Flow-mediated inhibition of contraction frequency is blunted in metabolic syndrome (MetSyn) thoracic ducts (TDs) despite a comparable response to exogenous NO. While the NOS inhibitor l-NAME abolished this difference, the ROS scavenging molecule tempol did not restore flow responses in MetSyn TDs. A significant reduction in eNOS in Metsyn TDs is found.
Keywords: metabolic syndrome, lymphatic vessel contraction, lymph flow, nitric oxide synthase, lymphatic endothelial cells
Abstract
Shear-dependent inhibition of lymphatic thoracic duct (TD) contractility is principally mediated by nitric oxide (NO). Endothelial dysfunction and poor NO bioavailability are hallmarks of vasculature dysfunction in states of insulin resistance and metabolic syndrome (MetSyn). We tested the hypothesis that flow-dependent regulation of lymphatic contractility is impaired under conditions of MetSyn. We utilized a 7-wk high-fructose-fed male Sprague-Dawley rat model of MetSyn and determined the stretch- and flow-dependent contractile responses in an isobaric ex vivo TD preparation. TD diameters were tracked and contractile parameters were determined in response to different transmural pressures, imposed flow, exogenous NO stimulation by S-nitro-N-acetylpenicillamine (SNAP), and inhibition of NO synthase (NOS) by l-nitro-arginine methyl ester (l-NAME) and the reactive oxygen species (ROS) scavenging molecule 4-hydroxy-tempo (tempol). Expression of endothelial NO synthase (eNOS) in TD was determined using Western blot. Approximately 25% of the normal flow-mediated inhibition of contraction frequency was lost in TDs isolated from MetSyn rats despite a comparable SNAP response. Inhibition of NOS with l-NAME abolished the differences in the shear-dependent contraction frequency regulation between control and MetSyn TDs, whereas tempol did not restore the flow responses in MetSyn TDs. We found a significant reduction in eNOS expression in MetSyn TDs suggesting that diminished NO production is partially responsible for impaired flow response. Thus our data provide the first evidence that MetSyn conditions diminish eNOS expression in TD endothelium, thereby affecting the flow-mediated changes in TD lymphatic function.
NEW & NOTEWORTHY
Flow-mediated inhibition of contraction frequency is blunted in metabolic syndrome (MetSyn) thoracic ducts (TDs) despite a comparable response to exogenous NO. While the NOS inhibitor l-NAME abolished this difference, the ROS scavenging molecule tempol did not restore flow responses in MetSyn TDs. A significant reduction in eNOS in Metsyn TDs is found.
proper lymphatic function is critical for interstitial fluid and macromolecule homeostasis and the prevention of edema. Lymphatic function is critically dependent on the contractile activity of muscularized lymphatic vessels to propel lymph centripetally back to the blood circulation (46). Lymphatic contractility has both pump and conduit functions that are intimately controlled by physical stimuli such as stretch and shear stress. Failure of the lymphatic system results in a chronic and progressive disease, lymphedema. Recently, morbid obesity has been associated with a high risk of developing “spontaneous lymphedema” described as massive localized lymphedema (14), and obesity is a well-documented risk factor for postsurgical lymphedema in cancer patients (1). The mechanisms underlying the association of lymphatic failure and metabolism require further investigation.
Obesity is the primary driver of multiple metabolic impairments that are described as the metabolic syndrome (MetSyn) that affects over a third of the U.S. population (22). Additionally, the MetSyn phenotype is characterized as a mild but chronic inflammatory state with elevated production of reactive oxygen species (ROS) and blood vascular dysfunction (16). Elevations in ROS, insulin resistance, dyslipidemia, and inflammatory cytokines have linked obesity-associated hypertension to blood vascular endothelial dysfunction as a function of impaired vasodilator and elevated vasoconstrictor pathways (6, 13, 28, 33). Specifically, blood vessel endothelial dysfunction is associated with reduced bioavailability of the potent vasodilator molecule nitric oxide (NO) (11, 24, 28, 37). Multiple mechanisms for reduced NO bioavailability in blood vessels have been described such as a reduction in the expression of endothelial nitric oxide synthase (eNOS), the oxidation of the critical cofactor tetrahydrobiopterin, and direct ROS reactivity with NO to form peroxynitrite. We have previously demonstrated that mesenteric lymphatics from a high fructose-fed rat model of MetSyn exhibit significant negative chronotropic effects at all transmural pressures that effectively reduced the intrinsic flow generating capacity of these vessels by ∼50% (49). However, the shear-dependent regulation and involvement of NO in lymphatic contractility under conditions of MetSyn have not been addressed.
NO is also a critical regulator of lymphatic contractility and is the primary signaling molecule in the flow/shear-mediated regulation of lymphatic contractility. While the lymphatic system must propel lymph against a net pressure gradient, certain situations can result in passive flow. Under conditions of imposed flow, lymphatic contraction frequency and vessel tone decrease in a primarily NO-dependent manner (19). Both reductions in frequency and vessel tone decrease the vessel resistance to flow as a function of average vessel diameter (46). Additionally, NO is also generated in response to the intrinsic lymph pump flow produced during the lymphatic contraction cycle as a self-regulatory mechanism (21).
In this study we have determined a role for insufficient NO production as a factor in the impaired flow-mediated responses of thoracic ducts (TDs) isolated from MetSyn rats. We employed an isolated TD vessel preparation to assess the effects of pressure (stretch) and flow (shear) on contractility and found a significant blunting in the flow-mediated inhibition of contractility in MetSyn TDs. The results demonstrated that TDs have endothelial dysfunction in the MetSyn state as a function of reduced eNOS expression, indicating reduced NO production in response to flow stimulated shear stress.
METHODS
Ethical approval.
Male Sprague-Dawley rats (150–180g) from Charles River were used in this study. All animals were housed in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and maintained in accordance with the policies defined by the Public Health Service Policy for the Humane Care and Use of Laboratory Animals and the United States Department of Agriculture's Animal Welfare Regulations, and all protocols were approved by the Scott & White Animal Care and Use Committee.
Model of MetSyn.
Rats were given ad libidum access to water and either control rodent chow or high-fructose feed diet (60% fructose; ID.89247 Harlan Teklad; 66% caloric content from fructose) for 7 wk except during the immediate preexperiment (16 h) fasting period. Blood was collected in fasted (16 h) rats via the lateral saphenous veins at the start and end of the 7-wk diet period. The lower leg was shaved clean, the lateral saphenous vein was punctured using a 27-gauge needle, and blood was collected in a nonheparinized tube. Blood was allowed to clot for 1 h at room temperature and spun for 10 min at 3,000 g. Serum was then frozen and stored at −80°C. MetSyn was confirmed through evaluating triglyceride and insulin concentrations, assessed via commercially available kits (colorimetric kit Bioassays ETGA-200, and an insulin ELISA kit Linco EZMRI-13k, respectively).
Lymphatic TD isolation.
