Activation of mitochondrial calpain within intermembrane space cleaves apoptotic-inducing factor. In the present study, activation of mitochondrial calpain sensitizes mitochondrial permeability transition pore opening, impairs complex I activity, and decreases pyruvate dehydrogenase content. Thus activation of mitochondrial calpain not only cleaves apoptotic-inducing factor, but also damages metabolic enzymes in the matrix.
Keywords: mitochondria, complex I, MPTP, PDH, calcium
Abstract
Calpain 1 (CPN1) is a ubiquitous cysteine protease that exists in both cytosol and cardiac mitochondria. Mitochondrial CPN1 (mit-CPN1) is located in the intermembrane space and matrix. Activation of mit-CPN1 within the intermembrane space increases cardiac injury by releasing apoptosis-inducing factor from mitochondria during ischemia-reperfusion (IR). We asked if activation of mit-CPN1 is involved in mitochondrial injury during IR. MDL-28170 (MDL) was used to inhibit CPN1 in buffer-perfused hearts following 25-min ischemia and 30-min reperfusion. MDL treatment decreased the release of lactate dehydrogenase into coronary effluent compared with untreated hearts, indicating that inhibition of CPN1 decreases cardiac injury. MDL also prevented the cleavage of spectrin (a substrate of CPN1) in cytosol during IR, supporting that MDL treatment decreased cytosolic calpain activation. In addition, MDL markedly improved calcium retention capacity compared with untreated heart, suggesting that MDL treatment decreases mitochondrial permeability transition pore opening. In addition, we found that IR led to decreased complex I activity, whereas inhibition of mit-CPN1 using MDL protected complex I. Pyruvate dehydrogenase content was decreased following IR. However, pyruvate dehydrogenase content was preserved in MDL-treated mitochondria. Taken together, MDL treatment decreased cardiac injury during IR by inhibiting both cytosolic and mit-CPN1. Activation of mit-CPN1 increases cardiac injury during IR by sensitizing mitochondrial permeability transition pore opening and impairing mitochondrial metabolism through damage of complex I.
NEW & NOTEWORTHY
Activation of mitochondrial calpain within intermembrane space cleaves apoptotic-inducing factor. In the present study, activation of mitochondrial calpain sensitizes mitochondrial permeability transition pore opening, impairs complex I activity, and decreases pyruvate dehydrogenase content. Thus activation of mitochondrial calpain not only cleaves apoptotic-inducing factor, but also damages metabolic enzymes in the matrix.
calpain 1 (cpn1, μ-calpain) and calpain 2 (CPN2, m-calpain) are two ubiquitous Ca2+-dependent cysteine proteases (20). CPN1 and CPN2 are well-known cytosolic proteins (20, 37, 50). Activation of cytosolic calpains contributes to cardiac injury during ischemia-reperfusion (IR) (7, 8, 18, 23, 53). The potential mechanisms whereby activation of cytosolic calpains increase cardiac injury during IR include, but are not limited to, enzymatic degradation of α-fordin (23), Na+-K+-ATPase (25, 44), Na+/Ca2+ exchanger (5, 29), and cleavage of bid to its activated form (truncated bid, t-bid) (8).
Recently, CPN1 has been found in heart, liver, and brain mitochondria (27, 37). CPN2 is also identified in liver mitochondria (38), as well as in rat heart mitochondria (42). However, CPN2 is not present in purified mouse heart mitochondria (11, 14). Mitochondrial CPN1 (mit-CPN1) has been identified in the intermembrane space (IMS) (14). Activation of mit-CPN1 increases cardiac injury during IR by cleaving and facilitating the translocation of AIF (apoptosis-inducing factor), which is also located within the IMS, from mitochondria into cytosol and nucleus (14). In addition to the IMS, recent work showed that mit-CPN1 is also located within the mitochondrial matrix. However, the function of mit-CPN1 within the matrix is not clear.
The mitochondrial permeability transition pore (MPTP) is a nonselective pore located on the inner mitochondrial membrane (IMM) (21, 51). MPTP opening increases the permeability of the IMM that leads to loss of the IMM potential and impaired energy production that, in turn, increased cell injury during IR (21, 51). The opening of MPTP also results in mitochondrial swelling that eventually ruptures the outer mitochondrial membrane and leads to the loss of proteins located the IMS, including cytochrome c and AIF (21, 51). A release of cytochrome c and AIF from mitochondria into cytosol increases cardiac injury during IR (4, 14, 34). Thus MPTP opening contributes a pivotal role in cell injury during IR (21, 51). Although the exact composition of the MPTP remains under investigation, several components, including voltage-dependent anion channel, adenine nucleotide translocase, and c-subunit of F1F0-ATPase, have been suggested (1). In addition, cyclophilin D in the matrix contributes a critical role in regulation of MPTP opening (3). Since mit-CPN1 is located in both the IMS and the matrix, we propose that activation of mit-CPN1 sensitizes to MPTP opening during IR through interaction with mitochondrial components.
IR damages the mitochondrial electron transport chain (ETC), leading to cardiac injury (10, 34). IR leads to decreased complex I (12, 32, 40, 47) and complex III activities (33). The mechanisms of complex I damage during IR involved posttranslational modification and complex I subunit damage (6, 24, 28, 43). We evaluated the role of activation of mit-CPN1 as a mechanism of complex I damage during IR. In addition to the ETC damage, IR also impairs metabolic enzyme activities within the mitochondrial matrix, including PDH (pyruvate dehydrogenase) (31, 49). In buffer-perfused mouse hearts, IR dramatically decreases the protein content of PDH in mitochondria (54). The mit-CPN1 exists in the matrix. We studied the potential contribution of the activation of mit-CPN1 within the matrix to PDH degradation during IR.
METHODS
Preparation of mouse hearts for perfusion.
