Fractal measures showed self-similarity in coronary branching. New: Local myocardial blood flows are correlated with near neighbors in two different patterns with similar fractal dimension: pattern 1, when metabolically driven, vasomotion persists; pattern 2, after adenosine stops vasomotion, vasodilated structure dominates.
Keywords: myocardial blood flow heterogeneity, fractal spatial correlation, vasomotor tone, catecholamine-stimulated metabolism
Abstract
Regional myocardial blood flows are markedly heterogeneous. Fractal analysis shows strong near-neighbor correlation. In experiments to distinguish control by vascular anatomy vs. local vasomotion, coronary flows were increased in open-chest dogs by stimulating myocardial metabolism (catecholamines + atropine) with and without adenosine. During control states mean left ventricular (LV) myocardial blood flows (microspheres) were 0.5–1 ml·g−1·min−1 and increased to 2–3 ml·g−1·min−1 with catecholamine infusion and to ∼4 ml·g−1·min−1 with adenosine (Ado). Flow heterogeneity was similar in all states: relative dispersion (RD = SD/mean) was ∼25%, using LV pieces 0.1–0.2% of total. During catecholamine infusion local flows increased in proportion to the mean flows in 45% of the LV, “tracking” closely (increased proportionately to mean flow), while ∼40% trended toward the mean. Near-neighbor regional flows remained strongly spatially correlated, with fractal dimension D near 1.2 (Hurst coefficient 0.8). The spatial patterns remain similar at varied levels of metabolic stimulation inferring metabolic dominance. In contrast, adenosine vasodilation increased flows eightfold times control while destroying correlation with the control state. The Ado-induced spatial patterns differed from control but were self-consistent, inferring that with full vasodilation the relaxed arterial anatomy dominates the distribution. We conclude that vascular anatomy governs flow distributions during adenosine vasodilation but that metabolic vasoregulation dominates in normal physiological states.
NEW & NOTEWORTHY
Fractal measures showed self-similarity in coronary branching. New: Local myocardial blood flows are correlated with near neighbors in two different patterns with similar fractal dimension: pattern 1, when metabolically driven, vasomotion persists; pattern 2, after adenosine stops vasomotion, vasodilated structure dominates.
there is marked heterogeneity of regional blood flows in the heart. The SDs of the distributions of the flows divided by the mean flow, the relative dispersion (RD) are 20–30% of the mean. (RD is the coefficient of variation.) Myocardial perfusion heterogeneity was recognized in early studies of myocardial regional blood flows by investigators using microspheres in anesthetized dogs (20, 56), in isolated blood-perfused dog hearts (6, 64), and in awake, resting, or exercising animals (22, 23, 35). In awake baboons, King et al. (35), using 15-μm carbonized microspheres in pieces weighing ∼0.6% of the total mass of the heart, found that the RDs for the left ventricular (LV) flow distributions were 26 ± 7%. This variation is real, since only a small component of it is due to methodology: estimates using the deposited tracer “molecular microsphere” iodinated desmethyl imipramine showed method variation of 2–3% while the microsphere method variation is 5–8% (10), affirming the earlier estimates of method error appropriate to the numbers of spheres per piece and the counting statistics (1, 6, 20, 25, 35, 48, 42).
It is a truism that the finer the spatial resolution of the observations, the more heterogeneity is revealed. However, it is not a truism that in the heart the diminution of variance with enlarging tissue piece size is a power law relationship, a fractal relationship (7, 9). When the fractal dimension D > 1.5, it means that flows in nearby regions are positively correlated with one another: in healthy hearts the nearest-neighbor correlation coefficients are ∼0.6 (12, 57) and diminish with further separation.
Why should there be such broad heterogeneity of local flows in the heart where there is such a simple well-coordinated function, a spreading of excitation followed by contraction, that the vascular system serves by providing nutrients and removing metabolites? Because the methodological sources of variation are all small compared with the observed variation, the explanations must lie in the physiology or the anatomy, not merely in chance. What are the roles of microcirculatory regulation vs. vascular tree structure vs. random fluctuations in microcirculatory flows?
Experimental data contradict the idea that the heterogeneity is attributable to random temporal fluctuations. King et al. (35, 36) reported that in awake baboons the individual regions retained their relative ranking of flows (from low to high) over a range of modest physiological stresses, i.e., the relative flows were stable. Few myocardial regions changed their flow significantly relative to the mean flow; regions with flows near 0.5 times the mean flow did not reach flows equal to the mean during exercise or heat stress. Likewise, regions with flows initially ∼1.5 times the mean never diminished their relative flows to be as low as the mean. The suggestion of Yipintsoi et al. (64) that “twinkling,” temporal fluctuations in local flows, contributes to the RD is correct, but King and Bassingthwaighte's (36) data on regional flows collected for up to 24 h showed that twinkling variance alone cannot explain the magnitude of the heterogeneity in awake animals with intact vasomotor regulation.
Abolishing vasomotor tone creates a different situation. Sestier et al. (48) found an increase in dispersion of relative regional myocardial flows in dogs, from 22 to 31%, with an adenosine-induced fourfold flow increase while coronary pressure was held constant. Austin et al. (2, 3) showed with intracoronary adenosine infusion in dogs, elevating flow by 5 to 10 times, that the RD of LV regional flows increased from 20% during the control state to 30%, using piece size resolution of 1% of LV mass. At the same time, the “tracking” or the maintenance of rank position in relative regional flow was almost abolished by hypoxia and by adenosine administration. At the same time there was a strong spatial correlation between regional myocardial blood flows during adenosine infusion and during hypoxia (2). Given that both hypoxia and adenosine administration cause vasodilation, one might surmise that the fully relaxed vascular network anatomy defines the distribution of flows in those states. In the physiologically normal heart there is vasoregulation, influenced presumably by the local metabolic requirements of the tissue, even if not by local adenosine release (63). In anesthetized open-chest dogs Prinzen and Bassingthwaighte (44) observed that a change in the location of the initial ventricular depolarization led to changes in local myocardial flows, thus the physiological situation has an influence. Regions first activated, contracting while the rest of the ventricle was relaxed, shortened rapidly and almost isotonically, and reduced their blood flow, while late-activated regions, contracting more nearly isometrically, against a rising LV pressure and a stiffened septum, increased their regional flow. Similar phenomena were observed in dogs paced chronically, asymmetrically, to mimic the activation pattern of left bundle branch block (59, 60) and also resulted in remodeling of the ventricular mass.
Does vasoregulatory control over regional flow distributions persist with exercise? Is it totally abolished with adenosine vasodilation? Is the basis of the near-neighbor correlation merely the result of supply from a common upstream vessel, i.e., purely anatomic? We use the tracking of regional flows from one state to another to help answer these questions. In each state the flow in each small piece of myocardium is measured relative to the mean flow. When the relative flow, fi, in the ith piece remains the same (i.e., remains with the same range of relative flows) after a change in the physiological state, then it is tracking, and we interpret this to imply that the processes governing the distribution of the myocardial blood flows did not change when the physiological state changed, i.e., the balance of vasomotor vs. metabolic control was not changed. On giving a metabolic stimulus, or alternatively, a vasodilator, or both together, the flows to individual myocardial pieces that lie in any particular range of flows during the control state may be changed in particular ways and thereby give us insight into mechanism. The results encourage us to believe that in the completely vasodilated states with adenosine administration the flow distributions are dominated by the structure of the vasodilated vascular network, i.e., by anatomical rather than by physiological regulation. In contrast, the data we describe below show that with metabolic drive (without added adenosine) the spatial distributions of flows retain their similarity from resting state to high-flow states of heightened workload. Such observations are consistent with control in accord with local metabolic rate, not vascular anatomy.
METHODS
Adult mongrel dogs fasted overnight were premedicated with morphine (3 mg/kg), anesthetized with α-chloralose (100 mg/kg iv), and maintained by additional chloralose when necessary. Following intubation, catheters were placed by cut down or fluoroscopy: 1) in the coronary sinus for sampling of oxygen, blood gases, pH, and lactate; 2) in the descending thoracic aorta to obtain a reference blood sample while the radioactive microspheres were injected for the estimation of regional myocardial blood flow; 3) in the abdominal aorta for blood gases, oxygen content, lactate, and hematocrit; 4) in the right femoral vein for infusion of fluid or drugs to alter metabolism, and, finally, 5) following a left lateral thoracotomy, a catheter was placed in the left atrium for the injection of microspheres.
Protocol
Observations of physiological variables and of myocardial microsphere distributions were made during an initial control state (control C1), during low metabolic drive (LM or LoMetab), during high metabolic drive (HM or HiMetab), during HM together with adenosine infusion (HM + Ado), during the infusion of adenosine alone (Ado), and during a final control state 15–30 min after the infusions were stopped (control C2).
Atropine (0.25 mg/kg body wt) was given intravenously to keep the heart rate at a high level during the simulated exercise states (LM, HM, and HM + Ado) (30). It was given only one time since the study ended within an hour after its administration. Adenosine solution (6.7 mg/ml) was infused intravenously at rates from 9 to 20 mg/min (∼400–700 μg·min−1·kg−1). To produce LM we infused a solution containing 4 μg/ml norepinephrine and 1 μg/ml epinephrine in normal saline at 1 ml/min to enhance contractility and increase heart rate. To produce HM we infused the same solution at 2.5 ml/min.
Experimental Procedure
C1 was obtained after an initial period of 20–30 min during which the animal was stable with respect to anesthesia. Blood pressure and heart rate were measured, and samples of arterial and coronary sinus blood were withdrawn for measuring Po2, Pco2, pH, hematocrit, and hemoglobin saturation, and lactate. Regional myocardial and total coronary blood flows and cardiac output were measured using radioactively labeled carbonized microspheres injected in the left atrium and sampling the reference blood from the aorta. The microspheres were vigorously ultrasonicated to prevent aggregation until just before they were infused in 5 ml over 15 s in the left atrium followed by a 20-ml saline flush over 5 s. The sampling for the arterial reference started 15 s before the injection and continued for a minute after the end of the flush. The blood loss at each intervention was ∼50 ml.
To simulate moderate exercise (LM state, LoMetab) atropine was injected and catecholamine infusion was started, following the procedure of Haidet et al. (30), so causing a combination of a high heart rate over two times the control, due mainly to the atropine, and increased systolic blood pressure to 150–160 mmHg. After hemodynamic stability was attained 15–40 min after C1, the second set of microspheres was injected, and blood samples were taken.
For simulating heavier exercise (HM, HiMetab), with a stable higher heart rate and blood pressure, the catecholamine dose was increased, and a third set of microspheres was infused.
For inducing maximal vasodilatation together with high metabolism (HM + Ado state), the catecholamine solution was infused at 2.5 ml/min, and Ado was infused at a rate adjusted to keep the mean aortic blood pressure toward the control level (∼100 mmHg). When pressure and heart rate had stabilized the fourth set of microspheres was injected. The drug infusions were then stopped.
