress mediates sitting-induced leg endothelial dysfunction.
Keywords: physical inactivity, blood flow, endothelial function
Abstract
We and others have recently reported that prolonged sitting impairs endothelial function in the leg vasculature; however, the mechanism(s) remain unknown. Herein, we tested the hypothesis that a sustained reduction in flow-induced shear stress is the underlying mechanism by which sitting induces leg endothelial dysfunction. Specifically, we examined whether preventing the reduction in shear stress during sitting would abolish the detrimental effects of sitting on popliteal artery endothelial function. In 10 young healthy men, bilateral measurements of popliteal artery flow-mediated dilation were performed before and after a 3-h sitting period during which one foot was submerged in 42°C water (i.e., heated) to increase blood flow and thus shear stress, whereas the contralateral leg remained dry and served as internal control (i.e., nonheated). During sitting, popliteal artery mean shear rate was reduced in the nonheated leg (pre-sit, 42.9 ± 4.5 s−1; and 3-h sit, 23.6 ± 3.3 s−1; P < 0.05) but not in the heated leg (pre-sit, 38.9 ± 3.4 s−1; and 3-h sit, 63.9 ± 16.9 s−1; P > 0.05). Popliteal artery flow-mediated dilation was impaired after 3 h of sitting in the nonheated leg (pre-sit, 7.1 ± 1.4% vs. post-sit, 2.8 ± 0.9%; P < 0.05) but not in the heated leg (pre-sit: 7.3 ± 1.5% vs. post-sit, 10.9 ± 1.8%; P > 0.05). Collectively, these data suggest that preventing the reduction of flow-induced shear stress during prolonged sitting with local heating abolishes the impairment in popliteal artery endothelial function. Thus these findings are consistent with the hypothesis that sitting-induced leg endothelial dysfunction is mediated by a reduction in shear stress.
NEW & NOTEWORTHY
Data from the present study reveal that preventing the reduction of shear stress during prolonged sitting with local heating abolishes the impairment in popliteal artery endothelial function. Therefore, this study provides evidence that a reduction in shear stress mediates sitting-induced leg endothelial dysfunction.
the augmented propensity to atherosclerosis in the vasculature of the lower extremities, relative to the arms, is well recognized (1, 15, 16, 30, 32); however, the factors contributing to this heterogeneous distribution of vascular disease remain to be delineated. We (28) and others (17, 36) have recently shown that prolonged sitting impairs endothelial function in the leg vasculature, including the femoral and popliteal arteries. This is noteworthy in that endothelial dysfunction is implicated as a central feature of the initiation and progression of atherosclerotic lesions (26, 29, 38). Given the high prevalence of sedentary behavior in modern cultures and that sitting time is an independent risk factor for cardiovascular disease (4, 5, 8, 13, 19), it seems plausible that the increased susceptibility of the leg vasculature to atherosclerosis may be related to the effects of prolonged sitting on the vascular endothelium of lower limbs.
The mechanism(s) by which sitting impairs endothelial function in the legs are unknown. In our previous sitting study, we (28) showed that popliteal artery blood flow and shear rate were markedly reduced during sitting, similar to findings obtained by Thosar et al. (36) in the superficial femoral artery. Because flow-induced shear stress is an important physiological signal for maintaining endothelial health, it is reasonable to propose that sustained reductions of shear stress during sitting mediate leg endothelial dysfunction; however, this hypothesis has not been tested.
Accordingly, herein we examined whether preventing the reduction in leg blood flow and shear stress during sitting would abolish the detrimental effects of sitting on popliteal artery endothelial function. Bilateral measurements of popliteal artery flow-mediated dilation (FMD) were performed before and after a 3-h sitting period during which one foot was submerged in 42°C water (i.e., heated) to increase blood flow and thus shear stress, whereas the contralateral leg remained dry and served as an internal control (i.e., nonheated). It has been previously shown that local heating at 42°C is an effective stimulus for dilating the skin circulation and increasing limb vascular conductance and thus shear stress without producing major systemic cardiovascular effects (21, 23–25). We hypothesized that sitting-induced endothelial dysfunction would occur in the nonheated control leg but prevented by offsetting the reduction in leg blood flow and shear stress with local heating in the heated leg.
