Abstract
The role of capillaries is to serve as the interface for delivery of oxygen and removal of metabolites to/from tissues. During the past decade there has been a proliferation of studies that have advanced our understanding of angiogenesis, demonstrating that tissue capillary supply is under strict control during health but poorly controlled in disease, resulting in either excessive capillary growth (pathological angiogenesis) or losses in capillarity (rarefaction). Given that skeletal muscle comprises nearly 40% of body mass in humans, skeletal muscle capillary density has a significant impact on metabolism, endocrine function, and locomotion and is tightly regulated at many different levels. Skeletal muscle is also high adaptable and thus one of the few organ systems that can be experimentally manipulated (e.g., by exercise) to study physiological regulation of angiogenesis. This review will focus on the methodological concerns that have arisen in determining skeletal muscle capillarity and highlight the concepts that are reshaping our understanding of the angio-adaptation process. We also summarize selected new findings (physical influences, molecular changes, and ultrastructural rearrangement of capillaries) that identify areas of future research with the greatest potential to expand our understanding of how angiogenesis is normally regulated, and that may also help to better understand conditions of uncontrolled (pathological) angiogenesis.
Keywords: exercise, human, rodent, training
this article is part of a collection on Pan American Congress of Physiological Sciences Meeting 2014. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
There is wide recognition (17, 38, 126) and a surfeit of epidemiologic evidence that links the benefits of exercise training with a decreased risk of cardiovascular diseases. The reasons are manifold, but one important factor is that the peripheral microvasculature adapts in structure and function to this stimulus. The role of the microcirculation, in particular of the capillaries, is to serve as the interface for delivery of oxygen and removal of metabolites to/from tissues. In this context, the assumption has always been that “more is better” and that greater capillary surface area results in increased potential for diffusion of oxygen and metabolite clearance, thereby improving endurance and aerobic capacity (65). Indeed, the literature supports a correlation between capillary density (CD) and the proportion of oxidative fibers or average mitochondrial volume density among different skeletal muscles (63), and exercise performance or maximal aerobic capacity correlate well with skeletal muscle CD in animals (65).
Given that skeletal muscle comprises nearly 40% of body mass in humans, altering muscle CD has a significant impact on metabolism, endocrine function, and locomotion. Whereas an increased skeletal muscle CD likely indicates capillary growth (angiogenesis) linked with functional improvements, decreased skeletal muscle CD (i.e., capillary rarefaction) is associated with poor health outcomes and declining prognosis in association with many chronic diseases, such as peripheral arterial disease (77, 87), diabetes (79, 80), cachexia (7), and chronic obstructive pulmonary disease (74). It is therefore important to understand the mechanisms involved in the regulation of muscle capillaries to both optimize physiological outcomes and ameliorate pathological consequences.
In contrast to vasculogenesis, which is the de novo formation of blood vessels essential in the initial development of the microcirculation in embryos, angiogenesis is the formation of capillaries from existing blood vessels. Angiogenesis plays a key role throughout all stages of life (e.g., tissue injury/wound repair, the endometrial cycle, and striated muscle adaptation to stress/exercise). During the past decade there has been a proliferation of studies that have advanced our understanding of the angiogenic processes, demonstrating that capillary growth in skeletal muscle is a process that is tightly regulated at many different levels [see reviews (29, 59, 101)]. This regulation follows the principles of a control circuit. For example, when the microcirculation is inadequate to fully meet the metabolic demands imposed on the muscle by cellular and physical influences (see below), these influences exert an upstream effect on the capillary network to proliferate (upstream input). The physical influence(s) are sensed by muscle fibers and endothelial cells (ECs) and translated into an appropriate gene expression profile (control variable), which ultimately alter the EC phenotype (downstream output) and lead to capillary growth. Angiogenesis is thought to terminate once metabolic homeostasis is reestablished (feedback). This, however, is a multifactorial and highly orchestrated process for which we are only beginning to understand the molecular underpinnings.
This review will focus on the methodology to determine CD and highlight the concepts in health and disease that are reshaping our understanding of the angioadaptation process. We will use an integrative review to assess the impact of, but also responses to, altered muscle activity and summarize selected new findings that identify areas of future research with the greatest potential to expand our understanding of the regulation of skeletal muscle angiogenesis and thus improve therapeutic efficacy.
Methodology
As the number of studies on skeletal muscle angiogenesis continues to grow, it is increasingly important to have consistency and methodological commonality in quantitative determination of CD, to avoid confounding technical and analytical issues that can produce ambiguous results. Among the issues to consider are 1) the direction of skeletal muscle sectioning–it is critically important to have a reference orientation, but this is sometimes neglected; 2) appropriate structural indicators for characterization of capillarity; and 3) regional heterogeneity of fiber type and therefore muscle capillarity within a given muscle, which can lead to unintentional bias in the capillary data reported. The latter is perhaps most widely recognized within the gastrocnemius (15) and tibialis anterior muscles (which are well known “mixed muscle” phenotypes), but all skeletal muscle exhibit varying degrees of muscle fiber type and capillary heterogeneity (5, 27, 30, 111).
Skeletal muscle is an anisotropic tissue, meaning its major cellular components are not randomly arranged. The microvasculature run largely in parallel with the longitudinal axis of muscle fibers. However, the two entities may be loosely connected, with capillaries meandering over the surface of fibers (Fig. 1A). Hence, a potentially greater functional relevant index may be achieved by calculating capillary length density [the length of capillaries per unit volume of muscle fibers, in stereological terminology denoted as JV(c,f)], as this takes into account the additional surface area of each capillary offered by greater tortuosity (91). This is 7–64% higher than that estimated from CD alone in transverse tissue sections due to the tortuosity and branching anastomoses seen in skeletal muscles (90, 91). However, such quantification requires a more time-consuming and technically demanding methodology than routine estimates of CD, so unfortunately it is not commonly performed. When only muscle cross sections are evaluated, as is most often the case, appropriate values are most readily obtained with true transverse sections (i.e., perpendicular to the muscle fiber axis, as shown in Fig. 1B). It is important to avoid oblique sections, which lead to erroneously high-fiber area and/or counting multiple points of the same tortuous capillary undulating in and out of the horizontal plan of the muscle section. However, it should be noted that with an appropriate sampling design, it is possible to get estimates of CD from longitudinal sections, but this too is not often performed.
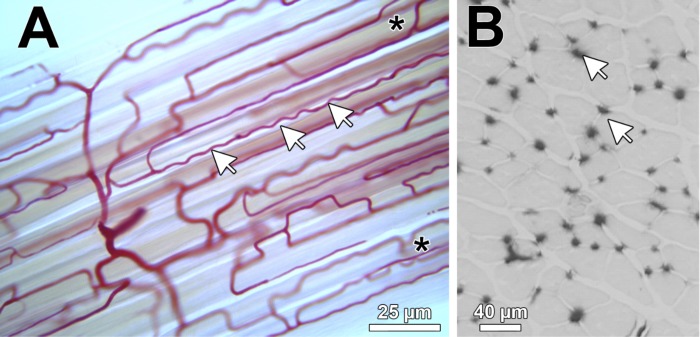
Fig. 1.
Light microscopic representation of capillaries in skeletal muscle. A: 60-μm-thick longitudinal section of a porcine skeletal muscle injected with colored gelatin. Note the tortuous course of the capillaries (arrows) and anastomoses (asterisks). B: light microscopic representation of a 10-μm-thick cryosection from a mouse extensor digitorum longus subjected to alkaline phosphatase histochemistry. The capillaries are visualized as black dots (arrows) surrounding the skeletal muscle fibers.
The number of muscle fiber and capillary profiles within a given area can be used to calculate the numerical capillary-to-fiber ratio (C:F) [in stereological notation, NN(c,f)]. This should not be confused with the number of capillaries surrounding individual fibers [i.e., N(c,f) or NCAF] or the derived index of capillary contacts, which are both quite insensitive to changes during tissue remodeling and hence have limited utility as a quantitative index of angiogenesis (30). In addition, the number of capillary profiles per cross-sectional area of muscle fibers is used to determine the CD [i.e., capillaries mm−2]. This measure is also frequently referred to as microvessel density, which can add ambiguity to the literature when studies include terminal arterioles and collecting venules within this definition. In stereological terms CD is denoted as NA(c,f) in striated muscle and provides a useful index of the functional capillary supply. However, it is important to recognize that CD depends on the mean cross-sectional area of muscle fibers. As mean cross-sectional area of muscle fibers is often modulated in response to changes in physical demand or the metabolic environment, CD should not be relied on as the sole metric used to quantify the extent of angiogenesis. Rather, inclusion of the C:F is often recommended since it is less sensitive to the issue of scaling (32).