Rats were anesthetized with Innovar-Vet (0.3 ml/kg im), which is a combination of a droperidol-fentanyl solution (20 mg/ml droperidol and 0.4 mg/ml fentanyl), and diazepam (2.5 mg/kg im). Once an anesthetic plane was reached, an incision was made through the chest ventral wall and euthanasia reached through a bilateral thoracotomy. The lymphatic TD was then carefully isolated and cut into two 1-cm segments. One segment was snap frozen in liquid nitrogen and stored at −80°C for protein collection. The remaining TD segment was maintained in albumin-supplemented physiological saline solution [APSS, in mM: 145.0 NaCl, 4.7 KCl, 2 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 sodium pyruvate, 0.02 EDTA, and 3.0 3-(N-morpholino) propanesulfonic acid (MOPS) and 1% wt/vol bovine serum albumin] at pH 7.4 at 38°C. After tissue removal, the rats were euthanized with an overdose of anesthetic.
Isolated vessel preparation.
The TD segment was then cannulated onto resistance-matched pipettes in a CH-1 chamber (Living Systems) and attached to independently adjustable pressure reservoirs. The TD was given ∼30 min to stabilize at an intramural pressure of 1 cmH2O, and spontaneous contractions were consistent. Each TD was exposed to pressures of 1, 3, and 5 cmH2O, and 5-min video recordings and diameter tracings were made at each pressure. In some vessels this pressure response was repeated in the presence of 100 μM SNAP after a 15-min equilibration period at a pressure of 1 cmH2O. Other vessels were subjected to a imposed flow by raising input and lowering output reservoirs equally such that transmural pressure was maintained at 3 cmH2O. This pressure and flow protocol was then repeated after a 20-min equilibration with either 100 μM l-NAME or 1 mM tempol, and their responses to each pressure and flow were recorded. Each experiment concluded with a 20-min equilibration in calcium-free APSS, and maximal diameters at each pressure were determined for vessel tone calculation.
Isolated vessel video analysis and statistics.
Lymphatic diameter traces were made for each 5-min video with a vessel wall-tracking program developed and provided by Dr. Michael Davis (12). Outer lymphatic vessel diameters were tracked 30 times per second providing a trace of diameter changes throughout periods of systole and diastole. The following lymphatic contractile parameters were derived: systolic/diastolic diameters, lymphatic tonic index, ejection fraction (EF), frequency, and fraction pump flow as previously described (5). Normalized frequency, normalized EF, and normalized tone for pressure responses were calculated by normalizing the response after pharmacological intervention to the APSS baseline values at that specific pressure. Flow responses were normalized to values obtained at a pressure of 3 cmH2O within each group and treatment.
Protein isolation and Western blotting.
Snap frozen TD sections were thawed in 60 μl of chilled NuPage sample buffer with protease inhibitor cocktail, phosphatase inhibitor cocktail, and 1× NuPage reducing agent. TDs were then sonicated for 3 min in a water bath sonicator and iced for 5 min. This process was repeated three times and then each sample was refrozen at −80°C. Samples were then thawed on ice, spun down at 1,000 g to remove insoluble material, and heated for 10 min at 70°C, as per the manufacturer's instructions. TD samples were loaded in alternating fashion (n = 6) in a 4–20% NuPage gel. Gels were run at 150 V and then transferred to 0.45-μm nitrocellulose overnight. Nitrocellulose membranes were stained with Ponceau S to ensure proper protein transfer and integrity. The blot was cut to allow simultaneous probing of eNOS and β-actin (βAct) and blocked in 5% milk for 3 h. Antibodies against βAct (Sigma A2228) and eNOS (BD Bioscience 610297) were utilized at 1:10,000 and 1:1,000 dilutions, respectively, overnight at 4°C. Blots were rinsed with Tris-buffered saline (TBS, pH 7.4) and probed with goat anti-mouse antibody conjugated with horseradish peroxidase at 1:10,000 and 1:2,000 dilutions, respectively, for 3 h at room temperature. Blots were imaged using SuperSignal West Dura Chemiluminescent Substrate (Pierce) on an ImageQuant LAS-4000 (GE) System with linear scale adjustment to minimize background noise. Signal intensity was quantified using ImageJ and values normalized to βAct.
Statistics and reporting.
Statistical significance for parameters of lymphatic TD function was determined through two-way ANOVA with Boneferrroni's post hoc analysis using the Statplus (Analyst soft) statistical software package. Data are represented as means ± SE, and significance was determined at P < 0.05. The eNOS-to-βAct ratios were assessed with two-tailed Student's t-test for statistical significance.
RESULTS
Normal stretch response in MetSyn TDs.
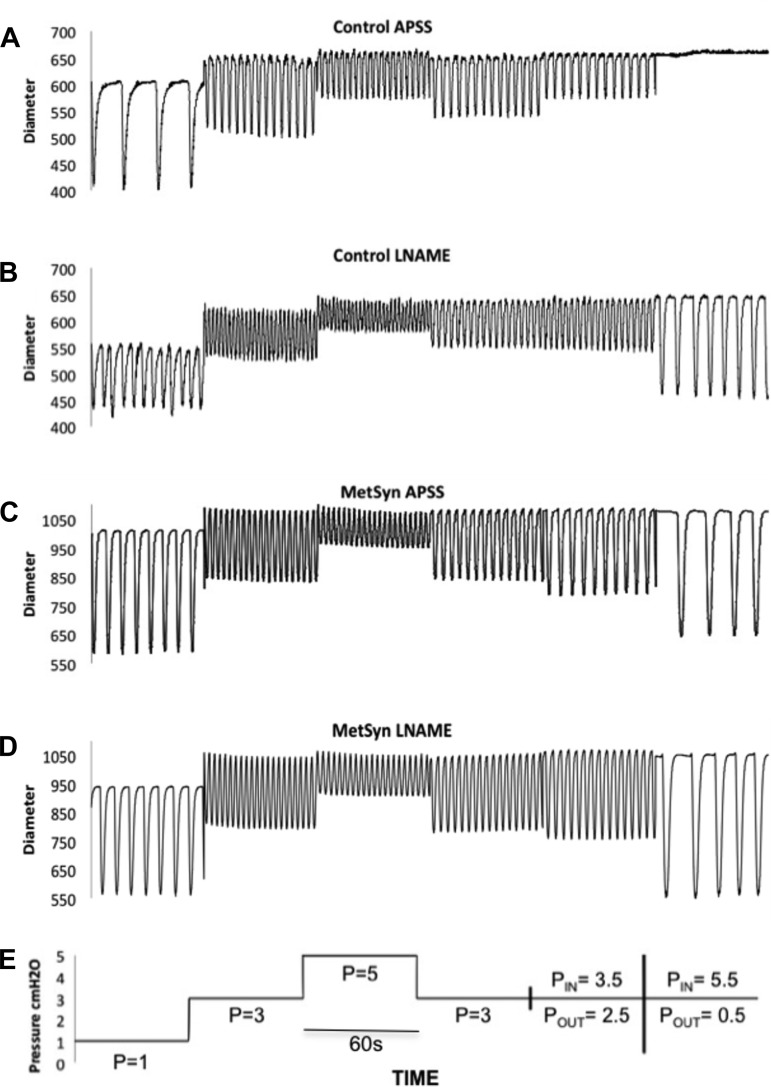
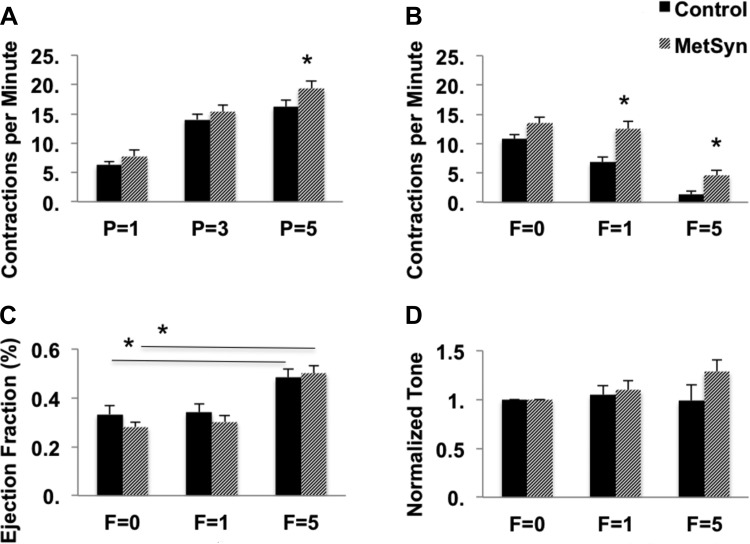
Figure 1 provides a schematic of the different pressure and flow step protocols used in this study and representative vessel traces. There were no significant differences observed in the TD diastolic diameters between MetSyn and control groups. However, there was a large variability of TD diameters within both the control and MetSyn cohorts with values ranging from 550 μm to over 1 mm. Such a large range in diameters will have significant effect on the magnitudes of contraction amplitude, stroke volume, and lymph pump flow. As such, we have focused solely on the normalized contractility parameter EF to reduce the variability in volume-based metrics. There was a mild but significant increase in lymphatic frequency in the MetSyn TDs observed at pressure of 5 cmH2O (Fig. 2A). MetSyn TDs also displayed a mild but significant reduction in EF at 5 cmH2O (Table 1). Calculated fractional pump flow was not statistically different at any pressure between control and MetSyn TDs as the differences in EF and frequency were mild and offsetting (data not shown). In addition MetSyn TD lymphatics did not exhibit a significant difference in tone at any pressure compared with control vessels (Table 1).
