Using intravital microscopy, we demonstrate that a brief exposure of the resistance vasculature to vasoconstrictors initiates inward remodeling processes, in vivo, via mechanism(s) that include the activation of transglutaminases and the reorganization of the actin cytoskeleton. This is the first study to examine the process in vivo, in real time.
Keywords: inward remodeling, cytoskeleton, hypertension, vasospasm, vasoconstriction, nitric oxide
Abstract
Inward remodeling of the resistance vasculature is strongly associated with life-threatening cardiovascular events. Previous studies have demonstrated that both actin polymerization and the activation of transglutaminases mediate early stages of the transition from a structurally normal vessel to an inwardly remodeled one. Ex vivo studies further suggest that a few hours of exposure to vasoconstrictor agonists induces inward remodeling in the absence of changes in intraluminal pressure. Here we report that a short, 10-min, topical exposure to serotonin (5-HT) + Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) was sufficient to initiate inward remodeling processes in rat cremasteric feed arterioles (100–200 μm lumen diameter), in vivo. Addition of the transglutaminase inhibitor, cystamine, blocked the in vivo remodeling. We further demonstrate that, in isolated arterioles, 5-HT + l-NAME activates transglutaminases and modulates the phosphorylation state of cofilin, a regulator of actin depolymerization. The 5-HT + l-NAME-induced remodeling process in isolated arterioles was also inhibited by an inhibitor of Lim Kinase, the kinase that phosphorylates and inactivates cofilin. Therefore, our results indicate that a brief vasoconstriction induced by 5-HT + l-NAME is able to reduce the passive structural diameter of arterioles through processes that are dependent on the activation of transglutaminases and Lim kinase, and the subsequent phosphorylation of cofilin.
NEW & NOTEWORTHY
Using intravital microscopy, we demonstrate that a brief exposure of the resistance vasculature to vasoconstrictors initiates inward remodeling processes, in vivo, via mechanism(s) that include the activation of transglutaminases and the reorganization of the actin cytoskeleton. This is the first study to examine the process in vivo, in real time.
inward remodeling of the resistance vasculature is a hallmark feature of a number of cardiovascular diseases including hypertension (31). The marked increase in the risk for life-threatening cardiovascular events associated with inward remodeling (6, 34, 42) highlights its clinical importance. In addition, inward eutrophic remodeling of the resistance vasculature is believed to play an important role in increasing peripheral vascular resistance and decreasing tissue blood flow during the development and maintenance of essential hypertension (15). Inward remodeling is defined as a decrease in the luminal diameter of blood vessels under passive conditions (38). In essential hypertension, this remodeling is eutrophic; that is, there is no significant change in the cross-sectional area of the vascular wall, which indicates there is no change in the amount of wall material. As for the etiology of the remodeling process, substantial evidence in isolated vessels suggests that prolonged agonist-induced vasoconstriction and not the presence of elevated intravascular pressure is the main stimulus that induces inward remodeling. In accordance with these observations, clinical evidence has shown that pharmacological intervention to ameliorate hypertension also reverses inward remodeling when treatments promote vasodilation, in contrast with interventions that only reduce blood pressure (which do not reverse remodeling), such as those that decrease cardiac output (9, 33). Therefore, it is of particular clinical interest to decipher the biochemical/physiological mechanism(s) that are activated in response to vasoconstriction and initiate the transition from a structurally normal resistance vasculature to an inwardly remodeled one to find new avenues to prevent, treat, or reverse the remodeling process and reduce its life-threatening cardiovascular consequences.
