This study supports microglia as a key cellular source of ANG II-mediated ROS production within the paraventricular nucleus and provides evidence for Toll-like receptor 4 as a key underlying molecular mechanism. These findings support a functional interaction between the innate immunity and central renin-angiotensin system in the regulation of sympathetic output from the paraventricular nucleus.
Keywords: angiotensin II, paraventricular nucleus, microglia, Toll-like receptor, angiotensin II type 1 receptor
Abstract
ANG II is thought to increase sympathetic outflow by increasing oxidative stress and promoting local inflammation in the paraventricular nucleus (PVN) of the hypothalamus. However, the relative contributions of inflammation and oxidative stress to sympathetic drive remain poorly understood, and the underlying cellular and molecular targets have yet to be examined. ANG II has been shown to enhance Toll-like receptor (TLR)4-mediated signaling on microglia. Thus, in the present study, we aimed to determine whether ANG II-mediated activation of microglial TLR4 signaling is a key molecular target initiating local oxidative stress in the PVN. We found TLR4 and ANG II type 1 (AT1) receptor mRNA expression in hypothalamic microglia, providing molecular evidence for the potential interaction between these two receptors. In hypothalamic slices, ANG II induced microglial activation within the PVN (∼65% increase, P < 0.001), an effect that was blunted in the absence of functional TLR4. ANG II increased ROS production, as indicated by dihydroethidium fluorescence, within the PVN of rats and mice (P < 0.0001 in both cases), effects that were also dependent on the presence of functional TLR4. The microglial inhibitor minocycline attenuated ANG II-mediated ROS production, yet ANG II effects persisted in PVN single-minded 1-AT1a knockout mice, supporting the contribution of a non-neuronal source (likely microglia) to ANG II-driven ROS production in the PVN. Taken together, these results support functional interactions between AT1 receptors and TLR4 in mediating ANG II-dependent microglial activation and oxidative stress within the PVN. More broadly, our results support a functional interaction between the central renin-angiotensin system and innate immunity in the regulation of neurohumoral outflows from the PVN.
NEW & NOTEWORTHY
This study supports microglia as a key cellular source of ANG II-mediated ROS production within the paraventricular nucleus and provides evidence for Toll-like receptor 4 as a key underlying molecular mechanism. These findings support a functional interaction between the innate immunity and central renin-angiotensin system in the regulation of sympathetic output from the paraventricular nucleus.
angiotensin ii (ANG II) plays a critical role in fluid balance and hemodynamic regulation (25). Within the central nervous system (CNS), ANG II acting through ANG II type 1a (AT1a) receptors (AT1aR) has been shown to be implicated in the regulation of sympathetic and neuroendocrine outputs from the brain (45, 49, 50). The paraventricular nucleus (PVN) of the hypothalamus has been recognized as a critical neuronal substrate mediating central ANG II actions, thus playing a critical role in ANG II-mediated neurohumoral responses (23, 78). Indeed, a large body of evidence supports enhanced angiotensinergic actions within the CNS, including the PVN, as a key pathophysiological mechanism involved in cardiovascular diseases, including hypertension and heart failure (37, 60, 78, 80, 88). However, despite its importance, the precise mechanisms and cellular/molecular targets underlying ANG II signaling within the CNS are incompletely understood.
Inflammation and oxidative stress have emerged as novel mechanisms by which central ANG II mediates its deleterious effects. For example, studies have shown that pressor and sympathetic responses to central ANG II are mediated by superoxide within the PVN (8, 22). Likewise, several studies have supported ANG II-mediated generation of ROS within the subfornical organ, PVN, and rostral ventrolateral medulla as an important contributor to sympathoexcitation in cardiovascular diseases (10, 33, 91, 92). Additionally, ANG II is known as a strong proinflammatory signal (46, 66). Systemic administration of ANG II stimulates expression of NF-κB and various proinflammatory cytokines, including IL-1β, IL-6, and TNF-α (11, 33), within the PVN. In addition, ANG II-mediated inflammation is now also recognized as an important pathophysiological mechanism in hypertension (65, 90). Thus, while both oxidative stress and inflammation are critical ANG II-mediated events within the PVN with major pathophysiological consequences, whether they are causally related and what the precise cellular and molecular targets mediating these actions are remain unknown.
Microglia are the resident immune cells in the brain and the first responders to injury and infection, turning into an activated, proinflammatory state (68), resulting in the release of a variety of proinflammatory and neuroactive substances, including cytokines, chemokines, and ROS (68). Importantly, ANG II has been shown to promote microglial cell activation in the PVN (32, 71).
Along with microglia, Toll-like receptor (TLR)4 is a signaling receptor that plays a major role in innate immunity (3, 76) and has been shown to also contribute to neuroinflammatory diseases (7, 13, 79). TLR4 is primarily expressed in microglial cells, and its activation leads to microglial cell activation and the release of proinflammatory signals (38, 47, 68). Moreover, TLR4 activation can lead to ROS production in neutrophils (1), and in vitro and in vivo studies have supported a functional/molecular link between ANG II-TLR4 in ANG II-mediated inflammation (2, 18, 82, 84). Based on these previous studies, we performed a series of experiments to test the hypothesis that microglial TLR4 constitutes a pivotal molecular link causally connecting ANG II-mediated microglial cell activation and oxidative stress within the PVN. Our results show that ANG II induces microglial activation within the PVN, an effect that is blunted in the absence of functional TLR4. We also demonstrate that TLR4 protein and mRNA and AT1a mRNA are expressed in hypothalamic microglia. In addition, we found that ANG II increased PVN ROS production, an effect that was also blunted in the absence of functional TLR4, was attenuated by the microglial cell inhibitor minocycline and, whereas it was dependent on AT1Rs, it did not involve receptors expressed in neurons. Taken together, the results from this work support a functional interaction between microglial AT1R and TLR4 in mediating ANG II-dependent microglial activation and oxidative stress within the PVN, ultimately suggesting a functional interaction between the central renin-angiotensin system and innate immunity in the regulation of neurohumoral outflows from the PVN.
METHODS
Animals and Experimental Groups
Male Wistar rats were purchased from Harlan Laboratories. Male TLR4-deficient C3H/HeJ and TLR4-sufficient C3H/OuJ mice were kindly donated by Dr. Dhandapani (Georgia Regents University) or purchased from The Jackson Laboratory. PVN single-minded (Sim)1-AT1a knockout (KO) mice and littermate control mice (AT1a flox/flox) were donated by Dr. Krause (University of Florida); in these mice, AT1a receptors were specifically deleted from PVN neurons through Cre recombinase-dependent excision driven by the Sim1 promoter (Sim1Cre mice were bread with AT1a flox mice as previously described) (19). All male animals used in this study were 7–12 wk old at the time experiments began. All procedures were approved by the Institutional Animal Care and Use Committee of Georgia Regents University.
Acute Hypothalamic Slices and Ex Vivo ANG II Stimulation
Coronal hypothalamic slices (200 μm thick) containing the PVN were obtained using a vibroslicer. Ice-cold standard artificial cerebrospinal fluid (aCSF) (6, 57) was used in the preparation process. The hemispheres were separated and placed in two holding chambers at 32°C for 60 min. After this time, the chambers were transferred to room temperature, and aCSF or aCSF in the presence of ANG II (1 μM, Sigma-Aldrich) was added to each of the chambers for another 60 min. Sections were then postfixed in 4% paraformaldehyde (PFA) for 24 h, and immunohistochemistry was performed for the microglial marker ionized calcium-binding adaptor molecule (IBA)1.
ROS Assessment
A modified dihydroethidium (DHE) staining method was used to evaluate in situ production of ROS (34, 81). Transverse sections (16 μm) of hypothalamic frozen tissue were collected on glass slides and equilibrated in either fresh aCSF, losartan (2 μM), or minocycline (100 μM, all Sigma-Aldrich) for 10 min in a humidified chamber at 37°C. Slides were then placed in an inverted Zeiss LSM780 confocal microscope stage (atmosphere and temperature controlled, Carl Zeiss), and sections were incubated with DHE (1 μM, Invitrogen) in the presence of either aCSF, ANG II (0.01–1 μM), or ANG II associated with losartan or minocycline. Immediately after the beginning of incubation, images were acquired every 2 min for a total of 10 min. Zeiss LSM780 software was used for analyses. Forty randomly selected cells per slice were manually traced, and the intensity of fluorescence of each cell was plotted over time. aCSF was used as the vehicle for all drug dilution.