The final “control” (C2) blood sampling and the last microsphere injection were 10 min after stopping the drug infusions. Because of atropine's continuing effect, the heart rates remained higher, so the C2 conditions did not match the C1 conditions.
The animal was killed with intravenous saturated KCl solution; the heart was removed, perfused with 10% formaldehyde, and then frozen until it was sectioned to assess the microsphere deposition densities. (The methods and protocols were reviewed and approved by the University of Washington's Institutional Animal Care and Use Committee.)
Radioactive Carbonized Microspheres
The radioactive carbonized microspheres were 15 ± 1 μm in diameter (purchased from NEN, Biotech Division, Wilmington, DE) and were labeled with 141Ce, 51Cr, 103Ru, 95Nb, or 46Sc with specific activities from 5 to 10 mCi/g and suspended in dextran with Tween 80 (0.01%). Before injection the solution of suspended microspheres was sonicated for ∼10 min, and just before injection the solution was also vigorously shaken with a vortex mixer. We injected 10–18 million spheres in the left atrium, resulting in 3–12 thousand microspheres/g of heart muscle. By counting the activity in the syringe before and after injection, the exact dose was calculated and used to estimate cardiac output. The dose of microspheres caused no observable increase in blood pressure or heart rate. This is as expected since Baer et al. (4) saw no effects from 4 serial injections totaling 48 million spheres in the whole body, and Monroe et al. (40) did not see deleterious effects from the injections of 10-μm-diameter spheres until they had injected ∼256 million spheres in the isolated blood-perfused heart.
Microsphere Blood Flows
The methods are described well by Heymann et al. (31) and by King et al. (35). The excess fat and epicardial vessels were trimmed off the surface of the partially frozen heart. The heart was not merely sampled at particular locations but rather was sectioned completely, without loss of any parts. It was first cut into “rings” or slices 0.5–0.7 cm thick using ∼8–10 slices from base to apex. Each ring of LV tissue was cut into 16 radial sectors. The anatomic location of these sectors followed the notation of King et al. (35); each sector was cut parallel to the surface into four to six layered pieces from endocardium to epicardium. The right ventricular (RV) free wall was cut similarly, but only into inner and outer pieces.
The pieces were weighed, placed in plastic vials, and capped. Subsequently these and the reference arterial blood samples were counted on a γ-well (Baird Atomic/Tracor Northern) for 10–30 min or up to at least 10,000 counts so that the counting error was ≤1%/isotope. The data reduction consisted of corrections for isotopic decay back to the time of microsphere injection, for background, and for the spillover. Repeated counting of the same sample gave results in counts per minute always within 1% of the first count. These data provided the isotopic counts in counts per minute per gram in every piece of the LV and RV myocardium, from which the absolute and relative flows were calculated. We used the reference arterial blood collected during microsphere injection and calculated absolute coronary flow and absolute myocardial tissue blood flows in milliliter blood per minute per gram and cardiac output in liters per minute. Flows in atrial pieces were not measured.
The absolute flows are reported in Table 1. The relative flow in each piece of tissue (fi = Fi/F) for the moment of each microsphere injection is the counts per minute per gram in the piece divided by the counts per minute per gram for the ventricles, the sum of all the counts in the pieces divided by the total heart (RV + LV) weight. This simplifies the comparisons of flows in the tissue pieces for the various interventions. A general review of this methodology is that of Prinzen and Bassingthwaighte (44); this provides an assessment of the accuracy of the various microsphere deposition techniques: the methods used here have SDs of 5–7% on myocardial regions in dog hearts of ∼0.5–1% of the LV mass.
Table 1.
Measured parameters during each of the interventions
| n | AoP, mmHg | HR, min−1 | DP Work | CBF LV, ml·g−1·min−1 | CBF RV, ml·g−1·min−1 | CBF/CO, % | MRO2 LV, μl O2·g−1·min−1 | RDLV, % | RDRV, % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 control 1 | 4 | Mean ± SD | 97 ± 12 | 79 ± 12 | 9.4 ± 0.4 | 0.54 ± 0.14 | 0.37 ± 0.12 | 3.1 ± 0.8 | 45 ± 6 | 25.4 ± 3.4 | 28.4 ± 9.2 |
| Range | 21–32 | 20–42 | |||||||||
| LM LoMetab | 3 | Mean ± SD | 131 ± 22 | 173 ± 58 | 26.2 ± 8.5 | 1.56 ± 0.56 | 1.18 ± 0.33 | 5.3 ± 1.4 | 144 ± 19 | 22.9 ± 1.4 | 28.0 ± 13.1 |
| Range | 22–24 | 25–40 | |||||||||
| HiMetab | 4 | Mean ± SD | 171 ± 22 | 200 ± 38 | 36.5 ± 8.6 | 2.41 ± 0.92 | 1.61 ± 0.66 | 7.0 ± 1.2 | 206 ± 49 | 24.5 ± 3.2 | 28.8 ± 5.3 |
| Range | 21–28 | 24–37 | |||||||||
| HM + Ado | 4 | Mean ± SD | 107 ± 8 | 185 ± 22 | 25.0 ± 2.4 | 4.20 ± 0.91 | 3.70 ± 0.68 | 11.9 ± 2.4 | 179 ± 75 | 20.0 ± 5.4 | 15.9 ± 1.8 |
| HiMet Vasod | Range | 13–26 | 15–20 | ||||||||
| C2 | 3 | Mean ± SD | 101 ± 5 | 181 ± 10 | 20.9 ± 1.6 | 0.98 ± 0.16 | 0.64 ± 0.25 | 4.5 ± 0.6 | 71 ± 16 | 25.6 ± 4.2 | 27.3 ± 6.9 |
| Control 2 | Range | 21–30 | 22–36 | ||||||||
| Ado Vasodi | 1 | Mean | 102 | 147 | 19.7 | 4.17 | 3.23 | 12.6 | 110 | 20.4 | 18.1 |
Values are means ± SD and ranges; n, no. of experiments.
AoP, mean aortic pressure; HR, heart rate; DP, “double product” work = heart rate times systolic aortic pressure; CBF, coronary blood flow to left ventricle (LV) and right ventricle (RV); CBF/CO, the percentage of total LV + RV coronary blood flow (ml/min)/cardiac output also in ml/min; MRO2, LV myocardial oxygen consumption; RDLV and RDRV, %relative dispersions of the distribution of regional blood flow to the left and right ventricles, respectively; C1, initial control; LM, low-level catecholamines; HM, high-level catecholamines; HM + Ado, high-level catecholamines + adenosine; C2, final control; Ado, adenosine alone was infused following the initial control in heart 4.
Analysis of the Behavior of Regional Myocardial Flows with the Interventions
Method 1, linear regression.
To compare the relative regional blood flows at one state vs. the control or other state we plotted the flows in all the individual pieces in one state vs. the other and calculated the linear regression assuming equal random error in X and in Y to avoid the bias introduced by the standard Y-on-X regression (10). The weighted least-squares regression accounted for inequalities in the masses of individual pieces. A high linear correlation between relative regional myocardial flows from two physiological states with the slope approaching unity indicates a high degree of tracking of regional blood flows.
Method 2, relative flow classification stability.
Regional flows of individual pieces were classified within categories defined by the mean and ±n SDs above or below the mean. Low-flow pieces (LFP) are those with initial relative flows between 2 SD and 1 SD below the mean of the initial control state; high-flow pieces (HFP) are those with initial relative flows >1 SD and <2 SD above the mean. These categorized pieces were followed with regard to their subsequent relative flows. [If the probability density function (PDF) of regional flow were exactly Gaussian there would be 34% of the pieces with flows within either ±1 SD of the mean, 13.5% between 1 and 2 SD both below and above the mean. This gives ∼95% of the tissue having flow within 2 SD of the mean.]
Figure 1 provides an example in which the tracking between two states is poor. The PDF of relative flows during adenosine infusion is given by the high-peaked curve. The mean LV flow was 4.17 ml·min−1·g−1, over 10 times the control (C1) flow of 0.37 ml·min−1·g−1. Two smaller histograms are shown on the same axis. The one labeled LFP represents the distributions of flows during adenosine infusion, to those pieces which during the control (C1) state had flows between 2 and 1 SD below the mean. In the case illustrated the adenosine vasodilation shifted both the low-relative-flow and high-relative-flow pieces closer to the mean. The changes were not proportional to the change in mean flow, and the tracking was poor. The tendency was to randomize the local relative flows compared with the control relative flows.
Fig. 1.
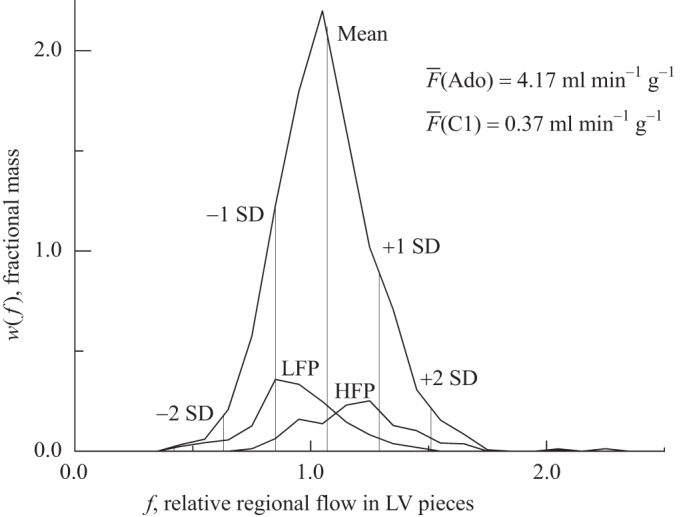
Example of the method of flow classification stability in a case with poor stability or tracking after a change from the control state to adenosine infusion inducing >10-fold increase in total left ventricular (LV) flow compared with control. The highest curve indicates PDF of relative flows in the vasodilated LV myocardium during adenosine (Ado) infusion. The mean flow (F) for the whole heart is 1.0; for the LV the mean is 1.06. The vertical lines divide the distribution into segments of 1 SD each from the LV mean. Pieces during control 1 (C1) that were defined as low-flow (LFP) had flows lying between −1 SD and −2 SD, whereas those defined as high-flow (HFP) had flows between +1 SD and +2 SD. The smaller curves labeled LFP and HFP represent the distributions during adenosine infusion of pieces that were LFP and HFP, respectively, during C1. The LFP comprised 14.4% of the LV weight and the HFP 12.4%. During adenosine infusion, of the LFP, 21.4% remained in their original class, 5.4% decreased, and 73.2% increased to a higher flow class (24% jumping to above the mean). Of the HFP, 24.5% remained at the original class, 7.3% increased to higher classes, and 68.2% decreased to lower classes (30.1% jumped to less than the mean). This is an example of poor tracking, almost random. [Also see Table 2 for linear regression, LV no. 4, adenosine (Ado).] Relative regional myocardial blood flows (fi) = Fi/F.