In isolated vessels ex vivo, it has been demonstrated that prolonged (4 h+) exposure of resistance arteries to vasoconstrictors initiates the inward remodeling process via pathways that include both the polymerization of actin and the activation of transglutaminases, specifically transglutaminase 2 (5, 32, 45). In vivo studies investigating the role of the actin cytoskeleton on inward remodeling within the resistance vasculature are limited. In comparison, a number of in vivo studies have shown a role for transglutaminases in resistance vessel remodeling in response to changes in flow (1, 3); in response to a 1-wk infusion of the nitric oxide (NO) synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) (41); and in response to a 1-wk infusion of phenylephrine (13). An important limitation in the majority of these in vivo studies is that the experimental designs did not allow for differentiation between the effects produced by blood pressure and vasoconstriction on the development of inward remodeling. Nevertheless, the cumulative ex vivo and in vivo evidence suggests that both intracellular changes in the cytoskeleton and modifications of the extracellular matrix (ECM) are associated with transglutaminase activity and represent key early events in the process that leads to inward eutrophic remodeling of the resistance vasculature.
To date, much of our insight into the mechanism(s) that initiate and control the inward remodeling process in resistance vessels comes from both in vitro and ex vivo studies performed on cultured cells and isolated vessels. Although mechanistically informative, these approaches lack the natural dynamics of intact tissues functioning within the body as a whole. The in vivo data are primarily derived from experimental protocols in which animals are exposed to pharmacological agents/vasoactive stimuli, and following a prolonged period of treatment, resistance vessels are excised and analyzed for structural and functional changes. The duration of these treatment protocols extends from days to weeks, thus at the time of tissue isolation, the derived structural/functional data represent but a single snapshot in the progression of vascular structural changes. Moreover, it has been demonstrated ex vivo that remodeling can occur over a relatively short span of time, i.e., over a span of hours. Currently available in vivo data are, therefore, inherently limited in defining the transitional mechanism(s) that occur during onset of the remodeling process.
To more thoroughly investigate the temporal aspects of the early remodeling process as well as verify the applicability of ex vivo studies on the mechanisms of inward remodeling of the resistance vasculature to an in vivo setting, we used an intravital microscopy approach. With the use of this technique, vasoactive agents were applied topically and locally to intact exposed tissues in the absence of systemic changes in blood pressure. We hypothesized that in vivo topical application of vasoconstrictor agonists for a short duration would initiate inward remodeling processes in resistance vessels, and that inward remodeling would be dependent on tranglutaminase activity and the activation of actin polymerization pathways.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats ≈200 g (Jackson Laboratories, Bar Harbor, ME) were used in all experiments. The Animal Care Quality Assurance office and the Animal Care and Use Committee at the University of Missouri-Columbia approved all animal protocols and procedures used during these studies. Before experimentation, rats were housed in pairs under a 12-h per day light/dark cycle and provided with ad libitum access to standard rat chow and water.
Intravital microscopy.
Rats were initially anesthetized with 5% isoflurane (Vet One, Boise, ID). Mean blood pressure was assessed via catheterization of the left femoral artery and maintained constant in each animal by modulation of percent isoflurane inhalation by using an anesthesia machine (Mouse Anesthesia Suite; Kent Scientific, Torrington, CT). Overall mean arterial pressure was 86 ± 0.6 mmHg. Minute volume respiration was delivered at a constant 230 ml. The left cremaster muscle in anesthetized rats was exteriorized and prepared as previously described (20, 21), with minor modifications. Briefly, an incision was made through the skin to expose the muscle, and the surrounding connective tissue was gently pulled from the muscle. A cautery (Geiger Model 150; Geiger Medical Technologies, Council Bluffs, IA) was used to open the cremaster longitudinally along its ventral surface, from the apex to the inguinal canal. The deferential arteriole and venule were then gently separated from the cremaster using the cautery, and care was taken to minimize bleeding. The testis and epididymis were moved into the abdominal cavity. The cremaster muscle was gently stretched radially, across a flat pedestal, and pinned at its edges. Grease (Dow Corning High Vacuum Grease; Dow Corning, Midland, MI) was added to encircle the cremaster and form a dam to facilitate continual perfusion of the tissue with a Krebs buffer containing (in mM) 111 NaCl, 25.7 NaHCO3, 4.9 KCl, 2.1 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 11.5 glucose, 10 HEPES (pH = 7.4 at 34°C) equilibrated with 5% CO2-95% N2. Tubing, carrying the perfusate, was fed through an aluminum block, heated with a ribbon heater connected to an Omega Temperature Controller (Omega, Stamford, CT) to maintain a cremasteric temperature of 34°C. Perfusion flow rate was 2 ml/min. All treatments were performed in this buffer. Rat body temperature was maintained at 37°C by using an in-house built heated platform controlled by an Omega Temperature Controller. The cremasteric first order arteriole (1A) was imaged using a Zeiss ACM microscope with a 10× objective connected to a Hitachi video feed (Model KP-208U). A videocaliper (Colorado Video, Boulder, CO) was used to determine vessel diameter.