Isolation of Microglia
Microglia from the hypothalamus were isolated by Percoll density gradient as previously described (21, 24). Animals were transcardially perfused with sterile saline, and the hypothalamus was macrodissected and homogenized using a Tenbroeck homogenizer with Dulbecco's PBS (dPBS) supplemented with 2% glucose. The homogenate was filtered using a 40-μm cell strainer, and cell pellets were obtained by brief centrifugation. The Percoll gradient was created by suspending the cell pellet in 70% isotonic Percoll followed by additional layers of 30% isotonic Percoll and dPBS. Gradients were centrifuged at 1,200 g for 55 min to separate mononuclear cells from the other CNS elements, and the resulting 70%–30% interface was collected. The resultant suspension was washed in dPBS, and the cell pellet was acquired by centrifugation at 1,000 g for 15 min. The resulting pellet was resuspended in 100 μl dPBS. Cell counting and morphological analysis was performed using a handheld automatic cell counter (Scepter instrument, EMD Millipore). Fifty thousand cells were then used for PCR experiments. In a subset of animals, to account for viability after isolation of microglia, 50,000 cells were stained with the live cell DNA dye Vybrant DyeCycle green for 2 h under a CO2 incubator (1:1,000 in DMEM with 10% FBS, Invitrogen). After this incubation, fluorescence and phase-contrast images were taken in a Deltavision high-resolution microscope (Applied Precision). In addition, to determine the purity of microglia and whether there was contamination of astrocytes and neurons, immunohistochemistry in isolated cells was performed and imaged as described below.
Fluorescence Immunohistochemistry
Standard immunofluorescence was performed as previously described (4, 6).
Immunofluorescence in hypothalamic tissue.
Animals were transcardially perfused with 0.01 mol/l PBS (150 ml) and 4% PFA (350 ml). Brains were dissected and postfixed overnight in 4% PFA followed by cryoprotection in PBS containing 30% sucrose for 3 days at 4°C. Sections (30 μm) containing the hypothalamus were collected. Acute hypothalamic slices were subjected to immunohistochemistry after fixation in 4% PFA. After a preincubation in 5% horse blocking serum for 1 h, the following respective primary antibodies were used: 1) anti-rabbit IBA1 (1:2,000, Wako), 2) anti-rabbit TLR4 (1:100, Santa Cruz Biotechnology), and 3) anti-guinea pig (Arg8)-vasopressin (1:20,000, Bachem, used as an anatomic marker).
Isolated microglia.
Approximately 50,000 cells were fixed in 4% PFA for 30 min followed by a brief rinse with dPBS and placed to adhere in a coated glass slide. Cells were immunostained with microglia-specific (IBA1 anti-rabbit, 1:200), neuron-specific (NeuN, anti-mouse, 1:200), and astrocyte-specific (glial fibrillary acidic protein, anti-chicken, 1:200) primary antibodies in dPBS, 0.05% Tween, and 5% horse serum overnight. Hoechst counterstaining (1 μg/ml) was performed after the complete immunohistochemistry reaction.
Immunofluorescence for tissue and cells was revealed by a secondary reaction with anti-mouse Cy3 (1:250); anti-guinea pig Cy3 (1:250), anti-rabbit Cy3 or FITC (1:250), or anti-chicken CY5 (1:50), accordingly (all secondary antibodies from Jackson Immunoresearch). The specificity of each antibody was tested by the omission of the primary antibody.
Confocal Imaging Acquisition
Immunofluorescence was examined with an upright Zeiss LSM510 confocal microscope as previously described (4). Images from consecutive optical focal planes were taken (for hypothalamic 200-μm sections: 40 images, 2-μm intervals; for 30-μm sections: 20 images, 1 μm-intervals; for isolated microglial cells: 6 images, 1-μm intervals), and a projection image of the sections was then generated. Each channel was acquired sequentially to minimize crossover artifacts among the channels. In hypothalamic slices, we quantified IBA1 density within the PVN based on a threshold paradigm as previously described (4–6), and the results were expressed as IBA percent areas. Images were evaluated with ImageJ software (National Institutes of Health), and the ImageJ plugin (http://rsbweb.nih.gov/ij/plugins/colocalization.html), which detects and displays pixels containing signals from both channels, was used to determine the colocalization between signals (5).
Real-Time RT-PCR
Microglial cells.
cDNA from 50,000 cells/animal were acquired using the Power SYBR green cells-to-Ct kit (Ambion, Life Technologies). Lysis solution was added to the cells, and reverse transcription was performed using a RT master mix according to the manufacturer's instructions. PCR amplification of cDNA was also performed using the Power SYBR green cells-to-Ct kit: cDNA (4 μl) was added to a PCR cocktail with gene-specific primers (200 nM each of forward and reverse primers), and PCR cycling conditions were set up as per the manufacturer's instructions.
PVN punches.
Brains from TLR4-deficient and -sufficient mice (n = 3 mice/group) were collected and immediately frozen in isopentane. Subsequent hypothalamic sections (200 μm) containing the PVN were obtained using a cryostat. The PVN was bilaterally microdissected using a Harris aluminum micropunch needle (1.0 mm, Ted Pella) following The Mouse Brain in Stereotaxic Coordinates (PVN: −0.82 to −1.22 from the bregma; Paxinos and Franklin, 2nd edition, 2001). Samples were collected with RNAlater reagent (Ambion), and total RNA was isolated using TRI reagent (Sigma-Aldrich). The RNA concentration was determined by a NanoDrop spectrophotometer (Thermo Scientific). cDNA synthesis was performed using 250 ng RNA for each sample using an iScript cDNA Synthesis Kit (Bio-Rad) per the manufacturer's instructions. cDNA (1 μl) was added to a PCR cocktail with gene-specific primers (200 nM each of forward and reverse primers), and PCR cycling conditions were set up as per manufacturer's instructions.
The following primer sequences were used: 1) CD11b (GenBank Accession No. NM_012711.1, forward 5′-ATCCGTAAAGTAGTGAGAGAAC-3′ and reverse 5′-TCTGCCTCAGGAATGACATC-3′), 2) AT1a (GenBank Accession No. NM_030985.4, forward 5′-CCTGTCACTCCACCTCAAA-3′ and reverse 5′-AACCCTCTGTTCTACGGC-3′), and 3) TLR4 (GenBank Accession No. NM_019178.1, forward 5′- GATTGCTCAGACATGGCAGTTTC-3′ and reverse 5′-CACTCGAGGTAGGTGTTTCTGCTAA-3′). β-Actin (GenBank Accession No. NM_031144.3, forward 5′-CACAGCCTGGATGGCTACGTA-3′ and reverse 5′-GACCCAGATCATGTTTGAGACCTT-3′) served as a housekeeping gene. All primers were obtained from Integrated DNA Technologies. For each experimental sample, duplicate reactions were conducted. The formation of the PCR product was monitored in real time using the 7500 Fast Real Time PCR System (Applied Biosystems). AT1a mRNA expression levels were expressed as percentages of AT1a mRNA levels relative to TLR4-sufficient mice.
Statistical Analyses
Data are presented as means ± SE. Unpaired Student t-tests or one-way or two-way ANOVAs followed by post hoc multiple-comparison tests were used as indicated (GraphPad Prism 5). P values of <0.05 were considered to indicate statistical significance.