METHODS
Ten young healthy men recruited from the University of Missouri campus and surrounding Columbia, MO, area participated in this study (age, 26 ± 1 yr; height, 178.1 ± 3.3 cm; weight, 85.7 ± 6.1 kg; and body mass index, 26.8 ± 1.3 kg/m2). All experimental procedures and measurements conformed to the Declaration of Helsinki and were approved by the University of Missouri Health Sciences Institutional Review Board. Before participating in the study, each subject provided written informed consent. Subjects were recreationally active, nonsmokers, with no history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease as determined from a detailed medical health history questionnaire. No subjects were using prescribed or over-the-counter medications.
Experimental procedures.
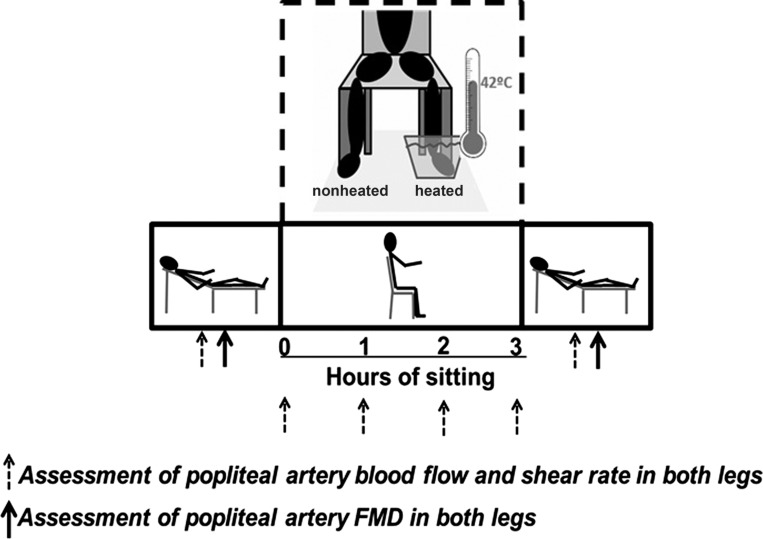
A schematic of the study design is presented in Fig. 1 illustrating the sequence of events and various positions in which measurements were made. Subjects were 2-h postprandial upon arrival to the laboratory. All study visits were performed in a temperature-controlled room kept at 21–22°C. Upon arrival to the laboratory, subjects were placed in a supine position and instrumented with an automated sphygmomanometer (SphygmoCor XCEL, AtCor Medical, Itasca, IL) for periodic measurements of arterial blood pressure (BP) after resting quietly for 10 min. Popliteal artery diameter and blood velocity were measured using duplex-Doppler ultrasound (Logiq P5; GE Medical Systems, Milwaukee, WI). An 11-MHz linear array transducer was placed over the popliteal artery just distal to the popliteal fossa. Simultaneous diameter and velocity signals were obtained in duplex mode at a pulsed frequency of 5 MHz and corrected with an insonation angle of 60°. Sample volume was adjusted to encompass the entire lumen of the vessel without extending beyond the walls, and the cursor was set at midvessel. Popliteal artery FMD was assessed in both legs as previously described (2, 28). Briefly, a rapid inflating cuff was placed on the lower leg ∼5 cm distal to the fibular head. Two minutes of baseline hemodynamics were recorded, and the cuff was then inflated to a pressure of 220 mmHg for 5 min. Continuous diameter and blood velocity measures were recorded for 30 s before and 3 min following cuff deflation. Recordings of all vascular variables were analyzed off-line using specialized edge-detection software (Cardiovascular Suite, Quipu srl, Pisa, Italy).
Fig. 1.
Schematic diagram of experimental protocol and positional changes over the course of study. Measurements taken at the time points of 0, 1, 2, and 3 h were made while the subject was in the seated position, whereas pre-sit and post-sit measurements were taken while the subject was in the supine position. FMD, flow-mediated dilation.