The following experimental protocol was used to determine the duration and magnitude of vasoconstriction observed in response to different vasoactive agonists and to assess remodeling over the course of the experiment. An initial 10-ml bolus of 10−4 M adenosine + 10−4 M papaverine + 10−5 M sodium nitroprusside (SNP) was added to the perfusate to determine maximum passive diameter. This was washed away with a 10-min perfusion in Krebs buffer, followed by a 4-h perfusion with vasoconstrictors and a subsequent 10-min Krebs wash. Then, a 10-ml bolus of 10−4 M adenosine + 10−4 M papaverine + 10−5 M SNP was administered to reassess maximal passive diameter and remodeling for 10 min. Vessels that did not constrict to at least 65% of their maximum passive diameter after exposure to the vasoconstrictor agonists were excluded from the study.
The same protocol was used to determine whether the vasoconstrictors, capable of inducing inward remodeling after 4 h, were able to induce remodeling after only 10 min of exposure. In this acute exposure protocol, the final determination of passive diameter following the Krebs wash to remove the vasoconstrictors consisted of a continuous perfusion of 10−4 M adenosine + 10−4 M papaverine + 10−5 M SNP for 30 min. Maximal passive diameter in this vasodilator cocktail was continuously monitored, and remodeling was determined at 10 and 30 min into the vasodilator cocktail perfusion. To determine the role of transglutaminase activity on the remodeling process, a series of experiments was performed with 10−3 M cystamine (transglutaminase inhibitor) added to all solutions. For comparisons of treatment regimes, vessel diameters were normalized to maximum pretreatment passive diameter. Percent remodeling was calculated using the following equation: 1 − (post-treatment passive diameter/pretreatment passive diameter) × 100%. Percent constriction was calculated as follows: 1 − (smallest diameter in vasoconstrictor/ pretreatment passive diameter) × 100%.
Vessel isolation for ex vivo experiments.
Rats were anesthetized with an intraperitoneal injection (100 mg/kg) of pentobarbital sodium. After the absence of spinal reflexes was confirmed, cremaster muscles were excised and placed in 4°C physiological saline solution (PSS) containing (in mM) 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.0 MgSO4, 1.2 NaH2PO4, 0.02 EDTA, 2.0 pyruvic acid, 5.0 glucose, and 3.0 MOPS, pH = 7.4. The 1A feed arterioles from each cremaster were isolated, cannulated, and pressurized as previously described (29). Briefly, arteriolar segments of ∼1 mm in length were cannulated onto glass micropipettes mounted in an observation chamber (Living Systems Instrumentation, Burlington, VT) and filled with PSS. The arterioles were pressurized without flow to 60 mmHg using a Pressure Servo System (Living Systems Instrumentation) and PSS containing 0.15 mM bovine serum albumin. The observation chamber containing the cannulated vessel was transferred to an inverted microscope equipped with a video display and video caliper system (Living Systems Instrumentation) to record measurements of wall thickness and luminal diameter. All experiments were performed at 34.5°C. Percent remodeling was calculated as in the intravital experiments.