The Animal Care and Use Committees of the McGuire VA Medical Center and Virginia Commonwealth University approved the study. Male C57BL/6 mice (2–3 mo) were anesthetized with pentobarbital sodium (100 mg/kg ip) and anticoagulated with heparin (1,000 IU/kg ip). The mouse heart was quickly excised and perfused retrogradely via the aorta in the Langendorf mode with modified Krebs-Henseleit (K-H) buffer (115 mM NaCl, 4.0 mMKCl, 2.0 mM CaCl2, 26 mM NaHCO3, 1.1 mM MgSO4, 0.9 mM KH2PO4, and 5.5 mM glucose), gassed with 95% O2/5% CO2 to adjust pH to 7.35–7.45 (12). Hearts were paced at 420 beats/min. The cardiac function was monitored with a balloon inserted into the left ventricle, and data were recorded digitally with Powerlab (AD Instruments, Colorado Springs, CO). In the untreated IR group, hearts were perfused for 15-min equilibration with K-H buffer, including 0.001% DMSO as vehicle, followed by 25-min global ischemia at 37°C and 30-min reperfusion. In the MDL-28170 (MDL)-treated hearts, hearts were first perfused with K-H buffer, including 10 μM MDL for 15 min. Then hearts underwent 25-min global ischemia and 30-min reperfusion. MDL was also present in K-H buffer during reperfusion (Fig. 1A). In time control group, hearts were only buffer perfused without IR. Coronary effluent was collected during the entire reperfusion period in IR and MDL groups and during the last 30-min perfusion period in time control group for lactate dehydrogenase (LDH) measurement (12). At the end of the experiment, the heart was harvested for mitochondrial isolation.
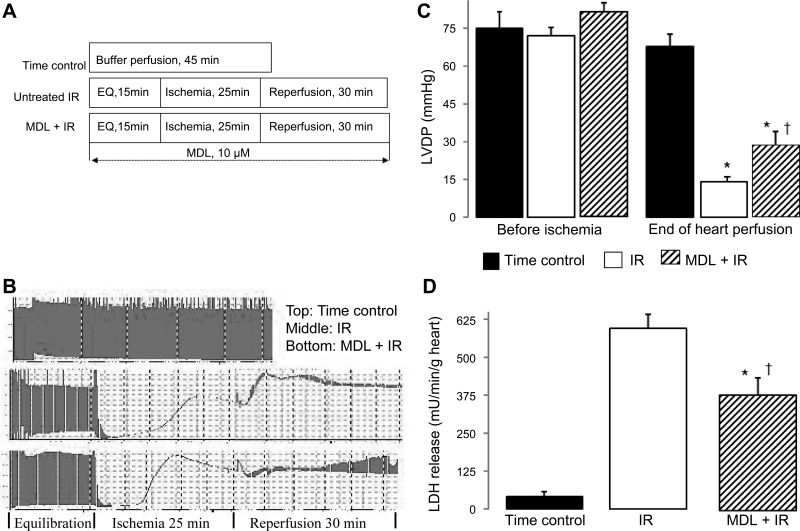
Fig. 1.
Administration of calpain inhibitor [MDL-28170 (MDL)] decreases cardiac injury in mouse hearts following ischemia-reperfusion (IR). Mouse hearts were buffer perfused following 25-min ischemia and 30-min reperfusion. MDL (10 μM) was present during the entire perfusion time. A: hearts in the time control (TC) group were buffer-perfused without IR. B: an original tracing of hemodynamic data in each group. (Time scale was changed when the original tracing was fitting into the figure.) C: cardiac contractility [reflected by left ventricular developed pressure (LVDP); mmHg] was decreased following IR. MDL treatment improved contractile function compared with untreated hearts. D: MDL treatment decreased lactate dehydrogenase (LDH) release into coronary effluent compared with untreated hearts. Values are means ± SE; n = 5–7 in each group. *P < 0.05 vs. TC. †P < 0.05 vs. untreated hearts. EQ, equilibration.
Isolation of mouse heart mitochondria.
The mouse heart was placed in cold buffer A {composition in mM: 100 KCl, 50 MOPS [3-(N-morpholino)propanesulfonic acid], 1 EGTA, 5 MgSO4, and 1 mM ATP}. The heart was blotted dry, weighed, and homogenized using a polytron tissue homogenizer at 10,000 rpm for 2.5 s with trypsin (5 mg/g tissue). The homogenate was incubated for 15 min at 4°C, then the same volume of buffer B (buffer A + 0.2% bovine serum albumin) was added, and the mixture was centrifuged at 500 g for 10 min. The supernatant was again centrifuged at 3,000 g to pellet mitochondria. The mitochondrial pellet was first washed with buffer B, then resuspended in KME (100 mM KCl, 50 mM MOPS, 0.5 mM EGTA), and centrifuged at 3,000 g to yield the final mitochondrial pellet. Mitochondria were resuspended in KME for study (47).
Mitochondrial oxidative phosphorylation.
Oxygen consumption in mitochondria was measured using a Clark-type oxygen electrode at 30°C, as previously described (47). Mitochondria were incubated in 80 mM KCl, 50 mM MOPS, 1 mM EGTA, 5 mM KH2PO4, and 1 mg defatted, dialyzed bovine serum albumin/ml at pH 7.4. Glutamate (20 mM) + malate (10 mM) (complex I substrate), succinate (20 mM) plus 7.5 μM rotenone (complex II substrate), and N,N,N′,N′ tetramethyl-p-phenylenediamine (1 mM)-ascorbate (10 mM, complex IV substrate) + rotenone were used. After incubation of mitochondria with substrates, ADP (0.2 mM) was added to stimulate respiration to measure rate of state 3. The rate of state 4 was recorded when ADP was converted to ATP. ADP (2 mM) was used to determine the maximal rate of ADP-stimulated respiration.
Calcium retention capacity in isolated mitochondria.