Following the initial pre-sit measures, subjects were moved into a seated position for 3 h. Subjects were instructed to refrain from any leg movement during the sitting period, and a study representative monitored the subject during the entire sitting period to ensure that no leg movement occurred. The experimental conditions of the legs comprised of one foot being submerged up to the ankle in a commercially available foot spa (Kendal Foot Spa, New Shining Image, Middletown, NY) with temperature of the water maintained at 42°C (heated), whereas the contralateral foot remained in open air (nonheated). It has been previously shown that local heating at 42°C is an effective stimulus for dilating the skin circulation and increasing limb vascular conductance and thus flow-induced shear stress without producing major systemic cardiovascular effects (21, 23–25). Both feet were equally supported over the course of the sitting period, and study personnel closely monitored the temperature of the water. Right and left legs were randomly assigned the condition of nonheated or heated. During the course of the sitting period, popliteal artery blood flow measurements were taken within the first 10 min upon sitting (0 h) as well as at 1, 2, and 3 h on both the nonheated and heated legs (Fig. 1). Following the 3-h sitting period, subjects were manually lifted and placed back into the supine position to avoid any muscle activity of the legs. FMD assessments were repeated, and the order of assessments was randomized between nonheated and heated leg within each subject.
Data analysis.
Mean blood flow was calculated from continuous diameter and mean blood velocity recordings at each of the experimental time points using the following equation: 3.14·(diameter/2)2·mean blood velocity·60. Hyperemic blood flow area under the curve (AUC) was calculated for the entire period in which blood flow was above baseline values using the sum of trapezoids method (20). Popliteal artery FMD percent change was calculated using the following equation: %FMD = (peak diameter − base diameter)/(base diameter)·100. Shear rate was defined as 8·mean blood velocity/diameter (22). Hyperemic shear rate AUC up to peak diameter was calculated as stimulus for FMD, as previously described (2, 33).
Statistical analysis.