Assessing transglutaminase activation via fluorescent cadaverine transamidation.
To determine relative transglutaminase activity, isolated arteriolar sections were incubated with the transglutaminase substrate Alexa Fluor 488 cadaverine (Invitrogen A30676), as previously described (7), with slight modifications. Briefly, rat cemasteric 1A arterioles were isolated and dissected in PSS at 4°C. Vessels were then transferred to tubes containing PSS + 10−5 M cadaverine and incubated overnight at 4°C to ensure that fluorescently labeled cadaverine was able to enter the cells, since we were particularly interested in the intracellular transamidating activity of transgutaminase. The next day, vessels were warmed to 34°C and incubated in one of the following treatments: PSS + 10−5 M cadaverine (Control), PSS + 10−4 M serotonin (5-HT) + 10−4 M l-NAME + 10−5 M cadaverine (5-HT + l-NAME), PSS + 10−4 M 5-HT + 10−4 M l-NAME + 10−3 M cystamine + 10−5 M cadaverine (5-HT + l-NAME + cystamine) for 10–30 min at 34°C. All subsequent steps were performed at 4°C. Vessels were washed twice in phosphate buffered saline (PBS) to remove unbound fluorescently labeled cadaverine and then fixed for 1 h in 4% paraformaldehyde. Vessels were washed twice in PBS and then incubated for 1 h in 2 μM 4M,6-diamidino-2-phenylindole (DAPI) in PBS + 10% BSA to stain nuclei. Vessels were washed 2× in PBS and subsequently imaged using a Leica SPE confocal microscope with a 63× water objective. Cadaverine was excited with a laser tuned to emit at 488 nm. DAPI was excited at 405 nm. Images were processed and quantitated using Imaris software. For quantification, a region of interest (ROI ≈ 50 μm × 50 μm × 10 μm) was randomly selected to contain predominantly vascular smooth muscle cells. Two ROIs per vessel were assessed. The mean fluorescence intensity - background per μm3 was determined for the ROI and averaged for each vessel. For publication, all three representative images were equally adjusted for brightness/contrast.
Determining phosphorylated-cofilin/total-cofilin ratio.
Freshly isolated 1A arterioles were transferred to Krebs and warmed to 34°C for 30 min. Vessels were then incubated for 10 min at 34°C in Krebs with vehicles (Controls), 5-HT + l-NAME, 5-HT + l-NAME + cystamine, or 5-HT + l-NAME + Lim Kinase inhibitor (LimKi). Following treatment, vessels were immediately frozen to −20°C, and cell lysates obtained via dounce homogenization in 50 mM PIPES, pH 6.9; 50 mM NaCl; 5 mM MgCl2; 5 mM EGTA; 5% (vol/vol) glycerol; 0.1% Nonidet P-40; 0.1% Triton X-100; 0.1% Tween 20; 0.1% 2-mercapto-ethanol; 0.001% AntifoamC; + 100 μM ATP + HALT Protease Inhibitors. For treatments, Control, 5-HT + l-NAME, and 5-HT + l-NAME + cystamine, samples were centrifuged at 100,000 g for 1 h to separate all membranes from cytosolic components. Because we did not see any evidence for cofilin partitioning/localization in membrane fractions (data not shown), subsequent samples (Controls and 5-HT + l-NAME + LimKi) were centrifuged at 15,000 g for 10 min to remove cellular debris. Supernatant from a single treated vessel was loaded into individual lanes on a 10% gel, separated by electrophoresis, and then transferred to nitrocellulose membranes. Blots were probed with 1/500 dilution of phosphorylated-cofilin (P-cofilin; Sigma) antibody and imaged with a ChemiDOC XRS+ (Bio-Rad). Bands were quantified and the blots stripped and reprobed with 1/500 dilution total-cofilin antibody. The P-cofilin/total-cofilin ratio was determined and normalized to controls.
Determining F- to G-actin ratios in cultured vascular smooth muscle cells.