Finally, skeletal muscle displays a varying degree of fiber-type heterogeneity that can have a direct and significant influence on the quantitative assessment of muscle capillarity indexes. For example, the gastrocnemius and tibialis anterior muscles are frequently studied because of their active role in locomotion and exercise. But these and other skeletal muscle represent a “mixed” muscle type containing a heterogeneous distribution of myofiber phenotypes (27, 116) with concomitant regional heterogeneity in capillary supply (Fig. 2). Thus rigorous procedural attention is required when selecting histological images for counting to avoid sampling bias within any muscle sample. Indeed, unbiased counting rules defining randomness of sampling and counting procedures are essential and obviate the need to analyze the entire muscle when the rules of stereology are strictly followed (31, 34).
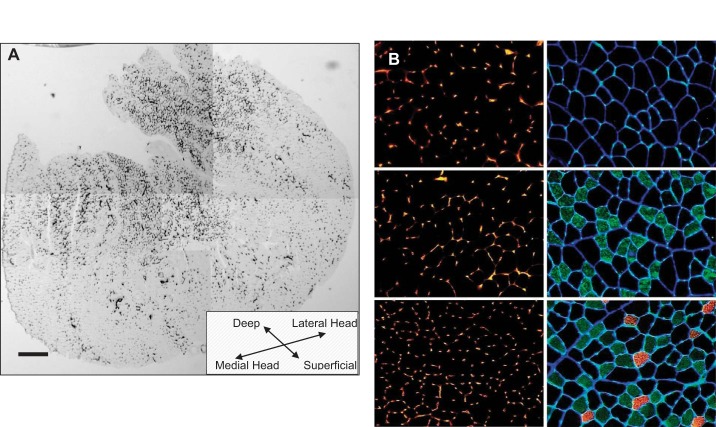
Fig. 2.
Capillary and fiber-type heterogeneity within skeletal muscle. Depicted here is capillary heterogeneity within the gastrocnemius (A) and extensor digitorum longus (B) skeletal muscle. A: montage of 5 overlapping images obtained using light microscopy (5×) to depict the entire transverse (or horizontal) view of a single mouse gastrocnemius muscle stained with alkaline phosphatase (capillaries appear as dark spots within the muscle section). Scale bar = 400 μm. B: images obtained using light microscopy (20×) from transverse section of mouse extensor digitorum longus muscle. B, left: images stained with rhodamine-labeled lectin (capillaries appear as bright spots within the muscle section). B, right: adjacent images stained for fiber types (and which are obtained from same region corresponding to images at left). Immunolabeling reveals fibers appearing black (dark) are type IIB, green-appearing fibers are type IIA, and orange-colored fibers are type I. Blue stain is laminin used to delineate fiber boundaries. A & B: significant heterogeneity in muscle capillarity depending on region of sampling within a muscle and that (as seen in B) this heterogeneity corresponds to muscle fiber-type heterogeneity found in muscles with mixed fiber-type composition. Scale bar = 200 μm. (Kissane and Egginton, unpublished.)
Physical Influences
Physiological regulation of CD (i.e., during adaptive remodeling) involves a significant influence of the physical environment (Fig. 3). For example, mechanical factors such as increased shear stress exerted on the capillary lumen by functional hyperemia, or elevated tissue strain as a consequence of repetitive contraction/relaxation cycles may stimulate capillary growth in both cardiac and skeletal muscle (65).
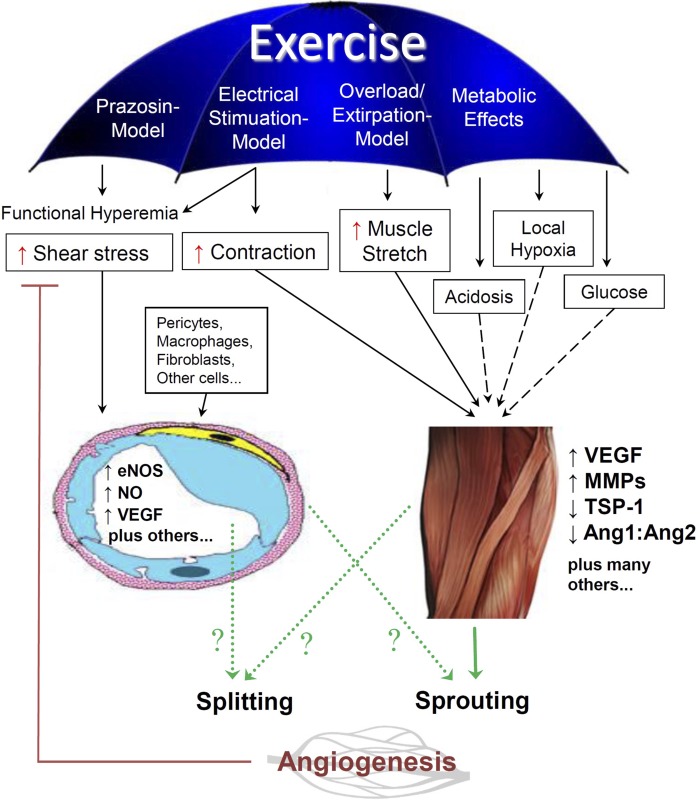
Fig. 3.
Overview of the physical and metabolic stimuli contributing to angiogenesis in skeletal muscle in response to endurance exercise. Exercise leads to an increase in blood flow through capillaries (functional hyperemia), as well as metabolic and mechanical (physical) alterations of the skeletal muscle fibers that may modulate the gene expression patterns of angiogenic factors, and subsequently, change the capillary ultrastructure (e.g., pericytes, protrusions, etc.) that determines angiotype (e.g., splitting and sprouting angiogenesis) in skeletal muscle. Solid lines (–––) represent the physical stimuli that are believed to influence exercise-induced skeletal muscle angiogenesis. Dashed lines (− − −) represent metabolic stimuli that have also been reported to have either primary or secondary influences on exercise-induced skeletal muscle angiogenesis. Dotted green lines (···) represent the potential sources releasing molecular factors responsible for angiogenesis. eNOS, endothelial nitric oxide (NO) synthase; MMP, matrix metalloproteinase; TSP-1, thrombospondin-1.
The in vivo control of angiogenesis involves a complex array of stimuli and an ever-growing multifactorial list of candidates associated with muscle exercise activity [see reviews (29, 59, 101)], which invites a reductionist approach to better define potential contributors. Functional hyperemia increases microvascular shear stress (because of low compliance of capillaries) and chronically elevated blood flow is capable of inducing angiogenesis in the absence of any change in muscle phenotype, which demonstrates a potential disconnection between the cellular feedback from host tissue that has usually been deemed essential for angiogenesis. For example, when muscles were exposed to targeted hindlimb dilatation, raising average capillary wall shear stress by nearly fourfold, there was a 30% increase in C:F (24).
At rest, blood flow to skeletal muscle is low, but during contractile activity, it can be enhanced up to 100-fold to accommodate the large increase in oxygen demand (3). Blood flow to the muscle is determined by perfusion pressure and vascular conductance. The latter is well balanced and regulated by sympathetic vasoconstriction, sympatholytic compounds, and vasodilators, which are locally produced in the muscle (primarily by EC, red blood cells, and skeletal muscle fibers). Regulation of vascular conductance involves flow-mediated, conducted, and red blood cell-mediated dilatation, as well as interaction between several vasodilators, including nitric oxide (NO), prostacyclin, adenosine, and ATP (114). NO and prostaglandins have been found to be central in blood flow regulation, as the vasodilatory effect of many other compounds (e.g., acetylcholine, adenosine, ATP, and bradykinin) as well as mechanical signals (shear stress) are mediated through the formation of these compounds (55). The vascular system is therefore particularly sensitive to alterations in NO and prostaglandin bioavailability. This may be particularly important in the context of capillary adaptation, since negative angiogenic regulators, such as thrombospondin-1 (TSP-1), which is discussed later, are also known to inhibit NO activity (71, 109).