We used the anatomic location of these pieces to determine whether or not a particular region or set of regions showed a predilection for certain directional changes.
Correlation Structure in Regional Flow Distributions
The relationship between the fractal dimension D and the Hurst coefficient H in any Euclidean dimension space (E) is D + H = E + 1. Previous studies (9, 10, 35, 36) revealed no consistent patterns of relative regional flow profiles beyond the difference between RV and LV. Thus the LV flows had to be regarded as isotropic with respect to spatial correlations in local flow. On this assumption the analysis ignored the three-dimensional geometry and only considered distances between pieces, i.e., considered E to be 1, a one-dimensional space. Thus D + H = 2. The more D exceeds 1, the rougher (more fragmented or noisy) the signal, where the signal here is the local relative flow fi vs. distance. A value of D = 1.5 is the expectation for uncorrelated flows, random variation, while a D close to 1.0 implies smooth contours in regional flows, and high near-neighbor correlation. Thus, conversely, a high H (0.75 < H < 1) means relatively smooth contours. An H of 0.5 means random uncorrelated near-neighbor flows, and H < 0.5 means inversely correlated near-neighbor flows. These relationships are the same for the characterization of temporal signals, e.g., time-varying flows (24).
Whether or not there were changes in the degree of correlation in the spatial distributions of flows was assessed by two types of statistical evaluations. The first was to use dispersional analysis (10) to determine the fractal dimension D or the Hurst coefficient (H) where H = 2 − D for a one-dimensional measure. The method to obtain H is to calculate the SD of the regional flows at several different levels of spatial resolution. The different levels are obtained by aggregating adjacent individual tissue pieces into larger composite pieces (whose mean flow is therefore the mass-weighted average). The data are fitted with the regression, SD(m)/SD(m0) = (m/m0)H − 1, where m is the mean aggregate mass at any particular level of resolution and m0 is an arbitrary reference aggregate size, e.g., 1 g. The use of dispersional analysis (Disp) has been shown by Caccia et al. (21) to estimate H accurately.
The fractal roughness characterization is independent of the magnitude of the flow heterogeneity. Consider a PDF of flows in a LV such as in Fig. 1 or a more Gaussian distribution. The distribution could be composed of an infinite number of arrangements of regional flows. One extreme case would be a point of minimum flow at the apex (or in the middle of the septum) and then increasingly higher flows at increasing distances from the apex, gradually and smoothly until the local flow reaches a maximum at some point on the opposite side of the heart. A second way of providing the same PDF of flow would be to have random flows in the small pieces, as if plucked from the same distribution by a truly random number generator. A third way would be to similarly pluck numbers from the same PDF, but instead of letting them be random, allow the flow in the next neighboring piece to be less than some fraction of a different SD. All three distributions have identical PDFs. The second has a Hurst coefficient of 0.5 and a fractal dimension of 1.5. The first one, the unimodally increasing spatially distributed flow, has an H nearly 1 and D just above 1, and high positive correlations between neighbors. The third has a lower level of positive correlation between neighbors than does the first, a faster diminution in correlation with increasing separation, and is more like our data. While negatively correlated neighbors have never been observed in mammalian hearts so far as we know, one could imagine a local “steal” phenomenon whereby the vasodilation of one daughter branch from a parent artery steals the flow from the other daughter branch, so their flow changes are negatively correlated, but it would be difficult to see this happening over the whole ventricle.
The second measure is to look for self-similarity in the spatial autocorrelation function, i.e., to determine the correlation between adjacent tissue pieces, next-neighbors but one, etc., at two or more levels of resolution, as described by Bassingthwaighte and Beyer (12). The correlation coefficient rn between nth neighbors in a spatial fractal is:
| (1) |
The test for self-similarity is to see whether or not this function is the same at different levels of spatial resolution, e.g., comparing piece size m with size 2m–4m, etc. (13), assuming the correlation to be isotropic, not dependent on direction. If the analysis for single piece-to-piece correlation follows Eq. 1 that infers, only infers, that the relationship is a long memory process. However, if it also holds for aggregates of pairs, triples, quadruples, of near neighbors, then it is showing self-similarity independent of scale, and is clear evidence of a fractal process.
RESULTS
The four dogs weighed 20–26 kg. The average (±1 SD) LV weight was 128.9 ± 15.6 g and the RV was 43.5 ± 6.0 g. The hearts were cut into an average 643 ± 160 LV pieces and 192 ± 59 RV pieces. The average LV piece weights ranged from 0.17 to 0.24 g for each heart, thus each piece weighed 0.1–0.2% of the total LV weight. (In a fifth dog to be described in the discussion, the only interventions were two adenosine infusions, at two different levels, to provide a comparison between control and vasodilated states without any metabolic stimulus.) In these four hearts the LV and RV were divided into a total of 3,328 pieces with either four or five different radioisotopically labeled microsphere types in each, a total of 16,029 observations.
The physiological states are described by the data shown in Table 1, left nine columns. The two right columns are the RD of the LV and RV regional flows, each calculated relative to its own mean. (In two instances, the observations of either LM or C2 were omitted in order to gather data on interventions with Ado or HM + Ado.) With step increases in catecholamine stimulation, mean aortic blood pressure and the double product (systolic blood pressure times heart rate) increased stepwise, whereas the coronary blood flow and the oxygen consumption in both ventricles increased three times during low metabolic stimulus (LM) and then by five times with the greater stimulus (HM). The flows were nearly doubled by the addition of adenosine to the high dose of catecholamines, even though mean aortic pressure decreased to control levels. With return to C2, the flows and the oxygen consumptions remained elevated at about two times that of C1; the heart rate elevation was due to the prolonged action of the atropine.
RDLV and RDRV (Table 1) were ∼25% except during adenosine with catecholamines or adenosine alone when the RD fell to 20%. The range of RDs was from 12 to 26% during adenosine infusions compared with the range of 21–33% in LV and even over 40% in RV at other conditions. RDRV were larger than the RDLV except during adenosine infusion.
Changes in Flow Distributions
Examples of changes in flow distributions are shown in Fig. 2 for one study. The regional flows, in milliliters per gram per minute, were categorized with respect to their position relative to the mean flow for the two ventricles and colored according to the number of SDs above or below the mean. The color coding for the individual pieces in the control state was kept the same for the subsequent states so that the original flows are identifiable. The positions of a chosen color during C2 differed from their original categorization in C1: pieces that had flows far from the mean tended to shift toward the mean; a few crossed over the mean. For pieces with flows near the mean the only possibility is a shift away from the mean, so tracking these is not so informative.
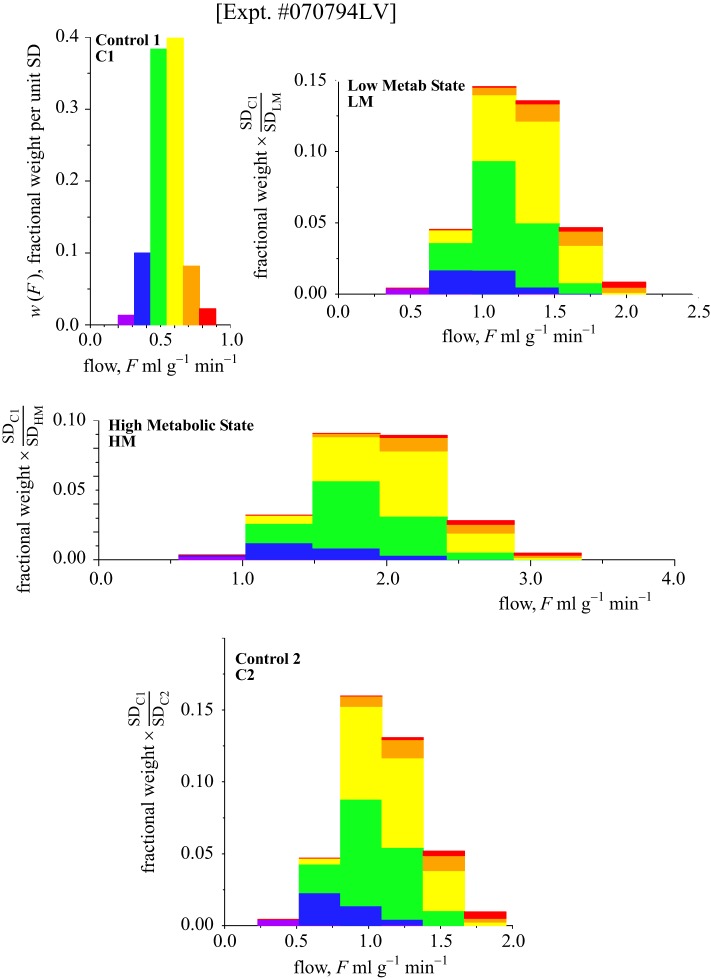
Fig. 2.
Probability density functions [w(F)] for regional myocardial blood flows in an anesthetized dog. Top left, C1; top right, low metabolic stimulation with catecholamine infusion (LM); middle, high metabolic stimulation with catecholamine (HM); bottom, control 2 (C2), which is the second control state, after the catecholamine infusion was stopped, although with a persisting high heart rate. The histograms are color coded for each class with width 1 SD from the first control (C1). The pieces retain their original color code throughout the series of interventions as they change their flows and their relative positions within the histogram. The abscissa is the same in all panels. The area under each probability density function is unity. The ordinate scales are different in each panel. The order of the series was C1, LM, HM, and C2 (at 90 min after C1).
Correlation between Flow Distributions in Different States
To begin with the strong and clear result, we found that with catecholamine stimulation at two different levels the PDFs of regional flows were similar, and that the relative flows in each piece remained stable. Figure 3 shows a plot of the relative flow in each piece during HM stimulus vs. the flow in the same piece at LM stimulus. The linear regression has a slope near 1.0; the high correlation coefficient, 0.95, fits with the observations that the pieces “tracked” closely, i.e., fell into the same SD class in the distribution despite the >50% increase in mean LV flow from 1.22 to 1.92 ml·g−1·min−1. The slope near 1.0 and the intercept near 0 indicate that flows increased almost purely proportionately in going from LM to HM states. The result indicates that the factors governing the regional flows in these two states did not change. By later making comparisons between the metabolically stimulated states and states produced by the perfusion of adenosine in addition, we can infer that the vasoregulatory control in the HM and LM states was maintained.
Fig. 3.