A two-way (time × leg), repeated-measures ANOVA with Tukey post hoc testing was performed on all dependent variables. Values for FMD are presented as percent change and corrected for hyperemic shear-rate AUC. FMD was adjusted for hyperemic shear-rate AUC via ANCOVA to statistically control for the influence of shear stimulus on FMD response. ANOVA tests were completed using SigmaStat software (version 12.2), and ANCOVA test was performed using SPSS software (version 23). Significance was accepted at P ≤ 0.05. Data are expressed as means ± SE. Based on data from our previous sitting study (28), we performed a power calculation and determined that eight subjects would be needed to detect a statistically significant (P < 0.05) effect of sitting on leg endothelial function with a power of 0.8.
RESULTS
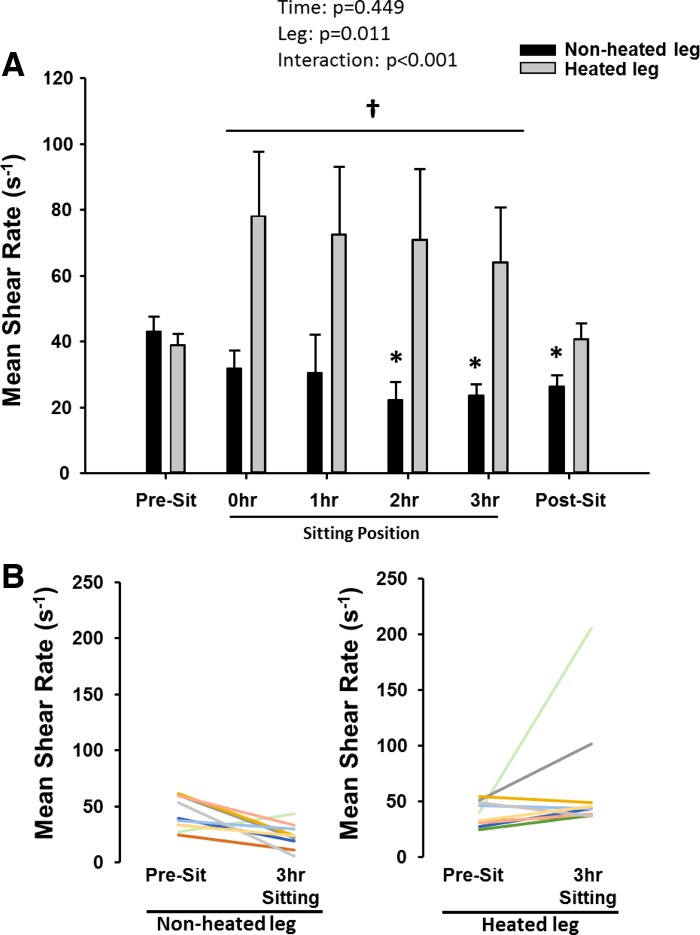
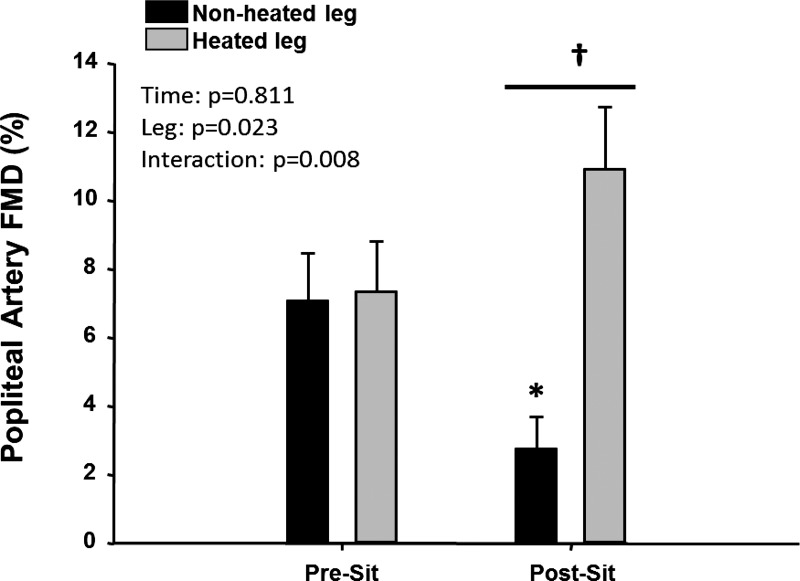
Over the course of the sitting period, popliteal artery blood flow (Table 1) and shear rate (Fig. 2A) were progressively reduced under the nonheated control condition (P < 0.05). In contrast, blood flow and shear rate in the heated leg were not reduced during sitting and were higher than in the nonheated leg (Fig. 2A, P < 0.05). These results indicate the effectiveness of limb heating in preventing the decline in blood flow and shear rate during the sitting period. As illustrated in Fig. 2B, several subjects actually exhibited an increase in blood flow and shear rate during sitting in the heated leg with the magnitude of this increase being variable among subjects. Nonetheless, the impairment in popliteal artery FMD after 3 h of sitting in the nonheated leg (7.1 ± 1.4 to 2.8 ± 0.9%; Cohen's d = 1.16; P < 0.05) was prevented in the heated leg (7.3 ± 1.5 to 10.9 ± 1.8%; Cohen's d = 0.68; P > 0.05; Fig. 3). Hyperemic shear rate AUC and blood flow AUC were similar between legs before sitting and reduced in both legs after sitting (Table 1). FMD corrected for hyperemic shear rate AUC using ANCOVA did not affect the interpretation of the main findings (Table 1). No changes were observed in popliteal artery diameter over time, and no differences between legs were detected across time points (Table 1). BP was unaffected by the period of sitting (pre-sit: systolic BP = 123.6 ± 2.8 mmHg, diastolic BP = 76.4 ± 3.6 mmHg, and mean arterial pressure = 92.1 ± 3.1 mmHg; and post-sit: systolic BP = 125.6 ± 2.7 mmHg, diastolic BP = 75.8 ± 2.5 mmHg, and mean arterial pressure = 92.4 ± 2.4 mmHg).
Table 1.