Fig. 1.
Representative diameter tracings from paired albumin supplemented physiological saline solution (APSS) and l-nitro-arginine methyl ester (l-NAME) responses in control with the corresponding pressure (P) and flow. Representative diameter traces over a 1-min period for lymphatic thoracic ducts (TDs) isolated from control (A) and metabolic syndrome (MetSyn) rats (C) and their responses to l-NAME (B and D) respectively. Schematic of a pressure step protocol used in this study (E) for the traces displayed.
Fig. 2.
Effects of pressure (P) and imposed flow (F) on lymphatic TD function from control and MetSyn rats. TD contractions per min were counted at each experimental pressure and imposed flow (A and B, respectively). Ejection fraction (EF; C) and normalized vessel tone (D) for control and MetSyn TDs exposed to imposed flow were calculated as described in materials and methods. Data are presented as means ± SE; n = 23 for A; n = 20 for B–D. *P < 0.05, significance comparing MetSyn to control values at the same pressure by two-way ANOVA.
Table 1.
Contractile parameters for control and MetSyn TDs
| Cohort | P = 1 | P = 3 | P = 5 | F = 0 | F = 1 | F = 5 |
|---|---|---|---|---|---|---|
| Outer diastolic diameter | ||||||
| Control | 751 ± 27.7 | 792 ± 24.6 | 817 ± 27.1 | 779.6 ± 30.3 | 783.0 ± 36.3 | 783.3 ± 30.9 |
| MetSyn | 776 ± 24.9 | 822 ± 25.7 | 831 ± 25.8 | 838 ± 29.5 | 836 ± 29.3 | 833.4 ± 28.7 |
| Frequency | ||||||
| Control | 6.27 ± 1.08 | 14.0 ± 1.23 | 16.2 ± 1.4 | 10.8 ± 0.77 | 6.84 ± 0.93 | 1.29 ± 0.59 |
| MetSyn | 7.76 ± 1.02 | 15.4 ± 1.1 | 19.3 ± 1.4 | 13.5 ± 1.05 | 12.5 ± 1.22 | 4.61 ± 0.81 |
| Control SNAP | 3.07 ± 0.75 | 10.6 ± 1.79 | 13.2 ± 1.38 | nd | nd | nd |
| MetSyn SNAP | 4.54 ± 2.07 | 6.94 ± 2.80 | 7.63 ± 2.91 | nd | nd | nd |
| Control l-NAME | 8.81 ± 1.96 | 13.5 ± 1.94 | 16.3 ± 1.98 | 14.1 ± 1.68 | 13.6 ± 1.73 | 6.23 ± 1.53 |
| MetSyn l-NAME | 12.9 ± 2.41 | 22.4 ± 1.57 | 25.0 ± 2.18 | 20.1 ± 2.11 | 21.8 ± 3.30 | 13.4 ± 2.03 |
| Control tempol | 7.03 ± 0.52 | 14.1 ± 1.54 | 15.3 ± 1.95 | 11.8 ± 1.46 | 9.3 ± 1.11 | 1.17 ± 0.05 |
| MetSyn tempol | 10.0 ± 1.96 | 16.9 ± 2.1 | 17.7 ± 3.7 | 12.4 ± 2.48 | 12.3 ± 1.99 | 4.2 ± 1.41 |
| Ejection Fraction | ||||||
| Control | 0.52 ± 0.02 | 0.34 ± 0.03 | 0.23 ± 0.03 | 0.33 ± 0.04 | 0.34 ± 0.04 | 0.48 ± 0.04 |
| MetSyn | 0.49 ± 0.02 | 0.28 ± 0.02 | 0.15 ± 0.02 | 0.28 ± 0.02 | 0.30 ± 0.03 | 0.50 ± 0.03 |
| Control SNAP | 0.61 ± 0.02 | 0.37 ± 0.03 | 0.22 ± 0.03 | nd | nd | nd |
| MetSyn SNAP | 0.33 ± 0.05 | 0.14 ± 0.02 | 0.07 ± 0.01 | nd | nd | nd |
| Control l-NAME | 0.27 ± 0.04 | 0.28 ± 0.03 | 0.17 ± 0.03 | 0.25 ± 0.03 | 0.28 ± 0.04 | 0.42 ± 0.05 |
| MetSyn l-NAME | 0.53 ± 0.05 | 0.32 ± 0.04 | 0.16 ± 0.03 | 0.30 ± 0.03 | 0.30 ± 0.05 | 0.44 ± 0.07 |
| Control tempol | 0.53 ± 0.05 | 0.40 ± 0.03 | 0.27 ± 0.02 | 0.39 ± 0.05 | 0.43 ± 0.05 | 0.48 ± 0.06 |
| MetSyn tempol | 0.49 ± 0.07 | 0.29 ± 0.06 | 0.16 ± 0.05 | 0.30 ± 0.05 | 0.29 ± 0.06 | 0.52 ± 0.04 |
| Tonic index | ||||||
| Control | 4.86 ± 0.56 | 5.26 ± 0.68 | 5.48 ± 0.74 | 3.56 ± 0.18 | 3.12 ± 0.16 | 3.31 ± 0.17 |
| MetSyn | 5.68 ± 0.59 | 3.98 ± 0.43 | 4.42 ± 0.55 | 3.37 ± 0.17 | 3.57 ± 0.18 | 3.87 ± 0.19 |
| Control SNAP | 2.00 ± 0.50 | 4.00 ± 1.04 | 3.97 ± 1.19 | nd | nd | nd |
| MetSyn SNAP | 1.75 ± 0.53 | 1.18 ± 0.29 | 0.82 ± 0.21 | nd | nd | nd |
| Control l-NAME | 11.2 ± 0.83 | 9.62 ± 1.87 | 9.27 ± 2.07 | 7.82 ± 1.57 | 7.28 ± 1.61 | 3.14 ± 0.97 |
| MetSyn l-NAME | 6.99 ± 1.35 | 6.30 ± 1.45 | 8.02 ± 1.57 | 5.40 ± 1.36 | 5.58 ± 1.62 | 4.90 ± 1.12 |
| Control tempol | 4.24 ± 0.69 | 4.10 ± 0.96 | 3.64 ± 0.95 | 3.60 ± 0.88 | 3.06 ± 0.67 | 2.23 ± 0.45 |
| MetSyn tempol | 5.06 ± 0.72 | 4.19 ± 1.08 | 4.69 ± 1.07 | 3.27 ± 0.83 | 2.38 ± 0.62 | 2.97 ± 0.52 |
Values are means ± SE in cmH2O.