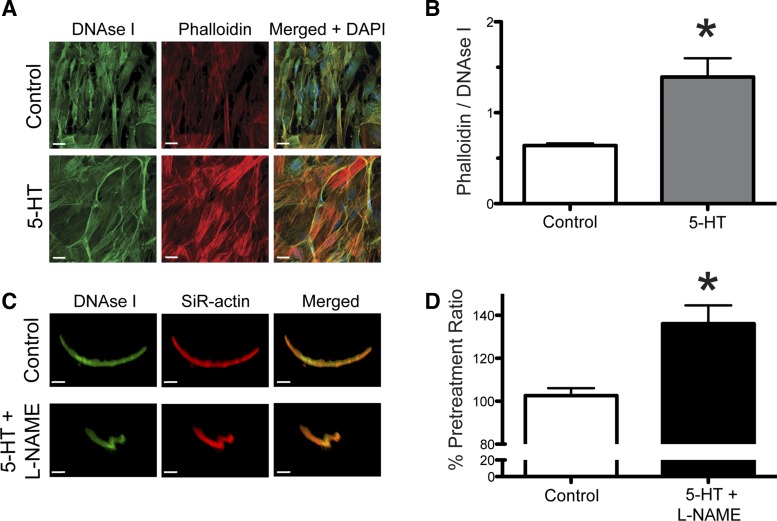
Rat cremasteric vascular smooth muscle cells, passage 3, were grown to confluence on cell culture plates. Growth media (DMEM media + 10% fetal bovine serum) was removed, and the cells were treated for 10 min with DMEM ± 10−4 M 5-HT. Cells were immediately fixed in 4% paraformaldehyde for 10 min at room temperature. The fixative was quenched with 0.1 M glycine in PBS, followed by 2 washes in PBS. Cells were permeabilized for 1 h in 0.1% Triton X-100 + 1.0% BSA in PBS at 4°C. Cells were blocked in 10% goat serum + 0.05% Triton X-100 + 1.0% BSA in PBS. Cells were incubated overnight at 4°C with 300 nM Alexa Fluor 488 Deoxyribonuclease (DNAse) I (D12371; Life Technologies) to stain G-actin + 150 nM Alexa 568 Phalloidin (A12380; Life Technologies) to stain F-actin in 2% goat serum + 0.05% Triton X-100 + 1.0% BSA in PBS. Before imaging, cells were coated with Prolong Diamond antifade mountant + DAPI (P36962; Life technologies). A Leica SPE confocal microscope with a 63× water objective was used to acquire images. Laser excitation wavelengths used were 405 nm (DAPI), 488 nm (Alexa Fluor 488 DNAse I), and 561nm (Alexa Fluor 568 Phalloidin). Acquired images were analyzed with Imaris software to determine the mean intensity of Alexa Fluor 568 Phalloidin and Alexa Fluor 488 DNAse I in a region of interest (344 μm × 344 μm × 7 μm). Data are expressed as mean intensity of Phalloidin/mean intensity of DNAse I.
Determining F- to G-actin ratios in isolated vessels.
