INTRODUCTION
The vein and ligament of Marshall (VOM and LOM) have been the focus of attention of basic and clinical electrophysiologists for several decades. Their relevance extends beyond that of being mere embryological remnants of a left superior vena cava, since they have been implicated in the pathogenesis of atrial fibrillation (AF), both as a source of initiating triggers,1 as well as a vehicle of parasympathetic2 and sympathetic3 innervations that modulate electrical properties of atrial tissue and contribute to AF maintenance.4 These properties make the VOM an attractive target during ablation of atrial arrhythmias. Here we review the anatomical and physiological underpinnings of VOM-related arrhythmogenesis and the data that support targeting it for therapeutic purposes.
ANATOMY AND PHYSIOLOGICAL FUNCTION
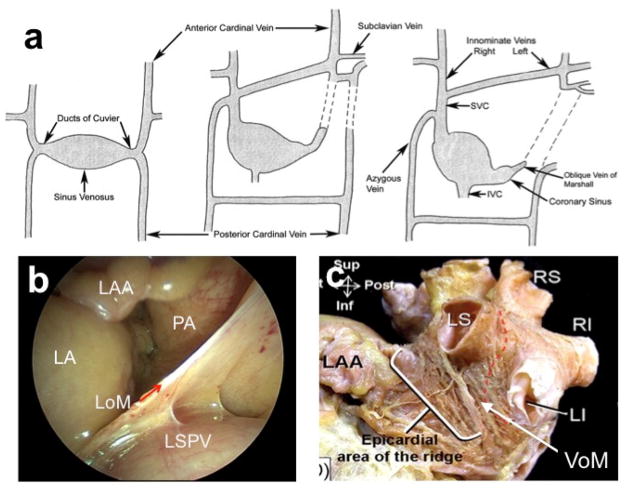
The LOM is a vestigial fold that results from the embryonic obliteration of the left anterior cardinal vein during the transition from a symmetric venous system to right-sided one. The left anterior cardinal vein connects caudally with the duct of Cuvier-later the coronary sinus (CS)- and with the subclavian/innominate vein in the cephalad direction (Figure 1A).5 It may remain open as the normal variant of persistent left superior vena cava, but most commonly only its intracardiac portion remains patent as the VOM (also known as oblique vein of the left atrium (LA)), connected with the CS at the level of the valve of Vieussens. In the adult, the LOM contains portions of the VOM, a myocardial sleeve (the Marshall bundle [MB]), and autonomic nerves6. It is important to recognize the distinction between the extracardiac LOM (Figure 1B, 1C), a remnant of the left superior vena cava, and the intracardiac VOM.7 The VOM is located in the epicardial aspect of the left lateral ridge. Figure 1 illustrates the embryology of the VOM and its adult anatomical location.
Figure 1.
Embryology and anatomy of the VOM/LOM. A, Embryological development of left-sided superior vena cava (SVC). Initially, the anterior cardinal vein drains into the primitive sino-atrial chamber (left image). The left cardinal vein later obliterates (center image), leaving only the oblique vein of Marshall as a remnant (right image). The failure of obliteration leads to a ‘persistent’ left-sided superior vena cava (dotted lines)5. B, The adult extracardiac LOM can be easily visualized during cardiac surgery, and continues as the intracardiac VOM (C), posterior to the left atrial appendage (LAA) and anterior to the left superior (LS) and inferior (LI) pulmonary vein (LSPV).44
Neural content
Early functional experiments by Scherlag et al8 had shown that left cardiac sympathetic nerve stimulation in dogs could lead to an ectopic atrial rhythm arising from the LOM area, thus suggesting a neural connection between atrial tissue and the sympathetic cardiac innervation via the LOM. Later on, Kim et al demonstrated that the human LOM contained abundant sympathetic innervation as shown by tyrosine hydroxylase (TH) staining.3 Additionally, TH-negative staining and nerve ganglia were identified. Ulphani and colleagues were able to show-using acetylcholinesterase staining-abundant parasympathetic innervation in the LOM-VOM area,2 and demonstrated its functional relevance by eliminating vagal stimulation-induced decreases in effective refractory period after ablating the LOM.
The LOM can be conceived as a connecting pathway between intrathoracic cardiac ganglia and intrinsic cardiac ganglia (ICG), specifically the inferior left ganglion, which is located below the left inferior pulmonary vein (LIPV), and would coincide more closely with the VOM as it connects with the CS. Finally, it is important to recognize that the anatomical location of the LOM-VOM in the LA ridge coincides with the ventro-lateral cardiac nerve, destined to provide innervation to the posterolateral left ventricle9. Figure 2 illustrates the neural content of the LOM and VOM.
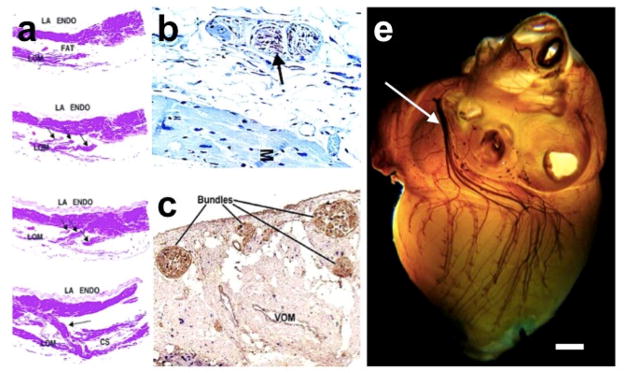
Figure 2.