The large increase in blood flow to muscle during exercise will induce a significant increase in shear stress in the capillary bed and thereby promote angiogenesis. Similarly, several vasodilators (including NO, prostaglandins, and adenosine) are also implicated in the regulation of muscle angiogenesis (1, 8, 106), e.g., NO simultaneously induces vasodilatation and enhances vascular endothelial growth factor (VEGF) expression (11). Not surprisingly, there is a close association between the magnitude of blood flow in tissues and the level of capillarization (127). In addition to the usual increase in VEGF, high shear stress also leads to upregulation of endothelial NO synthase (eNOS) mRNA and protein expression, possibly leading to feedback promotion of VEGF expression (8). However, the level of shear stress induced by pharmacological vasodilatation is likely in excess of that associated with functional hyperemia induced by electrical stimulation in rodents. But, it must be noted that direct measurements of capillary shear stress during exercise in humans are lacking.
Muscle stretch, either during normal duty cycle or overload (induced with synergistic muscle extirpation), is also a potent angiogenic stimulus accompanying muscle hypertrophy (25, 121). In resistance training mitochondrial volume density decreases in proportion to muscle hypertrophy, whereas with stretch, capillary supply increases in response to an anabolic (aerobic) environment caused by imposition of a sustained strain of 20%, where C:F increased by some 45% (131). In this case higher VEGF levels were accompanied by elevated matrix metalloproteinase (MMP) activity associated with extracellular matrix remodeling (49). Interestingly, MMP inhibition prevented EC migration but not proliferation during muscle stimulation, demonstrating differential control over individual elements of the angiogenic cascade (32).
Indirect electrical stimulation of skeletal muscle is one of the most potent angiogenic stimuli known, and in rodent hindlimb extensors, it can increase C:F by 50% (36). In this experimental model, the multifactorial consequences of such activity–hemodynamic forces (shear stress, transmural pressure) and dynamic duty cycles (muscle stretch and compression)–initiate remodeling of the vasculature by activating endothelial mechanotransduction mechanisms. These are likely mediated by integrins and associated GTPases. Subsequent signal transduction acts through phosphorylation of kinases (e.g., FAK, c-Src, Akt kinase, phosphatidylinositol 3-kinase, MLCK, and MAPK) to elicit a response appropriate to the stimulus and tissue (29). But it should also be noted that electrical stimulation and its indirect effect of increasing shear stress can result in damage to the luminal glycocalyx that may also potentiate the endothelial response (32). Nevertheless, it is thought that the angiogenic stimuli elicited via electrical stimulation is likely the most similar to that experienced during endurance exercise.
There is great potential to exploit these findings in developing specific angiotherapies, in particular the differential response of muscle and microcirculation. However, there is a growing awareness of the need to better understand the time course of adaptation and/or the intensity of the stimulus. For example, whereas forced treadmill running typically requires at least 6 wk to elicit a significant increase in skeletal muscle angiogenesis, voluntary wheel running in mice has been shown to increase skeletal muscle CD starting after only 5–7 days (99). Likewise, functional hyperemia and overload stimuli achieve similar responses in 2 wk, whereas electrical stimulation can do so in only 1 wk (32). Based on data from whole body exercise, it is becoming evident that the volume of exercise (i.e., time spent exercising) also has an influence capillary adaptation that is currently underappreciated. For example, rodents given access to running wheels voluntarily run between 5–10 km/day and rarely exceed 25% of their maximal effort, but yet achieve capillary expansion rapidly (i.e., 5–7 days). In contrast, forced treadmill running regimes typically attain <2 km/day but often impose exercise above 50% of maximum effort and usually take 6 wk or more for changes in muscle capillarity to occur. These data suggest that the combination of high-volume, low-intensity exercise (i.e., voluntary wheel running) elicits a quicker angioadaptive response than that occurring with low-volume, high-intensity exercise programs. Similar time course experiments are not available in humans, but training studies involving different exercise intensities indicate that the angiogenic response to a period of training at moderate intensity is similar to that of high-intensity training in previously untrained individuals (21, 60, 72). However, there are indications that very high-intensity training can actually lead to a negative effect on VEGF levels (43, 61). In addition, the recognition that mechanical factors may play as potent a role in microvascular remodeling as chemical or metabolic factors (such as local tissue hypoxia, acidosis, glucose, etc.) has led to the concept of “passive” training, which shows an elevation of angiogenic factor expression in muscle without active work being performed (56). Thus it is tempting to speculate that low-intensity, high-volume exercise regimes may produce a stronger angiogenic stimulus and perhaps use intussusception/longitudinal splitting (i.e., a mode of angiogenesis with less energetic requirements compared with sprouting, discussed further in following section) and therefore result in more rapid capillary adaptation compared with capillary sprouting (that is expected from high-intensity, low-volume exercise frequently used with forced treadmill running in rodents). At present, however, the potential differential stimuli and associated molecular responses between exercise modalities are still only poorly understood and will require more deliberate investigation to unravel. Indeed, future studies aimed at directly assessing these responses are needed, as they may also help to devise and improve modes of exercise to maximize the benefits from rehabilitation therapies in the clinic.
Capillary Ultrastructure
Endothelial cells.
Microvessel in skeletal muscles manifest a characteristic ultrastructure, which is best seen by transmission electron microscopy. Shown in Fig. 4 is a transmission electron microscopy image where the outer (abluminal) surface of capillary ECs is covered by a dense basement membrane frequently containing profiles of one or more perivascular pericytes. It is interesting to note, this image appears to show that both cell types communicate extensively during angiogenesis (9, 66). In response to elevated luminal shear stress (prazosin-treated rats), a higher proportion of intraluminal irregularities, projections, and septa combined with extensive cytoplasmic vacuolization of EC were observed in skeletal muscle capillaries, which are interpreted as signs of a longitudinal splitting form of angiogenesis with little evidence for EC proliferation (130). When the canonical sprouting angiogenesis was induced in rats by increased stretch of skeletal muscles due to overload (131) or during chronic electrical stimulation (51), the proportion of abluminal sprouts was higher in capillaries during active angiogenesis, whereas the labeling index (bromodeoxyuridine incorporation) was higher than in the control animals, suggesting that this angiotype is associated with proliferation of ECs (131). In addition, endurance exercise is accompanied by thinning of the pericapillary basement membrane (125), which is presumably induced by the higher expression/activity of MMPs and their proteolytic actions (110). Indeed, in studies with rodents, hallmarks for changes in the ultrastructural phenotype of capillaries in response to angiogenic stimuli have been identified (36).
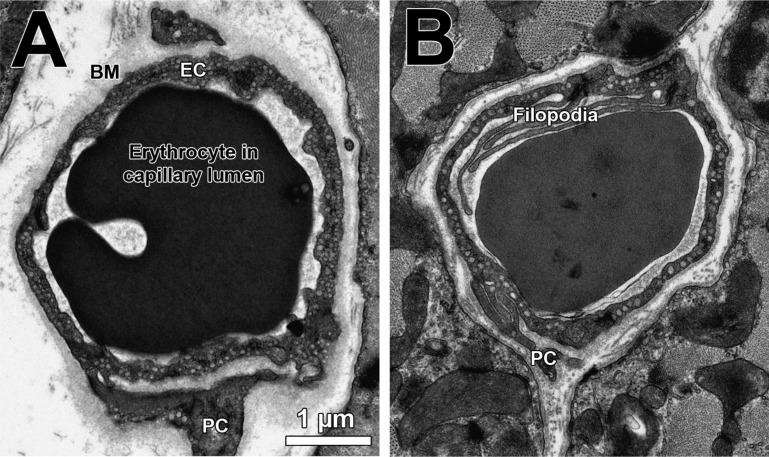
Fig. 4.
Characteristic ultrastructure of a capillary from human (A) and mouse (B) skeletal muscles depicted by transmission electron microscopy. In general, capillaries from humans are larger, exhibit a thicker basement membrane (BM), and form less filopoda than those from mice. EC, endothelial cell; PC, pericytes. Same scale applies to both images.