RESULTS
ANG II-Mediated PVN Microglial Activation Requires Functional TLR4
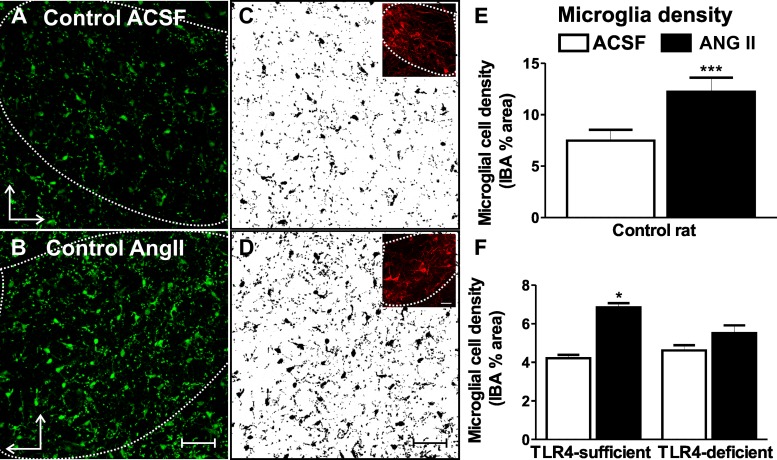
Previous studies have shown that ANG II stimulates microglial cell activation within the PVN (32, 71). Here, we investigated whether this effect involves TLR4 and whether this leads to the production of superoxide. To this end, we performed ex vivo assays using acute hypothalamic slices containing the PVN, which were obtained from control rats as well as from TLR4-sufficient C3H/OuJ (TLR4-sufficient) and TLR4-deficient C3H/HeJ (TLR4-deficient) mice (56). We found that ANG II (1 μM) significantly increased the degree of activated microglia (shown as significantly enhanced staining density for IBA1 within the PVN) (15, 71) in rat slices compared with control aCSF (∼65% increase from 9.8 ± 1.1% to 16.2 ± 1.4%, n = 10, P < 0.001; Fig. 1, A–E). Similarly, we found that ANG II increased IBA1 staining in TLR4-sufficient mice (∼65% increase from 3.9 ± 0.1% to 6.5 ± 0.3%, n = 5, P < 0.05; Fig. 1F), effects that were blunted in TLR4-deficient mice (∼20% increase from 4.3 ± 0.1% to 5.2 ± 0.5%, n = 3, Fig. 1F). To rule out the possibility that basal staining of microglia was different between TLR4 mouse strains, we measured basal IBA1 in a separate set of animals. In this case, we failed to observe significant differences in IBA1 staining between TLR4-sufficient mice (6.5 ± 0.5%) and deficient mice (7.2 ± 0.6%, n = 4 mice/group). Taken together, these results are consistent with the notion that ANG II-mediated microglial activation requires the presence of functional TLR4.
Fig. 1.
ANG II induces microglial activation within the paraventricular nucleus (PVN) of the hypothalamus of control rats and mice, an effect that is blunted in the absence of functional Toll-like receptor (TLR)4. A and B: confocal images of microglial marker ionized calcium-binding adaptor molecule (IBA)1 staining in the presence of artificial cerebrospinal fluid (aCSF; A) and ANG II (1 μM, 60 min; B) in control rats. C and D: IBA1 signal-subtracted threshold used for quantification. Insets show vasopressin stain used as an anatomic marker to trace the region of interest (PVN). E and F: summary data showing the ANG II-induced increase in microglial cell density in control rats (n = 10; E) and TLR4-sufficient mice (n = 5; F). Microglial cell density was not altered in TLR4-deficient mice in the presence of ANG II (n = 3; F). ***P < 0.0001 and *P < 0.05 vs. aCSF. The vertical arrow points dorsally and the horizontal arrow points medially. Scale bars = 50 μm.
PVN Microglial Cells Express AT1aRs and TLR4
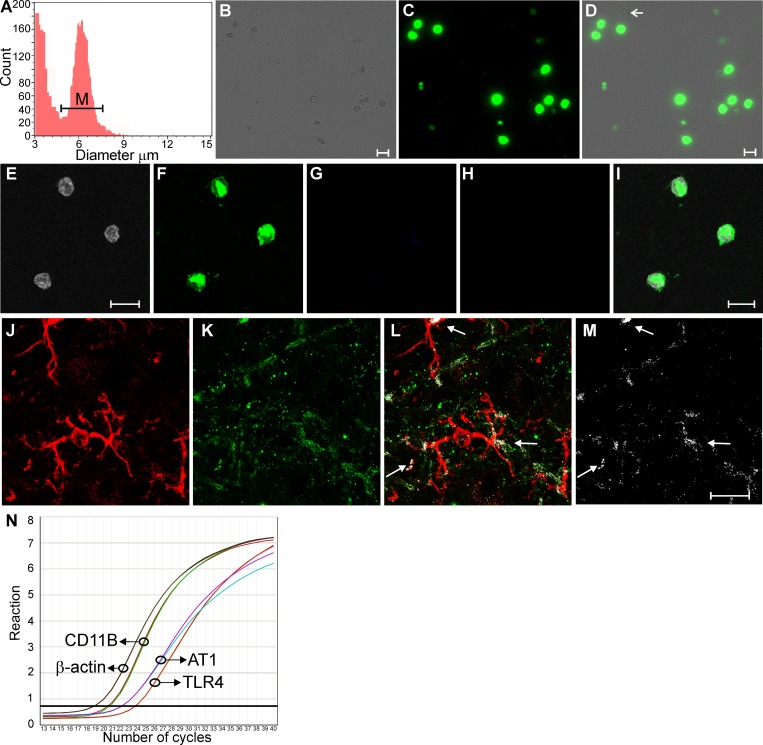
While these results support a contribution of TLR4 to ANG II-mediated microglial activation, whether PVN microglia express both ANG II receptors and TLR4 is at present unknown. To address this, we performed real-time PCR in isolated microglial cells from the hypothalamus along with immunohistochemistry in hypothalamic slices. Hypothalamic microglial cells were isolated following conventional Percoll density gradient procedures (see methods). The viability, identity, and purity of the extracted population were then assessed. To confirm the phenotype of the isolated cells, we used an automatic cell counting approach, which uses morphometry (i.e., cell body diameter) to differentiate isolated cells from debris (Fig. 2A). As previously reported, isolated microglia had a cell body diameter of ∼ 6–9 μm (21). Analysis of viable living cells following the procedure was measured using a DNA-selective stain that is cell permeable and emits a fluorescent signal proportional to the DNA mass after binding double-stranded DNA of living cells (Vybrant DyeCycle green; Fig. 2, B–D). We found that in our samples, ∼90% of cells were alive. In addition, isolated cells were stained with antibodies against microglia (IBA1), astrocytes (glial fibrillary acidic protein), and neurons (NeuN). As shown in Fig. 2, E–I, cells identified by Hoechst counterstaining (Fig. 2E) were positively immunostained for IBA1 (Fig. 2F), whereas no staining was observed for astrocytes (Fig. 2G) or neurons (Fig. 2H), indicating that the cells collected were mainly microglia.
Fig. 2.
Hypothalamic microglia express ANG II type 1 (AT1) receptors (AT1Rs) and TLR4. A: automatic measurement of cell number and diameter from the extracted cell population by Percol gradient permitting the distinction between microglia (M) and debris. B and C: live isolated cells (phase contrast; B) were stained with Vybrant DyeCycle (C). D: merged image. The arrow points to a representative dead cell. Manual counting showed ∼90% live cells in our samples. E–H: isolated cells (Hoechst counterstaining; E) showed positive immunostaining for microglia (IBA1; F) but not for astrocytes [glial fibrillary acidic protein (GFAP); G] or neurons (NeuN; H). I: merged images from E–H. J and K: confocal photomicrographs showing the microglial marker IBA1 (red; J) and TLR4 (green; K). L: merged image showing the signal colocalization (white pixels). M: only colocalized pixels are shown. N: real-time PCR detection of microglia (CD11B), AT1a, and TLR4 mRNA in isolated hypothalamic microglia. Scale bars = 10 μm (isolated cells) and 20 μm (tissue).
As shown in Fig. 2N, real-time PCR performed on cDNA obtained from the isolated hypothalamic microglial cell population showed robust amplification for the microglial marker CD11B (as well as for IBA1; not shown). Moreover, both AT1a and TLR4 mRNAs were also detected in the same samples of microglia. Similar results were observed in six independently performed experiments. Likewise, using immunohistochemistry, we found immunoreactivity and costaining for the microglial marker IBA1 and TLR4 (Fig. 2, J–M). Taken together, these experiments support the presence of mRNA for AT1a and mRNA and protein for TLR4 in hypothalamic microglia.