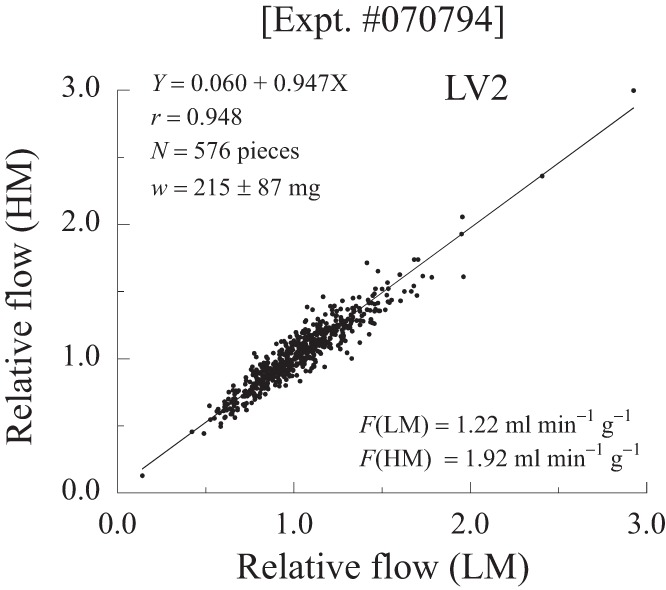
Relationship between relative regional myocardial blood flows (fi) in 576 pieces of myocardium in a dog given a HM stimulus with catecholamine and atropine (ordinate) vs. fi in the same piece at LM stimulus. The higher stimulus increased the flows by a similar proportion in all pieces. (Dog 2, LV pieces only).
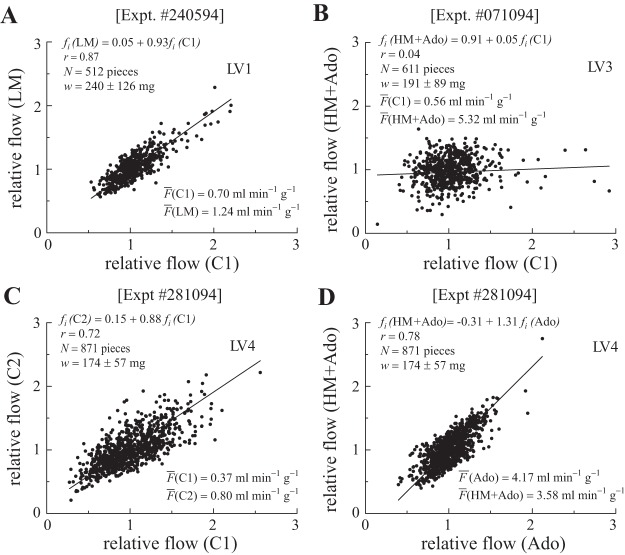
Table 2 lists the linear regression relationships between the relative regional blood flows during C1 and the subsequent interventions for each experiment where a metabolic stimulus was used. The regressions assumed equal error in ordinate and abscissa (and so are actually the bisector of the angle between the Y-on-X and the X-on-Y regressions). Figure 4 illustrates some of these. The degree of correlation is different among the different hearts as seen by the high correlation with LM in LV1 (Table 2 and Fig. 4A) and the low correlation with LM in LV3 (Table 2). Note as well that the RV patterns paralleled that of the LV. With added adenosine (HM + Ado) or alone (LV4 in Table 2), the correlation decreased and the slope decreased in accord with the decrease in RD (Fig. 4B). Another feature is the return, after intervening states, to a higher correlation between the initial (C1) and the final (C2) controls (Fig. 4C) despite the absence of correlation with the flow distributions during the physiologically contrasting intervening states. Higher correlations were seen between LM or HM and C2: the correlation coefficient (r) ranged from 0.66 to 0.91; the correlation averaged 0.76 for LM with C2, but was 0.87 for HM with C2. This is higher than between C1 and either LM or HM states. In a single study we compared adenosine alone with adenosine plus catecholamines (Fig. 4D), and found a high correlation although there was an intervening state with high catecholamine alone.
Table 2.
Linear regression values of interventions against C1
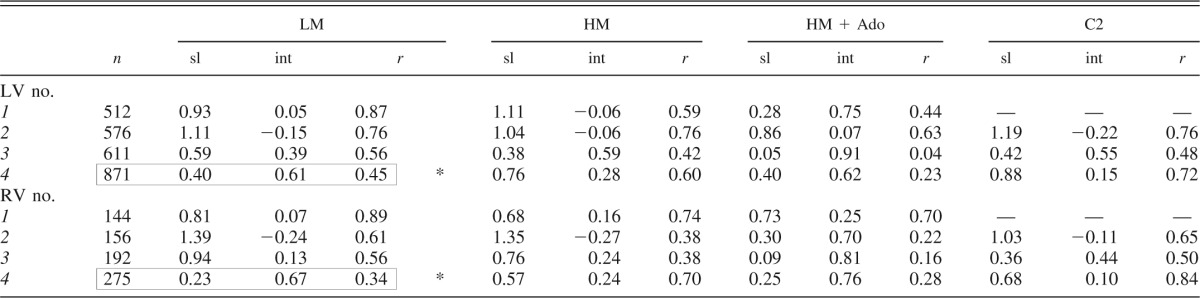
sl, Slope; int, intercept; r, correlation coefficient.
In heart 4 the regression line is for Ado vs. C, not LM vs. C1; data are boxed to mark the deviation from the table format.
Fig. 4.
Weighted regressions between regional LV blood flows in different states. A: flows in LM (35 min after C1) vs. C1 for dog 1. The relative dispersions (RDs) were 22.3 and 23.1%. This was the closest relationship between the two states among the four hearts. Data from LV1 show 512 LV pieces (N) weighing (w) 0.24 + 0.15 g. B: in dog 3 adenosine added to high catecholamine (HM + Ado) destroyed any correlation with C1 (HM + Ado was 37 min after C1, with 2 other states in between). The RD decreased from 28.0 to 22.3%. This reduction in correlation was seen with adenosine in all four hearts (Table 2). C: in dog 4 C2 flows were proportional to C1 flows even though the cardiac output and heart rate still remained elevated. The RD values were 32.8 and 29.4%. There were three interventions (LM, HM, HM + Ado) during the 52 min between C1 and C2. D: in dog 4 close correlation occurred between adenosine alone (Ado) and catecholamine with Ado (HM + Ado) although both differed from C1 (see Table 2). The RD values were 20.4% (Ado alone) and 26.0% (HM + Ado). Twenty minutes separated the two measurements. LV number refers to data from LV of dog number: dog 1, Expt 240594 (A); dog 3, Expt 071094 (B); and dog 4, Expt 281094 (C & D).
Tracking Local Flows Through Changes in Physiological State
Tables 3 and 4 summarize the tracking of relative regional flows (fi) of myocardial pieces from one flow category to another. In Table 3, C1 Ø LM are not necessarily in sequence, since LM and HM and Ado + HM were in pseudorandom order. As for the next three rows of Tables 3 and 4, the changes in flow class (defined as a shift to a higher or lower class or remaining in the same SD class, unchanged) are compared with the initial control C1. The bottom three rows show the changes in flow category for sequential physiological states. The low-flow pieces comprised 11–12% of the LV mass (Table 3). Forty-five to 48% by weight of LFP remained in their original classification, except with Ado. While fewer than 5% of LFP reduced their relative flows into the next lower class, 37–49% moved toward the mean (i.e., increased) and 9–15% increased to above the mean of the new distribution. When adenosine was added, there was an even wider excursion among categories of regional flows (as shown in Fig. 1), as is expected when the RD during adenosine narrows.
Table 3.
Tracking of LFP (−1 to −2 SD below mean) in LV
| n | Mass fraction of LV,% | Decrease, % of mass | Unchanged, % of mass | Increase, % of mass | Increase >2 SD, % of mass | Dt, min | |
|---|---|---|---|---|---|---|---|
| C1→LM | 3 | 12 ± 2 | 1 ± 1 | 45 ± 9 | 45 ± 4 | 9 ± 6 | 25 ± 12 |
| C1→HM | 4 | 12 ± 2 | 3 ± 3 | 38 ± 11 | 41 ± 9 | 18 ± 4 | 38 ± 11 |
| C1→HM + Ado | 4 | 12 ± 2 | 4 ± 2 | 24 ± 13 | 38 ± 4 | 34 ± 11 | 48 ± 12 |
| C1→C2 | 3 | 11 ± 2 | 2 ± 2 | 48 ± 9 | 41 ± 5 | 9 ± 4 | 58 ± 15 |
| LM→HM | 3 | 13 ± 1 | 1 ± 1 | 71 ± 13 | 26 ± 13 | 2 ± 2 | 13 ± 3 |
| HM→HM + Ado | 4 | 14 ± 2 | 6 ± 5 | 38 ± 4 | 42 ± 5 | 15 ± 7 | 11 ± 3 |
| HM + Ado→C2 | 3 | 12 ± 2 | 1 ± 1 | 35 ± 11 | 50 ± 11 | 14 ± 1 | 14 ± 4 |
Values are means ± SD; n, no. dogs in the comparison. Mass fraction of LV, weight of low-flow pieces (LFP) as a fraction of the LV mass; decrease, increase, and unchanged, percent of mass that decreased or increased by 1 SD, or remained within the original flow-class; increase or decrease >2 SD, increased or decreased >1 SD so much that its flow increased or decreased to the opposite side of the mean; Dt, time interval in min between microsphere injections.
Table 4.
Tracking of HFP (+1 to +2 SD above mean) in LV
| n | Mass fraction of LV,% | Increase, % of mass | Unchanged, % of mass | Decrease, % of mass | Decrease >2 SD, % of mass | Dt, min | |
|---|---|---|---|---|---|---|---|
| C1→LM | 3 | 10 ± 2 | 7 ± 3 | 37 ± 5 | 45 ± 6 | 11 ± 7 | 25 ± 12 |
| C1→HM | 4 | 10 ± 2 | 6 ± 2 | 31 ± 3 | 45 ± 4 | 18 ± 5 | 38 ± 11 |
| C1→HM + Ado | 4 | 10 ± 2 | 3 ± 2 | 19 ± 6 | 40 ± 13 | 37 ± 15 | 48 ± 12 |
| C1→C2 | 3 | 9 ± 2 | 9 ± 2 | 35 ± 3 | 43 ± 4 | 14 ± 7 | 58 ± 15 |
| LM→HM | 3 | 12 ± 2 | 6 ± 1 | 53 ± 7 | 34 ± 4 | 7 ± 4 | 13 ± 3 |
| HM→HM + Ado | 4 | 13 ± 2 | 4 ± 2 | 37 ± 5 | 44 ± 4 | 15 ± 5 | 11 ± 3 |
| HM + Ado→C2 | 3 | 13 ± 2 | 11 ± 3 | 32 ± 10 | 39 ± 3 | 18 ± 5 | 14 ± 4 |
Values are means ± SD; n, no. of dogs in the comparison. Mass fraction of LV, weight of the high-flow pieces (HFP) as a fraction of the LV mass.
Analogous trends can be seen for the high-flow pieces: 34–41% remained unchanged in their relative SD position, 4–6% increased while 42–43% decreased one SD step, toward the mean, and 11–19% shifted to less than the mean. During adenosine infusion, these changes also showed a wider excursion, i.e., a smaller fraction remained unchanged.