Calcium retention capacity (CRC) was used to assess calcium-induced MPTP opening in isolated mitochondria (39). CRC was evaluated in mitochondria (125 μg/ml) incubated in medium containing 150 mM sucrose, 50 mM KCl, 2 mM KH2PO4, 5 mM succinate, in 20 mM Tris·HCl, pH 7.4, by sequential pulses of 5 nmol calcium. Extra-mitochondrial Ca2+ concentration was recorded with 0.5 μM calcium Green-5N (Life Technologies), and fluorescence was monitored with excitation and emission wavelengths set at 500 and 530 nm, respectively (39).
Inhibition of the mit-CPN1 using MDL in isolated and purified mitochondria.
Administration of MDL to buffer perfused hearts inhibits both cytosolic and mitochondrial calpains (37). To avoid the effect of cytosolic calpain activation on MPTP and PDH, trypsin-purified mouse heart mitochondria were used to test if inhibition of the mit-CPN1 with MDL decreased MPTP opening and protected PDH in mitochondria treated with exogenous calcium.
Measurement of mitochondrial inner membrane potential to reflect MPTP opening in purified mitochondria with exogenous calcium incubation.
Since exogenous calcium was used to activate the mit-CPN1, this calcium exposure affects the calcium content calculation in the CRC determination in incubated mitochondria. Thus mitochondrial inner membrane potential (Δψ), rather than the CRC, was used to reflect MPTP opening in exogenous calcium-treated mitochondria. The Δψ was measured using the fluorogenic indicator TMRM (tetramethylrhodamine methyl ester) (13, 41). Freshly isolated mitochondria (0.2 mg/ml) from nonischemic mouse hearts were incubated with exogenous calcium (50 and 100 μM) for 20 min at 30°C in the presence or absence of MDL or cyclosporine A. Succinate was used to energize the mitochondria during incubation. At the end of incubation, mitochondria were sedimented using 5,000 g and resuspended in KME for the Δψ measurement. The Δψ was reflected by change of the TMRM fluorescence intensity before and after ADP addition (13).
Measurement of mitochondrial calpain activity.
The mit-CPN1 activity was determined using a commercially available kit: (QIA 120, Calbiochem, San Diego, CA) (9, 37). Succ-Leu-Tyr-AMC (7-amino-4-methylcoumarin) was cleaved by calpain to form a fluorescent product that is quantified (excitation: 380 nm; emission: 460 nm) (8). Mitochondria (0.5 mg/ml) were incubated in reduced buffer in the presence of 5 mM CaCl2 and 20 μM Succ-Leu-Tyr-AMC for 30 min at 30°C. MDL (3 and 10 μM) was used as a CPN1 inhibitor. The intensity of fluorescence was determined (Victor 2, PerkinElmer, Waltham MA) (14, 37).
Statistical analysis.
Data are expressed as the mean ± SE (45). For all analyses, differences between groups were compared by one-way ANOVA. For cardiac functional analysis, differences between groups were compared by two-way ANOVA. When a significant F-value was obtained, means were compared using the Student-Newman-Keuls test of multiple comparisons. Statistical significance was defined as a value of P < 0.05.
RESULTS
Inhibition of calpain decreases cardiac injury in buffer-perfused hearts during IR.
Cardiac injury was dramatically increased in buffer-perfused mouse hearts following 25-min ischemia + 30-min reperfusion compared with time control, as shown by the decrease in left ventricular developed pressure (Fig. 1, B and C) and increased LDH release into coronary effluent (Fig. 1D). In contrast, MDL treatment markedly improved left ventricular developed pressure (Fig. 1, B and C) and reduced LDH release during reperfusion (Fig. 1D) compared with untreated hearts, supporting that inhibition of calpain decreases cardiac injury in isolated mouse hearts during IR.
Administration of MDL prevents cytosolic calpain activation during IR.
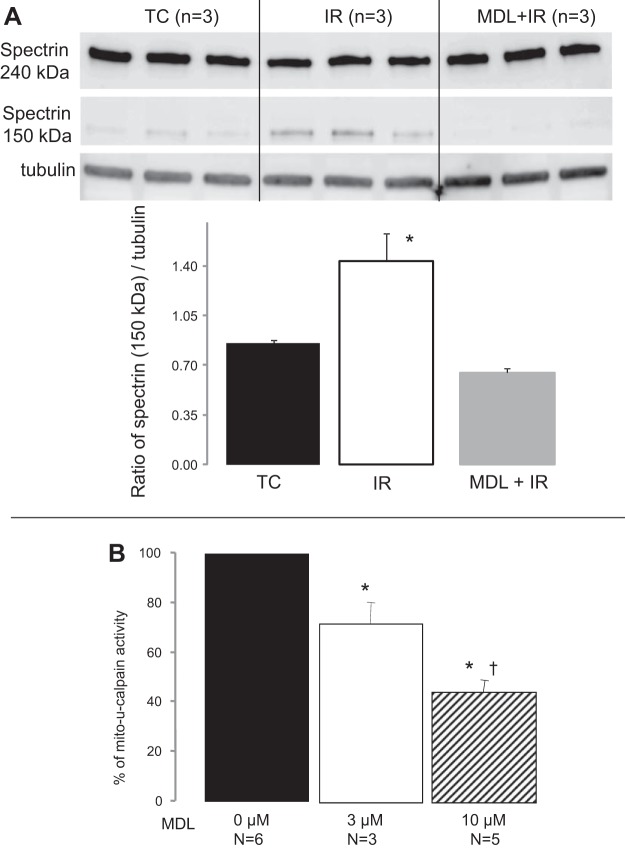
IR activates cytosolic calpains in a number of animal models. The cleaved product of spectrin is commonly used as a marker of cytosolic calpain activation. IR increased the formation of the cleaved spectrin compared with time control (Fig. 2A), supporting that cytosolic calpains are activated during IR. MDL treatment decreased the formation of the cleaved spectrin compared with untreated hearts (Fig. 2A); supporting that MDL treatment attenuates cytosolic calpain activation.