While the sprouting form of angiogenesis is characterized by hallmarks of EC proliferation and migration (110), nonsprouting angiogenesis can be divided into 1) longitudinal splitting, involving cytoplasmic invagination and bridging over the lumen, and 2) intussusception that occurs with infiltration of stromal cells (36). It is thought that as nonsprouting angiogenesis involves a much lower extent of EC proliferation, this may be an energetically efficient and potentially more rapid way of extending the microcirculation.
Surprisingly, it has not yet been determined whether sprouting or splitting/intussusception is used to realize physiological angiogenesis in skeletal muscles in response to exercise or whether this could explain the temporal difference in capillary adaptation between voluntary wheel running versus forced treadmill exercise. It is interesting to note that EC production of VEGF appears not to be essential to elicit angiogenesis, since selective deletion of VEGF in ECs does not appear to alter CD in a variety of organs (83). However, it should be emphasized that tight regulation of angiogenic activity still requires involvement of EC, as well as influence of perivascular cell types within the interstitium. Indeed, this cell-cell contact suggests other signaling routes may be operative (32), perhaps in the context of basement membrane remodeling, potentially balancing activity of angiogenic inhibitors and stimulators.
Pericytes.
Because of their close spatial relationship to ECs, it was speculated that pericytes contribute to the regulation of angiogenesis (41, 57), involving a combination of tight junction electrical communication and paracrine influence, e.g., VEGF (62). Indeed, there was a significant reduction in pericyte coverage of ECs during early phases of angiogenesis in skeletal muscles of rats subjected to chronic electrical stimulation, which suggested an antiangiogenic impact of pericytes in this experimental model (33). In contrast, long-term peripheral vasodilation (prazosin model) or prolonged stretch (extirpation model) was accompanied by no change or an increase in pericytes coverage, respectively (35). Thus the role of pericytes in controlling physiological angiogenesis seems context dependent, reflecting the nature of the initial stimulus (35), although the contribution of pericytes subtypes to different angiotypes has yet to be characterized (12).
Fibroblasts, mast cells, macrophages, and circulating cells.
Mesenchymal fibroblasts are cells within the interstitium that synthesize the ECM and connective tissue of the stroma. They are perhaps best known as the origin of pathological conditions such as fibrosis but also aid inflammation and tumor growth. Their physiological role is to support cell-cell interaction and cell migration and may mediate angiogenesis chemically (via expression of angiogenic factors) and mechanically (via disruption of connective tissue and ECM deposition) (67). In sprouting angiogenesis, the number of fibroblasts increases in close proximity to sites of neovascularization following increased muscle activity and are responsible for much of the mitotic activity evident with light microscopy (36), although whether activated EC attract motile fibroblasts or activated fibroblasts stimulate an EC response remains unclear (32).
Mast cells of the hematopoietic lineage are terminally differentiated in peripheral tissue, where activation causes release of growth factors and inflammatory cytokines that may stimulate angiogenesis in a pathological environment such as found in tumors. In muscle they are found within the interstitial, often close to capillaries, where they are thought to be responsive to both mechanical deformation and/or metabolite release. Although mast cells may be important in cardiac muscle remodeling, their activation appears not to be an important regulator of angiogenesis as a result of increased skeletal muscle activity (28).
Similarly, macrophages are ubiquitous constituents of tissue but are also thought to mainly play a role in inflammation-mediated angiogenesis, as a result of secreted factors under pathological conditions. However, the resident population may be renewed by turnover or by infiltration of other macrophages. Interestingly, an increase in these VEGF-positive cells within the interstitium appears to be an early sign of angiogenesis in response to increased muscle activity (18), perhaps an indication that part of the exercise-induced remodeling has an inflammatory basis (107).
There is increasing interest in the potential role of circulating cells within the local vasculature for regulation of angiogenesis. Perhaps the best known are the hematopoietic stem cells that support vigorous angiogenesis within solid tumors and may supplement endothelial turnover during tissue repair. Other cell types will require further investigation. However, increased leukocyte concentration around venules (the supposed site of new capillary growth) within skeletal muscle suggest they may have some regulatory influence, but in physiological angiogenesis this appears to be unlikely (105). Intriguingly, however, circulating platelets appear to have a differential effect on physiological angiogenesis within skeletal muscle, likely influencing endothelial migration by cyclooxygenase signaling (105). Platelets are also a major site for the production of TSP-1, which exerts a negative influence on EC production and migration (19), but thus far there is little evidence that circulating plasma/serum TSP-1 levels have any direct influence on skeletal muscle angiogenesis.
Molecular Changes
The orchestration of molecular information at various levels is required to generate the appropriate extent of angiogenesis in skeletal muscles. Although details are still to be finalized, it is evident that this involves sensitive feedback control and disruption of such signaling is likely a component of hyperproliferative or ischemic vascular diseases. There is an ever-growing list of pro- and antiangiogenic factors that must remain in balance to effect microvascular homeostasis [see reviews (53, 101)]. We will focus on the description of those that have emerged as the major players and some new factors (Table 1) that may provide significant impact on our understanding of physiologically controlled angiogenesis. This does not diminish the potential importance of the factors not discussed here but points out that our current understanding for many of the positive and negative angiogenic regulators remains circumstantial and in some cases context dependent.
Table 1.
Emerging regulators and their actions and responses
| Emerging Regulators | Action/Response | References |
|---|---|---|
| FoxO1 | Modulate TSP-1 effects | (94, 112, 118) |
| Nucleolin | Interacts wiht VEGF and MMP9. | (37, 64) |
| EC surface receptor for endostatin. | (97, 98, 117) | |
| Ephrin B2 | Increased by stretch and hypoxic stress. | (75, 128) |
| Polymerae δ-interacting protein 2 (Poldip2) | Necessary for vascular integerity, EC proliferation, inhibition of MMPs, and cell adhesion. | (120) |
| PGC-1α and Secreted phosphoprotein 1 | Transcriptional coactivator responsible in the function of mitochondrial biology, electron transport chain, δ oxidation, and influence on angioregulatory mitogens (e.g., VEGF). | (4, 96, 112) |
| Vasoinhibin-1 | Anti-angiogenic mitogen | (76, 78, 101) |
| Promotes vessel stablization and maturation. | ||
| Endosialin (CD248) | Required for PDGFRβ-dependent capillary sprouting angiogenesis. | (95) |
FoxO1, Forkhead Box O 1; TSP-1, thrombospondin-1; MMP, matrix metalloproteinase; EC, endothelial cell; PDGFR, PDGF receptor.
Vascular endothelial growth factor.
VEGF exists in several homodimeric isoforms: VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206 (85). Although several of these isoforms likely play a role in angiogenesis, VEGF165 has been found to be the most central proangiogenic factor in skeletal muscle. Some of the early evidence for the importance of VEGF in angiogenesis was that the training-induced increase in capillary growth was partially reduced with VEGF receptor blockade (86). Moreover, treatment of rodents with a VEGF sequestor revealed that VEGF is required for both shear stress- and overload-induced angiogenesis (124). Studies on genetically modified mice lacking muscle-specific VEGF have demonstrated that basal capillarization is markedly reduced compared with wild-type mice (103) and that the training-induced increases in capillary growth is abolished (26, 104), providing solid evidence for the importance of VEGF in muscle. Additional studies using myocyte-specific, VEGF-deficient mice revealed that skeletal muscle angiogenesis does not occur in response to prazosin-mediated shear stress (122) or extirpation-mediated muscle stretch (i.e., muscle overload) (45), which seems to clearly establish that muscle fiber-derived VEGF is essential to initiate the angiogenic process within skeletal muscle.
An acute exercise bout leads to a transient, severalfold increase in expression of VEGF mRNA (16, 48, 108) with levels returning to baseline after 4–6 h of recovery (46, 73). Levels of VEGF protein also increase after an acute exercise bout in rodents (99) and are elevated during the first few weeks of aerobic exercise training in young healthy individuals (47) and animals (99), whereas in humans VEGF protein levels appear to return to baseline after 4 wk of training (60). This pattern of VEGF protein changes appears to be different in individuals with lifestyle-related diseases (52) and ageing (22, 44), where muscle VEGF levels are lower in elderly than in young individuals at baseline but are enhanced with exercise training.