ANG II-Mediated ROS Within the PVN Involves Activated Microglia
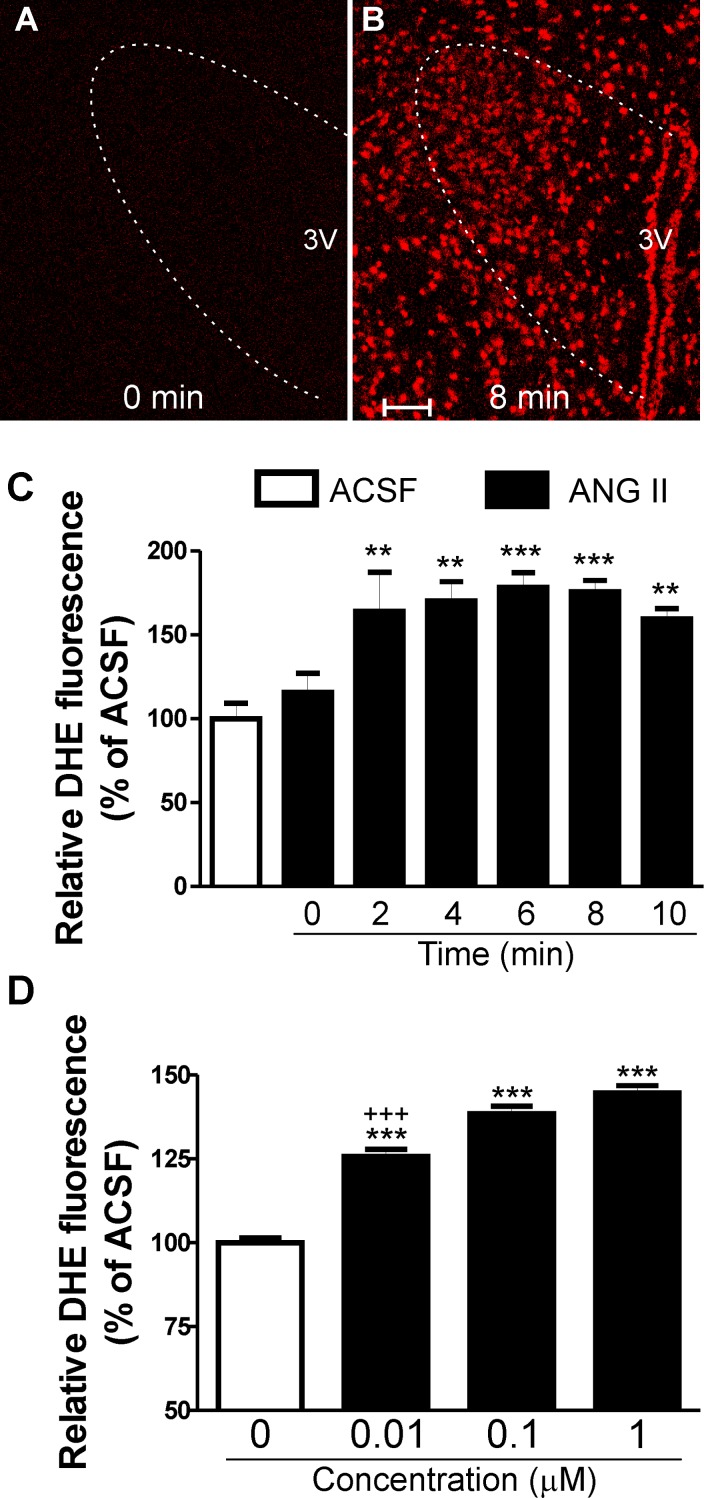
We used DHE staining to evaluate in situ production of ROS (34, 81) and to determine whether microglia and TLR4 contributed to ANG II-mediated ROS production within the PVN. Experiments were run in pairs of slices: one slice incubated with regular aCSF and another slice in the presence of ANG II (0.01–1 μM).
As previously observed, we found that even in the presence of aCSF, there was a progressive increase in background DHE fluorescence levels during imaging acquisition (89). Thus, to quantify the effect of ANG II, we calculated proportional changes (percentages of aCSF) in DHE staining fluorescence intensity at each particular time point and plotted this proportional change over time. We found that ANG II significantly increased DHE staining within the PVN of rats in a time-dependent manner (F = 15.3, P < 0.0001 by one-way ANOVA, Fig. 3, A–C) and concentration-dependent manner (F = 129.3, P < 0.0001 by one-way ANOVA, data obtained 10 min after the beginning of the different concentrations of ANG II incubation; Fig. 3D). The proportional changes of ANG II-dependent ROS production started within 2 min of incubation in ANG II, peaked at 4–8 min, and decreased thereafter (Fig. 3C).
Fig. 3.
ANG II-mediated ROS within the PVN of rats and mice. A and B: photomicrographs of dihydroethidium (DHE) staining in the presence of ANG II (1 μM) obtained at time 0 (A) and 8 min (B) within the PVN of TLR4-sufficient mice. C: mean data representing relative DHE fluorescence (percentage of ACSF) acquired from time 0 to 10 min, sampled every 2 min after ANG II (1 μM) incubation. D: mean data representing relative DHE fluorescence (percentage of ACSF) acquired at 10 min after the beginning of different concentrations of ANG II (0.01, 0.1, and 1 μM) incubation. 3V, third ventricle. Scale bars = 50 μm. n = 8/group. ***P < 0.0001 and **P < 0.001 vs. aCSF; +++P < 0.0001 vs. ANG II (0.1 and 1 μM).
Based on our data described above, we then evaluated whether activated microglia contribute to ANG II-evoked ROS production in the PVN. To this end, we performed similar DHE experiments in slices incubated in either 1) regular aCSF, 2) aCSF + ANG II (1 μM), or 3) aCSF + ANG II + the microglial inhibitor minocycline (100 μM) (15, 86). As previously observed, ANG II significantly increased DHE staining compared with control aCSF (n = 4/group, Fig. 4, A–D), an effect that was partially blunted in the presence of minocycline (n = 4, P < 0.0001 vs. ANG II).
Fig. 4.
Microglial inhibition decreases ANG II-increased ROS production within the PVN of rats. A–C: confocal images showing hypothalamic slices incubated in DHE in the presence of aCSF (A); ANG II (1 μM; B) and ANG II + minocycline (Mino; 100 μM; C). D: summary data showing increased ROS production in the presence of ANG II and the blunted effect observed in slices pretreated with minocycline. Confocal images represent data obtained 8 min after the beginning of the acquisition. Scale bars = 50 μm. n = 4 rats/group. ***P < 0.0001 vs. aCSF and +++P < 0.0001 vs. ANG II at each given time point. (Main effect by two-way ANOVA: treatment: F = 17.3, P < 0.0001; time: F = 4.5, P < 0.0001; interactions: F = 2.93, P < 0.0001.)