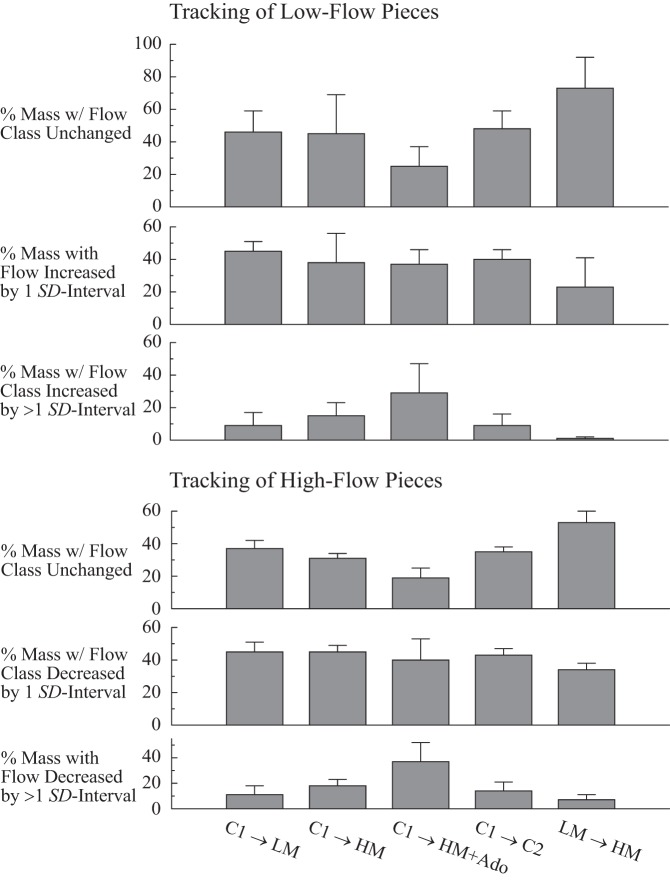
The degree of tracking in the various states is summarized from Tables 3 and 4 in Fig. 5. Tracking is defined as having changes in flow roughly proportional to the mean and no change in category relative to the mean.
Fig. 5.
Tracking probabilities for LFP (top) and HFP (bottom). “Close tracking” is measured by the percent of the heart weight with relative flows unchanged, i.e., the top row for LFP, and the fourth row for HFP. The highest percentage tracking was observed between LM and HM stimuli, the right-most column (LM Ø HM) in rows 1 and 4. The tracking tendency toward the mean is represented in rows 2 and 5; the tendency to change to the opposite side of the mean is less, as shown in rows 3 and 6.
From Fig. 5, top, it can be seen that for LF pieces the closest tracking is between the two metabolically stimulated states, LM and HM (rightmost column, row on top), with over 70% of the mass tracking. The poorest tracking is between C1 and the metabolically stimulated, adenosine-vasodilated state, HM + Ado (column in middle, row on top), with ∼25% tracking. Tracking for low-flow pieces tends to be toward the mean with any subsequent intervention, since ∼40% of the LFP moved into the next higher category (Fig. 5, row 2). For HFP, the tracking again tends to be toward the mean (Fig. 5, row 5, with 40% of the mass decreasing flow into the next lower category); similarly to the LFP, the closest tracking of HFP is between the two metabolically stimulated states.
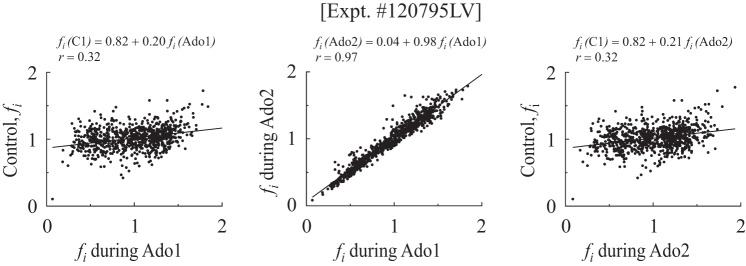
Further statistical testing was done to ascertain if any particular direction of shift in the flow rankings occurred at particular anatomic sites in the LV. Examination of subendocardial or subepicardial pieces or the free wall of the LV or the septum revealed no trends. In an exploratory study in a fifth dog more directly comparable to those of Austin et al. (3), comparisons were made between two runs with adenosine alone before and after a single control run; this dog is not reported in results except for the Hurst coefficients in Table 5. First was a 10-min adenosine infusion (Ado1) with the microspheres injected during the steady state of the 10th min. After 26 min without Ado infusion, microspheres were injected for control (C1). Next, the second Ado infusion was begun, and after 33 min the spheres were injected (Ado2). The correlation between Ado1 and Ado2 was high (see Fig. 6, middle); the regression was fi(Ado2) = 0.04 + 0.98 fi(Ado1) (r = 0.97, N = 761 pieces averaging 157 ± 54 mg, with LV weight = 119.4 g). Comparing the two periods of adenosine infusion (Fig. 6), 52% by weight of the low-flow and 61% of the high-flow regions remained in their flow categories. Neither of the Ado runs correlated closely with the intervening control run: the correlation coefficients were 0.31 and 0.30, and the slopes were 0.15. (The aortic blood pressures were 100/25 in both Ado states, but 115/70 in the control state.) The control RD was 16.5%, whereas those for Ado1 and Ado2 were 35 and 33.7%, respectively. This RD of 16.5% during control was different from that in all the animals used in the studies of the metabolic stimulus, is smaller than in all previous studies at this level of resolution in baboons, sheep, and rabbits, and so should be considered a probable artifact due to the preceding Ado infusion, and might even be due to regional myocardial recovery from a relative vascular steal where local metabolism was compromised due to flow reduction during Ado and where flow rose when Ado infusion was stopped. (All of the points above and to the left of the line of identity in Fig. 6, leftmost or rightmost panels, represent this possibility.)
Table 5.
Hurst coefficients (each is the average from RDs and rn values)
| Dog No. | C1 | C2 | LM | HM | HM + Ado | Ado1 | Ado2 |
|---|---|---|---|---|---|---|---|
| LV1 | 0.75 | 0.82 | 0.83 | 0.81 | |||
| LV2 | 0.61 | 0.78 | 0.75 | 0.76 | 0.71 | ||
| LV3 | 0.77 | 0.81 | 0.76 | 0.79 | 0.79 | ||
| LV4 | 0.78 | 0.79 | 0.82 | 0.86 | 0.77 | ||
| LV5 | 0.67 | 0.91 | 0.91 | ||||
| Mean ± SD | 0.73 ± 0.08 | 0.76 ± 0.06 | 0.77 ± 0.04 | 0.80 ± 0.03 | 0.79 ± 0.06 | 0.84 ± 0.09 | 0.91 ± 0 |
Ado1, first adenosine injection; Ado2, second adenosine injection.
Fig. 6.
fi in one dog given adenosine infusion first (Ado1), returned to control (after 26 min), and then given a second adenosine infusion (Ado2) at 59 min. Left, flows at C1 plotted against flows with Ado1; middle, Ado2 plotted against Ado1; right, flows at C1 plotted against flows with Ado2. The mean flows were: F(Ado1) = 2.31 ml·g−1·min−1, F(control) = 1.59 ml·g−1·min−1, and F(Ado2) = 1.51 ml·g−1·min−1 (N = 761 pieces averaging 157 ± 54 mg; heart weight = 119 g).
The interesting point is that, no matter what the control showed, the flow distributions in the two Ado states (Fig. 6, middle) were almost identical despite an hour separation; thus, the adenosine-induced state is based on a reproducible situation, presumably the anatomy of the fully vasodilated network, without vascular tone.
Affirming the Fractal Nature of Near-Neighbor Correlations by Self-Similarity
Self-similarity is demonstrated by power law relationships in RD, and in the superposition of the correlation falloff at different resolutions. Both relate to the texture of the spatial heterogeneity in flows. Both roughness and correlation measures use successive aggregation, lumping two of more pieces together to incorporate an evaluation of “scale independence,” meaning that the ratio of a change in the measure is the same for the successive aggregations. The physiological question is whether or not an intervention disturbs the spatial relationships.
The Hurst coefficient for each state was taken as the average from the two methods used, Disp and rn, and is listed in Table 5. Disp and the spatial autocorrelation methods give mutually consistent evaluations: the sum of the D from Disp and the H from fitting rn should theoretically be 2.0 for each condition; the average sum was 1.95 ± 0.04 with a range from 1.89 to 2.04, indicating the absence of any large bias. The application of this fractal analysis therefore appears robust and practical.
Near-neighbor correlation in all of these hearts was high, giving an average Hurst coefficient H for all conditions of 0.80; this is equivalent to a fractal dimension D of 1.2 and a nearest neighbor correlation coefficient of r1 = 22H − 1 − 1 = 0.516.
The results for the different physiological conditions showed small but consistent differences. The correlation and the value of H was slightly lower in the first control state than any other state (for C1 the average H = 0.73), whereas HM stimulation with or without adenosine showed correlation (average H = 0.80) that was the highest found, except for the runs with adenosine alone (right two columns of Table 5). The high Hurst coefficients are fractal measures characterizing the degree of nonuniformity and the falloff in correlation over distance.
Dispersional analyses for one heart are shown for the five states in Fig. 7.
Fig. 7.