Popliteal artery hemodynamics in nonheated and heated legs before, during, and after sitting for 3 h
| Basal Diameter, cm |
Basal Blood Flow, ml/min |
Hyperemic Blood Flow AUC, arbitrary units |
Hyperemic Shear Rate AUC, arbitrary units |
ANCOVA-Corrected FMD, % |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonheated | Heated | Nonheated | Heated | Nonheated | Heated | Nonheated | Heated | Nonheated | Heated | ||
| Supine | Pre-sit | 0.59 ± 0.03 | 0.59 ± 0.03 | 58.9 ± 11.2 | 47.1 ± 5.0 | 45,990 ± 8,986 | 42,552 ± 6,725 | 34,259 ± 6,155 | 30,640 ± 4,018 | 6.9 ± 1.5 | 7.2 ± 1.5 |
| Sitting | 0 h | 0.58 ± 0.02 | 0.57 ± 0.02 | 36.2 ± 5.5*† | 79.4 ± 13.7 | ||||||
| 1 h | 0.59 ± 0.02 | 0.57 ± 0.02 | 32.3 ± 6.7*† | 79.5 ± 16.3 | |||||||
| 2 h | 0.57 ± 0.02 | 0.57 ± 0.02 | 23.8 ± 3.7*† | 72.8 ± 15.5 | |||||||
| 3 h | 0.57 ± 0.02 | 0.56 ± 0.02 | 25.1 ± 3.3*† | 64.7 ± 12.4 | |||||||
| Supine | Post-sit | 0.58 ± 0.02 | 0.59 ± 0.03 | 30.7 ± 4.7*† | 56.2 ± 11.8 | 20,715 ± 5,171* | 28,749 ± 8,253 | 18,281 ± 3,846* | 16,807 ± 4,531* | 2.9 ± 1.5† | 11.0 ± 1.5 |
| ANOVA | Time, P = 0.598 | Time, P = 0.474 | Time, P = 0.002 | Time, P < 0.001 | Time, P = 0.944 | ||||||
| Leg, P = 0.648 | Leg, P = 0.003 | Leg, P = 0.664 | Leg, P = 0.485 | Leg, P = 0.006 | |||||||
| Interaction, P = 0.526 | Interaction, P < 0.001 | Interaction, P = 0.399 | Interaction, P = 0.798 | Interaction, P = 0.011 | |||||||
Values are means ± SE. ANCOVA-corrected flow-mediated dilation (FMD) data are adjusted for hyperemic shear rate area under the curve (AUC).
P < 0.05, vs. pre-sit;
P < 0.05, between legs.
Fig. 2.
A: popliteal artery mean shear rate in nonheated and heated legs before, during, and after sitting for 3 h. B: mean shear rate individual subject responses from pre-sit to 3 h of sitting for both nonheated and heated legs. Data are expressed as means ± SE; *P < 0.05 vs. pre-sit; †P < 0.05, between legs.
Fig. 3.
Popliteal artery %FMD in nonheated and heated legs before and after sitting for 3 h. %FMD data are unadjusted for hyperemic shear rate area under the curve. Data are expressed as means ± SE; *P < 0.05 vs. pre-sit; †P < 0.05, between legs.
DISCUSSION
The major novel finding of the present study is that leg endothelial dysfunction caused by prolonged sitting is mediated by a reduction in shear stress. Indeed, we found that preventing the reduction of flow-induced shear stress during sitting with local heating abolishes the impairment in popliteal artery endothelial function. This finding supports the hypothesis that reduced leg vascular shear stress is an underlying physiological mechanism by which prolonged sitting causes endothelial dysfunction in the lower limbs.
The finding that prolonged sitting impairs endothelial function in the lower limbs might be of significance in light of evidence demonstrating that the leg vasculature is highly susceptible to atherosclerosis, relative to other disease-resistant vasculatures such as the brachial artery (1, 15, 16, 30, 32). Our previous observation that sitting causes endothelial dysfunction in the popliteal, but not brachial, artery (28) stimulates the idea that increased vulnerability of the leg vasculature to atherosclerosis may be partly attributable to the direct detrimental effects of prolonged sitting on that vasculature. As such, identification of the mechanism(s) contributing to leg vascular dysfunction with sitting requires attention. Given our earlier finding that sitting was accompanied with a marked reduction in popliteal artery blood flow and shear rate (28), our next logical step was to test the hypothesis that the sustained reduction of shear stress during sitting was the mediator of leg endothelial dysfunction. This was accomplished by increasing leg vascular conductance with local heating of one foot during sitting in an experimental setting where the contralateral leg served as an internal control. Consistent with our hypothesis, we provide evidence that leg endothelial dysfunction following sitting can be abrogated by preventing the decrease in shear during sitting.
The notion that maintenance of shear stress is critical for sustaining optimal vascular health is supported by a plethora of both in vitro and in vivo studies. For example, studies using the proatherogenic apolipoprotein-E null mice demonstrate that chronic experimental reduction of carotid artery shear stress impairs endothelial function and promotes atherosclerotic lesions (3, 18). Similarly, studies in humans also demonstrate that short-term (i.e., 30 min) experimental induction of low (and oscillatory) conduit artery shear stress by inflation of a distal cuff blunts FMD in both upper and lower extremities and causes endothelial inflammation (11, 12, 31, 34, 37). Notably, recent data indicate that these effects of low shear stress on brachial artery endothelial function persist when the reduction in shear stress via forearm compression is maintained for 2 wk (35), thus further emphasizing the importance of shear stress for maintaining optimal endothelial health.