Fig. 2.
MDL prevents cytosolic calpain activation during IR. A: spectrin is a substrate of calpain, and an increase of its cleavage product is commonly used as an marker of calpain activation in cytosol. IR increased the cleaved spectrin content (150 kDa) in cytosol compared with TC. In contrast, MDL treatment decreased spectrin cleavage during IR compared with untreated hearts. Values are means ± SE; n = 3 in each group. *P < 0.05 vs. TC or MDL-treated mitochondria. B: in isolated control mouse heart mitochondria, MDL decreased mitochondrial calpain 1 (mit-CPN1) activity in a dose-dependent manner. The fluorescence intensity in mitochondria without MDL is 5,471 ± 185 arbitrary units (n = 6), and this value was used as 100%. Values are means ± SE; n = 3 and 5 in MDL 3 μM and 10 μM groups, respectively. *P < 0.05 vs. mitochondria without MDL treatment. †P < 0.05 vs. 3 μM MDL group.
MDL inhibits mit-CPN1 activity in a dose-dependent manner.
Exogenous calcium was used to activate mit-CPN1 in isolated control mouse heart mitochondria. Incubation of MDL with control mitochondria markedly decreased mit-CPN1 activity (Fig. 2B). These results indicate that MDL can effectively inhibit mit-CPN1 in isolated mouse heart mitochondria.
Administration of MDL improves oxidative phosphorylation in mouse heart mitochondria following IR.
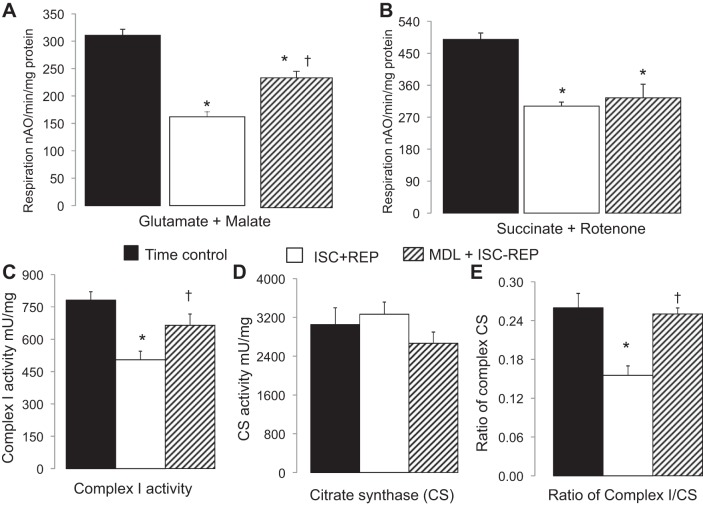
The rate of oxidative phosphorylation was decreased in intact mouse heart mitochondria following IR. Compared with time control, the rate of glutamate + malate oxidation and succinate oxidation was decreased by 47% (Fig. 3A) or 34% (Fig. 3B), respectively, following IR. In MDL-treated hearts, the rate of glutamate + malate oxidation dramatically improved compared with untreated hearts (Fig. 3A). However, the succinate oxidation was not improved (Fig. 3B). These results indicate that inhibition of calpain during IR attenuates the IR-induced impairment of the proximal ETC.
Fig. 3.
MDL treatment improved oxidative phosphorylation and complex I activity in mouse mitochondria following IR (ISC+REP). A and B: IR decreased the rate of oxidative phosphorylation using glutamate + malate as complex I substrate (A), and succinate + rotenone as complex II substrates (B) compared with TC. MDL treatment improved oxidative phosphorylation with complex I substrates (A), but not with complex II substrates (B) compared with untreated mitochondria. C–E: IR decreased complex I activity (C) without alteration of citrate synthase (CS) activity (CSA) (D) compared with TC. This led to a decreased ratio of complex I to CS (E). MDL mitigated complex I damage during IR compared with untreated mitochondria, as shown by improved complex I activity and the ratio of complex I to CS. Values are means ± SE; n = 5–7 in each group. *P < 0.05 vs. TC. †P < 0.05 vs. untreated.
Administration of MDL improves complex I activity in mouse heart mitochondria following IR.
The activities of complex I and citrate synthase (CS) were determined in detergent-solubilized mitochondria. The complex I activity was significantly decreased in mitochondria following IR compared with time control (Fig. 3C). MDL treatment dramatically improved complex I activity compared with untreated heart mitochondria following IR (Fig. 3C). There were no differences in CS activity between groups (Fig. 3D). The decreased ratio of complex I to CS in IR-damaged mitochondria further supported that complex I defect was not due to the loss of mitochondrial content (Fig. 3E).
Administration of MDL decreases the MPTP opening in mouse heart mitochondria following IR.
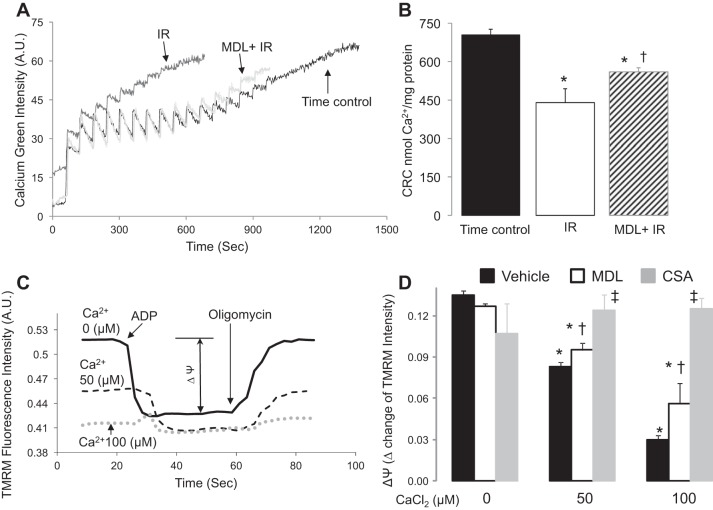
CRC was used to assess the susceptibility to MPTP opening in isolated heart mitochondria (39). Cardiac mitochondria were isolated from time control, untreated, and MDL-treated hearts at the end of perfusion. A representative tracing of CRC measurement is shown in Fig. 4A. Compared with time control, the CRC in untreated mitochondria following IR was decreased by 38% (Fig. 4B). MDL treatment significantly improved CRC compared with untreated hearts (Fig. 4B). These results indicate that activation of calpains during IR sensitizes to MPTP opening in mouse heart mitochondria.