Neural and myocardial content of the LOM/VOM. A, human serial (top to bottom) sections showing LOM isolated from the left atrial wall (top), and with discrete insertions in the left atrial wall and coronary sinus (hematoxylin and eosin [H&E] stain x 10).3 B, Immunohistochemical staining for tyrosine hydroxylase showing positively in nerve (brown staining—arrow). M = myocardium. C, Dual staining for DBH (blue) and ChAT (brown) shows a predominance of cholinergic nerve fibers within the nerve bundles of the LOM (x 5 magnification)2. D, Histochemical whole canine heart staining of parasympathetic innervation. The ventrolateral cardiac nerve traverses the fold anterior to the pulmonary veins on its way to innervate the inferior left ventricle. (Courtesy of Dainius Pauza, MD).
A histological study on human hearts by Makino et al10 found that the parasympathetic ganglia are mainly located at the VOM-CS junction and the parasympathetic neural elements progressively diminish along the LOM from the CS toward the left superior PV. The density of the sympathetic nerve decreases progressively in the opposite direction, which is consistent with the electrophysiological findings presented in the study of Lin et al.11 Using LOM stimulation, they demonstrated a gradient of AF inducibility along the LOM, from LOMCS (lowest) toward LOMLSPV (highest). Remarkably, in their study, high-frequency stimulation (HFS) applied to the LOM also induced accelerated junctional rhythm, premature ventricular beats, and accelerated idioventricular rhythm, particularly at the LOMLSPV and LOMLS-LIPV areas. We showed that in humans, the VOM and its neighboring atrial myocardium contain intracardiac nerves that can reach the AV node and induce parasympathetic responses.12
Myocardial fibers
The LOM-VOM contains myocardial fibers connecting the ligament itself with the CS musculature, the underlying atrial myocardium, and the PV musculature.10 Discrete connections were first presented by Kim et al3 in human specimens. In vivo in humans, Han et al documented three different types of MB-LA connections in 72 patients who underwent MB mapping and ablation for AF:13 CS junction, LA-left PVs junction, and LA in between CS and PVs with wide multiple connections. Interestingly, rapid and fractionated activations were most commonly observed in the MB that had multiple LA connections. Therefore, it is plausible to conceptualize the LOM as a direct electroanatomical conduit between the left lateral ridge and the CS muscle sleeve. Animal studies have suggested that electrical connections through the LOM contribute to the LA activation during sinus rhythm –connecting the LA to the CS- and the genesis of the electrocardiographic P wave.14
ROLE OF LOM AS ARRHYTHMOGENIC SOURCE AND POTENTIAL THERAPEUTIC TARGET
Atrial fibrillation
Canine and human studies have shown that the VOM and LOM is a potential cause of AF and a therapeutic target.15, 16 Scherlag et al8 demonstrated an inducible ectopic rhythm arising from the ligament area upon left cardiac sympathetic nerve stimulation. Doshi et al17 went on to specifically demonstrate the role of the LOM in adrenergic atrial tachycardia (AT). HFS in the LOM (without exciting the atrial myocardium) leads to induction of AF, and this induction is inhibited by both esmolol and atropine, suggesting autonomic mediation.11, 18 Ectopic beats from the VOM have been reported to originate paroxysmal AF in a plethora of case reports13, 16, 19–22 and in experimental models of persistent AF.23
However, despite the potential arrhythmogenic role of the VOM, the current role of VOM ablation in the setting of AF ablation is not established. It has been proposed that if the earliest activation of ectopic beats or AF is in the mid or distal CS, and double potentials are present at those sites, then LOM mapping should be considered.6,15 In addition, if the earliest endocardial activation is located inside the left PVs but the PV potential during triggered beats precedes the LA potential by < 45 ms, then LOM mapping should be considered. Finally, if electrophysiological study after complete PV isolations shows that a left PV premature atrial contraction seems to have triggered AF, but no left PV trigger can be found despite careful mapping, then LOM mapping may be needed to identify the focus. 6, 15
Atrial tachycardias and failed AF ablations
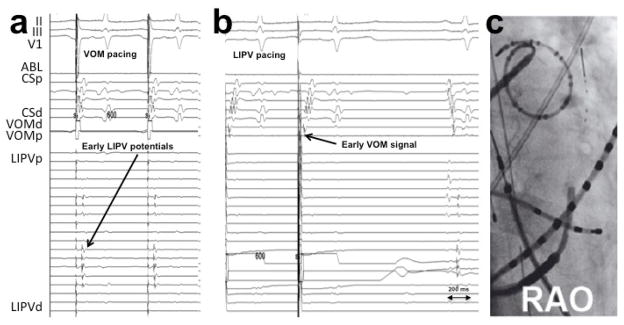
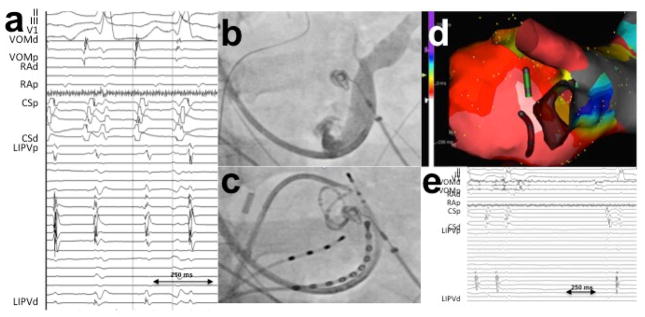
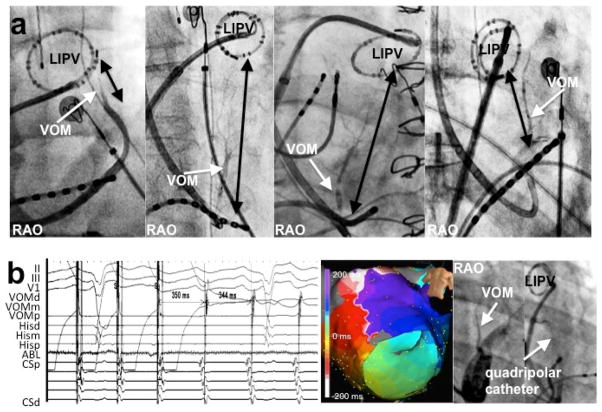