ANG II-Mediated ROS Production Within the PVN Is Dependent on TLR4
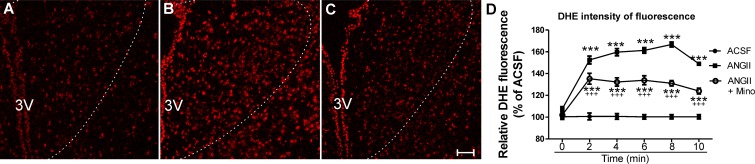
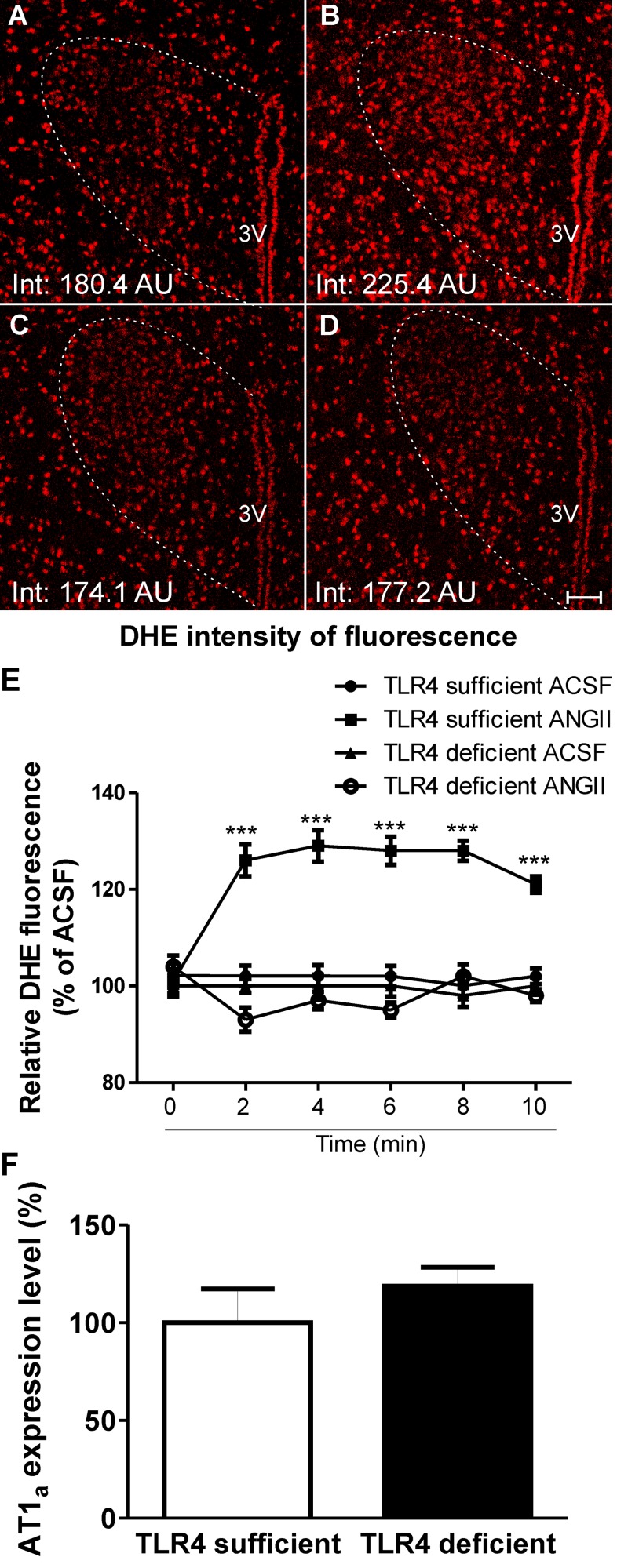
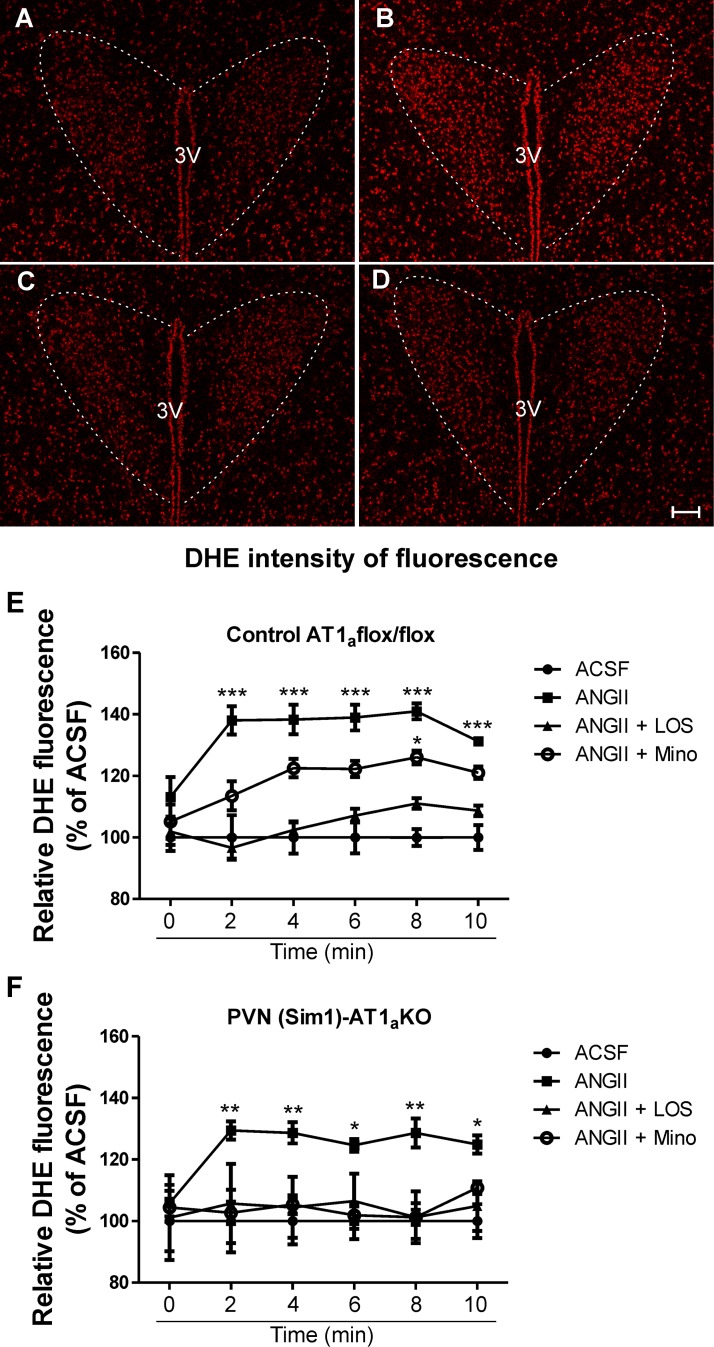
To determine whether TLR4 contributes to ANG II-mediated ROS production in the PVN, experiments were repeated in TLR4-sufficient and -deficient mice. We observed that similar to our previous results in rats, ANG II (1 μM) significantly increased DHE staining in TLR4-sufficient mice (n = 4, P < 0.0001; Fig. 5, A, B, and E), an effect that was, however, absent in TLR4-deficient mice (n = 5; Fig. 5, C–E). To exclude the possibility that the results observed were due to basal differences in the expression pattern of AT1aRs between TLR4 mouse strains, we measured AT1a mRNA expression within the PVN of TLR4-sufficient and -deficient mice. We did not observe significant differences in AT1a mRNA expression between strains (TLR4-sufficient mice: 100 ± 17.3% and TLR4-deficient mice: 118 ± 9.8%, n = 3; Fig. 5F). Taken together, our results support a major contribution of microglia and TLR4 to ANG II-mediated ROS production within the PVN. However, in addition to their presence in microglia [as shown in the present study and elsewhere (38, 68)], TLR4 as well as AT1aRs have also been shown to be expressed in neurons (40, 48, 64). Thus, to further test the relative contribution of microglia versus neurons as cell targets mediating ANG II effects on PVN ROS production, we performed similar experiments in mice in which AT1aRs were specifically deleted from PVN neurons [(Sim1)-AT1a KO mice; see methods]. In this set of experiments, we again observed increased PVN DHE fluorescence intensity in the presence of ANG II (1 μM) compared with aCSF both in control AT1a flox/flox and PVN (Sim1)-AT1a KO mice (P < 0.0001 and 0.001; Fig. 6, A, B, E, and F). While this effect was partially blunted by minocycline in control AT1a flox/flox mice, it was completely prevented in PVN (Sim1)-AT1a KO mice (Fig. 6, C, E, and F). In both groups, however, losartan (2 μM) completely prevented ANG II (1 μM) effects (Fig. 6, D–F). These results indicate that even in the absence of neuronal AT1Rs, ANG II continues to stimulate ROS production, likely via the activation of microglia signaling cascades.
Fig. 5.