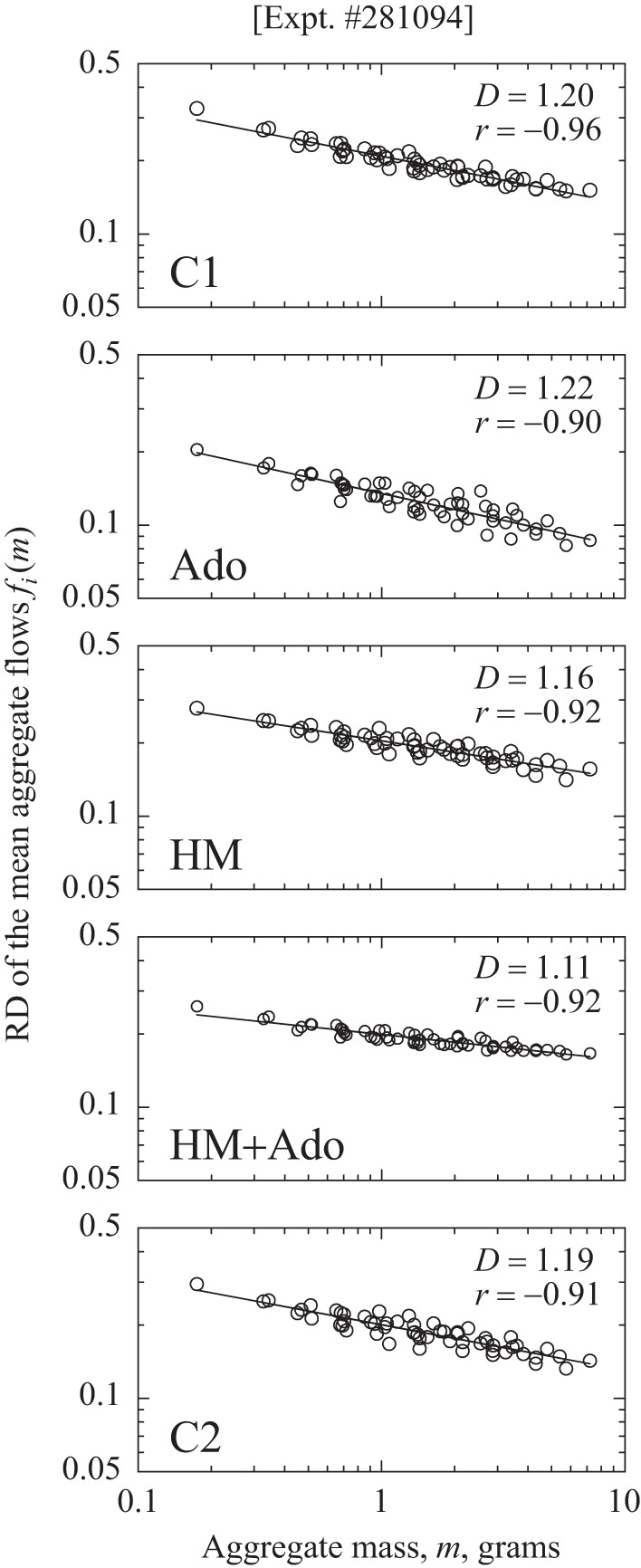
Power law relationships for RD of regional flows fi(m) vs. the sizes (m) of aggregates of near-neighbor pieces of dog LV myocardium (heart 4). The sequence of studies was C1, Ado, HM, HM + Ado, C2, from top to bottom. D, fractal dimension; r, correlation coefficient for log RD vs. log m. Lower slopes and lower fractal D values indicate stronger near-neighbor correlation and higher values of H, the Hurst coefficient.
The leftmost point on each plot is the RD (= SD/mean) of the relative flows fi for the 871 pieces of LV in the heart for a particular state; the points for the aggregates of higher mass show the RDs for various numbers of pieces aggregated together. (There is only one estimate of RD using all of the unaggregated pieces, but there were two ways to aggregate pairs of pieces, therefore, 2 points near 0.25 g; 4 ways to aggregate pairs of pairs of pieces into ∼0.5 g aggregates, therefore, 4 estimates, and so on.) The slope of the regression of log RD vs. log m, where m is the mass of the aggregate, is theoretically 1 − D. The linearity of the log-log plots in Fig. 7 indicates that a power-law fractal descriptor for the flow heterogeneity is appropriate.
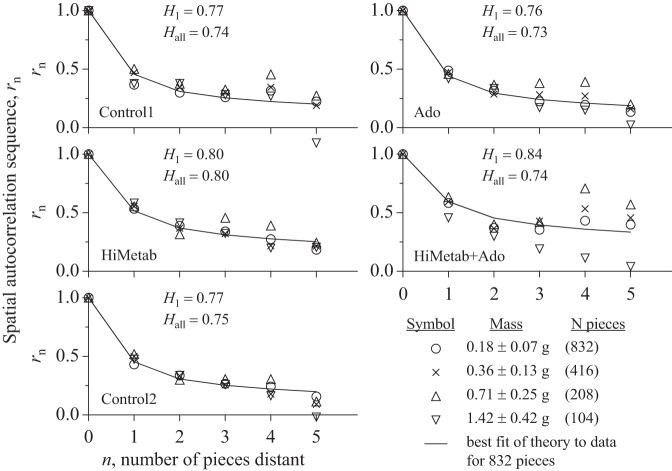
The spatial autocorrelation function rn is based on calculating the series, rn, of correlation coefficients and then fitting the one-parameter equation, rn = 0.5[(n + 1)2H − 2n2H + (n − 1)2H], to the series to obtain H. To obtain the series, rn is first calculated for individual pieces (open circles in Fig. 8), calculating r1 for correlation with its nearest neighbors, then neighbors separated by one, two, and three pieces to get r2, r3, and r4. In the three-dimensional matrix of rectangular pieces there are six neighbors for internal pieces and five for pieces at a surface, so r1 (o) at n = 1 represents the average correlation of 832 × 6/2 flows, similarly for r2–r5. The same operation was done on aggregates of two pieces (x symbols), then four pieces (open triangles), and eight pieces (open inverted triangles). Larger aggregates cannot be used because the data become too sparse. The analysis is shown in Fig. 8 for the same heart (no. 4) that was used for Fig. 7. The fractal self-similarity, independent of scale, is evident in that the series rn remains much the same for the sets of aggregates as for the single pieces (open circles) despite the sparser data. As expected, because of the separation by two or three intervening pieces, the results are more scattered for points farther apart, r4 and r5 than for r1 and r2.
Fig. 8.
Spatial autocorrelation function (rn) for different conditions in LV #4. The series was: C1, Ado, HM, HM + Ado, C2. The nth neighbor correlation coefficients were obtained for individual pieces (○), pairs of pieces (x), quadruples of pieces (△), and octuplets of pieces (▽). The continuous line is the best fit of the theoretical model, Eq. 1, to the data for the individual pieces. Given in each panel is H1 for smallest pieces, and then Hall for the best fit to all of the aggregated pieces, regardless of size. (There were 871 pieces in this heart since it was cut originally; the 39 smallest pieces were lumped with nearest neighbors to minimize the range of piece weights, ending up with 832 pieces for the spatial analysis for near-neighbor aggregation by 2, 4, and 8 exactly.)
The analyses shown in Figs. 7 and 8 do affirm the fractal nature of the flow heterogeneity, but do not define its basis. The data in Table 5 summarize the estimates by the two methods, RDs vs. aggregate size, and the correlation algorithm, Eq. 1. The changes in H and D with an intervention are modest, raising the question of whether or not the fractal characterization helps in figuring out the physiological basis for the responses. The coronary anatomy is fractal, so, as expected, the fractal relationship is strong when there is full vasodilation. The Hurst coefficient does not provide a way to distinguish the nature of the heterogeneity with normal vasomotor control vs. that with adenosine vasodilation. The scatter of the relationship between the local flows with adenosine infusion and any of the other states (C1, C2, LM, or HM) was basically a scatter plot, uncorrelated, as exemplified by Fig. 4, top right. While this contrasts with the high correlation among C1, C2, LM, and HM, the near-neighbor correlation was high in all situations, and even higher with Ado than in the other settings. Therefore, it cannot be supposed that the situations where there is vasomotor control are just modulations of those in the Ado state, they are really different, as shown by Fig. 4B, open inverted triangles.
DISCUSSION
Starling's concept (49) that the heart uses oxygen in proportion to its workload would be expected to apply locally as well as globally. Evidence at the local level is slim, because it is so difficult to measure local stress simultaneously with local oxygen uptake or ATP utilization. Local oxygen consumption estimated from 15O2 imaging by positron emission tomography was correlated with local flows from microsphere data (14). The idea is that the cardiac metabolic system operates as an impedance-matched system such that local work requires proportional metabolic substrate turnover, which in turn requires proportional substrate transport capacity of the cell membranes, and therefore requires proportional delivery of substrates and oxygen by flow (11, 22). Given that fatty acid is the prime substrate for the heart and that its transport is at least partially mediated by a facilitated transport (8, 27, 51, 58), it makes sense for a transporter protein to be expressed in proportion to the need for substrate locally. The correlation principle (impedance-matching idea) is supported by the observations of Caldwell et al. (23) that regional fatty acid transport capacity in the hearts of dogs during exercise on a treadmill ramp is proportional to the regional flows. These data feed into the development of integrative schema of the relationships between myocardial local mechanics, metabolism, and regional flows (15, 41). The eventual goal is to define the cell-to-tissue-to-organ biophysics in terms of the mechanical and electrical work and energy consumption in accord with first principles as expressed by Beard and Qian (19).
This current study provides data to test the principles over a range of vasodilatory conditions, and uses adenosine as a vehicle for testing the nature of the responses. Kroll and Feigl (37) determined that adenosine is not the physiological regulator of local myocardial blood flows, information amplified later by Tune et al. (53, 54, 55). Gorman et al. (29) suggest that adenine nucleotide release is probably more relevant to the physiological vasoregulation than is nucleoside. Stepp et al. (50) found the vasodilatory response to adenosine was quantitatively reproducible, with a Km of 2 μM, but a high Hill coefficient (∼5). This steep smooth muscle receptor response clearly differs from that of the adenosine transendothelial transporter (46, 47) whose response is standard first-order low-affinity binding with a Km of ∼112 μM but high capacity.
Metabolic stimulation increased flow by about fourfold (see Table 1), in contrast to the baboon studies of King et al. (35) using heat stress or mild bicycle exercise that only about doubled coronary blood flow. Our results differ from those of Austin et al. (2) who used metabolic depression with hypoxia or vasodilation with adenosine along with some metabolic depression. We found that the degree of correlation between states, the magnitude and directions of tracking, and the maintenance of spatial correlation were quite comparable from heart to heart. The strong trend was for the low-flow or high-flow regions to remain in their relative flow positions or ranks with metabolic interventions from control to control and even with the varied doses of catecholamines plus atropine. Thirty to fifty percent of the low-flow or high-flow pieces (as defined) remained in their relative ranking during these interventions (Fig. 5). Similar percentages were seen when initial and final controls were compared (C1 vs. C2, Tables 3 and 4 and Fig. 5). This occurred despite the intervening pharmacological perturbations, including vasodilators, during the hour between C1 and C2.
The stability of the relative flows is the dominant pattern, most strongly evident in going directly from a low-catecholamine dosage (LM) to the higher level (HM): 71% of LFP did not change category (Table 3: LM→HM, unchanged) and 53% of HFP were unchanged (Table 4: LM→HM, unchanged). Large excursions in flow relative to the mean were uncommon even with large changes in mean flows. Changes of >2 SD to the opposite side of the mean flow (increases of LFPs in Table 3 or decreases of HFPs in Table 4) occurred in only 9–19% of the tissue mass with metabolic drive, but were greater with the maximal vasodilatation using Ado. C1 and C2 differed by twofold in the metabolic rate of oxygen utilization and coronary blood flow; even so, in C2 only 9 and 14% of LF and HF pieces changed >1 SD, moving across the mean. The variation contrary to strict proportionality to the mean flow is thus relatively minor.