Fig. 4.
MDL treatment inhibited mitochondrial permeability transition pore (MPTP) opening in mouse heart mitochondria following IR. CRC (calcium retention capacity) was used to measure MPTP opening in mouse heart mitochondria following IR. A: a representative original tracing in each group is shown. B: IR markedly decreased the CRC compared with TC, whereas MDL treatment improved the CRC compared with untreated. These results support that activation of calpains contributes to MPTP opening during IR. Values are means ± SE; n = 5–7 in each group. *P < 0.05 vs. TC. †P < 0.05 vs. untreated hearts. Inner mitochondria membrane potential (Δψ) was used to reflect MPTP opening in mitochondria already incubated with exogenous calcium. C: the original tracing in each group is shown. D: exogenous calcium dose-dependently depolarized the Δψ in mouse heart mitochondria. Cyclophilin A prevented the calcium-induced depolarization of the Δψ, supporting that the depolarization of the Δψ was due to MPTP opening. MDL treatment decreased the extent of the Δψ depolarization compared with untreated mitochondria, suggesting that activation of mit-CPN1 sensitizes to MPTP opening. Values are means ± SE; n = 4 in each group. *P < 0.05 vs. noncalcium-treated mitochondria. †P < 0.05 vs. non-MDL-treated mitochondria. ‡P < 0.05 vs. calcium-treated mitochondria in the presence or absence of MDL. TMRM, tetramethylrhodamine methyl ester; AU, arbitrary units.
Administration of MDL inhibits MPTP opening in isolated mitochondria.
Administration of MDL in buffer-perfused hearts inhibits both cytosolic and mitochondrial calpain. Thus the purified cardiac mitochondria that avoid cytosolic contamination were used to test if MDL treatment can decrease MPTP opening in isolated mitochondria. The opening of the MPTP results in a permeation of the IMM that leads to the loss of Δψ. Thus Δψ was used to reflect the MPTP opening in mitochondria incubated with exogenous calcium. The Δψ was determined in isolated mitochondria using glutamate + malate as substrates. ADP (2 mM) was used to induce mitochondrial inner membrane depolarization. The Δψ was reflected by the change of the intensity of TMRM fluorescence before and after ADP addition (Fig. 4C). The Δψ was depolarized in mitochondria exposed to exogenous calcium in a dose-dependent manner (Fig. 4, C and D). Cyclosporine A, a known MPTP inhibitor, prevented the depolarization of the Δψ in calcium-treated mitochondria (Fig. 4D), supporting that the depolarized Δψ was due to MPTP opening. MDL treatment improved the Δψ in calcium-treated cardiac mitochondria, suggesting that activation of mit-CPN1 contributes to MPTP opening.
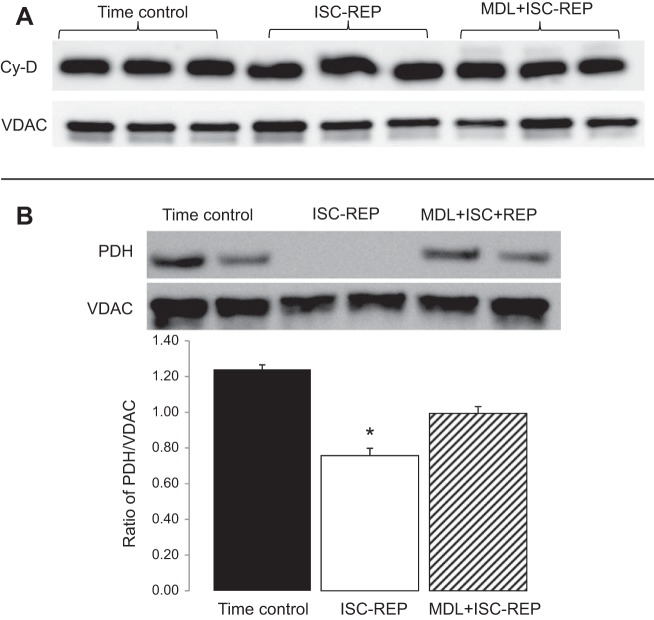
Administration of MDL did not alter cyclophilin D content in mitochondria following IR.
Cyclophilin D is a key factor to regulate MPTP opening. Compared with time control, IR did not markedly alter cyclophilin D content in mouse heart mitochondria. There was no difference in cyclophilin D content between untreated and MDL-treated mitochondria following IR (Fig. 5A). These results suggest that the decreased MPTP opening in MDL-treated mitochondria is not due to altered cyclophilin D content.
Fig. 5.
MDL treatment preserved pyruvate dehydrogenase (PDH) content in mouse heart mitochondria following IR. IR did not alter cyclophilin D (Cy-D) content compared with TC. A: there was no difference in Cy-D content between untreated and MDL-treated mitochondria following IR. IR markedly decreased the content of PDH α1-subunit compared with TC. B: MDL treatment preserved PDH content compared with untreated heart mitochondria following IR. Voltage-dependent anion channel (VDAC) was used as protein loading control. Values are means ± SE; n = 4 in each group. *P < 0.05 vs. TC or MDL treatment.