Absence of functional TLR4 blunts ANG II-mediated ROS production within the PVN of mice. A–D: confocal images showing DHE staining in the presence of aCSF (A and C) and ANG II (1 μM; B and D) in TLR4-sufficient mice (A and B) and TLR4-deficient mice (C and D). E: summary data showing increased ROS production in the presence of ANG II in TLR4-sufficient mice but not in TLR4 deficient mice. F: summary data showing lack of statistical differences in basal PVN AT1a mRNA expression between TLR4-sufficient and -deficient mice (expressed as the percentage of AT1a mRNA levels in TLR4-sufficient mice). AU, arbitrary units. Scale bars = 50 μm. n = 4 and 5 mice/group for ROS measurements and 3 mice/group for mRNA measurements. ***P < 0.0001 vs. aCSF at each given time point. (Main effect by two-way ANOVA: treatment: F = 11.9, P < 0.0001; time: F = 0.4, P < 0.05; interactions: F = 3.12, P < 0.0001.)
Fig. 6.
ANG II-mediated ROS production within the PVN is not dependent on neuronal AT1aRs. A–D: confocal images showing DHE staining in the presence of aCSF (A), ANG II (1 μM; B), ANG II + minocycline (100 μM; C), and ANG II + losartan (Los; 2 μM; D) in PVN single-minded (Sim1)-AT1a knockout (KO) mice. E and F: summary data showing increased ROS production in the presence of ANG II (1 μM) in control AT1a flox/flox and PVN (Sim1)-AT1a KO mice and partial inhibition of ANG II + minocycline in control AT1a flox/flox mice. No differences were observed in DHE staining in slices incubated with ANG II + losartan in both groups. Scale bars = 50 μm. n = 4 PVN (Sim1)-AT1a KO mice and 5 wild-type mice. ***P < 0.0001, **P < 0.001, and *P < 0.05 vs. aCSF at each given time point. (Main effect by two-way ANOVA for E: treatment: F = 6.97, P < 0.0001; time: F = 0.9, P < 0.001; interactions: F = 0.7, P = 0.2; and main effect by two-way ANOVA for F: treatment: F = 1.4, P < 0.0001; time: F = 0.1, P = 0.7; interactions: F = 0.2, P = 0.9.)
DISCUSSION
The findings from the present study provide evidence that microglial TLR4 is a molecular candidate causally linking ANG II-AT1aR-mediated microglial cell activation and oxidative stress within the PVN. Our results show that 1) ANG II-induced microglial activation within the PVN is dependent on the presence of TLR4; 2) TLR4 and AT1a mRNA are expressed in hypothalamic microglia; 3) ANG II via AT1aRs induces ROS production within the PVN, in part from activated microglia; and 4) functional TLR4 is necessary for ANG II-induced ROS production within the PVN.
Functional TLR4 Is Required for ANG II-Induced Microglial Activation Within the PVN
Previous studies have demonstrated that overactivation of ANG II-mediated signaling within the PVN plays a major role in microglial activation, with the consequent production of proinflammatory cytokines (32, 71). Accordingly, we found in our preparation an increase in the degree of activated microglia in the presence of ANG II. However, the molecular targets mediating these actions remain incompletely understood. Because microglia are the major cell types expressing TLR4 within the CNS (38, 51, 54) and ANG II has been implicated in the induction of the inflammatory response via the TLR4 pathway (17, 29, 82), we further assessed the contribution of TLR4 to ANG II-induced microglial activation within the PVN.
ANG II, via AT1Rs, has been shown to induce inflammatory responses by increasing TLR4 mRNA and protein expression in mesangial cells, smooth muscle cells, and mesothelial cells in vitro and in vivo (29, 82, 84). Likewise, AT1R blockers reduced TLR4 protein and mRNA expression, with a subsequent decrease in proinflammatory effects in human monocytes (18). Within the CNS, gene and protein expression for TLR4 were found to be upregulated within the PVN of ANG II-infused rats, in which chronic blockade of brain TLR4 attenuates inflammatory molecules (17). In the present study, we found that ANG II-mediated microglial activation was blunted in mice lacking functional TLR4, supporting TLR4 as a molecular candidate by which ANG II mediates microglial activation. These data are in accordance with recent work showing that direct TLR4 activation via lipopolysaccharide, a specific ligand for TLR4 (44), promotes microglial activation within the PVN (47).
We need to acknowledge the possibility of microglial cell priming due to possible tissue damage during the tissue slicing procedure. This was minimized, however, by slicing the tissue under very cold conditions. This is a standard procedure that we and others have conventionally used to monitor live neuronal and astrocyte activity in brain slices (6, 28, 62, 72). Even if microglia were partially activated, and considering that all experimental groups were subjected to the same dissection/slicing procedures, this potential drawback did not prevent us from detecting significant differences among groups. To provide anatomic evidence for ANG II-TLR4 cross talk within PVN microglia, we performed real-time PCR in isolated microglia of the hypothalamus. The expression of functional AT1Rs has been previously demonstrated in amoeboid cultured microglia in postnatal rats based on RT-PCR and lectin stain (41, 52). Expression of the AT1R, however, has been shown to progressively decrease with the transformation of amoeboid into ramified forms of microglia (41), and other studies have reported lack of AT1R protein and mRNA expression in adult microglia under normal conditions (52, 83). In our hands, however, besides finding TLR4 protein expression within PVN microglia, we found the presence of TLR4 and AT1a mRNA expression in isolated hypothalamic microglia. The discrepancies observed among these studies could be due in part to the material used (cultured cells vs. brain tissue or cells extracted in vivo). While we also observed AT1aR immunoreactivity in PVN microglia (not shown), the commercially available antibodies for AT1Rs are unreliable due to lack of specificity (26). Thus, given these limitations, we based our conclusions regarding the presence of functional AT1aRs on PVN microglial cells on the combined results we obtained from the PCR experiments along with our functional and pharmacological experiments. In addition, although most of our isolated cells were positively stained with IBA1 (>90%) or showed robust expression of IBA1 and CD11B mRNA, we acknowledge that these markers are not able to distinguish microglia from macrophages. Thus, our experiments did not differentiate between innate microglia or possible infiltrating macrophages.
TLR4 in Microglia Is Involved in ANG II-Mediated ROS Within the PVN
ROS has been highlighted as a critical mechanism by which ANG II exerts its deleterious effects within the CNS (10, 35, 91, 92). For example, ANG II-mediated ROS production within the PVN contributes to cardiovascular diseases with high sympathetic drive, such as hypertension and heart failure (9, 43, 59, 74). However, the cellular sources and molecular targets involved in ANG II-ROS signaling dysregulation have not been fully elucidated. Because both ANG II-AT1R-mediated signaling and TLR4-mediated signaling share in common several downstream products, including the generation of proinflammatory and neuroactive substances such as ROS (1, 68), we sought to determine whether these two signaling mechanisms were interrelated.
As previously reported (22, 74), we found that ANG II stimulated ROS production within the PVN. Moreover, our results support that microglia significantly contributed to this effect. First, we found that in the presence of the microglial inhibitor minocycline (77, 86), ANG II-mediated ROS production was significantly blunted. The unblocked ROS production in the presence of minocycline may be indicative of an incomplete block by minocycline or, more likely, that an alternative cellular source (i.e., neuronal) also contributes to ANG II-ROS production in the PVN (see more below).
Interestingly, minocycline has been considered neuroprotective not only for inhibiting microglial activation itself but also to inhibit NADPH and proinflammatory cytokine expression, including nitric oxide synthase expression and IL-1α, from activated microglia (16, 84). Moreover, minocycline has also been shown to have cytoprotective effects by inhibiting enzymatic activity of MAPKs (31, 73), which may have also contributed to some of the effects observed here.