For muscle fiber-derived VEGF to promote angiogenesis, secretion to the extracellular space is required, and mechanical stimulus induced by passive movement of the muscle (56) or active muscle contraction (39, 58) leads to increased muscle interstitium VEGF levels. While increases in extracellular VEGF can originate from different cell types including ECs, fibroblast, and pericytes, evidence (noted above) points to myocytes being the most important source (62, 73). Muscle fibers contain high levels of VEGF stored in vesicles (62), and synthesis of VEGF does not appear to be required for secretion but likely occurs after contraction to replenish muscle stores (62). The exercise-induced increase in muscle interstitial VEGF does not appear to be altered by exercise training in healthy individuals (39, 60) but may be improved by training in individuals with lifestyle-related disease (52). At present, the mechanisms underlying VEGF secretion from skeletal muscle, and how this is altered by disease and exercise, has yet to be fully determined. While there is robust evidence that VEGF is required for the angiogenesis process, it must be emphasized that VEGF cannot act alone, and other factors are also essential and need to be co-regulated to elicit angiogenesis.
Nitric oxide.
The gaseous, radical NO (NO·= NO) is generated by the catalytic activity of three intracellular NO synthases (NOSs). While the existence of the inducible NOS (iNOS) in skeletal muscle is not confirmed, eNOS is found in ECs of all vascular segments including capillaries (115), whereas neuronal NOS (nNOS) is present at high concentrations in the sarcolemma of skeletal muscle fibers in close proximity to the microcirculation (119).
A modulatory impact of NO on angiogenesis has been shown in rodents. Administration of NOS inhibitors, such as NG-nitro-l-arginine (l-NNA) and Nω-nitro-l-arginine methyl ester (l-NAME), blunts angiogenesis in skeletal muscle in response to chronic electrical stimulation (35) and whole body exercise (40). Furthermore, the increased expression of VEGFR-2 and VEGF proteins in skeletal muscles was abrogated in rats electrically stimulated for 2 and 4 days but not for 7 days (93). These data implied that chronic electrical stimulation leads to induction of two peaks of VEGF and VEGFR-2 expression: an early first NO-dependent increase required for the onset of angiogenesis and a later, NO-independent rise that may aid maintenance of the expanding capillary bed (93).
However, chemical NOS inhibitors used do not typically discriminate between NOS isoforms, and understanding the differential impact of each one on angiogenic events requires studies with NOS-deficient knockout (KO) mice. The VEGF increase in ECs (23) and angiogenesis (8, 123) were blunted in skeletal muscles of eNOS-KO mice after prazosin administration, suggesting that eNOS-derived NO produced represents an obligate upstream signal for shear stress-induced angiogenesis (8). This angiotype depends on functional hyperemia and the vasodilatory potential of arteries, which is significantly lower in eNOS-KO than WT mice (81, 129). In contrast, overload-induced sprouting was not prevented in eNOS-KO mice, and there was no effect on either splitting or sprouting angiogenesis in nNOS-KO mice (10, 123). Hence, the influence of NO on expression of VEGF might depend on the site of NO at which it is generated and the context of the physical stimuli (29).
In humans, a direct role of NO for skeletal muscle angiogenesis has not been determined; however, eNOS is upregulated in muscle in response to acute exercise, exercise training, and passive movement leading to increased shear stress (21, 54, 56). A similar role for eNOS and NO in angiogenesis in humans, as seen in rodents, therefore seems likely.
Thrombospondin-1.
The first reports that acute exercise increased, but that exercise training decreased, skeletal muscle TSP-1 expression (102) and that increasing shear stress decreased EC expression of TSP-1 (14) provided an incentive to understand the role of negative regulators of angiogenesis in this context (53, 101). Indeed, whereas a single exercise bout increased skeletal muscle TSP-1 and VEGF mRNA expression up to fourfold, repeated daily bouts of exercise reduces the TSP-1 (but not VEGF) response to exercise (102). More recently, the transcription factor Forkhead Box O 1 (FoxO1) has been shown to transcriptionally regulate EC production of TSP-1 (112). Whereas increasing FoxO1 can restrain angiogenesis in ischemic skeletal muscle (94, 112), gene deletion of FoxO1/FoxO3a abolished the increase in TSP-1 expression following acute exercise and induced a more rapid exercise-induced expansion in muscle capillarity (118). Studies examining skeletal muscle expression of TSP-1 in response to varying duration and intensity of exercise training (60, 68, 69, 82, 88, 89) indicate that it likely plays a key regulatory role in controlling the onset of exercise-induced angiogenesis. Thus, while VEGF-A is perhaps the most central angiogenic mitogen whose secretion is an absolute requirement for the initiation of skeletal muscle angiogenesis, it appears that TSP-1 may be essential for the necessary physiological constraint on capillary expansion (100).
Responses following physical deconditioning (i.e., detraining) in rodents demonstrate that capillary regression seems to occur in temporal correlation to muscle TSP-1 protein expression, even in the face of elevated muscle VEGF expression (97). Hence, while VEGF is necessary to initiate angiogenesis, it appears that TSP-1 (and likely other antiangiogenic factors) may exert principal control on the growth process, determining when and possibly where angiogenesis takes place. Indeed, there is also ample evidence from gain and loss of function studies involving TSP-1 that support the importance of TSP-1 in regulating muscle angiogenesis under both physiological (6, 89) and pathological (70, 80, 92) conditions.
Other pro- and antiangiogenic factors.
While much attention has focused on VEGF-A, and increasingly TSP-1, numerous other factors are known to exhibit either proangiogenic or anti-angiogenic properties in other tissue. These include growth factor/cognate receptor pairings such as the angiopoietins/TIE receptors, ephrins/Ephs, FGFs/FGF receptors, PDGFs/PDGF receptors, and delta-like ligand 4/notch, in addition to endothelial function modulators such as nNOS and eNOS. There is a growing realization that these factors need to be presented in spatially and temporally balanced sequences for the formation and maintenance of functional vessels in skeletal muscle [reviewed in Olfert and Birot (101)]. Furthermore, various proteins without previously established function in vascular biology, such as secretion of phosphoprotein 1 (113), polymerase δ-interacting protein 2 (2), nucleolin (97, 98), as well as peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), all appear to have influence on capillarity in skeletal muscles.
Notably, PGC-1α is a key transcriptional coactivator that regulates expression of genes involved in mitochondrial biogenesis and energy metabolism (50, 99). Recent evidence from muscle-specific PGC-1α KO mice suggest that this factor is an important mediator of exercise-induced angiogenesis (4). The molecular mechanisms behind this effect include exercise-dependent β-adrenergic signaling that induces transcription of an additional isoform (PGC-1α2) from an alternative promoter, which in turn increased expression of angiogenic factors such as VEGF-A and angiopoietin 2 (4, 20, 84). The use of this alternative PGC-1α promotor also seems important for the response of skeletal muscle to exercise in humans (96). Angiogenic activity of PGC-1α might involve its downstream ligand peroxisome proliferator-activated receptor-β/δ (13). Translation of PGC-1α2 mRNA leads to two PGC-1α protein variants with slightly different NH2-terminus (PGC-1α2 and PGC-1α3) (20). A recent study suggests that PGC-1α2 is the most responsive to a single exercise bout in human skeletal muscle (42). Despite remaining questions, it is clear that PGC-1α and its isoforms likely play an important role in regulating skeletal muscle angiogenesis.
Notwithstanding that the expanding list of factors that are now known to have an impact on this physiological process, their roles often remain only partially understood and need to be characterized in more detail. A vital and important arena for future research is the specific roles and particularly the interplay of these gene products during angiogenesis in skeletal muscle.
Summary and Relevance
Beyond growth and development, physiologically controlled angiogenesis is largely limited to 1) the female reproductive cycle, i.e., menses, involving smooth muscle, and 2) skeletal muscle adaptation to exercise stimuli. Therefore, studies aimed at understanding angioadaptation and regulation of angiogenesis in muscle provide a great opportunity to investigate and understand the complexity and highly structured choreography essential for controlled regulation of angiogenesis. Given the number of diseases that involve aberrant regulation of angiogenesis, resulting in either uncontrolled vessel growth or capillary rarefaction, it is imperative that a fundamental understanding of the mechanisms that work to effectively control angiogenesis is attained, before we can truly fully understand pathological dysregulation. Indeed, lessons learnt from the past decade reinforce the need for a more comprehensive understanding of the temporal control and degree of interaction between stimulators and inhibitors of angiogenesis that goes beyond single gene and protein responses. It is here that use of multiplexing technology, including but not limited to -omic levels approaches, will allow a more global assessment angiogenic-related factors which provide the greatest opportunities to fully understand the complex interplay between stimulators and inhibitors of angiogenesis. From this foundation we will be better equipped to develop tools and/or therapies that effectively combat uncontrolled vessel growth (such as that seen in cancer/tumor development) or capillary loss often associated with many chronic diseases (such as diabetes, hypertension, Alzheimer's, osteoporosis, etc.).