The second pattern observed was the nature of alteration induced by adenosine: the RD (= SD/mean) diminished, tracking was poor (Fig. 5, middle), and correlation with the control state was abolished. This is just as Austin et al. (2, 3) had observed with either intracoronary or systemic adenosine, except that they found that adenosine increased the RD (increased the heterogeneity) when there was a four to eight times increase in coronary flow. In both of their studies (2, 3), the RD on the anterior LV wall during adenosine had values around 30% compared with the control RD of 24.3% (2) or 20.5% (3). Their myocardial sample sizes averaged 100 or 140 mg, which were smaller than ours (170–240 mg). Sestier et al. (48), perfusing just the left coronary artery and sampling pieces averaging 738 mg, reported that, with intracoronary adenosine or dipyridamole, the RD increased from 21.7 to 30.8% if the perfusing pressure was held constant and flow was allowed to rise. The RD was 34.3% (not statistically different from 30.8%) when flow was kept relatively constant while diastolic coronary pressure was allowed to fall to 42 mmHg compared with 97 mmHg during control. Austin et al. (2, 3) took 1.5–2 min to inject their microspheres while Sestier et al. (48) took 4 s. These varying techniques, which resulted in increases of RD following adenosine and dipyridamole, suggested that the techniques alone cannot explain our differing result. Coronary cannulation itself had no effect (2, 3): for example, in two open-chest dogs of Austin et al. (2) the distributions as seen on the linear regression plots appear unchanged after cannulation compared with the control state. Rembert et al. (45) showed, although they did not evaluate regional myocardial flow distribution in terms of RD, the endocardial-to-epicardial flow ratios increased to twofold or greater with adenosine, which implies a widening of the RD. Their conclusion was that adenosine had a greater vasodilatory effect in subendocardial than subepicardial vessels, which is compatible with the general observation that adenosine preferentially dilates smaller resistance vessels. Gorman et al. (29) suggested that the widening or narrowing of the RD really depends on the interaction between flow and perfusing pressure: when a lowered pressure accompanies adenosine vasodilation, the RD widens, but if the perfusion pressure is maintained, then with adenosine the RD narrows. Their studies, however, were on electrolyte-perfused guinea pigs hearts. Although the apparent disparity between the results found with vasodilators alone (29, 2) and ours may be attributable to the accompanying catecholamines, this remains unproven since in one of these dogs where adenosine alone was given, the RD was reduced from 33% during control to 20% (first and last lines of Table 1).
Presumably the distributions shown in Fig. 6 under adenosine infusion are governed by the resistances through a completely vasodilated coronary arterial network, i.e., basically limited by the arterial network resistances in the maximally dilated microvascular network (5, 16, 17). Austin et al. (2) also noted the stability occurring with maximal loss of coronary tone. Likewise, the regional flows during asphyxia were closely correlated with those during adenosine infusion, but not to the regional flows obtained during the control state. Hence, under maximum vasodilatation (adenosine) and loss of autoregulation (hypoxia), it appears that the regional flows are governed by the same state of the vasculature, presumably the fully vasodilated anatomy of the arterial network.
It was disappointing that even though the spatial correlations differed after vasodilation with adenosine from those during control, e.g., as in Fig. 4, top right, the Hurst coefficient itself is not helpful in distinguishing the vasodilated state from the vasoregulated state: the H's in the HM and HM + Ado columns of Table 5 do not differ. The conclusion on this point is that the vasodilated vascular structure gives locally coordinated flows, presumably via the commonality of parents to daughter vessels in the region. Likewise, in the control and exercise states this is a commonality, but it is quite different from that in the adenosine-vasodilated state, and the difference is mediated through vasoregulation. While we presume that the vasoregulation is tuned to match local metabolic requirements, these studies offer no direct evidence. A further shortcoming in the design of the study was that it provides no information on sheet and fiber direction for each piece within the heart; such data could be used in reconstructing the three-dimensional structure and relating that to the regional strains, stresses, and flows, extrapolating from work to metabolic demands. The variegated local flows and mechanical stresses are almost surely closely related to each other, since they are a product of the routes of excitation, and contraction in a heart developed through the tissue foldings during early cardiac development (52). Having the kind of data that Vinnakota and Bassingthwaighte (61) integrated with respect to the average tissue composition can help with relating tissue metabolite abundances with concentrations and fluxes, but to relate the local energetics to the anatomy will require comprehensive local measures of the anatomy and the strain patterns.
Our data show that in anesthetized open-chest dogs close tracking occurs between LV regional flows in control and metabolically stimulated states, and even more strikingly between LM and HM states. This points to a persistence of a coordinated distribution of vasomotor tone through these states. In the past, fractal analysis has provided statistical measures of self-similarity in the branching anatomy of the coronary arteries by the constancy of parent-to-daughter segment lengths and diameters. Now we observe that the regional blood flows are shown to be correlated in a three-dimensional fractal field with self-similarity independent of the spatial resolution. While this is reassuring from the point of view of measurement and meaning, there is a problem: the fractal dimension, by itself, fails to distinguish the correlation in those with vasomotor tone from those in fully vasodilated states. The distinction is shown by the stability of the different associations in the two states (vasodilated vs. metabolically driven), i.e., the pattern of highest near-neighbor correlations is the same in the various metabolically stimulated states, but these differ from the patterns in the fully vasodilated states, as in Fig. 6, Ado1 vs. Ado2.
The cause of the close relationship between local flows and local myocardial function or metabolism is not revealed by this study. The data do imply that low-flow regions are not metabolically deprived, or deficient in vascularity, for they respond proportionately (or even more) to increases in metabolic demand. This fits, too, with the data of Loncar et al. (39) showing that, unless coronary stenosis is induced, there is normal aerobic metabolism (without excess lactate production in low-flow regions). This fits the observation that the coronary flows in the control states were found to be proportional to the local capacity for fatty acid uptake (23). Franzen et al. (26) had the idea even earlier and sought the relation between local flow and the concentration of enzymes or solutes of metabolic importance; Prinzen et al. (43) showed that regions doing more contractile work had higher flows. Their studies in chronically paced dogs (59, 60) showed also that the early activated LV septal region, where shortening occurred against a reduced load, atrophied. The late-activated LV free wall, which was prestretched a little by the septal contraction, had to do almost all of the work of ejection, and so became hypertrophied. By increasing the muscle mass this caused a transient reduction in capillary density (nos./g) in the early weeks; it took some months to renormalize the regional capillarity in both septum and free wall. While we used adenosine to vasodilate fully, it is not a normal regulator of vasomotion; more likely influences are combinations of ATP and nitric oxide release from red blood cells (RBC), as with hypoxia (34) or exercise (32), and, to a lesser extent, ATP leakage with RBC hydrolysis. Building on models of ion regulation, excitation-contraction coupling, and mitochondrial energy transduction, Beard (18) and Wu et al. (62) have brought diverse observations together through systematic quantitative modeling of the combined influences of excitatory spread, contractile tension development, substrate utilization, and ATP generation and consumption. Howard et al. (33) found that the Starling effect (greater force generation with prestretch) was still evident in failing hearts in both early and late-activated regions; they formulated a three-dimensional cardiac model of cardiac contraction that fit these observations of strain patterns. What remains to be done are experiments with simultaneous data acquisition of metabolic and energetic fluxes and the regional mechanics.
Modern clinical imaging techniques are now approaching the levels of spatial definition that reveal the ever-present heterogeneity. Thus recognition that there are normally some low-flow regions, not at all ischemic, helps to avoid false diagnoses of local ischemia. With the use of positron emission tomography, 15O-labeled water for flow and 15O2-labeled oxygen for metabolism can only be done in a few centers with a cyclotron because of the 2-min half-life, but 82Rb, 13N-labeled ammonia, and other deposited tracers now give ∼1 cm resolution and allow the heart to be segmented into 20 or more regions for flow measurement. Likewise, magnetic resonance imaging (MRI) contrast agents like Gd-DPTA or Gd-BOPTA (in Europe) provide similar or higher resolution when region-of-interest time-course data are analyzed using mathematical model accounting for flow and exchanges. X-ray computed tomography with iodinated contrast agents can be analyzed similarly. These methods will before long reach the spatial resolution of microsphere deposition and tissue analysis. Putting together MRI flows with tagged MRI for local strain patterns would be useful to demonstrate the flow-work relationships regionally, especially in either ischemic heart disease or in heart failure where receptor densities are so heterogeneous.
Putting these observations together, one may reasonably conclude that, since flow distributions are similar in the varied physiological states but differ from the fully vasodilated Ado states, vasoregulation is normally always present in the various physiological states where metabolism is the important determinant of myocardial flow distributions. Only when vasodilation is abolished by pharmacological intervention or hypoxia is vascular network structure the limiting determinant.
GRANTS
This research has been supported by National Institutes of Health Grants HL-19139, HL-50238, and HL-49822 for the experimental studies and by EB-8407 and 1-P50-GM-094503 for parts of the analysis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.Y., K.K., and J.B.B. conception and design of research; T.Y., K.K., and J.B.B. performed experiments; T.Y., K.K., and J.B.B. analyzed data; T.Y., K.K., and J.B.B. interpreted results of experiments; T.Y. and J.B.B. edited and revised manuscript; J.B.B. prepared figures; J.B.B. drafted manuscript; J.B.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for the assistance of Stephanie Belanger and James Ploger for technical assistance and Richard van Bibber for surgical assistance.