P, pressure; F, flow; MetSyn, metabolic syndrome; TDs, thoracic ducts; SNAP, S-nitro-N-acetylpenicillamine; l-NAME, l-nitro-arginine methyl ester; nd, not determined.
Blunted flow-mediated inhibition of contractility in MetSyn TD.
As expected, the frequency decreased sharply in control TDs in response to imposed flows of 1 and 5 cmH2O. In contrast, the flow-mediated inhibition of contractility was significantly blunted in the MetSyn TDs whose contraction frequency was significantly higher at both experimental imposed flows (Fig. 2B). Control TD contraction frequency was reduced by 36.6 and 88.1% at F = 1 and F = 5, respectively. MetSyn TD contraction frequency was only reduced by 7.4 and 65.8% at F = 1 and F = 5. This suggests that 25% of the flow-mediated inhibition of frequency is lost in MetSyn TDs. The changes in frequency were not associated with any change in EF between control and MetSyn TDs (Fig. 2C). However, EF significantly increased in response to 5 cmH2O flow in both control and MetSyn TDs compared with their no flow values respectively. Normalized vessel tone was not significantly different between control and MetSyn (Fig. 2D).
Lymphatic TDs from MetSyn rats are responsive to NO.
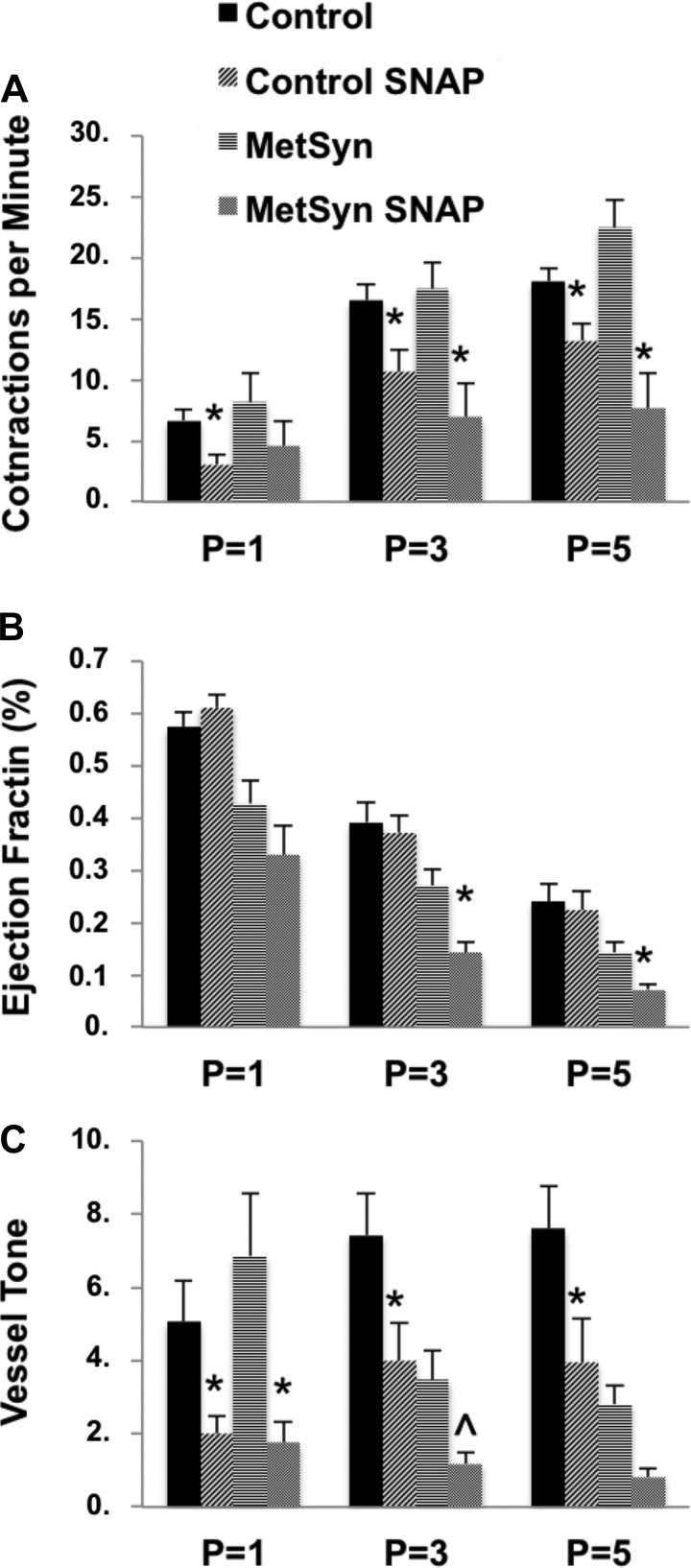
The blunted flow response in MetSyn TDs appears consistent with alterations in the normal NO-mediated flow response in rat TD (20, 21, 42, 44). To determine whether the blunted flow response in MetSyn TDs was due to insufficient production of NO or a loss of sensitivity to NO, vessels were exposed to the exogenous NO donor SNAP (100 μM) to assess lymphatic muscle response to NO at each pressure to limit the potential differences in basal NO production or NO-independent mechanisms that exist under conditions of imposed flow. Exposure to SNAP significantly reduced lymphatic frequency in both control and MetSyn vessels (Fig. 3A). EF was not significantly affected by SNAP in control TDs. In contrast, MetSyn TD EF was significantly reduced by SNAP at pressures of 3 and 5 cmH2O (Fig. 3B). In control TD tone slightly rose with increasing pressure and 100 μM SNAP significantly reduced vessel tone in control TDs at each experimental pressure. While in MetSyn tone fell with elevated pressures and was significantly lowered by SNAP only at a pressure of 1 cmH2O but was not statistically different at pressures of 3 or 5 cmH2O (Fig. 3C).
Fig. 3.