Freshly isolated 1A arterioles were incubated overnight at 4°C in a Ca2+-free PSS solution containing 10−4 M adenosine + 10−6 M verapamil + 100 nM of a silicon rhodamine conjugated jasplakinolide fluorophore (SiR-actin) (SC001; Cytoskeleton) to stain F-actin. The following day, DNAse I was added at a final concentration of 300 nM and incubated an additional 2 h at 4°C to stain G-actin. Vessels were cannulated and mounted in PSS, pressurized to 70 mmHg and warmed to 37°C until myogenic tone was established; the PSS buffer was replaced every 15 min. Vessels were imaged with a Leica SP-5 confocal microscope with a 63× water objective. Laser excitation wavelengths used were 488 nm (Alexa Fluor 488 DNAse I) and 633 nm (SiR-actin) and acquired simultaneously. Costained vascular smooth muscle cells within the vessel (≈1% of cells costained with both dyes) were identified and imaged to obtain a baseline SIR-actin/DNAse I ratio (pretreatment). The buffer was replaced with PSS, and following a 10-min incubation, the same cell was reimaged (Control). The PSS was replaced with PSS + 10−4 M 5-HT + 10−4 M l-NAME, and following a 10-min incubation the same cell was again imaged (5-HT + l-NAME). Acquired images were analyzed with Imaris software to define an ROI encompassing a single costained cell and determine the mean intensity of Alexa Fluor 488 DNAse I and 633 SiR-actin. This was achieved by applying a threshold to the 633 channel and masking all values that fell below threshold intensity. The same threshold was used for all three cell images (pretreatment, Control, and 5-HT + l-NAME) to define the ROI. The SiR-actin/DNAse I ratios following treatment were normalized to the pretreatment ratio. Ratios are expressed as percentage of pretreatment ratio.
Chemicals.
All chemicals and drugs used in this study were purchased from Sigma (St. Louis, MO), except for LimKi, which was acquired from EMD Millipore (EMD Millipore, San Diego, CA), and SiR-actin, which was purchased from Cytoskeleton (Denver, CO). A stock solution of LimKi was made in DMSO at a concentration of 20 mM and diluted in the buffer solution used as superfusate (i.e., PSS or Ca2+-free PSS). A concentration-response curve was assessed to determine a concentration (10−6 M) that did not affect constriction to 10−4 M 5-HT + 10−4 M l-NAME (data not shown). The final concentrations reported refer to final concentrations