Administration of MDL protects the PDH content in mouse heart mitochondria following IR.
PDH α1-subunit content in cardiac mitochondria was determined by immunoblotting. IR markedly decreased PDH content compared with time control. MDL treatment preserved the PDH content compared with untreated mitochondria following IR (Fig. 5B). These results suggest that activation of mit-CPN1 contributes to PDH degradation during IR.
DISCUSSION
In the present study, we show that mit-CPN1 exists in both IMS and matrix in mouse heart mitochondria. Administration of a calpain inhibitor decreases cardiac injury in buffer-perfused hearts during IR. Inhibition of calpain improves oxidative phosphorylation with complex I substrates following IR. Direct enzyme activity measurement shows that inhibition of calpain protects complex I activity. Inhibition of mit-CPN1 also attenuates the degradation of PDH during IR. These results suggest that activation of mit-CPN1 in the matrix inhibits metabolism by inducing complex I damage and PDH degradation during IR. Calpain inhibition improves the CRC following IR, suggesting that calpain activation contributes to MPTP opening. In purified heart mitochondria, administration of calpain inhibitor attenuates the exogenous calcium-mediated depolarization of the Δψ, supporting that activation of the mit-CPN1 contributes to MPTP opening.
Activation of mit-CPN1 contributes to cardiac injury during IR (11, 14). In a previous study, activation of mit-CPN1 within the IMS augments cardiac injury during reperfusion by inducing a translocation of AIF from mitochondria to cytosol and nucleus (11, 14, 52). In the present study, we found that activation of matrix-localized mit-CPN1 impairs energy generation and sensitizes to MPTP opening during IR. Thus, in addition to AIF release, activation of mit-CPN1 increases cardiac injury during IR through direct damage of the ETC. MPTP opening results in a release of the IMS proteins, including AIF and cytochrome c, into cytosol that increases cardiac injury through caspase-dependent and independent cell death pathways (14, 55).
Administration of calpain inhibitor to isolated rabbit hearts improves oxidative phosphorylation using complex I substrates (35, 48). In the present study, inhibition of calpain using MDL also improves oxidative phosphorylation in mouse heart mitochondria using complex I substrates, but not with complex II substrates. Direct measurement of complex I activity shows that IR decreases complex I activity. In contrast, inhibition of the mit-CPN1 using MDL improves the complex I activity in mitochondria following IR. These results suggest that activation of the mit-CPN1 damages complex I during IR.
In addition to the ETC damage, IR also decreases the content of multiple metabolic enzymes within the matrix, including PDH, malate dehydrogenase, and succinate dehydrogenase (16, 31, 49). In buffer-perfused mouse hearts, IR dramatically decreased the contents of PDH, malate dehydrogenase, and succinate dehydrogenase compared with time control. Protective modulation of mitochondrial respiration using amobarbital preserved these enzyme contents in mitochondria following IR (54). These results suggest that the mitochondrial respiratory chain is the source of injury that ultimately causes enzyme loss during IR. In the present study, we tested if activation of mit-CPN1 contributes to PDH loss during IR. PDH is selected as our target protein in the present study in that PDH is the gate keeper for metabolic production of NADH from glucose to flow into the ETC (49). MDL treatment preserves PDH content in mouse heart mitochondria following IR, supporting that activation of calpain within the matrix contributes to PDH damage during IR. In addition to mit-CPN1, mitochondrial CPN10 does exist within heart mitochondria (2). However, CPN10 is not sensitive to inhibition by MDL (11). These results support that activation of the matrix-located mit-CPN1 contributes to PDH damage in mouse heart mitochondria.
MPTP opening contributes a critical role in cardiac cell death during IR (46, 51). In cultured hepatocytes, administration of calpain inhibitors attenuates the microcystin-LR-induced depolarization of the Δψ (17), suggesting that activation of calpain contributes to MPTP opening. In the present study, administration of calpain inhibitor in buffer-perfused hearts also improves the CRC in cardiac mitochondria following IR, supporting that calpain activation is involved in MPTP opening. Since CPN1 exists in both cytosol and mitochondria, administration of a calpain inhibitor (or knockdown of protein content) at the organ or cell level is unable to differentiate the role of mit-CPN1 vs. cytosolic CPN1 (14). A recent study shows that cytosolic CPN1 can transfer into mitochondria during chronic lipopolysaccharide treatment (36). However, mit-CPN1 content is not altered in mouse heart mitochondria following acute IR (14). Thus activation of cytosolic CPN1 is less likely to directly access and cleave matrix and inner membrane proteins. Activation of the cytosolic calpain may increase MPTP opening through indirect mechanisms via myocyte calcium overload (15). Activation of cytosolic calpain during IR can lead to intracellular calcium overload by cleaving Na+-K+-ATPase, Ca2+-ATPase, H+/Na+ exchanger, and Na+/Ca2+ exchanger (23, 25, 26). The intracellular calcium overload will increase mitochondrial calcium concentration and trigger MPTP opening when the calcium concentration reaches the threshold. Therefore, administration of calpain inhibitor in buffer-perfused hearts can also decrease MPTP opening by inhibiting cytosolic calpains.
Mitochondrial calcium overload is one of the key factors to induce MPTP opening (51). To clarify the role of the mit-CPN1 in the MPTP opening, purified heart mitochondria without cytosolic contamination must be used. Our study shows that inhibition of mit-CPN1 using MDL attenuates the exogenous calcium-induced depolarization of the Δψ. In the present study, MDL dose-dependently inhibits mit-CPN1 activity in control mouse heart mitochondria. CPN10 is less sensitive to MDL inhibition (11). MDL treatment decreases the depolarization of the Δψ in calcium-treated liver mitochondria, indicating that activation of the mit-CPN1 sensitizes the MPTP opening (17). Thus our results provide the direct evidence that activation of the mit-CPN1 contributes to the MPTP opening in cardiac mitochondria.