The effect of exogenous nitric oxide (NO) on lymphatic TD function in control and MetSyn rats. Frequency (A), EF (B), and vessel tone (C) were determined at each experimental pressure for control and MetSyn TDS. Data are presented as means ± SE; n = 9 for control; n = 7 for MetSyn. *P < 0.05, and 0.05 < P̂ < 0.10, significance comparing within each cohort at each experimental pressure by two-way ANOVA.
Control TDs mimic MetSyn phenotype with NOS inhibition.
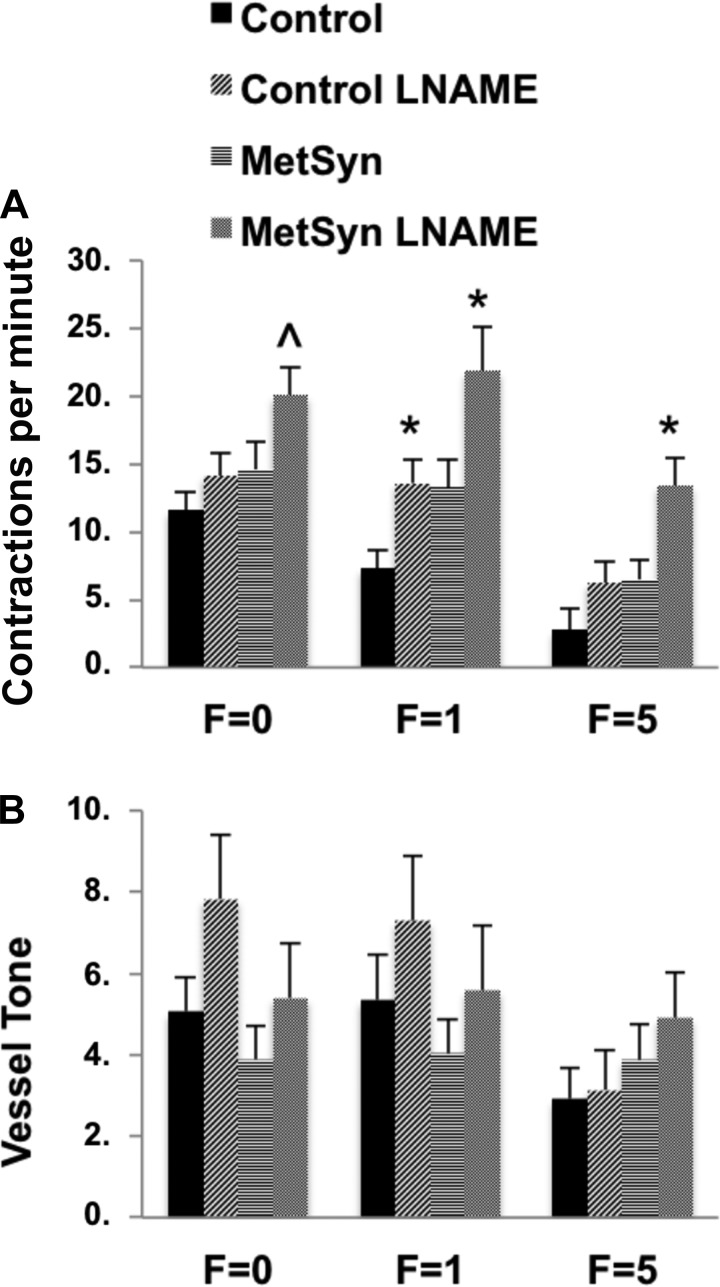
We used l-NAME (100 μM) to inhibit NOS and assess the role for NO in the regulation of MetSyn TDs contractility in response to both stretch (pressure) and shear (flow). Control vessel frequency was significantly increased following l-NAME application at a pressure of 3 cmH2O. l-NAME also significantly increased the frequency of MetSyn vessels at a pressure of 3 and 5 cmH2O. EF for control and MetSyn vessels was unaffected by l-NAME except for a reduction observed in control vessels at a pressure of 1 cmH2O (Table 1). Additionally, vessel tone was increased in response to l-NAME in control TDs at pressures of 3 and 5 cmH2O. l-NAME had no significant effect on vessel tone in MetSyn TDs (Table 1). Inhibition of NOS with l-NAME blunted the frequency flow response in control TDs and mimicked the MetSyn flow response (Fig. 4A). l-NAME increased frequency in the control TDs at imposed flow of 1 cmH2O but the effect was not significant at 5 cmH2O (Fig. 4A). l-NAME further exacerbated the poor flow response observed in MetSyn TDs. l-NAME significantly increased frequency in MetSyn TDs at both 1 and 5 cmH2O imposed flow. Surprisingly, l-NAME did not affect EF (Table 1) in either control or MetSyn TDs despite the observed effect on frequency (Fig. 4B).
Fig. 4.
The effect of nitric oxide synthase (NOS) inhibition with l-NAME on lymphatic TD imposed flow responses in control and MetSyn rats. TDs were equilibrated for 20 min in 100 μM l-NAME and their responses to imposed flow were recorded and analyzed for Frequency (A) and vessel tone (B). Data are presented as means ± SE; n = 7 for MetSyn; n = 10 for control. *P < 0.05, significance comparing within the cohort by two-way ANOVA.
A role for reduced NO production in MetSyn TD dysfunction.
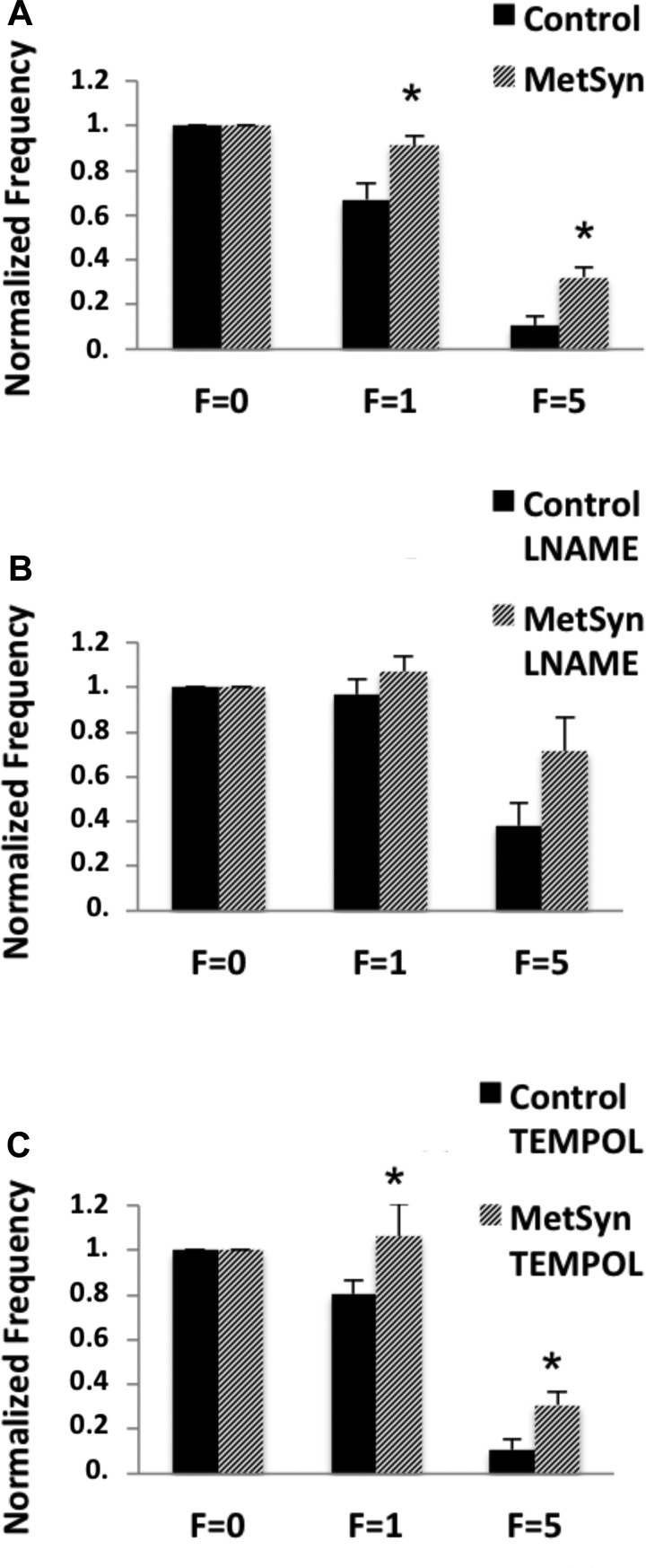
Since frequency tended to be higher in the MetSyn TDs, we normalized the values measured in the flow responses to that seen in each vessel at pressure “3 cmH2O flow = 0” to limit the effect of an elevated flow independent contractile drive. We have demonstrated a blunted flow response in MetSyn TDs (Fig. 5A) and that inhibition of NOS in control TDs mimics that seen in the MetSyn response (Figs. 4A and 5B). Since ROS are known to reduce NO bioavailability and promote endothelial dysfunction, we used the ROS scavenging molecule tempol (1 mM) to determine whether ROS production may decrease the NO bioavailability in the MetSyn TD. Tempol did not restore the flow response in the MetSyn TDs (Fig. 5C). In fact, 1 mM tempol did not have a significant effect on frequency, vessel tone, or EF under any experimental pressure or flow condition in control and MetSyn TDs (Table 1).