in the superfusate. SiR-actin was dissolved in DMSO to generate a 1-mM stock solution. All other drugs were prepared the day of the experiment and diluted into the appropriate buffer at the indicated concentrations. Antibodies for P-cofilin (c8736) and total-cofilin (c8992) were from Sigma. Alexa Fluor 488 cadaverine was from Invitrogen (Life Technologies, Grand Island, NY).
Data analyses.
Data are presented as mean values of multiple experiments ± SE (the number of experiments, n, is reported for each experimental series in the figure legends). One-way ANOVA followed by Fisher's protected least significant difference as well as paired and unpaired t-tests were used to make statistical comparisons between means. Differences were considered significant at values of P ≤ 0.05.
RESULTS
Topical exposure to 5-HT + l-NAME for 4 h maintains vasoconstriction and induces arteriolar inward remodeling in vivo.
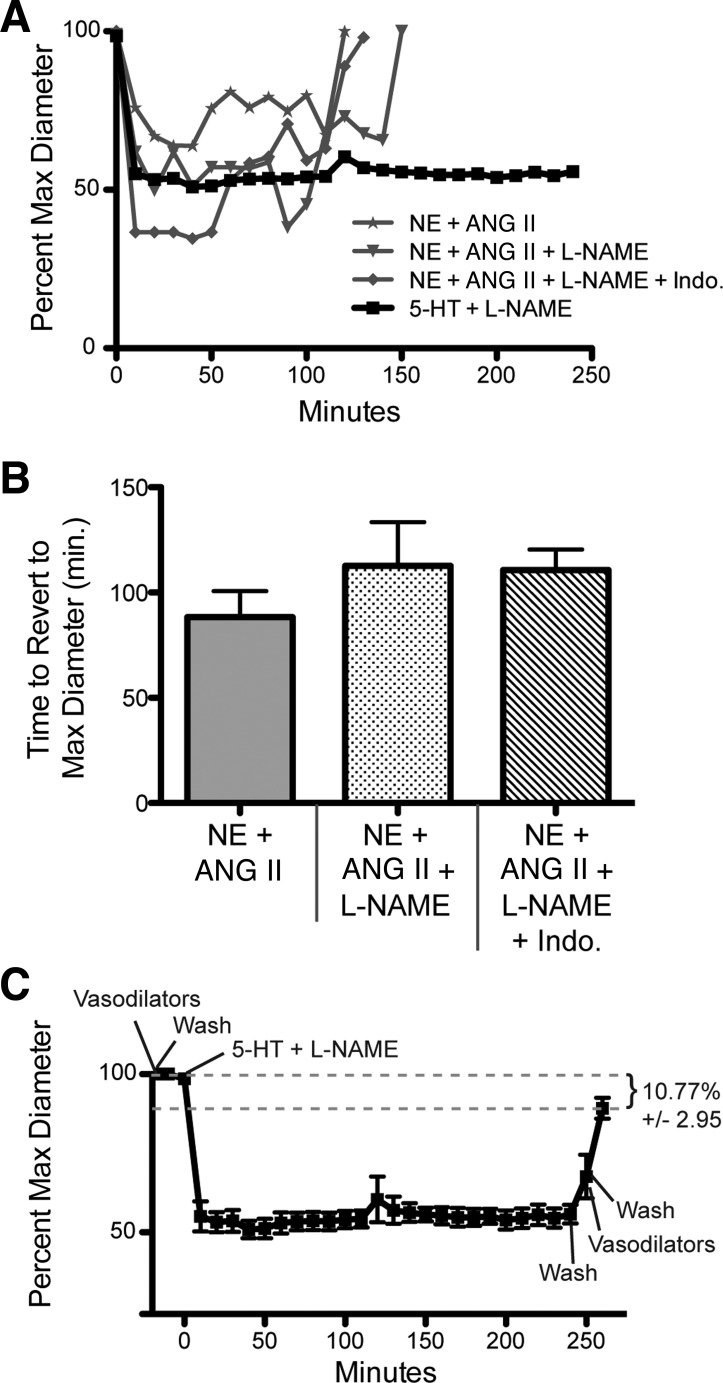
Topical application of 10−5.5 M norepinephrine (NE) + 10−7 M Ang II for 4 h to exposed cremasters in vivo was unable to maintain vasoconstriction in 1A arterioles over the course of exposure to the agonists. After an initial constriction of 44 ± 7%, vessels dilated back to original diameters in the presence of these vasoconstrictor agonists. To determine whether vessels failed to maintain constriction due to vasodilation induced by NO or prostaglandins, vessels were incubated with the vasoconstrictor agonists in the presence of l-NAME (10−4 M) to inhibit NOS or indomethacin (5 × 10−5 M) to inhibit cyclooxygenase activity. Inhibition of NOS or prostaglandin production increased vasoconstriction to 55 ± 6% and 65 ± 2%, respectively, but did not result in sustained constriction for the full 4-h exposure to the agonists. In contrast, exposure to 10−4 M 5-HT + 10−4 M l-NAME caused a 50 ± 4% vasoconstriction in 1A arterioles that was consistently maintained for 4 h (Fig. 1A) and caused vessels to remodel inwardly. The mean time for vessels to return to their passive diameter in the presence of 10−5.5 M NE + 10−7 M Ang II, 10−5.5 M NE + 10−7 M Ang II + 10−4 M l-NAME, or 10−5.5 M NE + 10−7 M Ang II + 10−4 M l-NAME + 5 × 10−5 M indomethacin was 88 min, 113 min, and 111 min, respectively (Fig. 1B). Vessels exposed to 10−4 M 5-HT + 10−4 M l-NAME for 4 h inwardly remodeled, with a reduced passive diameter of 10.7 ± 2.95% (Fig. 1C).
Fig. 1.