Chik et al24 described 5 patients with tachycardia involving the LOM region in a sample of 240 patients who underwent a “single ring” PV isolation ablation. Of note, both focal non-reentrant mechanisms 8, 17 and also macro-reentry electrical connections between the LOM/VOM and left atrial myocardium could underlie these arrhythmias 25. We addressed the role of VOM in patients with recurrent atrial arrhythmias (tachycardia and AF) after a previous AF catheter ablation.26 Unlike prior studies of this clinical scenario, we selectively cannulated the VOM for signal recording. VOM signals were consistently present in these cases, even after extensive prior endocardial ablation leading to endocardial scar in the LA ridge. However, VOM triggering of AF could only demonstrated in 1 patient (out of n=54). Direct connections between the left PV and VOM –a long-hypothesized concept – were only present in 5/32 reconnected LIPVs, confirming that although it is possible that the VOM may bridge the left PV with the LA–bypassing the usual endocardial ablation lines-, this scenario is rare. Figure 3 shows an example. VOM-tachycardia could be triggered by isoproterenol infusion for a total of 2 patients. Figure 4 shows an example.
Figure 3.
VOM-mediated LIPV reconnection. Differential pacing from the VOM and LIPV is performed.26 A, VOM-mediated LIPV reconnection: pacing from the VOM with advanced LIPV potentials—relative to the atrial signal—compared to sinus rhythm (last beat in B). Note that there is still delay from the stimulus artifact to the LIPV potentials, ruling out direct LIPV capture. B, VOM potentials during LIPV pacing preceding those of the coronary sinus. LIPV: left inferior pulmonary vein. VOM: vein of Marshall.
Figure 4.
Tachycardia arising from the VOM. A, Activation times were earliest in the VOM recordings. B and C, fluoroscopic images, showing a coronary sinus venogram, a circular catheter in the LIPV and a quadripolar catheter in the VOM. D, electroanatomical map of the tachycardia. E, Ethanol infusion led to tachycardia termination. 26 LIPV: left inferior pulmonary vein
VOM and the mitral isthmus
Perimitral flutter (PMF) is a common LA reentrant tachycardia occurring after AF ablation. Macroreentrant tachycardia involving the mitral annulus-PMF causes 33%–60% of post PV isolation atrial flutters.27–29 Catheter ablation of PMF involves, most commonly, the creation of a linear lesion from the mitral annulus to the LIPV, in the so-called mitral isthmus (MI).30 Achieving a complete ablation-defined as the creation of bidirectional block across the MI- is critical to obtain enduring success, but doing so can be technically difficult, with success rates that are consistently suboptimal (reported as 32%31, 64%32 or 71%).33 Anatomical features such as myocardial thickness and presence of pouches,34 isthmus length and relationship to the circumflex artery,32 high take-off of the LIPV, local coronary flow35 and acute edema in response to RF ablation have been described as possible obstacles to MI ablation. Additionally, an epicardial CS connection providing an epicardial bridge36 (localized in LOM area) could circumvent the MI and lead to recurrent PMF or failure to achieve MI block with endocardial ablation. Incomplete ablation of the MI is proarrhythmogenic,33,37 increasing the risk of recurrent flutter by up to 4 times.
The location of the VOM is similar to that of the MI, as it connects the CS –close to the mitral annulus-with the LA ridge, anterior to the left PVs. The VOM consistently lies in the re-entrant circuit of PMF as can be demonstrated by entrainment maneuvers (Figure 5). However the length of the MI (as measured from the CS to the LIPV) is quite variable. Figure 5A shows examples of the anatomical variations of the VOM in the MI area.
Figure 5.
VOM and mitral isthmus. A, Anatomical variability of the mitral isthmus length (black arrows from left inferior pulmonary vein to the coronary sinus in selective VOM venograms. Right (RAO) or left (LAO) anterior oblique views as indicated.43 B, Perimitral flutter response to entrainment from the VOM showing overt entrainment of counterclockwise perimitral flutter (intracardiac electrocardiogram, activation map and fluoroscopic image respectively.43
Accessory pathways
If an accessory pathway (AP) inserts directly into the MB or into the CS muscle sleeves near the LOM-CS junction, then it may conduct electrical impulse from the left ventricle into the LA, bypassing the auriculoventricular (AV) node and facilitating the initiation and maintenance of reciprocating tachycardia.38 Hwang et al reported in four patients the participation of MB in AV reciprocating tachycardias in whom RF ablation directed towards the MB eliminated the pathway conduction but conventional techniques targeted at the AV annulus failed.38 Hence, though they represent a minority of incidental cases, LOM mapping and potential route to deliver RF should be beard in mind when dealing with left free wall AP with previous failed ablation attempts.