Fig. 5.
Differences between control and MetSyn TD function in response to flow are abolished when NOS is inhibited but not when reactive oxygen species (ROS) is inhibited. Frequency was normalized within each cohort to zero flow (F = 0) to compare the role for NO and ROS in the MetSyn TD flow response. MetSyn TDs displayed blunted flow-mediated inhibition of contractility (A). Inhibition of NOS with 100 μM l-NAME abolishes the differences in normalized frequency (B). Inhibition of ROS with 1 mM tempol did not restore flow-mediated inhibition in MetSyn TD (C). Data are presented as mean ± SE; n = 20 for control and MetSyn (A); n = 7 for control and 10 for MetSyn for the l-NAME response (B); and n = 6 for both control and MetSyn for the tempol response (C). *P < 0.05, significance by two-way ANOVA.
eNOS insufficiency in the TDs isolated from MetSyn rats.
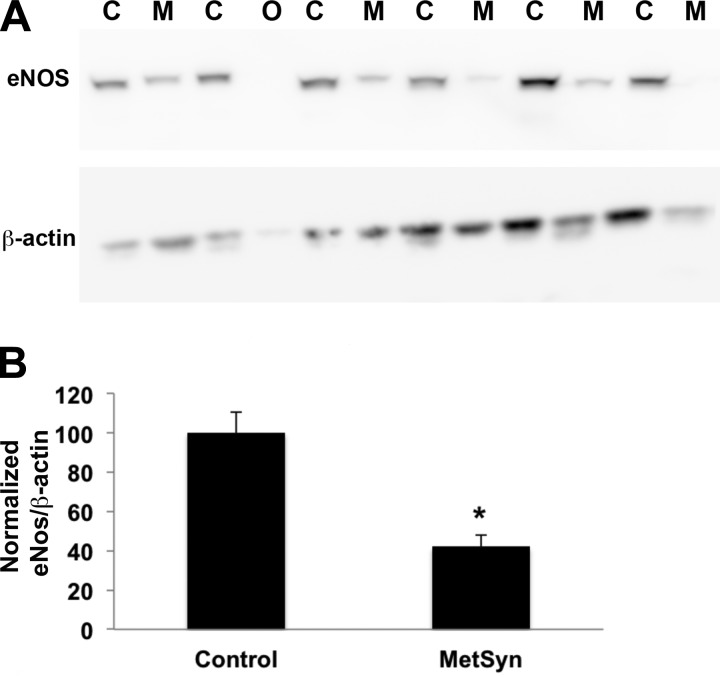
Protein was harvested from snap frozen TDs and eNOS expression was determined through Western blot. Protein from six control and six MetSyn TDs was loaded in alternating fashion and probed for eNOS and βAct (Fig. 6B). One MetSyn sample was excluded from quantitative analysis due to insufficient protein concentration. We normalized eNOS signal intensity to βAct and found a consistent and significant reduction (∼55%) in eNOS expression in the MetSyn TDs (Fig. 6B).
Fig. 6.
Reduced expression of eNOS in TDs isolated from MetSyn rats. Proteins were isolated from TDs isolated from control and MetSyn rats and used for Western blots to assess enothelial NOS (eNOS) expression. β-Actin (βAct) was used as a loading control. A: a representative Western blot; C, control sample; M, MetSyn sample; O, omitted sample in the analysis due to low amount of protein loading. B: quantitative analysis of eNOS expression. The eNOS-to-βAct ratios were normalized to control values. The values obtained from 3 different blots were used for the analysis. Data are presented as means ± SE; n = 5 for MetSyn and 6 for control. *P < 0.05, significance by two-way ANOVA.
DISCUSSION
The results presented in this study demonstrate the first evidence for endothelial dysfunction and impaired lymphatic TD function as a consequence of metabolic disease. Our data clearly indicate that flow-mediated inhibition of frequency is blunted in MetSyn TDs, even though they appear more sensitive to exogenous NO (SNAP response). Inhibition of NOS diminished the differences in the flow-mediated frequency regulation between control and MetSyn TDs, whereas tempol did not restore the flow responses in MetSyn TDs. Our Western blot data indicate a significant reduction in eNOS expression in MetSyn TDs. Collectively, these data suggest MetSyn conditions influence eNOS expression in TD, consequently affecting its functional characteristics.
We have previously demonstrated that lymphatic collecting vessels of the mesentery of high-fructose-fed MetSyn rats exhibit a significant reduction in the phasic contraction frequency and consequently impaired pump function (49). Similar findings have recently been observed using diet-induced mouse models of MetSyn (7, 45). The reduction in contractility demonstrated in those studies appears to be in contrast with the elevated contractility observed here in the TD. The lymphatic functional variations between these studies could be due to the existence of significant differences in the regional stretch- and shear-dependent regulation of lymphatic contractility (18). The TD is the largest vessel in the lymphatic vasculature and displays significant shear sensitivity indicative of a conduit function (17, 18, 32) as opposed to the “pump” function of the mesenteric collecting vessels.