Although the mechanism by which activation of mit-CPN1 increases MPTP opening is not clear, several potential factors may contribute to mit-CPN1-induced MPTP opening. Cyclophilin D within the matrix is a critical regulator of the MPTP opening. Posttranslational modification of the cyclophilin D, including acetylation, favors MPTP opening (19, 30). However, there is no difference in cyclophilin D content between MDL-treated and untreated mouse heart mitochondria following IR (Fig. 5A). There are also no cleaved bands of cyclophilin D detected in mouse heart mitochondria following IR. These results suggest that activation of mit-CPN1 is less likely to increase MPTP through cleavage of cyclophilin D. Genetic ablation of a complex I subunit sensitizes to MPTP opening in mouse heart mitochondria (30). The activity of mit-CPN1 is increased following IR (14). Administration of calpain inhibitor in buffer-perfused heart attenuates complex I damage. Thus activation of the mit-CPN1 may sensitize the MPTP opening by impairing complex I during IR. In a recent study, activation of mit-CPN1 has been shown to cleave ATP synthase α-subunit in mouse heart mitochondria (36), suggesting that subunits of ATP synthase are potential substrates of mit-CPN1. Although the c-subunit but not ATP synthase α-subunit is a potential component of MPTP (1), the interaction between mit-CPN1 activation and c-subunit of ATP synthase needs to be investigated.
MPTP opening in response to calcium overload during IR has been ascribed solely to the actions of cyclophilin D (3). The present work supports a contributing role for activation of mitochondria-localized calpains. Based on studies of isolated, intact mitochondria, it is likely that mit-CPN1 localized to the matrix enhances susceptibility to MPTP. Thus calcium-mediated mitochondrial injury not only is a consequence of calcium-activated MPTP opening via cyclophilin D (3, 21, 22), but involves activation of mitochondrial localized calpains as well (11, 17, 42).
Several limitations of the present study are relevant to the need for additional work. MDL is a classic CPN1 inhibitor, but it also has off-target effects, especially in high concentrations. Although our pharmacological approach provides a potential link between mit-CPN1 activation and MPTP opening, a genetic approach is needed to further clarify the role of mit-CPN1 activation in MPTP opening. CPN2 has been found in the rat heart mitochondria (42). However, only mit-CPN1 is found in the purified mouse heart mitochondria. Currently, CPN1 and CPN2 are identified in mitochondria mainly based on immunoblotting results. Since knockout of CPN2 is embryotic lethal, a genetic approach to develop a conditional knockout of CPN2 will be needed to further study the potential role of CPN2 in mouse heart mitochondria.
Reversible inhibition of complex I decreases cardiac injury during IR, indicating that the manipulation of complex I activity is a valuable approach to decrease cardiac injury (10). However, persistent complex I damage increases cardiac injury during reperfusion by impairing energy generation and increasing MPTP opening (30). Activation of mit-CPN1 leads to complex I damage and MPTP opening, indicating that targeting mit-CPN1 for inhibition is a novel strategy to decrease cardiac injury during reperfusion. In addition to the matrix-facing ETC subunits and cyclophilin D, multiple metabolic enzymes are located within the matrix. Investigation of the relationship between the activation of mit-CPN1 and the degradation of the metabolic enzymes also will provide new insights to understand the mechanism of IR injury. Our results will further help to develop focused approaches to decrease cardiac injury by manipulating the mit-CPN1 activity during IR.
GRANTS
This work was supported by a Scientist Development Grant (11SDG5120011) and a Grant-in-aid (15GRNT24480123) from the American Heart Association (Q. Chen); Virginia Commonwealth University's Clinical and Translational Science Award (UL1TR000058 from the National Institutes of Health National Center for Advancing Translational Science); the Center for Clinical and Translational Research Endowment Fund of the Virginia Commonwealth University (Q. Chen); the Office of Research and Development; Medical Research Service Merit Review Award (1IO1BX001355-01A1); Department of Veterans Affairs (E. J. Lesnefsky); and the Pauley Heart Center, Virginia Commonwealth University (Q. Chen, E. J. Lesnefsky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.T., Y.H., and Q.C. performed experiments; J.T., Y.H., E.J.L., and Q.C. analyzed data; J.T., Y.H., E.J.L., and Q.C. interpreted results of experiments; J.T., Y.H., E.J.L., and Q.C. approved final version of manuscript; E.J.L. and Q.C. conception and design of research; E.J.L. and Q.C. edited and revised manuscript; Q.C. prepared figures; Q.C. drafted manuscript.