GRANTS
I. M. Olfert received financial support, in part, by a grant to the West Virginia Clinical and Translational Science Institute (National Institute of General Medical Sciences Grant U54GM104942), American Heart Association Innovation Research Grant 13IRG14330015, West Virginia University Research Foundation PSCoR grant, and from West Virginia University School of Medicine. O. Baum recieved financial support by Swiss National Science Foundation Grant 320030-144167. Y. Hellsten recieved financial support from The Danish Council for Independent Research-Medical Sciences, The Lundbeck Foundation, Novo Nordisk Foundation, and the Danish Ministry of Culture. S. Egginton recieved financial support from the British Heart Foundation Grant PG/14/15/30691.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
I.M.O., O.B., Y.H., and S.E. worked together on the conception, design, interpretation and critical analysis of the data and literature reviewed; I.M.O. and O.B. prepared figures; and all authors participated in drafting the manuscript and approving the final version of manuscript.
REFERENCES
- 1.Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol 289: R283–R296, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Amanso AM, Lassegue B, Joseph G, Landazuri N, Long JS, Weiss D, Taylor WR, Griendling KK. Polymerase delta-interacting protein 2 promotes postischemic neovascularization of the mouse hindlimb. Arterioscler Thromb Vasc Biol 34: 1548–1555, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21: 51–55, 1973. [DOI] [PubMed] [Google Scholar]
- 6.Audet GN, Fulks D, Stricker JC, Olfert IM. Chronic delivery of a thrombospondin-1 mimetic decreases skeletal muscle capillarity in mice. PLoS One 8: e55953, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basic VT, Jacobsen A, Sirsjo A, Abdel-Halim SM. TNF stimulation induces VHL overexpression and impairs angiogenic potential in skeletal muscle myocytes. Int J Mol Med 34: 228–236, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Baum O, Da Silva-Azevedo L, Willerding G, Wockel A, Planitzer G, Gossrau R, Pries AR, Zakrzewicz A. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol 287: H2300–H2308, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Baum O, Gubeli J, Frese S, Torchetti E, Malik C, Odriozola A, Graber F, Hoppeler HH, Tschanz SA. Angiogenesis-related ultrastructural changes of capillaries in human skeletal muscle in response to endurance exercise. J Appl Physiol 119: 1118–1126, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Baum O, Vieregge M, Koch P, Gul S, Hahn S, Huber-Abel FA, Pries AR, Hoppeler H. Phenotype of capillaries in skeletal muscle of nNOS-knockout mice. Am J Physiol Regul Integr Comp Physiol 304: R1175–R1182, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Benoit H, Jordan M, Wagner H, Wagner PD. Effect of NO, vasodilator prostaglandins, and adenosine on skeletal muscle angiogenic growth factor gene expression. J Appl Physiol 86: 1513–1518, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307: C25–C38, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop-Bailey D. PPARs and angiogenesis. Biochem Soc Trans 39: 1601–1605, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Bongrazio M, Da Silva-Azevedo L, Bergmann EC, Baum O, Hinz B, Pries AR, Zakrzewicz A. Shear stress modulates the expression of thrombospondin-1 and CD36 in endothelial cells in vitro and during shear stress-induced angiogenesis in vivo. Int J Immunopathol Pharmacol 19: 35–48, 2006. [PubMed] [Google Scholar]
- 15.Brasseur JE, Curtis RL, Mellender JW, Rimm AA, Melvin JL, Sulaiman AR. Systematic distribution of muscle fiber types in the medial gastrocnemius of the laboratory mouse: a morphometric analysis. Anat Rec 218: 396–401, 1987. [DOI] [PubMed] [Google Scholar]
- 16.Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81: 355–361, 1996. [DOI] [PubMed] [Google Scholar]
- 17.British Heart Foundation. Put your heart into walking: British Heart Foundation, 2013. [Google Scholar]
- 18.Brown MD, Egginton S, Hudlická O, Milkiewicz M, Verhaeg J. Macrophages as a source of VEGF in activity-induced angiogenesis in rat skeletal muscle. XXXIVth IUPS Congress, Christchurch, New Zealand (pre-circulated abstract), 2001. [Google Scholar]
- 19.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol 19: 597–614, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci USA 106: 21401–21406, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe AM, Barker TA, Tipton KD, Wagenmakers AJ. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J Physiol 591: 641–656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croley AN, Zwetsloot KA, Westerkamp LM, Ryan NA, Pendergast AM, Hickner RC, Pofahl WE, Gavin TP. Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged vs. young women. J Appl Physiol 99: 1872–1879, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Da Silva-Azevedo L, Baum O, Zakrzewicz A, Pries AR. Vascular endothelial growth factor is expressed in endothelial cells isolated from skeletal muscles of nitric oxide synthase knockout mice during prazosin-induced angiogenesis. Biochem Biophys Res Commun 297: 1270–1276, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Dawson JM, Hudlicka O. The effects of long term administration of prazosin on the microcirculation in skeletal muscles. Cardiovasc Res 23: 913–920, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Degens H, Turek Z, Hoofd LJ, Van't Hof MA, Binkhorst RA. The relationship between capillarisation and fibre types during compensatory hypertrophy of the plantaris muscle in the rat. J Anat 180: 455–463, 1992. [PMC free article] [PubMed] [Google Scholar]
- 26.Delavar H, Nogueira L, Wagner PD, Hogan MC, Metzger D, Breen EC. Skeletal myofiber VEGF is essential for the exercise training response in adult mice. Am J Physiol Regul Integr Comp Physiol 306: R586–R595, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Doyle JL, Haas TL. The angiogenic response to skeletal muscle overload is not dependent on mast cell activation. Microcirculation 17: 548–556, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Egginton S. Invited review: activity-induced angiogenesis. Pflügers Arch 457: 963–977, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Egginton S. Morphometric analysis of tissue capillary supply. In: Advances in Comparative and Environmental Physiology: Vertebrate Gas Exchange. Berlin: Springer-Verlag, 1990, p. 73–141. [Google Scholar]
- 31.Egginton S. Numerical and areal density estimates of fibre type composition in a skeletal muscle (rat extensor digitorum longus). J Anat 168: 73–80, 1990. [PMC free article] [PubMed] [Google Scholar]
- 32.Egginton S. Physiological factors influencing capillary growth. Acta Physiol (Oxf) 202: 225–239, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Egginton S, Hudlicka O, Brown MD, Graciotti L, Granata AL. In vivo pericyte-endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc Res 51: 213–228, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Egginton S, Turek Z. Comparative distributions of numerical and areal indices of tissue capillarity. Adv Exp Med Biol 277: 161–169, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Egginton S, Zhou AL, Brown MD, Hudlicka O. The role of pericytes in controlling angiogenesis in vivo. Adv Exp Med Biol 476: 81–99, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Egginton S, Zhou AL, Brown MD, Hudlicka O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res 49: 634–646, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Fahling M, Steege A, Perlewitz A, Nafz B, Mrowka R, Persson PB, Thiele BJ. Role of nucleolin in posttranscriptional control of MMP-9 expression. Biochim Biophys Acta 1731: 32–40, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher GF, Balady G, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, Froelicher ES, Froelicher VF, Pina IL, Pollock ML. Statement on exercise: benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation 94: 857–862, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Gavin TP, Ruster RS, Carrithers JA, Zwetsloot KA, Kraus RM, Evans CA, Knapp DJ, Drew JL, McCartney JS, Garry JP, Hickner RC. No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol 585: 231–239, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD. Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol 88: 1192–1198, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314: 15–23, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Gidlund Ek Ydfors M, Appel S, Rundqvist H, Sundberg CJ, Norrbom JM. Rapidly elevated levels of PGC-1α-b protein in human skeletal muscle after exercise: exploring regulatory factors in a randomized controlled trial. J Appl Physiol 119: 374–384, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Gliemann L, Gunnarsson TP, Hellsten Y, Bangsbo J. 10-20-30 training increases performance and lowers blood pressure and VEGF in runners. Scand J Med Sci Sports 25: e479–e489, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Gliemann L, Olesen J, Bienso RS, Schmidt JF, Akerstrom T, Nyberg M, Lindqvist A, Bangsbo J, Hellsten Y. Resveratrol modulates the angiogenic response to exercise training in skeletal muscles of aged men. Am J Physiol Heart Circ Physiol 307: H1111–H1119, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Gorman JL, Liu ST, Slopack D, Shariati K, Hasanee A, Olenich S, Olfert IM, Haas TL. Angiotensin II evokes angiogenic signals within skeletal muscle through co-ordinated effects on skeletal myocytes and endothelial cells. PLoS One 9: e85537, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustafsson T, Ameln H, Fischer H, Sundberg CJ, Timmons JA, Jansson E. VEGF-A splice variants and related receptor expression in human skeletal muscle following submaximal exercise. J Appl Physiol 98: 2137–2146, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson T, Knutsson A, Puntschart A, Kaijser L, Nordqvist AC, Sundberg CJ, Jansson E. Increased expression of vascular endothelial growth factor in human skeletal muscle in response to short-term one-legged exercise training. Pflügers Arch 444: 752–759, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Gustafsson T, Sundberg CJ. Expression of angiogenic growth factors in hum,an skeletal muscle in response to a singular bout of exercise. Am J Physiol Heart Circ Physiol 279: H3144–H3145, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, Brown MD, Madri JA, Hudlicka O. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol 279: H1540–H1547, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27: 728–735, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Hansen-Smith FM, Hudlicka O, Egginton S. In vivo angiogenesis in adult rat skeletal muscle: early changes in capillary network architecture and ultrastructure. Cell Tissue Res 286: 123–136, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Hansen AH, Nielsen JJ, Saltin B, Hellsten Y. Exercise training normalizes skeletal muscle vascular endothelial growth factor levels in patients with essential hypertension. J Hypertens 28: 1176–1185, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Hellsten Y, Hoier B. Capillary growth in human skeletal muscle: physiological factors and the balance between pro-angiogenic and angiostatic factors. Biochem Soc Trans 42: 1616–1622, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Hellsten Y, Nielsen JJ, Lykkesfeldt J, Bruhn M, Silveira L, Pilegaard H, Bangsbo J. Antioxidant supplementation enhances the exercise-induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic Biol Med 43: 353–361, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol 590: 6297–6305, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R975–R982, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Hirschi KK, D'Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. EXS 79: 419–428, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Hoffner L, Nielsen JJ, Langberg H, Hellsten Y. Exercise but not prostanoids enhance levels of vascular endothelial growth factor and other proliferative agents in human skeletal muscle interstitium. J Physiol 550: 217–225, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoier B, Hellsten Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation 21: 301–314, 2014. [DOI] [PubMed] [Google Scholar]
- 60.Hoier B, Nordsborg N, Andersen S, Jensen L, Nybo L, Bangsbo J, Hellsten Y. Pro- and anti-angiogenic factors in human skeletal muscle in response to acute exercise and training. J Physiol 590: 595–606, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoier B, Passos M, Bangsbo J, Hellsten Y. Intense intermittent exercise provides weak stimulus for vascular endothelial growth factor secretion and capillary growth in skeletal muscle. Exp Physiol 98: 585–597, 2013. [DOI] [PubMed] [Google Scholar]
- 62.Hoier B, Prats C, Qvortrup K, Pilegaard H, Bangsbo J, Hellsten Y. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. FASEB J 27: 3496–3504, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Hoppeler H, Kayar S. Capillarity and oxidative capacity of muscles. Physiology 3: 113–116, 1988. [Google Scholar]
- 64.Huang Y, Shi H, Zhou H, Song X, Yuan S, Luo Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood 107: 3564–3571, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev 72: 369–417, 1992. [DOI] [PubMed] [Google Scholar]
- 66.Hudlicka O, Brown M, Egginton S. The microcirculation in skeletal muscle. In: Myology, Basic and Clinical (3rd ed), edited by Engel A and Franzini-Armstrong C. New York: McGraw-Hill, 2004, p. 511–533. [Google Scholar]
- 67.Hurley JR, Balaji S, Narmoneva DA. Complex temporal regulation of capillary morphogenesis by fibroblasts. Am J Physiol Cell Physiol 299: C444–C453, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huttemann M, Lee I, Malek MH. (−)-Epicatechin maintains endurance training adaptation in mice after 14 days of detraining. FASEB J 26: 1413–1422, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huttemann M, Lee I, Perkins GA, Britton SL, Koch LG, Malek MH. (−)-Epicatechin is associated with increased angiogenic and mitochondrial signalling in the hindlimb of rats selectively bred for innate low running capacity. Clin Sci (Lond) 124: 663–674, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol 28: 110–119, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA 102: 13141–13146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jensen L, Bangsbo J, Hellsten Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J Physiol 557: 571–582, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jensen L, Schjerling P, Hellsten Y. Regulation of VEGF and bFGF mRNA expression and other proliferative compounds in skeletal muscle cells. Angiogenesis 7: 255–267, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Jobin J, Maltais F, Doyon JF, LeBlanc P, Simard PM, Simard AA, Simard C. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil 18: 432–437, 1998. [DOI] [PubMed] [Google Scholar]
- 75.Katsu M, Koyama H, Maekawa H, Kurihara H, Uchida H, Hamada H. Ex vivo gene delivery of ephrin-B2 induces development of functional collateral vessels in a rabbit model of hind limb ischemia. J Vasc Surg 49: 192–198, 2009. [DOI] [PubMed] [Google Scholar]
- 76.Kerbel RS. Vasohibin: the feedback on a new inhibitor of angiogenesis. J Clin Invest 114: 884–886, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kikuchi R, Nakamura K, MacLauchlan S, Ngo DTM, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, Matsushita T, Murohara T, Gokce N, Bates DO, Hamburg NM, Walsh K. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med 20: 1464–1471, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kishlyansky M, Vojnovic J, Roudier E, Gineste C, Decary S, Forn P, Bergeron R, Desplanches D, Birot O. Striated muscle angio-adaptation requires changes in Vasohibin-1 expression pattern. Biochem Biophys Res Commun 399: 359–364, 2010. [DOI] [PubMed] [Google Scholar]
- 79.Kivela R, Silvennoinen M, Touvra AM, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J 20: 1570–1572, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Kondo H, Fujino H, Murakami S, Tanaka M, Kanazashi M, Nagatomo F, Ishihara A, Roy RR. Low-intensity running exercise enhances the capillary volume and pro-angiogenic factors in the soleus muscle of type 2 diabetic rats. Muscle Nerve 51: 391–399, 2015. [DOI] [PubMed] [Google Scholar]
- 81.Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics 2: 21–27, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Lee I, Hüttemann M, Kruger A, Bollig-Fischer A, Malek MH. (−)-Epicatechin combined with 8 weeks of treadmill exercise is associated with increased angiogenic and mitochondrial signaling in mice. Front Pharmacol 6: 43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130: 691–703, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JF, Hidalgo J, Pilegaard H. PGC-1alpha mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab 297: E92–E103, 2009. [DOI] [PubMed] [Google Scholar]
- 85.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309, 1989. [DOI] [PubMed] [Google Scholar]
- 86.Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am J Physiol Heart Circ Physiol 288: H759–H768, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2528–H2538, 2001. [DOI] [PubMed] [Google Scholar]