TARGETING THE LOM/VOM
LOM ablation
LOM can be ablated from the endocardial side. For this purpose, it is recommended to place a mapping catheter through the VOM, advanced it in the direction of the left lateral ridge and the left PVs according to the technique described by Hwang et al.19 This VOM catheter is used as the anatomical target to guide endocardial ablation and to confirm the successful ablation of the LOM. The mid portion of LOM can be ablated from the inferoanterior aspect of the left lateral ridge under the LIPV ostium. RF energy applied with an open irrigation catheter at that region eliminates the LOM potentials in more than 90% of the patients.15 However, in some cases, it is necessary to use higher power over the left lateral ridge and the septoatrial bundle, near the orifice of the left PV, and in the LA appendage to eliminate all LOM connections.15 The elimination of MB electrograms and the presence of exit block during pacing are both suggestive of successful MB ablation.
Surgical excision
Surgical (Cox-Maze-III) or minimally invasive atrial fibrillation surgery (MIAFS) can provide a potential successful therapy for cases with an expected low surgical mortality and paroxysmal AF who failed catheter ablations.39 During those approaches, the extracardiac LOM (not the vein as already pointed out) is usually excised. Some portions of the VOM may be ablated if an epicardial mitral isthmus ablation line is performed. Whether these contribute to superior outcomes compared to endocardial catheter ablation –as those reported in the FAST trial40 is unclear.
VOM ETHANOL INFUSION
The VOM as a true atrial vein
It has been generally understood that the left cardinal venous system becomes anatomically and functionally atretic during embryological development, and its connection to the coronary sinus becomes the LOM.5 Even if the ligament is patent as the VOM, whether the VOM provides any venous drainage function to the LA was unclear. We showed that the VOM serves a true venous function that communicated with the underlying myocardium and is therefore a viable vascular route for the delivery of therapeutic agents to the LA myocardium.41 Demonstration of echocardiographic contrast passage into the LA cavity (as shown in Figure 6) supports such transmyocardial transport, and highlights the relevance of a slow ethanol infusion to allow for rapid dilution as it reaches the systemic circulation. Additionally, occlusive VOM venograms demonstrate connections between the LSPV and, occasionally with the RSPV via collaterals.
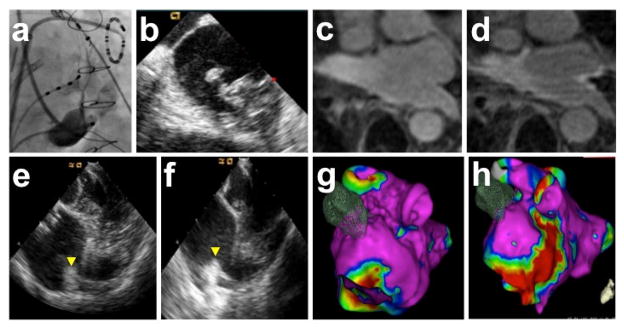
Figure 6.
VOM as an atrial vein and ethanol-induced scar. A, VOM venogram. B, Echocardiographic contrast injection in the VOM. Intracardiac echocardiographic snapshot of the left atrium, showing contrast appearance in the carina.41 C-H: scar created by VOM ethanol infusion demonstrated by pre- and post-ethanol imaging using gadolinium-enhanced MRI (C and D), intracardiac echocardiogram imaging of the left atrial ridge (E and F), and electro-anatomical mapping (G and H).
Procedural steps for VOM ethanol infusion
Retrograde infusion of ethanol in the VOM has been demonstrated to achieve rapid ablation of LA tissues. 12, 26, 41, 43 In our in toto experience of 165 patients has an overall success of VOM cannulation and ethanol delivery in 89% (unpublished data). The initial step includes CS cannulation with a suitably large sheath (usually 7F sheath) preferably via the right internal jugular vein, although femoral or left subclavian vein approaches can be successful. Once CS has been cannulated, direct contrast injection or a balloon occlusion CS venogram are then performed to assess the presence or absence of the VOM, which is identified as a branch of the CS directed posteriorly (best shown in a right-anterior oblique fluoroscopic projection) and superiorly. If present, subselective cannulation of the VOM can be attempted with the use of a left internal mammary artery (LIMA) angioplasty guide catheter or a subselective catheter for CS branch cannulation (for LV lead delivery), directing the catheter tip posteriorly while injecting radiographic contrast to verify engagement in the VOM. Once engagement is obtained, an angioplasty wire must be advanced into the VOM as far as possible to secure cannulation. A pre-loaded over-the-wire angioplasty balloon (most commonly 6 mm length, 1.5–2 mm nominal diameter) is then advanced into the proximal VOM, inflated and a selective VOM venogram is obtained by injecting contrast into the balloon lumen. Afterwards, between two and four (depending on the length of the VOM) separate injections of 1 cc 98% ethanol administered over 2 minutes each are performed, 2 minutes apart. Finally, VOM angiograms can be repeated to verify the integrity of the vein.