We assessed the contractile regulation of MetSyn TDs in response to both shear and stretch forces and specifically examined the role of NO in contractile regulation. There were modest and opposing differences between the stretch responses in control and MetSyn TDs. At 5 cmH2O of intramural pressure, frequency in MetSyn TD was significantly elevated while EF was significantly reduced. While these effects are mild and did not affect calculated pump flow, these data fit very well with the current model of contraction generated NO as a regulator of TD contractility through a PKG-dependent pathway (20) and our current hypothesis of insufficient NO production in MetSyn TD. NO levels fluctuate during the contraction cycle due to pulsatile flow (8, 9), and reduced production of NO would impair this autoregulatory function resulting in elevated frequency and reduced EF. More importantly, the TDs isolated from rats with MetSyn demonstrated a significant reduction in the flow-mediated inhibition of contractility at both experimental flows tested (1 and 5 cmH2O). Contractions under conditions of passive flow result in a smaller time averaged diameter and directly increase resistance to flow.
Three possible explanations for the impaired flow response are as follows: 1) reduced NO sensitivity, 2) reduced NO bioavailability, and/or 3) increased contractile drive. We directly tested the sensitivity to NO through the exogenous NO donor SNAP. SNAP significantly inhibited frequency and vessel tone in both control and MetSyn TDs (Fig. 3). Interestingly, only MetSyn TDs displayed a significant reduction in EF in response to SNAP, which is in stark contrast to the elevation in EF in response to flow. This may be a consequence of an elevated constant and global NO vs. pulsatile and localized NO that is produced in response to contractions and imposed flow (8, 9). Another possibility is that MetSyn TDs exhibit an increased sensitivity to NO. The lymphatic TDs express both PKG1α and PKG1β, although PKG1α expression is 10 times higher than the observed expression in the vena cava or aorta (20). PKG1α is also more sensitive to cyclic guanosine monophosphate but additional experiments are needed to support the notion that MetSyn may perturb the expression balance of these two isoforms. Nonetheless, we have demonstrated that MetSyn TDs are sensitive to exogenous NO.
To assess the role of NO production we treated both control and MetSyn vessels with 100 μM l-NAME to inhibit NO production. l-NAME significantly increased frequency in both control and MetSyn TDs in response to stretch suggesting the presence of basal NO production in response to contractions in MetSyn TDs. This recapitulates our previous data using l-NAME in the rat TD (21) and stands in contrast to the l-NAME response in the mouse popliteal that shows a reduction in frequency (36). It is likely that these differences are explainable by regional differences in vessel regulation, as we have seen earlier. We have observed similar l-NAME responses as those observed in mouse popliteal vessel (36) in the rat mesenteric vessels treated with l-NAME under stretch conditions (unpublished data). Additionally, our data support the notion that while NO is the predominant mediator of the flow response in lymphatic TDs it may not be the sole mediator (2, 19). Both control and MetSyn TDs demonstrated significantly lower contraction frequencies in response to high flow despite the presence of 100 μM l-NAME (Fig. 4). While outside the scope of this study, the identity of this NOS-independent flow-sensitive inhibitory pathway may be related to histamine production as has recently been described in the rat mesenteric vessels (30). Control TDs treated with l-NAME were resistant to the flow-mediated inhibition of frequency and mimicked the MetSyn phenotype. Surprisingly, l-NAME also further exacerbated the MetSyn phenotype and significantly increased frequency at both flows tested in MetSyn TDs. Taken together, these data suggest that reduced NO bioavailability is not sufficient to completely explain the MetSyn TD phenotype. Microvascular dysfunction often displays increased vasoconstrictor (endothelin-1, ROS, and angiotensin) action in addition to blunted vasodilation (31). To focus on solely the altered flow-induced NO response, we normalized the frequency at each experimental flow to their corresponding pressure “3 cmH2O flow = 0” within their respective treatment and group (Fig. 5). The normalized frequency differences in response to flow observed between MetSyn and Control TDs were no longer significant after treatment with l-NAME, despite a significant difference in the magnitude of frequency. Additionally, MetSyn TDs displayed had significantly higher contraction frequencies compared with their control counterparts to stretch after NOS inhibition. We interpreted this as a potential unmasking of an elevated contractile drive that was repressed by the presence of basal NO. However, alterations in a flow-mediated NO independent flow mechanisms of contraction regulations may also play a role in the altered flow response in MetSyn TDs and warrant further investigation. The persistence of a flow response despite l-NAME treatment further suggests that NO independent mechanism(s) also lends credence to this alternative interpretation. The regulation of lymphatic frequency is dependent on the depolarization of the lymphatic muscle (41), which is directly tied to the membrane potential and voltage-sensitive calcium channels (26). NO directly increases the hyperpolarization of the lymphatic muscle cells through PKG- and PKA-mediated activation of ATP-sensitive potassium channels (20, 40, 44). Resting membrane potentials and spontaneous depolarizations have been recorded in rat mesenteric vessels in response to stretch and pharmacological intervention to assess ionic channel contributions to contractility (26, 43). Similar studies under conditions of flow and a more detailed characterization of the lymphatic endothelial response to shear are further required to evaluate the role of NO in the regulation of lymphatic contractile frequency.
Degradation of NO by ROS and loss of eNOS expression/activity are two mechanisms that potentially could explain the reduced NO bioavailability in the MetSyn TDs. In addition to rapidly degrading NO, ROS are also important vasoactive molecules. Additionally, previous work in aged rats suggested a role for ROS in the impairment of mesenteric lymphatic function (38, 47, 48). We tested the hypothesis that ROS impair NO bioavailability through the use of 1 mM tempol to scavenge acute ROS production. Observed frequency was mildly but not significantly increased in response to tempol in control and MetSyn TDs under both stretch and shear conditions. Scavenging of ROS with tempol did not restore flow-mediated inhibition nor did it have any pronounced effect on TDs contractility.
Western blot data revealed a significant loss of eNOS expression by the MetSyn TDs, which correlates very strongly with the observed flow insensitivity. Downregulation of eNOS has been observed in other models of metabolic disruption (39) although eNOS activity is also regulated by multiple mechanisms such as phosphorylation, intracellular calcium, BH4 levels, and substrate availability (23). Phosphorylation of eNOS at serine 1177, the availability of l-arginine substrate for key enzymes, the presence of critical cofactors such as BH4, and the oxidative stress of the cell all feed into endothelial dysfunction (15). However, reduced eNOS expression is commonly observed in models of obesity and diabetes and can partly be explained through insulin resistance and the chronic inflammation. The role of insulin resistance and the mechanisms underlying the eNOS expression reduction in MetSyn lymphatics warrant further investigation.
The role for NO in the regulation of lymphatic EF, tone, and frequency is complex. There are different hypothesis concerning the role for NO in impairment of lymphatic function: 1) NO decreases frequency thus allowing greater time to fill, which results in a stronger contraction (positive lusitropy) (20, 21, 42, 44); or 2) NO directly inhibits both frequency and EF (36). Our data here provide more evidence for the former as opposed to the latter. EF rose significantly in response to high imposed flow while frequency dropped precipitously in both control and MetSyn TDs (Fig. 2). MetSyn TDs also demonstrated higher frequency and lower EF when exposed to pressure (5 cmH2O). Inhibition of EF was observed in the MetSyn TDs treated with SNAP; however, control TD EF was not affected (Fig. 3). Interestingly, l-NAME exposure blunted the flow-mediated inhibition of frequency although EF was not consistently affected (although it trended lower in association with the higher frequency).