- 88.Malek MH, Huttemann M, Lee I, Coburn JW. Similar skeletal muscle angiogenic and mitochondrial signalling following 8 weeks of endurance exercise in mice: discontinuous versus continuous training. Exp Physiol 98: 807–818, 2013. [DOI] [PubMed] [Google Scholar]
- 89.Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol 94: 749–760, 2009. [DOI] [PubMed] [Google Scholar]
- 90.Mathieu-Costello O, Hoppeler H, Weibel ER. Capillary tortuosity in skeletal muscles of mammals depends on muscle contraction. J Appl Physiol 66: 1436–1442, 1989. [DOI] [PubMed] [Google Scholar]
- 91.Mathieu O, Cruz-Orive LM, Hoppeler H, Weibel ER. Estimating length density and quantifying anisotropy in skeletal muscle capillaries. J Microsc 131: 131–146, 1983. [DOI] [PubMed] [Google Scholar]
- 92.Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg 124: 1880–1889, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milkiewicz M, Hudlicka O, Brown MD, Silgram H. Nitric oxide, VEGF, and VEGFR-2: interactions in activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol 289: H336–H343, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Milkiewicz M, Roudier E, Doyle JL, Trifonova A, Birot O, Haas TL. Identification of a mechanism underlying regulation of the anti-angiogenic forkhead transcription factor FoxO1 in cultured endothelial cells and ischemic muscle. Am J Pathol 178: 935–944, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naylor AJ, McGettrick HM, Maynard WD, May P, Barone F, Croft AP, Egginton S, Buckley CD. A differential role for CD248 (Endosialin) in PDGF-mediated skeletal muscle angiogenesis. PLoS One 9: e107146, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Norrbom J, Sallstedt EK, Fischer H, Sundberg CJ, Rundqvist H, Gustafsson T. Alternative splice variant PGC-1alpha-b is strongly induced by exercise in human skeletal muscle. Am J Physiol Endocrinol Metab 301: E1092–E1098, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Olenich SA, Audet GN, Roberts KA, Olfert IM. Effects of detraining on the temporal expression of positive and negative angioregulatory proteins in skeletal muscle of mice. J Physiol 592: 3325–3338, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olenich SA, Gutierrez-Reed N, Audet GN, Olfert IM. Temporal response of positive and negative angiogenic regulators in response to acute and chronic exercise training in mice. J Physiol 591: 5157–5169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olenich J, Kiilerich K, Pilegaard H. PGC-1alpha-mediated adaptations in skeletal muscle. Pflügers Arch 460: 153–162, 2010. [DOI] [PubMed] [Google Scholar]
- 100.Olesen IM. Physiological capillary regression is not dependent on reducing VEGF expression. Microcirculation. 2015 Dec 11. doi: 10.1111/micc.12263 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olfert IM, Birot O. Importance of anti-angiogenic factors in the regulation of skeletal muscle angiogenesis. Microcirculation 18: 316–330, 2011. [DOI] [PubMed] [Google Scholar]
- 102.Olfert IM, Breen EC, Gavin TP, Wagner PD. Temporal thrombospondin-1 mRNA response in skeletal msucle exposed to acute and chronic exercise. Growth Factors 24: 253–259, 2006. [DOI] [PubMed] [Google Scholar]
- 103.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587: 1755–1767, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olfert IM, Howlett RA, Wagner PD, Breen EC. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Regul Integr Comp Physiol 299: R1059–R1067, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Packham IM, Watson SP, Bicknell R, Egginton S. In vivo evidence for platelet-induced physiological angiogenesis by a COX driven mechanism. PLoS One 9: e107503, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pearce SC, Hudlicka O, Brown MD. Effect of indomethacin on capillary growth and microvasculature in chronically stimulated rat skeletal muscles. J Physiol 526: 435–443, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pedersen BK. Muscle as a secretory organ. Compr Physiol 3: 1337–1362, 2013. [DOI] [PubMed] [Google Scholar]
- 108.Richardson RS, Wagner H, Mudaliar SR, Henry R, Noyszewski EA, Wagner PD. Human VEGF gene expression in skeletal muscle: effect of acute normoxic and hypoxic exercise. Am J Physiol Heart Circ Physiol 277: H2247–H2252, 1999. [DOI] [PubMed] [Google Scholar]
- 109.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA 102: 13147–13152, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol 283: H1430–H1438, 2002. [DOI] [PubMed] [Google Scholar]
- 111.Rosenblatt JD, Kuzon WM Jr, Plyley MJ, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol 62: 85–92, 1987. [DOI] [PubMed] [Google Scholar]
- 112.Roudier E, Milkiewicz M, Birot O, Slopack D, Montelius A, Gustafsson T, Paik JH, DePinho RA, Casale GP, Pipinos II, Haas TL. Endothelial FoxO1 is an intrinsic regulator of thrombospondin 1 expression that restrains angiogenesis in ischemic muscle. Angiogenesis 16: 759–772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rowe GC, Raghuram S, Jang C, Nagy JA, Patten IS, Goyal A, Chan MC, Liu LX, Jiang A, Spokes KC, Beeler D, Dvorak H, Aird WC, Arany Z. PGC-1alpha induces SPP1 to activate macrophages and orchestrate functional angiogenesis in skeletal muscle. Circ Res 115: 504–517, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sarelius I, Pohl U. Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol (Oxf) 199: 349–365, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am J Physiol Heart Circ Physiol 277: H1167–H1177, 1999. [DOI] [PubMed] [Google Scholar]
- 116.Sher J, Cardasis C. Skeletal muscle fiber types in the adult mouse. Acta Neurol Scand 54: 45–56, 1976. [DOI] [PubMed] [Google Scholar]
- 117.Shi H, Huang Y, Zhou H, Song X, Yuan S, Fu Y, Luo Y. Nucleolin is a receptor that mediates antiangiogenic and antitumor activity of endostatin. Blood 110: 2899–2906, 2007. [DOI] [PubMed] [Google Scholar]
- 118.Slopack D, Roudier E, Liu ST, Nwadozi E, Birot O, Haas TL. Forkhead BoxO transcription factors restrain exercise-induced angiogenesis. J Physiol 592: 4069–4082, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001. [DOI] [PubMed] [Google Scholar]
- 120.Sutliff RL, Hilenski LL, Amanso AM, Parastatidis I, Dikalova AE, Hansen L, Datla SR, Long JS, El-Ali AM, Joseph G, Gleason RL, Taylor WR, Hart CM, Griendling KK, Lassègue B. Polymerase delta interacting protein 2 sustains vascular structure and function. Arterioscler Thromb Vasc Biol 33: 2154–2161, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomanek RJ, Torry RJ. Growth of the coronary vasculature in hypertrophy: mechanisms and model dependence. Cell Mol Biol Res 40: 129–136, 1994. [PubMed] [Google Scholar]
- 122.Uchida C, Nwadozi E, Hasanee A, Olenich S, Olfert IM, Haas TL. Muscle-derived vascular endothelial growth factor regulates microvascular remodelling in response to increased shear stress in mice. Acta Physiol (Oxf) 214: 349–360, 2015. [DOI] [PubMed] [Google Scholar]
- 123.Williams JL, Cartland D, Hussain A, Egginton S. A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J Physiol 570: 445–454, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Williams JL, Cartland D, Rudge JS, Egginton S. VEGF trap abolishes shear stress- and overload-dependent angiogenesis in skeletal muscle. Microcirculation 13: 499–509, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Williamson JR, Hoffmann PL, Kohrt WM, Spina RJ, Coggan AR, Holloszy O. Endurance exercise training decreases capillary basement membrane width in older nondiabetic and diabetic adults. J Appl Physiol 80: 747–753, 1996. [DOI] [PubMed] [Google Scholar]
- 126.World Health Organization. Global recommendations on physical activity for health. Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]
- 127.Wragg JW, Durant S, McGettrick HM, Sample KM, Egginton S, Bicknell R. Shear stress regulated gene expression and angiogenesis in vascular endothelium. Microcirculation 21: 290–300, 2014. [DOI] [PubMed] [Google Scholar]
- 128.Yamashita T, Ohneda K, Nagano M, Miyoshi C, Kaneko N, Miwa Y, Yamamoto M, Ohneda O, Fujii-Kuriyama Y. Hypoxia-inducible transcription factor-2alpha in endothelial cells regulates tumor neovascularization through activation of ephrin A1. J Biol Chem 283: 18926–18936, 2008. [DOI] [PubMed] [Google Scholar]
- 129.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA 102: 10999–11004, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou A, Egginton S, Hudlicka O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res 293: 293–303, 1998. [DOI] [PubMed] [Google Scholar]
- 131.Zhou AL, Egginton S, Brown MD, Hudlicka O. Capillary growth in overloaded, hypertrophic adult rat skeletal muscle: an ultrastructural study. Anatomical Rec 252: 49–63, 1998. [DOI] [PubMed] [Google Scholar]