In our experience, ethanol infusion is feasible and safe, and achieves rapid ablation of LA tissue. Other than occasional atrial extrasystoles there are no hemodynamic or clinical consequences of ethanol infusion. As a matter of fact, ethanol levels measured in mixed venous blood at the end of the procedure are undetectable in all patients. 12 In the long term, pleuro-pericarditis (Dressler’s syndrome) and subacute pericardial effusions were complications described when the procedure were noted in 2/165 cases (all performed concomitantly with RF ablation for PV isolation).
VOM ethanol and failed AF ablations
We used VOM ethanol infusion in cases of recurrent AF after a previous ablation in which the left pulmonary veins had reconnected. Up to four 1 cc infusions of 98% ethanol were delivered in the VOM. As described above, in a minority of PVs, the VOM was shown to provide epicardial LA-PV connections by differential pacing. Regardless of the reconnection pattern, ethanol infusion eliminated LIPV and LSPV reconnection in 23/32 and 13/30 patients, respectively. Ethanol terminated VOM and LIPV tachycardias in 2 patients. 26 These results support that chemical ablation via the VOM may assist in achieving lesion transmurality in this region, because regardless of the presence and patterns of VOM-PV connections, VOM ethanol infusion was helpful in achieving redisconnection of the left PVs in a substantial number of patients with reconnected veins.
VOM ethanol and parasympathetic denervation
Intracardiac nerve (ICN) ablation has been proposed as an adjunctive or stand-alone therapy for AF. As aforementioned, the LOM is considered part of the ICN. It was however not known whether the VOM could anatomically connect with the ICN associated with the LOM. The implications of such a connection are significant since it would imply that the VOM could be used as a vascular route to deliver therapeutic agents targeting the ICN. We addressed this question in humans undergoing AF ablation.12 A multipolar catheter was introduced in the VOM and used for HFS aimed at capturing ICN and eliciting parasympathetic responses. HFS was delivered either as short, P-wave-synchronized burst of 30 pulses, 10 ms apart, (SyncHFS) or as continuous HFS bursts for 3 to 10 seconds (BurstHFS). AV nodal conduction slowing (asystole >2 s or R-R interval prolongation >50%) and AF inducibility were assessed before and after VOM ethanol infusion. SynchHFS did not capture the atrium but induced AF in 8 of 8 patients. In 4 of 8 AF initiated spontaneously from VOM-initiating beats. Figure 7 shows an example. No parasympathetic responses were elicited by SynchHFS. BurstHFS was performed in 32 patients undergoing de novo AF ablation (group 1) and 40 patients undergoing repeat ablation (group 2). Parasympathetic responses were found in all 32 group 1 patients and in 75% of group 2 patients. Figure 8 shows an example. After VOM ethanol infusion, parasympathetic responses were abolished in all patients (both groups).
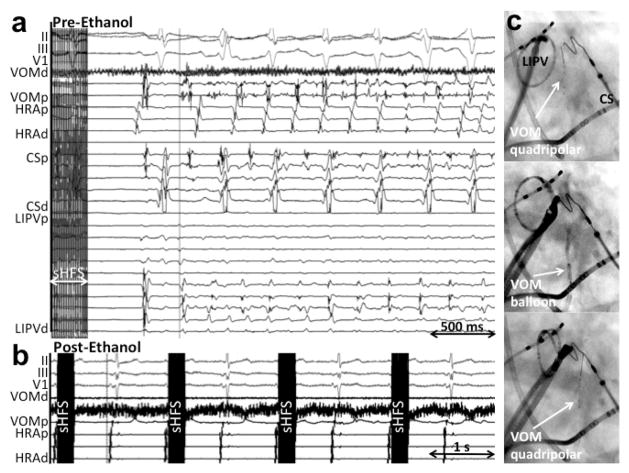
Figure 7.
VOM stimulation triggers VOM-initiated AF. A, Using Synch-High-frequency stimulation (see text), spontaneous AF was induced without atrial capture and initiating in the VOM. B, after ethanol, there was no AF induction.
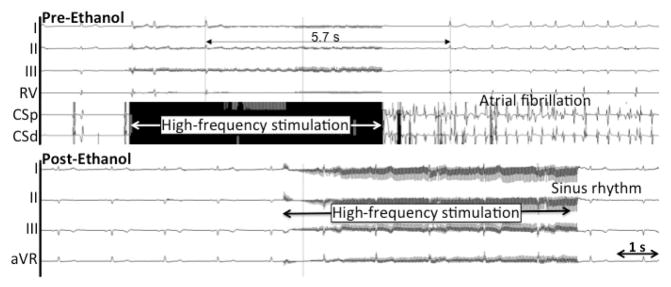
Figure 8.
VOM stimulation, parasympathetic AV nodal responses and ethanol-induced denervation. Top, a burst of high-frequency stimulation leads to induction of AF and asystole due to AV conduction slowing. Bottom, after ethanol, AF induction and asystole do not occur.
Eliciting a parasympathetic response in the AV node by VOM HFS proves not only local ICN stimulation but also the existence and activation of interneuronal communications between different ICN. As to the mechanism explaining induction of AF without local atrial capture, it could be conjectured that SynchHFS can selectively stimulate local ICN leading to acetylcholine release and action potential shortening. The relative inconsistency of AF induction by this SynchHFS (4 of 8 patients) may thus illustrate the multiple pathophysiological steps required in its mechanism. Finally, the fact that VOM ethanol infusion eliminated parasympathetic responses and AF induction could be consistent with regional LA denervation.