The regulation of lymph transport is complex as both external and intrinsic forces regulate lymph formation and flow, in addition to regional differences in lymphatic contractile regulation (46). The causative factors of lymphedema are still unclear (34); however, there is growing evidence for altered tissue remodeling and fibrosis in the face of poor fluid clearance from the tissue (4, 27). There is accumulating evidence for lymphatic dysfunction in human populations suffering from obesity (29), and often coincident MetSyn (22, 25), with the emergence of massive localized edema in the morbidly obese (3, 10) and identification of obesity as a major risk factor for postoperative lymphedema (1). We have previously reported on the role poor contractile behavior of mesenteric lymphatic vessels under conditions of MetSyn (49), and similar findings have been observed in the murine models as well (29, 35, 45). In addition to these findings of poor pump function in these smaller peripheral vessels, we have now presented data that the conduit function of large lymphatic vessels is also impaired under the conditions of MetSyn and that endothelial dysfunction is evident in the lymphatic vasculature. Failure of the lymphatic vessels to properly dilate and reduce contraction frequency in response to passive flow can greatly diminish the rate of fluid clearance due to elevated resistance as a function of radius to the fourth power. Utilizing one of the more severe MetSyn phenotypes we observed, the change in time averaged diameter as a function of the presence of contractions (11.2 contractions per min; 37% EF) increased resistance to flow by ∼24% when compared against the resistance of a vessel that had complete flow inhibition of phasic contraction frequency. Despite the blunted flow-mediated response, our data also demonstrated that NO was still a critical regulator of lymphatic contractions in response to flow at this stage in the MetSyn phenotype. Likely, as the MetSyn progresses further the lymphatic dysfunction phenotype will be exaggerated as consequence of greater lymphatic endothelial dysfunction.
Conclusions.
We report here a significant dysfunction in the TD regulation of contractility under conditions of MetSyn. Although eNOS expression was significantly diminished in MetSyn, the loss of NO by itself does not completely explain TD dysfunction in the MetSyn rats. Some questions arise in response to the observations here: is the reduced eNOS expression due to insulin resistance and/or elevated inflammatory cytokine loads? How do insulin and glucose concentrations regulate lymphatic endothelial eNOS and redox potential? Is there an elevated fatty acid flux into the lymph that reduces insulin sensitivity? In vitro analysis using tissue specific lymphatic cells holds promise for clarification of these issues. Additionally, other mechanisms of lymphatic contractility regulation must be explored and more studies are needed to fully describe lymphatic endothelial dysfunction in the MetSyn.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-99221 (to M. Muthuchamy and D. C. Zawieja).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D.Z., O.Y.G., D.C.Z., and M.M. conception and design of research; S.D.Z. and O.Y.G. performed experiments; S.D.Z., O.Y.G., and M.M. analyzed data; S.D.Z., O.Y.G., D.C.Z., and M.M. interpreted results of experiments; S.D.Z. and M.M. prepared figures; S.D.Z. and M.M. drafted manuscript; S.D.Z., O.Y.G., D.C.Z., and M.M. edited and revised manuscript; S.D.Z., O.Y.G., D.C.Z., and M.M. approved final version of manuscript.
REFERENCES
- 1.Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women's Health Study. Breast Cancer Res Treat 130: 981–991, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akl TJ, Nagai T, Cote GL, Gashev AA. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol 301: H1828–H1840, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asch S, James WD, Castelo-Soccio L. Massive localized lymphedema: an emerging dermatologic complication of obesity. J Am Acad Dermatol 59: S109–110, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, Bromberg J, Mehrara BJ. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 27: 1114–1126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Bigazzi R, Bianchi S. Insulin resistance, metabolic syndrome and endothelial dysfunction. J Nephrol 20: 10–14, 2007. [PubMed] [Google Scholar]
- 7.Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, Detmar M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One 9: e94713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol 301: H1897–H1906, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 297: H1319–H1328, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra K, Tadisina KK, Brewer M, Holton LH, Banda AK, Singh DP. Massive localized lymphedema revisited: a quickly rising complication of the obesity epidemic. Ann Plast Surg 74: 126–132, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Cozma A, Orasan O, Sampelean D, Fodor A, Vlad C, Negrean V, Rednic N, Zdrenghea D. Endothelial dysfunction in metabolic syndrome. Rom J Intern Med 47: 133–140, 2009. [PubMed] [Google Scholar]
- 12.Davis MJ. An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation 12: 361–372, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Diamant M, Tushuizen ME. The metabolic syndrome and endothelial dysfunction: common highway to type 2 diabetes and CVD. Curr Diab Rep 6: 279–286, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Fife C. Massive localized lymphedema, a disease unique to the morbidly obese: a case study. Ostomy Wound Manage 60: 30–35, 2014. [PubMed] [Google Scholar]
- 15.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–R12, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gashev AA. Lymphatic vessels: pressure- and flow-dependent regulatory reactions. Ann NY Acad Sci 1131: 100–109, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11: 477–492, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasheva OY, Gashev AA, Zawieja DC. Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct. J Physiol 591: 4549–4565, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89: 2595–2600, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Hoang HH, Padgham SV, Meininger CJ. L-arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr Opin Clin Nutr Metab Care 16: 76–82, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28: 1982–1988, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SK, Kim HJ, Hur KY, Choi SH, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr 79: 593–599, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Roizes S, von der Weid PY. Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol 592: 5409–5427, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch LL, Mendez U, Waller AB, Gillette AA, Guillory RJ 2nd, Goldman J. Fibrosis worsens chronic lymphedema in rodent tissues. Am J Physiol Heart Circ Physiol 308: H1229–H1236, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54: 1384–1392, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg 134: 154e–160e, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, Chatterjee V, Meininger CJ, Gashev AA. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 21: 640–648, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289: H813–H822, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Quick CM, Venugopal AM, Gashev AA, Zawieja DC, Stewart RH. Intrinsic pump-conduit behavior of lymphangions. Am J Physiol Regul Integr Comp Physiol 292: R1510–R1518, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 55: 928–934, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Rockson SG. Update on the biology and treatment of lymphedema. Curr Treat Options Cardiovasc Med 14: 184–192, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, Joseph WJ, Mehrara BJ. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol 307: H165–H172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol 591: 2139–2156, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes 48: 2437–2445, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Thangaswamy S, Bridenbaugh EA, Gashev AA. Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol 10: 53–62, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 116: 2791–2798, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von der Weid PY. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and beta-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol 125: 17–22, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von der Weid PY. Review article: lymphatic vessel pumping and inflammation–the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment Pharmacol Ther 15: 1115–1129, 2001. [DOI] [PubMed] [Google Scholar]
- 42.von der Weid PY, Crowe MJ, Van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol 493: 563–575, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von der Weid PY, Lee S, Imtiaz MS, Zawieja DC, Davis MJ. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphat Res Biol 12: 66–75, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol 280: H2707–H2716, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D, Mehrara BJ. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 8: e70703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol 7: 87–96, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zawieja DC, Davis KL. Inhibition of the active lymph pump in rat mesenteric lymphatics by hydrogen peroxide. Lymphology 26: 135–142, 1993. [PubMed] [Google Scholar]
- 48.Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. Am J Physiol Heart Circ Physiol 260: H1935–H1943, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 302: H643–H653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]