REFERENCES
- 1.Austin RE Jr, Hauck WW, Aldea GS, Flynn AE, Coggins DL, Hoffman JIE. Quantitating error in blood flow measurements with radioactive microspheres. Am J Physiol Heart Circ Physiol 257: H280–H288, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Austin RE Jr, Aldea GS, Coggins DL, Flynn AE, Hoffman JIE. Profound spatial heterogeneity of coronary reserve: Discordance between patterns of resting and maximal myocardial blood flow. Circ Res 67: 319–331, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Austin RE Jr, Smedira NG, Squiers TM, Hoffman JIE. Influence of cardiac contraction and coronary vasomotor tone on regional myocardial blood flow. Am J Physiol Heart Circ Physiol 266: H2542–H2553, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Baer RW, Payne BD, Verrier ED, Vlahakes GJ, Molodowitch D, Uhlig PN, Hoffman JIE. Increased number of myocardial blood flow measurements with radionuclide-labeled microspheres. Am J Physiol Heart Circ Physiol 246: H418–H434, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Bassingthwaighte JB, Yipintsoi T, Harvey RB. Microvasculature of the dog left ventricular myocardium. Microvasc Res 7: 229–249, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassingthwaighte JB, Malone MA, Moffett TC, King RB, Little SE, Link JM, Krohn KA. Validity of microsphere depositions for regional myocardial flows. Am J Physiol Heart Circ Physiol 253: H184–H193, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassingthwaighte JB. Physiological heterogeneity: Fractals link determinism and randomness in structures and functions. News Physiol Sci 3: 5–10, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassingthwaighte JB, Noodleman L, van der Vusse GHJ, Glatz JFC. Modeling of palmitate transport in the heart. Mol Cell Biochem 88: 51–58, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. Circ Res 65: 578–590, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassingthwaighte JB, Malone MA, Moffett TC, King RB, Chan IS, Link JM, Krohn KA. Molecular and particulate depositions for regional myocardial flows in sheep. Circ Res 66: 1328–1344, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassingthwaighte JB, Caldwell JH, Raymond GM, Kroll K, Martin GV. Are flow, transport capacities, and metabolic requirements matched regionally in the myocardium?. In: Cardiac Electrophysiology, Circulation and Transport, edited by Sideman S., Beyar R. and Kléber A. G., Dordrecht, The Netherlands: Kluwer, 1991, p. 281–293. [Google Scholar]
- 12.Bassingthwaighte JB, Beyer RP. Fractal correlation in heterogeneous systems. Physica D 53: 71–84, 1991. [Google Scholar]
- 13.Bassingthwaighte JB, Liebovitch LS, West BJ. Fractal Physiology. New York, NY: Oxford Univ Press, 1994. [Google Scholar]
- 14.Bassingthwaighte JB, Li Z. Heterogeneities in myocardial flow and metabolism: exacerbation with abnormal excitation. Am J Cardiol 83: 7H–12H, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassingthwaighte JB, Beard DA, Carlson BE, Dash RK, Vinnakota K. Modeling to link regional myocardial work, metabolism and blood flows. Ann Biomed Eng 40: 2379–2398, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beard DA, Bassingthwaighte JB. The fractal nature of myocardial blood flow emerges from a whole-organ model of arterial network. J Vasc Res 37: 282–296, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Beard DA, Bassingthwaighte JB. Modeling advection and diffusion of oxygen in complex vascular networks. Ann Biomed Eng 29: 298–310, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beard DA. A biophysical model of the mitochondrial respiratory system and oxidative phosphorylation. PLoS Comput Biol 1: 252–264, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beard DA, Qian H. Chemical Biophysics. Quantitative Analysis of Cellular Systems. Cambridge, UK: Cambridge Univ Press, 2008. [Google Scholar]
- 20.Buckberg GD, Luck JC, Payne BD, Hoffman JIE, Archie JP, Fixler DE. Some sources of error in measuring regional blood flow with radioactive microspheres. J Appl Physiol 31: 598–604, 1971. [DOI] [PubMed] [Google Scholar]
- 21.Caccia DC, Percival DB, Cannon MJ, Raymond GM, Bassingthwaighte JB. Analyzing exact fractal time series: evaluating dispersional analysis and rescaled range methods. Physica A 246: 609–632, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldwell JH, Martin GV, Link JM, Gronka M, Krohn KA, Bassingthwaighte JB. Iodophenylpentadecanoic acid - myocardial blood flow relationship during maximal exercise with coronary occlusion. J Nucl Med 30: 99–105, 1990. [PubMed] [Google Scholar]
- 23.Caldwell JH, Martin GV, Raymond GM, Bassingthwaighte JB. Regional myocardial flow and capillary permeability-surface area products are nearly proportional. Am J Physiol Heart Circ Physiol 267: H654–H666, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Eke A, Herman P, Bassingthwaighte JB, Raymond GM, Cannon M, Balla I, Ikrenyi C. Fractal analysis of physiological time series. Eur J Physiol 439: 403–415, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Falsetti HL, Carroll RJ, Marcus ML. Temporal heterogeneity of myocardial blood flow in anesthetized dogs. Circulation 52: 848–853, 1975. [DOI] [PubMed] [Google Scholar]
- 26.Franzen D, Conway RS, Zhang H, Sonnenblick EH, Eng C. Spatial heterogeneity of local blood flow and metabolite content in dog hearts. Am J Physiol Heart Circ Physiol 254: H344–H353, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Goresky CA, Stremmel W, Rose CP, Guirguis S, Schwab AJ, Diede HE, Ibrahim E. The capillary transport system for free fatty acids in the heart. Circ Res 74: 1015–1026, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Gorman MW, Wangler RD, Sparks HV. Distribution of perfusate flow during vasodilation in the isolated guinea pig heart. Am J Physiol Heart Circ Physiol 256: H297–H301, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Gorman M, Rooke G, Savage MV, Jayasekara M, Jacobsen K, Feigl EO. Adenine nucleotide control of coronary blood flow during exercise. Am J Physiol Heart Circ Physiol 299: H1981–H1989, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haidet GC, Musch TI, Ordway GA, Mitchell JH. Exercise, dobutamine, and combined atropine, norepinephrine, and epinephrine compared. J Appl Physiol 58: 2047–2053, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Heymann MA, Payne BD, Hoffman JIE, Rudolph AM. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis 20: 55–79, 1977. [DOI] [PubMed] [Google Scholar]
- 32.Holdsworth CT, Copp SW, Ferguson SK, Sims GE, Poole DC, Musch TI. Acute inhibition of ATP-sensitive K channels impairs skeletal muscle vascular control in rats during treadmill exercise. Am J Physiol Heart Circ Physiol 308: H1434–H1442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard EJ, Kerckhoffs RCP, Vincent KP, Krishnamurthy A, Villongco CT, Mulligan LJ, McCulloch AD, Omens JH. Myofiber prestretch magnitude determines regional systolic function during ectopic activation in the tachycardia-induced failing canine heart. Am J Physiol Heart Circ Physiol 305: H192–H202, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001. [DOI] [PubMed] [Google Scholar]
- 35.King RB, Bassingthwaighte JB, Hales JRS, Rowell LB. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ Res 57: 285–295, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King RB, Bassingthwaighte JB. Temporal fluctuations in regional myocardial flows. Pflügers Arch 413/4: 336–342, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroll K, Feigl EO. Adenosine is unimportant in controlling coronary blood flow in unstressed dog hearts. Am J Physiol Heart Circ Physiol 249: H1176–H1187, 1985. [DOI] [PubMed] [Google Scholar]
- 38.Little SE, Link JM, Krohn KA, Bassingthwaighte JB. Myocardial extraction and retention of 2-iododesmethylimipramine: A novel flow marker. Am J Physiol Heart Circ Physiol 250: H1060–H1070, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loncar R, Flesche CW, Deussen A. Coronary reserve of high- and low-flow regions in the dog heart left ventricle. Circulation 98: 262–270, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Monroe RG, LaFarge CG, Gamble WJ, Kumar AE, Manasek FJ. Left ventricular performance and coronary flow after coronary embolization with plastic microspheres. J Clin Invest 50: 1656–1665, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musters MWJM, Bassingthwaighte JB, van Riel NAW, van der Vusse GJ. Computational evidence for protein-mediated fatty acid transport across the sarcolemma. Biochem J 393: 669–678, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nose Y, Nakamura T, Nakamura M. The microsphere method facilitates statistical assessment of regional blood flow. Basic Res Cardiol 80: 417–429, 1985. [DOI] [PubMed] [Google Scholar]
- 43.Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol Heart Circ Physiol 259: H300–H308, 1990. [DOI] [PubMed] [Google Scholar]
- 44.Prinzen FW, Bassingthwaighte JB. Blood flow distributions by microsphere deposition methods. Cardiovasc Res 45: 13–21, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rembert JC, Boyd LM, Watkinson WP, Greenfield JC Jr. Effect of adenosine on transmural myocardial blood flow distribution in the awake dog. Am J Physiol Heart Circ Physiol 239: H7–H13, 1980. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz LM, Bukowski TR, Revkin JH, Bassingthwaighte JB. Cardiac endothelial transport and metabolism of adenosine and inosine. Am J Physiol Heart Circ Physiol 277: H1241–H1251, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz LM, Bukowski TR, Ploger JD, Bassingthwaighte JB. Endothelial adenosine transporter characterization in perfused guinea pig hearts. Am J Physiol Heart Circ Physiol 279: H1502–H1511, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Sestier FJ, Mildenberger RR, Klassen GA. Role of autoregulation in spatial and temporal perfusion heterogeneity of canine myocardium. Am J Physiol Heart Circ Physiol 235: H64–H71, 1978. [DOI] [PubMed] [Google Scholar]
- 49.Starling E. Linacre Lecture on the Law of the Heart: 1915. London, UK: Longmans, 1918. [Google Scholar]
- 50.Stepp DW, van Bibber R, Kroll K, Feigl EO. Quantitative relation between interstitial adenosine concentration and coronary blood flow. Circ Res 79: 601–610, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Stremmel W. Fatty acid uptake by isolated rat heart myocytes represents a carrier-mediated transport process. J Clin Invest 81: 844–852, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torrent-Guasp F, Kocica MJ, Corno AF, Komeda M, Carreras-Costa F. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg 27: 191–201, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Tune JD, Richmond KN, Gorman MW, Olsson RA, Feigl EO. Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am J Physiol Heart Circ Physiol 278: H74–H84, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Tune JD, Richmond K, Gorman M, Feigl E. KATP channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 280: H868–H875, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Tune JD, Richmond K, Gorman M, Feigl EO. Control of coronary blood flow during exercise. Exper Biol Med 227: 238–250, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Utley J, Carlson EL, Hoffman JIE, Martinez HM, Buckberg GD. Total and regional myocardial blood flow measurements with 25 m, 15 m, 9 m, and filtered 1–10 m diameter microspheres and antipyrine in dogs and sheep. Circ Res 34: 391–405, 1974. [DOI] [PubMed] [Google Scholar]
- 57.van Beek JHGM, Roger SA, Bassingthwaighte JB. Regional myocardial flow heterogeneity explained with fractal networks. Am J Physiol Heart Circ Physiol 257: H1670–H1680, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van der Vusse GJ, Glatz JFC, Van Nieuwenhoven FA, Reneman RS, Bassingthwaighte JB. Transport of long-chain fatty acids across the muscular endothelium. Skeletal Muscle Metabolism in Exercise and Diabetes. Adv Exp Med Biol 441: 181–191, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Oosterhout MFM, Prinzen FW, Arts T, Schreuder JJ, Vanagt WYR, Cleutjens JPM, Reneman RS. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation 98: 588–595, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Van Oosterhout MFM, Arts T, Bassingthwaighte JB, Reneman RS, Prinzen FW. Relation between local myocardial growth and blood flow during chronic ventricular pacing. Cardiovasc Res 53: 831–840, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Vinnakota K, Bassingthwaighte JB. Myocardial density and composition: A basis for calculating intracellular metabolite concentrations. Am J Physiol Heart Circ Physiol 286: H1742–H1749, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Wu F, Yang F, Vinnakota KC, Beard DA. Computer modeling of mitochondrial tricarboxylic acid cycle, oxidative phosphorylation, metabolite transport, and electrophysiology. J Biol Chem 282: 24525–24537, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Yada T, Richmond KN, Van Bibber R, Kroll K, Feigl EO. Role of adenosine in local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 276: H1425–H1433, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Yipintsoi T, Dobbs WA Jr, Scanlon PD, Knopp TJ, Bassingthwaighte JB. Regional distribution of diffusible tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ Res 33: 573–587, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]