VOM ethanol and mitral isthmus ablation
Part of the limitations found at the time of MI ablation could be potentially overcome by VOM ethanol infusion. Voltage maps of the LA after ethanol injection reveal41, 42 low-voltage areas corresponding to the anatomical localization of the VOM and its branches: lateral wall of the LA, between the CS and the left-sided PVs, and anterior to them (figure 6). These areas represent critical parts for the PMF circuit. With this background we assessed whether VOM ethanol infusion could help achieve MI block.43 Two patient groups were studied. Group 1 included 50 patients with a previous PV isolation undergoing repeat ablation, 30 of whom had had MI ablation and group 2 which included 21 patients undergoing de novo AF ablation. In group 1, VOM ethanol infusion acutely terminated PMF in 5 of 29 patients. Five patients had bidirectional MI block achieved solely by VOM ethanol infusion without RF ablation. In both groups, ablation after VOM ethanol infusion was required in the annular aspect of the MI. Bidirectional conduction block was achieved in all (total n=71) the patients in this study. RF ablation needed to achieve bidirectional MI block was 2.2 ± 1.6 and 2.0 ± 1.6 minutes respectively. In 1 of them, the VOM could have provided a potential mechanism as an epicardial conduction pathway bypassing the previous endocardial ablation line in the MI. During 16.2 ± 6.2 months of follow up, 1 out of 20 patients returning with recurrent atrial arrhythmias had recurrent conduction across the MI, and there was recurrence of PMF in only 1 patient due to recurrent conduction across the MI (at the anterior site ablated with RF). Repeat RF (21 seconds) restored bidirectional block.
These data support the clinical utility of VOM ethanol for PMF ablation. Further studies will be necessary in order to assess the optimal timing and selection of patients that could be benefited by this approach.
Rhythm control after VOM ethanol
The ultimate role of VOM ethanol in the management of AF can only be determined in a randomized controlled clinical trial. We are currently enrolling patients in two studies (clinicaltrials.gov NCT 01898221): randomizing patients with persistent AF to either standard ablation (pulmonary vein antral isolation, PVAI) or PVAI in addition to VOM ethanol infusion. VENUS-AF: (Vein of Marshall EthaNol in Untreated perSistent AF) will include patients undergoing their first, de novo ablation, and MARS-AF (vein of Marshall ethAnol for Recurrent perSistent AF) includes patients with prior ablation. The primary endpoint is freedom from AF or flutter on 1-month monitor at 12 months post procedure.
CONCLUSIONS
Targeting the VOM and its neighboring tissues is supported by mechanistic data and by the anatomical coincidence of the VOM with routinely ablated tissue. VOM ethanol infusion is feasible, safe, and achieves rapid ablation of LA tissue. Ongoing randomized studies will determine its role in the management of AF.
Acknowledgments
This work was funded by the NIH/NHLBI (R21HL097305 and R01 HL115003 (to MV), the Charles Burnett III endowment and the Antonio Pacifico, MD fellowship support. We gratefully acknowledge Dr. M. La Meir for his assistance with reviewing and revising the surgical part of the manuscript.
ABBREVIATIONS
- AF
Atrial Fibrillation
- CS
Coronary sinus
- LOM
Ligament of Marshall
- MB
Marshall bundle
- PV
Pulmonary Veins
- RF
Radiofrequency
- VOM
Vein of Marshall
Footnotes
Conflict of interest: none to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee SH, Tai CT, Hsieh MH, Tsao HM, Lin YJ, Chang SL, Huang JL, Lee KT, Chen YJ, Cheng JJ, Chen SA. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: Implication for catheter ablation. Journal of the American College of Cardiology. 2005;46:1054–1059. doi: 10.1016/j.jacc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Ulphani JS, Arora R, Cain JH, Villuendas R, Shen S, Gordon D, Inderyas F, Harvey LA, Morris A, Goldberger JJ, Kadish AH. The ligament of marshall as a parasympathetic conduit. American journal of physiology. 2007;293:H1629–1635. doi: 10.1152/ajpheart.00139.2007. [DOI] [PubMed] [Google Scholar]
- 3.Kim DT, Lai AC, Hwang C, Fan LT, Karagueuzian HS, Chen PS, Fishbein MC. The ligament of marshall: A structural analysis in human hearts with implications for atrial arrhythmias. Journal of the American College of Cardiology. 2000;36:1324–1327. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- 4.Kamanu S, Tan AY, Peter CT, Hwang C, Chen PS. Vein of marshall activity during sustained atrial fibrillation. Journal of cardiovascular electrophysiology. 2006;17:839–846. doi: 10.1111/j.1540-8167.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 5.Irwin RB, Greaves M, Schmitt M. Left superior vena cava: revisited. Eur Heart J Cardiovasc Imaging. 2012;13:284–291. doi: 10.1093/ehjci/jes017. [DOI] [PubMed] [Google Scholar]