Although not the purpose of this study, a discussion on potential mechanisms contributing to the reduction in leg blood flow during sitting is deserved. It is possible that increased hydrostatic pressure within the leg vasculature provokes blood to pool within the venous circulation. In this regard, in our previous study we found an increase in calf circumference during sitting (28), thus suggesting that blood pooling is indeed occurring in the lower limbs. This effect may be exacerbated by reduced skeletal muscle activity during sitting, which eliminates any contribution of the muscle pump in facilitating venous return (6). Other factors that may contribute to an increase in leg vascular resistance during an orthostatic stress, such as sitting, are venous distension-induced arterial constriction and increased hydrostatic pressure-induced myogenic constriction (14). In addition, given that muscle sympathetic nerve activity is greater in the upright position compared with supine (27), adrenergic vasoconstriction may also contribute to the increased leg vascular resistance. In light of the present findings that sitting-induced reduction in blood flow and shear mediate the impairment in endothelial function, further research is needed to determine the mechanisms by which sitting increases leg vascular resistance. A better understanding of these mechanisms can lead to the development of strategies that prevent increased leg vascular resistance and thus the reduction in blood flow and shear during sitting.
Another salient observation of the present study is that reactive hyperemia, indicative of microvascular dilator function, was reduced after sitting in both legs. The finding that local heating-induced limb blood flow did not prevent the decline in microvascular dilator function with sitting may suggest that the skeletal muscle resistance vasculature within the lower limb was not exposed to a robust increase in shear stress with local heating. It is likely that most of the increase in limb blood flow was directed to skin as the heated area was limited to the foot. Future research is needed to determine if increasing the limb surface area subjected to heat (i.e., encompassing the calf) during sitting produces an increase in muscle blood flow (9), thus also preventing the impairment in microvascular dilator function associated with sitting.
Discussion of several considerations for the overall interpretation of the current findings is warranted. First, this study included only healthy young men, thus the generalizability of the findings remain limited to this population. The lack of a nonsitting control condition could be considered a potential limitation of the study. However, other studies show that over a 3-h period when subjects interrupt their sitting time, there is no decline in leg vascular function (17, 36). Lastly, although we aimed to maintain shear with heating during the prolonged sitting period, we observed an increase in mean shear during this intervention, although not statistically significant. Careful review of the individual data indicate that two big responders primarily drive the increase in mean shear with heating (Fig. 2B). Nevertheless, overall, heating was fairly effective in preventing the decrease in shear with prolonged sitting in the majority of subjects studied. Thus, although it remains unclear as to whether maintaining or slightly enhancing shear would be better during sitting, the current findings clearly demonstrate that preventing the reduction in shear with prolonged sitting also prevents the impairments in leg endothelial function.
The clinical relevance of the present findings should be highlighted. It is possible that the decline in leg endothelial function associated with sitting contributes to the increased propensity of atherosclerosis in the lower extremities (1, 15, 16, 30, 32); however, more research is needed to determine the long-term vascular ramifications of too much sitting. Although the prognostic value of popliteal artery FMD remains unknown, the finding that sitting lowered FMD by ∼4% (absolute units) should be considered in light of epidemiological data suggesting that a 1% decrease in brachial artery FMD is associated with a 13% increase of cardiovascular events in low-risk and high-risk populations (7, 10). Importantly, the finding that preventing the decrease in popliteal artery shear rate during sitting via heating of the foot prevented the decline in leg endothelial function may stimulate creation of simple therapeutic strategies (e.g., local heating) used to offset or alleviate the detrimental vascular effects of prolonged sitting. These interventions could be particularly favorable in clinical populations that are susceptible to peripheral artery disease such as patients with type 2 diabetes or spinal cord injury, who are limited in their physical activity and spend a large portion of their day sitting.
In conclusion, the present study revealed that preventing the reduction of shear stress during prolonged sitting with local heating abolishes the impairment in popliteal artery endothelial function. Therefore, this study provides evidence that a reduction in shear stress mediates sitting-induced leg endothelial dysfunction.