Second, we found that ANG II-mediated ROS production was almost completely absent in TLR4-deficient mice compared with TLR4-sufficient mice. Since TLR4 is predominantly expressed in microglia (38, 51, 54), these studies further support a contribution of microglia to ANG II-mediated ROS production in the PVN. These results are in accordance with previous studies showing that ANG II promotes the involvement of microglia in NADPH-derived ROS in primary mesencephalic cultures (63) and that lipopolysaccharide failed to induce ROS production in bone marrow-derived macrophages cells from the same TLR4-deficient mice (67). Nonetheless, TLR4 in the brain is not exclusively expressed in microglia, and, along with AT1aRs, TLR4 has been shown to be also present in neurons (40, 48, 64). Similarly, the NADPH oxidase family, a primary source contributing to ANG II-mediated superoxide production, is present in both microglia as well as neurons (12, 55, 70, 75, 85). Thus, to determine to what extent PVN neurons contribute to ANG II-mediated ROS production, at least under our conditions, we repeated experiments in mice in which AT1aRs were specifically deleted from PVN neurons as previously characterized [Sim1Cre PVN (Sim1)-AT1a KO mice] (19). Similar to what we observed in rats, ANG II-mediated ROS production in wild-type mice was partially blunted by minocycline and, in this case, completely blocked by the AT1R blocker losartan. In PVN (Sim1)-AT1a KO mice, ANG II-mediated ROS production was slightly but not significantly lower than in control AT1a flox/flox mice (∼11% less), supporting a minor contribution of AT1aR-expressing PVN neurons to ANG II-mediated ROS production in the PVN. Moreover, minocycline almost completely prevented ANG II-mediated ROS production in PVN (Sim1)-AT1a KO mice. In addition to AT1aRs, ANG II can also activate brain AT1bRs. However, whether AT1bRs are present in the PVN is controversial, with studies supporting (14, 58) or arguing against (30, 39) its presence within the PVN. Since both AT1R subtypes share similar intracellular signaling pathways, we cannot rule out at present a possible role for AT1bRs in ANG II-mediated ROS production in the PVN. Taken together, these results support the notion that microglia, in a TLR4- and AT1aR-dependent manner, are the major cellular substrates contributing to ANG II-mediated ROS production in the PVN.
The nature of the ANG II-AT1aR-TLR4 interaction on stimulating ROS production remains unknown. Our results show that ANG II-mediated ROS production in microglia was completely blocked by losartan. This argues against direct activation of TLR4, supporting, conversely, the notion that ANG II stimulates TLR4 signaling through a cascade of intracellular signals linked to AT1Rs. In this sense, AT1R activation has been reported to promote the transactivation of EGF receptors (EGFRs) (69). EGFR signaling is required for TLR4-mediated activation of NF-κB in response to lipopolysaccharide (20), and its activation by ANG II can occur within a similar time course (in minutes) as the effect we describe here for ANG II-TLR4-ROS production. Another possibility is that ANG II induces the upregulation and activation of the cytokine transforming growth factor-β by trombospondin-1 (36), which, in turn, activates TLR4 pathways in macrophages to induce TNF-α production (42). Clearly, future studies are warranted to more precisely characterize the signaling mechanisms underlying the ANG II-AT1aR-TLR4 cross talk in mediating microglial ROS production in the PVN. Moreover, given that the present study focused on characterizing the mechanistic links between ANG II-AT1aR-TLR4 cross talk in PVN microglial cells, future in vivo studies are also warranted to elucidate the cardiovascular consequences of ANG II activation of this microglial receptor cross talk.
Functional Implications
It is well established that the renin-angiotensin system plays a critical role in cardiovascular homeostasis as well as in the development and maintenance of cardiovascular-related diseases such as hypertension and heart failure (27, 78). Within the PVN, ANG II has been associated with increased microglial activation and increased expression of proinflammatory cytokines and the transcription factor NF-κB during these pathological conditions (11, 61, 71, 87). In addition, we recently showed that ANG II contributes to blood-brain barrier disruption within the PVN during hypertension, promoting leakage of circulating ANG II, which targets microglia within this nucleus (5). Moreover, a growing body of evidence implicates TLR4 signaling within the CNS in exacerbated sympathoexcitation and in the development of cardiovascular diseases. For instance, it has been shown that TLR4 activation, via AT1Rs within the brain stem, contributes to sympathoexcitation drive in heart failure (53), and, recently, Dange et al. (17) have shown that brain TLR4 blockade improves cardiac function in ANG II-induced hypertensive rats. Thus, our study, along with previous studies (17, 53), supports a mechanistic cross talk between the central renin-angiotensin system and the microglia-TLR4 signaling cascade in critical brain areas involved in cardiovascular control. Given the importance of both ANG II and TLR4 in hypertension and heart failure, it will be important to investigate in the future whether the ANG II-AT1aR-TLR4-ROS pathway described here is exacerbated in these prevalent cardiovascular disorders.
GRANTS
This work was supported by American Heart Association Scientific Development Grant 14SDG204000015 (to V. C. Biancardi) and National Heart, Lung, and Blood Institute Grant R01-HL-112225 (to J. E. Stern).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.C.B., A.M.S., E.G.K., A.D.d.K., and J.E.S. conception and design of research; V.C.B. and A.M.S. performed experiments; V.C.B. analyzed data; V.C.B. and J.E.S. interpreted results of experiments; V.C.B. prepared figures; V.C.B. drafted manuscript; V.C.B., A.M.S., and J.E.S. edited and revised manuscript; V.C.B., A.M.S., E.G.K., A.D.d.K., and J.E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Krishnan Dhandapani (Georgia Regents University) for the kind donation of part of the male TLR4-deficient C3H/HeJ and TLR4-sufficient C3H/OuJ mice used in this study.
REFERENCES
- 1.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-κB. J Immunol 172: 2522–2529, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36: 857–870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Topics Microbiol Immunol 270: 109–120, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Biancardi VC, Campos RR, Stern JE. Altered balance of γ-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol 518: 567–585, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 63: 572–579, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biancardi VC, Son SJ, Sonner PM, Zheng H, Patel KP, Stern JE. Contribution of central nervous system endothelial nitric oxide synthase to neurohumoral activation in heart failure rats. Hypertension 58: 454–463, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem 114: 13–27, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 46: 533–539, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Campos RR, Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT. The role of oxidative stress in renovascular hypertension. Clin Exp Pharmacol Physiol 38: 144–152, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J Neurosci 32: 4878–4886, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κB in the paraventricular nucleus. Hypertension 59: 113–122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Case AJ, Li S, Basu U, Tian J, Zimmerman MC. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305: H19–H28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115: 1599–1608, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Liu-Stratton Y, Hassanain H, Cool DR, Morris M. Dietary sodium regulates angiotensin AT1a and AT1b mRNA expression in mouse brain. Exp Neurol 188: 238–245, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque CW. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron 85: 549–560, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SH, Lee DY, Chung ES, Hong YB, Kim SU, Jin BK. Inhibition of thrombin-induced microglial activation and NADPH oxidase by minocycline protects dopaminergic neurons in the substantia nigra in vivo. J Neurochem 95: 1755–1765, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Dange RB, Agarwal D, Masson GS, Vila J, Wilson B, Nair A, Francis J. Central blockade of TLR4 improves cardiac function and attenuates myocardial inflammation in angiotensin II-induced hypertension. Cardiovasc Res 103: 17–27, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Dasu MR, Riosvelasco AC, Jialal I. Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis 202: 76–83, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci 33: 4825–4833, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De S, Zhou H, DeSantis D, Croniger CM, Li X, Stark GR. Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc Natl Acad Sci USA 112: 9680–9685, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dey A, Hao S, Erion JR, Wosiski-Kuhn M, Stranahan AM. Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J Neuroimmunol 269: 20–27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdos B, Broxson CS, King MA, Scarpace PJ, Tumer N. Acute pressor effect of central angiotensin II is mediated by NAD(P)H-oxidase-dependent production of superoxide in the hypothalamic cardiovascular regulatory nuclei. J Hypertens 24: 109–116, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 89: 370–376, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: Immunophenotypic and functional characteristics. J Neurosci Methods 151: 121–130, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Hall JE. Control of blood pressure by the renin-angiotensin-aldosterone system. Clin Cardiol 14: IV6–IV21; discussion IV52–IV55, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 61: 253–258, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang BS, Leenen FH. The brain renin-angiotensin-aldosterone system: a major mechanism for sympathetic hyperactivity and left ventricular remodeling and dysfunction after myocardial infarction. Curr Heart Fail Rep 6: 81–88, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Iddings JA, Kim KJ, Zhou Y, Higashimori H, Filosa JA. Enhanced parenchymal arteriole tone and astrocyte signaling protect neurovascular coupling mediated parenchymal arteriole vasodilation in the spontaneously hypertensive rat. J Cereb Blood Flow Metab 35: 1127–1136, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Y, Liu J, Wang Z, Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem 23: 265–276, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Johren O, Inagami T, Saavedra JM. AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. Neuroreport 6: 2549–2552, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Jordan J, Fernandez-Gomez FJ, Ramos M, Ikuta I, Aguirre N, Galindo MF. Minocycline and cytoprotection: shedding new light on a shadowy controversy. Curr Drug Deliv 4: 225–231, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Jun JY, Zubcevic J, Qi Y, Afzal A, Carvajal JM, Thinschmidt JS, Grant MB, Mocco J, Raizada MK. Brain-mediated dysregulation of the bone marrow activity in angiotensin II-induced hypertension. Hypertension 60: 1316–1323, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-κB activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 82: 503–512, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim-Mitsuyama S, Yamamoto E, Tanaka T, Zhan Y, Izumi Y, Izumiya Y, Ioroi T, Wanibuchi H, Iwao H. Critical role of angiotensin II in excess salt-induced brain oxidative stress of stroke-prone spontaneously hypertensive rats. Stroke 36: 1083–1088, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 109: 2357–2362, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L. Angiotensin II sustains brain inflammation in mice via TGF-β. J Clin Invest 120: 2782–2794, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leenen FH. Actions of circulating angiotensin II and aldosterone in the brain contributing to hypertension. Am J Hypertens 27: 1024–1032, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA 100: 8514–8519, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenkei Z, Corvol P, Llorens-Cortes C. The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Brain Res Mol Brain Res 30: 53–60, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Leow-Dyke S, Allen C, Denes A, Nilsson O, Maysami S, Bowie AG, Rothwell NJ, Pinteaux E. Neuronal toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J Neuroinflamm 9: 230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li JJ, Lu J, Kaur C, Sivakumar V, Wu CY, Ling EA. Expression of angiotensin II and its receptors in the normal and hypoxic amoeboid microglial cells and murine BV-2 cells. Neuroscience 158: 1488–1499, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Qi X, Tong X, Wang S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol Immunol 10: 506–512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res 94: 402–409, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 42: 145–151, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Mangiapane ML, Simpson JB. Subfornical organ lesions reduce the pressor effect of systemic angiotensin II. Neuroendocrinology 31: 380–384, 1980. [DOI] [PubMed] [Google Scholar]
- 46.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29: 367–374, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Masson GS, Nair AR, Dange RB, Silva-Soares PP, Michelini LC, Francis J. Toll-like receptor 4 promotes autonomic dysfunction, inflammation and microglia activation in the hypothalamic paraventricular nucleus: role of endoplasmic reticulum stress. PLos One 10: e0122850, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003. [DOI] [PubMed] [Google Scholar]
- 49.McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl 3: S99–S104, 1996. [PubMed] [Google Scholar]
- 50.Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci USA 81: 1575–1579, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29: 359–370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T. Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor κB and activator protein-1 activation. Eur J Neurosci 27: 343–351, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Ogawa K, Hirooka Y, Kishi T, Sunagawa K. Brain AT1 receptor activates the sympathetic nervous system through toll-like receptor 4 in mice with heart failure. J Cardiovasc Pharmacol 58: 543–549, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173: 3916–3924, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol 303: R291–R300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Premer C, Lamondin C, Mitzey A, Speth RC, Brownfield MS. Immunohistochemical localization of AT1a, AT1b, and AT2 angiotensin II receptor subtypes in the rat adrenal, pituitary, and brain with a perspective commentary. Int J Hypertens 2013: 175428, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pyner S. The paraventricular nucleus and heart failure. Exp Physiol 99: 332–339, 2014. [DOI] [PubMed] [Google Scholar]
- 60.Qi J, Zhang DM, Suo YP, Song XA, Yu XJ, Elks C, Lin YX, Xu YY, Zang WJ, Zhu Z, Kang YM. Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol 13: 48–54, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Rana I, Stebbing M, Kompa A, Kelly DJ, Krum H, Badoer E. Microglia activation in the hypothalamic PVN following myocardial infarction. Brain Res 1326: 96–104, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Reis WL, Biancardi VC, Son S, Antunes-Rodrigues J, Stern JE. Carbon monoxide and nitric oxide interactions in magnocellular neurosecretory neurones during water deprivation. J Neuroendocrinol 27: 111–122, 2015. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez-Pallares J, Rey P, Parga JA, Munoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis 31: 58–73, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 9: 1081–1088, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Ruiz-Ortega M, Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, Carvajal G, Egido J. Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens 15: 159–166, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl: S12–S22, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Ryan KA, Smith MF Jr, Sanders MK, Ernst PB. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-κB and interleukin-8 expression. Infect Immun 72: 2123–2130, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11: 775–787, 2011. [DOI] [PubMed] [Google Scholar]
- 69.Saito Y, Berk BC. Transactivation: a novel signaling pathway from angiotensin II to tyrosine kinase receptors. J Mol Cell Cardiol 33: 3–7, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys 353: 312–321, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension 56: 297–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron 78: 1036–1049, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct 2011: 792639, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, Yang Q, Miao YW, Yu XJ, Qi J, Zhu Z, Zhu GQ, Kang YM. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol 276: 115–120, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res 96: 659–666, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Takeda K, Akira S. Roles of Toll-like receptors in innate immune responses. Genes Cells 6: 733–742, 2001. [DOI] [PubMed] [Google Scholar]
- 77.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21: 2580–2588, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 139: 191–202, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem 20: 947–956, 2007. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Seto SW, Golledge J. Angiotensin II, sympathetic nerve activity and chronic heart failure. Heart Fail Rev 19: 187–198, 2014. [DOI] [PubMed] [Google Scholar]
- 81.Wenceslau CF, Davel AP, Xavier FE, Rossoni LV. Long-term ouabain treatment impairs vascular function in resistance arteries. J Vasc Res 48: 316–326, 2011. [DOI] [PubMed] [Google Scholar]
- 82.Wolf G, Bohlender J, Bondeva T, Roger T, Thaiss F, Wenzel UO. Angiotensin II upregulates toll-like receptor 4 on mesangial cells. J Am Soc Nephrol 17: 1585–1593, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Wu CY, Zha H, Xia QQ, Yuan Y, Liang XY, Li JH, Guo ZY, Li JJ. Expression of angiotensin II and its receptors in activated microglia in experimentally induced cerebral ischemia in the adult rats. Mol Cell Biochem 382: 47–58, 2013. [DOI] [PubMed] [Google Scholar]
- 84.Wu J, Yang X, Zhang YF, Zhou SF, Zhang R, Dong XQ, Fan JJ, Liu M, Yu XQ. Angiotensin II upregulates Toll-like receptor 4 and enhances lipopolysaccharide-induced CD40 expression in rat peritoneal mesothelial cells. Inflamm Res 58: 473–482, 2009. [DOI] [PubMed] [Google Scholar]
- 85.Yin JX, Yang RF, Li S, Renshaw AO, Li YL, Schultz HD, Zimmerman MC. Mitochondria-produced superoxide mediates angiotensin II-induced inhibition of neuronal potassium current. Am J Physiol Cell Physiol 298: C857–C865, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA 96: 13496–13500, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu XJ, Suo YP, Qi J, Yang Q, Li HH, Zhang DM, Yi QY, Zhang J, Zhu GQ, Zhu Z, Kang YM. Interaction between AT1 receptor and NF-κB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovasc Toxicol 13: 381–390, 2013. [DOI] [PubMed] [Google Scholar]
- 88.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol 283: H423–H433, 2002. [DOI] [PubMed] [Google Scholar]
- 89.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008. [DOI] [PubMed] [Google Scholar]
- 90.Zhou J, Ando H, Macova M, Dou JT, Saavedra JM. Angiotensin II AT1 receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J Cereb Blood Flow Metab 25: 878–886, 2005. [DOI] [PubMed] [Google Scholar]
- 91.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002. [DOI] [PubMed] [Google Scholar]
- 92.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004. [DOI] [PubMed] [Google Scholar]