- 6.Hwang C, Chen PS. Ligament of marshall: Why it is important for atrial fibrillation ablation. Heart Rhythm. 2009;6:S35–40. doi: 10.1016/j.hrthm.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera JA, Ho SY, Climent V, Sanchez-Quintana D. The architecture of the left lateral atrial wall: A particular anatomic region with implications for ablation of atrial fibrillation. European heart journal. 2008;29:356–362. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 8.Scherlag BJ, Yeh BK, Robinson MJ. Inferior interatrial pathway in the dog. Circulation research. 1972;31:18–35. doi: 10.1161/01.res.31.1.18. [DOI] [PubMed] [Google Scholar]
- 9.Armour JA, Hageman GR, Randall WC. Arrhythmias induced by local cardiac nerve stimulation. Am J Physiol. 1972;223:1068–1075. doi: 10.1152/ajplegacy.1972.223.5.1068. [DOI] [PubMed] [Google Scholar]
- 10.Makino M, Inoue S, Matsuyama TA, Ogawa G, Sakai T, Kobayashi Y, Katagiri T, Ota H. Diverse myocardial extension and autonomic innervation on ligament of marshall in humans. Journal of cardiovascular electrophysiology. 2006;17:594–599. doi: 10.1111/j.1540-8167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Scherlag BJ, Lu Z, Zhang Y, Liu S, Patterson E, Jackman WM, Lazzara R, Po SS. Inducibility of atrial and ventricular arrhythmias along the ligament of marshall: Role of autonomic factors. Journal of cardiovascular electrophysiology. 2008;19:955–962. doi: 10.1111/j.1540-8167.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 12.Baez-Escudero JL, Keida T, Dave AS, Okishige K, Valderrabano M. Ethanol infusion in the vein of marshall leads to parasympathetic denervation of the human left atrium: Implications for atrial fibrillation. Journal of the American College of Cardiology. 2014;63:1892–1901. doi: 10.1016/j.jacc.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han S, Joung B, Scanavacca M, Sosa E, Chen PS, Hwang C. Electrophysiological characteristics of the marshall bundle in humans. Heart Rhythm. 2010;7:786–793. doi: 10.1016/j.hrthm.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan AY, Chou CC, Zhou S, Nihei M, Hwang C, Peter CT, Fishbein MC, Chen PS. Electrical connections between left superior pulmonary vein, left atrium, and ligament of marshall: Implications for mechanisms of atrial fibrillation. American journal of physiology. 2006;290:H312–322. doi: 10.1152/ajpheart.00369.2005. [DOI] [PubMed] [Google Scholar]
- 15.Hwang C, Fishbein MC, Chen PS. How and when to ablate the ligament of marshall. Heart Rhythm. 2006;3:1505–1507. doi: 10.1016/j.hrthm.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: Possible roles of the ligament of marshall. Journal of cardiovascular electrophysiology. 1999;10:636–648. doi: 10.1111/j.1540-8167.1999.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 17.Doshi RN, Wu TJ, Yashima M, Kim YH, Ong JJ, Cao JM, Hwang C, Yashar P, Fishbein MC, Karagueuzian HS, Chen PS. Relation between ligament of marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–883. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Scherlag BJ, Niu G, Lu Z, Patterson E, Liu S, Lazzara R, Jackman WM, Po SS. Autonomic elements within the ligament of marshall and inferior left ganglionated plexus mediate functions of the atrial neural network. Journal of cardiovascular electrophysiology. 2009;20:318–324. doi: 10.1111/j.1540-8167.2008.01315.x. [DOI] [PubMed] [Google Scholar]
- 19.Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–1505. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 20.Polymeropoulos KP, Rodriguez LM, Timmermans C, Wellens HJ. Images in cardiovascular medicine. Radiofrequency ablation of a focal atrial tachycardia originating from the marshall ligament as a trigger for atrial fibrillation. Circulation. 2002;105:2112–2113. doi: 10.1161/01.cir.0000016168.49833.ce. [DOI] [PubMed] [Google Scholar]
- 21.Keida T, Fujita M, Okishige K, Takami M. Elimination of non-pulmonary vein ectopy by ethanol infusion in the vein of marshall. Heart Rhythm. 2013;10:1354–1356. doi: 10.1016/j.hrthm.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Katritsis D, Giazitzoglou E, Korovesis S, Paxinos G, Anagnostopoulos CE, Camm AJ. Epicardial foci of atrial arrhythmias apparently originating in the left pulmonary veins. Journal of cardiovascular electrophysiology. 2002;13:319–323. doi: 10.1046/j.1540-8167.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu TJ, Ong JJ, Chang CM, Doshi RN, Yashima M, Huang HL, Fishbein MC, Ting CT, Karagueuzian HS, Chen PS. Pulmonary veins and ligament of marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation. 2001;103:1157–1163. doi: 10.1161/01.cir.103.8.1157. [DOI] [PubMed] [Google Scholar]
- 24.Chik WW, Chan JK, Ross DL, Wagstaff J, Kizana E, Thiagalingam A, Kovoor P, Thomas SP. Atrial tachycardias utilizing the Ligament of Marshall region following single ring pulmonary vein isolation for atrial fibrillation. Pacing Clin Electrophysiol. 2014;37:1149–1158. doi: 10.1111/pace.12423. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto T, Maruyama M, Seino Y, Shimizu W. Marshall bundle reentry: a novel type of macroreentrant atrial tachycardia. Heart Rhythm. 2014;11:1229–1232. doi: 10.1016/j.hrthm.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 26.Dave AS, Baez-Escudero JL, Sasaridis C, Hong TE, Rami T, Valderrabano M. Role of the vein of marshall in atrial fibrillation recurrences after catheter ablation: Therapeutic effect of ethanol infusion. Journal of cardiovascular electrophysiology. 2012;23:583–591. doi: 10.1111/j.1540-8167.2011.02268.x. [DOI] [PubMed] [Google Scholar]