GRANTS
This work was supported by the National Institutes of Health Grants K01-HL-125503 and R21-DK-105368 (to J. Padilla).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.M.R., L.K.W., L.A.M.-L., P.J.F., and J.P. conception and design of research; R.M.R., L.K.W., T.M., and J.R.V. performed experiments; R.M.R., J.R.V., and J.P. analyzed data; R.M.R., L.K.W., T.M., J.R.V., L.A.M.-L., P.J.F., and J.P. interpreted results of experiments; R.M.R. prepared figures; R.M.R., P.J.F., and J.P. drafted manuscript; R.M.R., L.K.W., T.M., J.R.V., L.A.M.-L., P.J.F., and J.P. edited and revised manuscript; R.M.R., L.K.W., T.M., J.R.V., L.A.M.-L., P.J.F., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the time and effort put in by all volunteer subjects.
REFERENCES
- 1.Aboyans V, McClelland RL, Allison MA, McDermott MM, Blumenthal RS, Macura K, Criqui MH. Lower extremity peripheral artery disease in the absence of traditional risk factors. The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 214: 169–173, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol 115: 1519–1525, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113: 2744–2753, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, Garcia L, Allison MA, Sims ST, LaMonte MJ, Johnson KC, Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women's Health Initiative. J Am Coll Cardiol 61: 2346–2354, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in US occupation-related physical activity and their associations with obesity. PloS One 6: e19657, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 162: 411–419, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Heinonen I, Brothers RM, Kemppainen J, Knuuti J, Kalliokoski KK, Crandall CG. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol 111: 818–824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins NT, Padilla J, Boyle LJ, Credeur DP, Laughlin MH, Fadel PJ. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension 61: 615–621, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson BD, Mather KJ, Newcomer SC, Mickleborough TD, Wallace JP. Vitamin C prevents the acute decline of flow-mediated dilation after altered shear rate patterns. Appl Physiol Nutr Metab 38: 268–274, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 41: 998–1005, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Kitano A, Shoemaker JK, Ichinose M, Wada H, Nishiyasu T. Comparison of cardiovascular responses between lower body negative pressure and head-up tilt. J Appl Physiol 98: 2081–2086, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kroger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology 50: 649–654, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Li MF, Ren Y, Zhao CC, Zhang R, Li LX, Liu F, Lu JX, Tu YF, Zhao WJ, Bao YQ, Jia WP. Prevalence and clinical characteristics of lower limb atherosclerotic lesions in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Diabetol Metab Syndr 6: 71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McManus AM, Ainslie PN, Green DJ, Simair RG, Smith K, Lewis N. Impact of prolonged sitting on vascular function in young girls. Exp Physiol 100: 1379–1387, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297: H1535–H1543, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M, Grenon SM. Short-term physical inactivity impairs vascular function. J Surg Res 190: 672–682, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol 96: 1019–1027, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker BA, Trehearn TL, Meendering JR. Pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol 107: 1357–1359, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97: 499–508, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Pyke KE, Hartnett JA, Tschakovsky ME. Are the dynamic response characteristics of brachial artery flow-mediated dilation sensitive to the magnitude of increase in shear stimulus? J Appl Physiol 105: 282–292, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Pyke KE, Poitras V, Tschakovsky ME. Brachial artery flow-mediated dilation during handgrip exercise: evidence for endothelial transduction of the mean shear stimulus. Am J Physiol Heart Circ Physiol 294: H2669–H2679, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med 105: 32S–39S, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol Heart Circ Physiol 264: H1–H7, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801–809, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Ross R, Wight TN, Strandness E. Thiele Human atherosclerosis B. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93, 1984. [PMC free article] [PubMed] [Google Scholar]
- 31.Schreuder TH, Green DJ, Hopman MT, Thijssen DH. Acute impact of retrograde shear rate on brachial and superficial femoral artery flow-mediated dilation in humans. Physiol Rep 2: e00193, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, and Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92: 1355–1374, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Thijssen DH, Schreuder TH, Newcomer SW, Laughlin MH, Hopman MT, Green DJ. Impact of 2-weeks continuous increase in retrograde shear stress on brachial artery vasomotor function in young and older men. J Am Heart Assoc 4: e001968, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc 47: 843–849, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Totosy de Zepetnek JO, Jermey TL, MacDonald MJ. Superficial femoral artery endothelial responses to a short-term altered shear rate intervention in healthy men. PloS One 9: e113407, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. [DOI] [PubMed] [Google Scholar]