REFERENCES
- 1.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO-ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111: 10580–10585, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol 291: C1159–C1171, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS Lett 541: 1–5, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brustovetsky T, Bolshakov A, Brustovetsky N. Calpain activation and Na+/Ca2+ exchanger degradation occur downstream of calcium deregulation in hippocampal neurons exposed to excitotoxic glutamate. J Neurosci Res 88: 1317–1328, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394: 627–634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Henderson GI, Freeman GL. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J Mol Cell Cardiol 33: 1919–1927, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem 276: 30724–30728, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 277: 29181–29186, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol 292: C137–C147, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Lesnefsky EJ. Heart mitochondria and calpain 1: location, function, and targets. Biochim Biophys Acta 1852: 2372–2378, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319: 1405–1412, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Paillard M, Gomez L, Li H, Hu Y, Lesnefsky EJ. Postconditioning modulates ischemia-damaged mitochondria during reperfusion. J Cardiovasc Pharmacol 59: 101–108, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, Lesnefsky EJ. Activation of mitochondrial mu-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun 415: 533–538, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Han H, Ren W, Huang G. Heat transfer modeling of an annular on-line spray water cooling process for electric-resistance-welded steel pipe. PLoS One 10: e0131574, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng N, Zhang J, Zong C, Wang Y, Lu H, Yang P, Wang W, Young GW, Wang Y, Korge P, Lotz C, Doran P, Liem DA, Apweiler R, Weiss JN, Duan H, Ping P. Phosphoproteome analysis reveals regulatory sites in major pathways of cardiac mitochondria. Mol Cell Proteomics 10: M110.000117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding WX, Shen HM, Ong CN. Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem Biophys Res Commun 291: 321–331, 2002. [DOI] [PubMed] [Google Scholar]
- 18.French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KK, Powers SK. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol 290: H128–H136, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Gharib A, De Paulis D, Li B, Augeul L, Couture-Lepetit E, Gomez L, Angoulvant D, Ovize M. Opposite and tissue-specific effects of coenzyme Q2 on mPTP opening and ROS production between heart and liver mitochondria: role of complex I. J Mol Cell Cardiol 52: 1091–1095, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta 1767: 1007–1031, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernando V, Inserte J, Sartorio CL, Parra VM, Poncelas-Nozal M, Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol 49: 271–279, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Hollander JM, Thapa D, Shepherd DL. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. Am J Physiol Heart Circ Physiol 307: H1–H14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inserte J, Garcia-Dorado D, Hernando V, Soler-Soler J. Calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion contributes to cell death after myocardial ischemia. Circ Res 97: 465–473, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Inserte J, Hernando V, Garcia-Dorado D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovasc Res 96: 23–31, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Joshi A, Bondada V, Geddes JW. Mitochondrial micro-calpain is not involved in the processing of apoptosis-inducing factor. Exp Neurol 218: 221–227, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang PT, Zhang L, Chen CL, Chen J, Green KB, Chen YR. Protein thiyl radical mediates S-glutathionylation of complex I. Free Radic Biol Med 53: 962–973, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kar P, Chakraborti T, Samanta K, Chakraborti S. mu-Calpain mediated cleavage of the Na+/Ca2+ exchanger in isolated mitochondria under A23187 induced Ca2+ stimulation. Arch Biochem Biophys 482: 66–76, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim N, Lee Y, Kim H, Joo H, Youm JB, Park WS, Warda M, Cuong DV, Han J. Potential biomarkers for ischemic heart damage identified in mitochondrial proteins by comparative proteomics. Proteomics 6: 1237–1249, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Lee HL, Chen CL, Yeh ST, Zweier JL, Chen YR. Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 302: H1410–H1422, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys 385: 117–128, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol 33: 1065–1089, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Neuhof C, Gotte O, Trumbeckaite S, Attenberger M, Kuzkaya N, Gellerich F, Moller A, Lubisch W, Speth M, Tillmanns H, Neuhof H. A novel water-soluble and cell-permeable calpain inhibitor protects myocardial and mitochondrial function in postischemic reperfusion. Biol Chem 384: 1597–1603, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Ni R, Zheng D, Wang Q, Yu Y, Chen R, Sun T, Wang W, Fan GC, Greer PA, Gardiner RB, Peng T. Deletion of CAPN4 protects the heart against endotoxemic injury by preventing ATP synthase disruption and inhibiting mitochondrial superoxide generation. Circ Heart Fail 8: 988–996, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozaki T, Tomita H, Tamai M, Ishiguro S. Characteristics of mitochondrial calpains. J Biochem 142: 365–376, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Ozaki T, Yamashita T, Ishiguro S. Mitochondrial m-calpain plays a role in the release of truncated apoptosis-inducing factor from the mitochondria. Biochim Biophys Acta 1793: 1848–1859, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol 46: 902–909, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol Heart Circ Physiol 244: H743–H748, 1983. [DOI] [PubMed] [Google Scholar]
- 41.Scaduto RC Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76: 469–477, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shintani-Ishida K, Yoshida KI. Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia-reperfusion. Int J Cardiol 197: 26–32, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh RB, Dhalla NS. Ischemia-reperfusion-induced changes in sarcolemmal Na+/K+-ATPase are due to the activation of calpain in the heart. Can J Physiol Pharmacol 88: 388–397, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Steel R, Torrie J. Principles and Procedures of Statistics. New York: McGraw-Hill, 1960. [Google Scholar]
- 46.Sulkin MS, Boukens BJ, Tetlow M, Gutbrod SR, Ng FS, Efimov IR. Mitochondrial depolarization and electrophysiological changes during ischemia in the rabbit and human heart. Am J Physiol Heart Circ Physiol 307: H1178–H1186, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ, Larner AC. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem 286: 29610–29620, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trumbeckaite S, Neuhof C, Zierz S, Gellerich FN. Calpain inhibitor (BSF 409425) diminishes ischemia/reperfusion-induced damage of rabbit heart mitochondria. Biochem Pharmacol 65: 911–916, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS, Dyck JR, Lopaschuk GD. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res 94: 359–369, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol 38: 78–100, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res 93: 292–301, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Xu A, Szczepanek K, Hu Y, Lesnefsky EJ, Chen Q. Cardioprotection by modulation of mitochondrial respiration during ischemia-reperfusion: Role of apoptosis-inducing factor. Biochem Biophys Res Commun 435: 627–633, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Yoshikawa Y, Zhang GX, Obata K, Ohga Y, Matsuyoshi H, Taniguchi S, Takaki M. Cardioprotective effects of a novel calpain inhibitor SNJ-1945 for reperfusion injury after cardioplegic cardiac arrest. Am J Physiol Heart Circ Physiol 298: H643–H651, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Younus M, Thompson J, Hu Y, Chen Q, Hollander JM, Hu Y, Lesnefsky EJ. Intermediary metabolism and fatty acid oxidation: novel targets of electron transport chain driven injury during ischemia and reperfusion. Mitochondrial Meeting Abstract, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A 103: 18314–18319, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]