- 27.Deisenhofer I, Estner H, Zrenner B, Schreieck J, Weyerbrock S, Hessling G, Scharf K, Karch MR, Schmitt C. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: Incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace. 2006;8:573–582. doi: 10.1093/europace/eul077. [DOI] [PubMed] [Google Scholar]
- 28.Knecht S, Veenhuyzen G, O’Neill MD, Wright M, Nault I, Weerasooriya R, Miyazaki S, Sacher F, Hocini M, Jais P, Haissaguerre M. Atrial tachycardias encountered in the context of catheter ablation for atrial fibrillation part ii: Mapping and ablation. Pacing Clin Electrophysiol. 2009;32:528–538. doi: 10.1111/j.1540-8159.2009.02315.x. [DOI] [PubMed] [Google Scholar]
- 29.Chugh A, Oral H, Lemola K, Hall B, Cheung P, Good E, Tamirisa K, Han J, Bogun F, Pelosi F, Morady F. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005;2:464–471. doi: 10.1016/j.hrthm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Jais P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 31.Pak HN, Oh YS, Lim HE, Kim YH, Hwang C. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 2011;8:199–206. doi: 10.1016/j.hrthm.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Yokokawa M, Sundaram B, Garg A, Stojanovska J, Oral H, Morady F, Chugh A. Impact of mitral isthmus anatomy on the likelihood of achieving linear block in patients undergoing catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2011;8:1404–1410. doi: 10.1016/j.hrthm.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Mountantonakis S, Frankel DS, Hutchinson MD, Dixit S, Riley M, Callans DJ, Garcia F, Lin D, Tzou W, Bala R, Marchlinski FE, Gerstenfeld EP. Feasibility of catheter ablation of mitral annular flutter in patients with prior mitral valve surgery. Heart Rhythm. 2011;8:809–814. doi: 10.1016/j.hrthm.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 34.West JJ, Norton PT, Kramer CM, Moorman JR, Mahapatra S, DiMarco JP, Mangrum JM, Mounsey JP, Ferguson JD. Characterization of the mitral isthmus for atrial fibrillation ablation using intracardiac ultrasound from within the coronary sinus. Heart Rhythm. 2008;5:19–27. doi: 10.1016/j.hrthm.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 35.Kurotobi T, Shimada Y, Kino N, Iwakura K, Inoue K, Kimura R, Tosyoshima Y, Mizuno H, Okuyama Y, Fujii K, Nanto S, Komuro I. Local coronary flow is associated with an unsuccessful complete block line at the mitral isthmus in patients with atrial fibrillation. Circulation Arrhythmia and electrophysiology. 2011;4:838–843. doi: 10.1161/CIRCEP.111.964478. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki S, Shah AJ, Haissaguerre M. Recurrent perimitral tachycardia using epicardial coronary sinus connection to bypass endocardial conduction block at the mitral isthmus. Circulation Arrhythmia and electrophysiology. 2011;4:e39–41. doi: 10.1161/CIRCEP.111.963157. [DOI] [PubMed] [Google Scholar]
- 37.Matsuo S, Yamane T, Date T, et al. Completion of mitral isthmus ablation using a steerable sheath: Prospective randomized comparison with a nonsteerable sheath. Journal of cardiovascular electrophysiology. 2011 doi: 10.1111/j.1540-8167.2011.02112.x. [DOI] [PubMed] [Google Scholar]
- 38.Hwang C, Peter CT, Chen PS. Radiofrequency ablation of accessory pathways guided by the location of the ligament of marshall. Journal of cardiovascular electrophysiology. 2003;14:616–620. doi: 10.1046/j.1540-8167.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- 39.Krul SP, Driessen AH, Zwinderman AH, van Boven WJ, Wilde AA, de Bakker JM, de Groot JR. Navigating the mini-maze: systematic review of the first results and progress of minimally-invasive surgery in the treatment of atrial fibrillation. Int J Cardiol. 2013;166:132–140. doi: 10.1016/j.ijcard.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M, Sandoval E, Calvo N, Brugada J, Kelder J, Wijffels M, Mont L. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125:23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 41.Valderrabano M, Liu X, Sasaridis C, Sidhu J, Little S, Khoury DS. Ethanol infusion in the vein of marshall: Adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009;6:1552–1558. doi: 10.1016/j.hrthm.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valderrabano M, Chen HR, Sidhu J, Rao L, Ling Y, Khoury DS. Retrograde ethanol infusion in the vein of marshall: Regional left atrial ablation, vagal denervation and feasibility in humans. Circulation Arrhythmia and electrophysiology. 2009;2:50–56. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baez-Escudero JL, Morales PF, Dave AS, Sasaridis CM, Kim YH, Okishige K, Valderrabano M. Ethanol infusion in the vein of marshall facilitates mitral isthmus ablation. Heart Rhythm. 2012;9:1207–1215. doi: 10.1016/j.hrthm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabrera JA, Sanchez-Quintana D, Ho SY, Medina A, Anderson RH. The architecture of the atrial musculature between the orifice of the inferior caval vein and the tricuspid valve: The anatomy of the isthmus. Journal of cardiovascular electrophysiology. 1998;9:1186–1195. doi: 10.1111/j.1540-8167.1998.tb00091.x. [DOI] [PubMed] [Google Scholar]