Topical exposure to 5-HT + Nω-nitro-l-arginine methyl ester (l-NAME) maintains arteriolar constriction for 4 h and induces inward remodeling in vivo. A: normalized diameter of 1A cremaster feed arterioles (expressed as percent maximum passive diameter) in response to a 4-h exposure to the indicated vasoconstrictors. Data are expressed as means. B: mean time for arterioles exposed to 10−5.5 M norepinephrine (NE) + 10−7 M Ang II, 10−5.5 M NE + 10−7 M Ang II + 10−4 M l-NAME, or 10−5.5 M NE + 10−7 M Ang II + 5 × 10−5 M indomethacin (Indo) to return to maximum passive diameter while in the presence of the indicated vasoconstrictors. Data are expressed as means ± SE. C: normalized diameter of 1A cremaster feed arterioles (expressed as percent maximum passive diameter) in response to a 4-h exposure to 5-HT + l-NAME. Data are expressed as means ± SE. Remodeling is expressed as a percent reduction from the maximal passive diameter obtained before exposure to the vasoconstrictor agonists ± SE. For A–C, n = 4 for NE + Ang II, n = 3 for NE + Ang II + l-NAME and NE + Ang II + l-NAME + indomethacin, and n = 5 for 5-HT + l-NAME. Max, maximum; min, minutes.
Topical exposure to 5-HT + l-NAME for 10 min induces arteriolar inward remodeling in vivo.
To determine whether a brief exposure to 5-HT + l-NAME was also able to reduce the passive diameter of arterioles, we exposed the cremaster muscle of rats in vivo to 10−4 M 5-HT + 10−4 M l-NAME for 10 min and subsequently measured the maximal passive diameter of arterioles for up to 30 min after removal of the vasoconstrictor. Topical application of 5-HT + l-NAME for 10 min caused a significant reduction in the maximal passive diameter of 1A arterioles as measured after up to 30 min of exposure to 10−4 M adenosine + 10−4 M papaverine + 10−5 M SNP. The reduction in passive diameter was prevented by the presence of 10−3 M cystamine, a broad inhibitor of transglutaminase activity. Moreover, the inward remodeling was specific to 5-HT, since exposure to 10−5.5 M NE + 10−7 M Ang II + 10−4 M l-NAME for 10 min did not reduce the passive diameter of arterioles (Fig. 2A). Vessels exposed to 10−4 M 5-HT + 10−4 M l-NAME had an 11.2 ± 1.9% reduction in passive diameter after 10 min in the vasodilators (Fig. 2C) and a 6.5 ± 1.1% reduction after 30 min under passive conditions (Fig. 2D). The degree of constriction was not significantly different between 10−4 M 5-HT + 10−4 M l-NAME and 10−4 M 5-HT + 10−4 M l-NAME + 10−3 M cystamine. Also, 10−5.5 M NE + 10−7 M Ang II + 10−4 M l-NAME significantly constricted vessels to a greater degree than the other two treatment regimens (Fig. 2B).
Fig. 2.
Topical exposure to 5-HT + l-NAME for 10 min induces arteriolar inward remodeling in vivo. A: normalized diameter of 1A cremaster feed arterioles (expressed as percent maximum passive diameter) in response to a 10-min exposure to control conditions, 10−5.5 M NE + 10−7 M Ang II, 10−4 M 5-HT + 10−4 M l-NAME, or 10−4 M 5-HT + 10−4 M l-NAME + 10−3 M cystamine (Cyst). Data are expressed as means ± SE; n = 6 for all treatments. B: percent mean constriction at smallest diameter in response to treatment regimes. Data are expressed as means ± SE. *P ≤ 0.05 vs. Control; **P ≤ 0.05 vs. 5-HT + l-NAME ± cystamine. C: percent remodeling expressed as percent reduction of the maximal passive diameter obtained before exposure to the vasoconstrictors as measured at 45 min after the start of the experiment (t = 45). Data are expressed as means ± SE. *P ≤ 0.05 vs. Control. D: percent remodeling expressed as percent reduction of the maximal passive diameter obtained before exposure to the vasoconstrictors as measured at 65 min after the start of the experiment (t = 65). Data are expressed as means ± SE. *P ≤ 0.05 vs. Control.
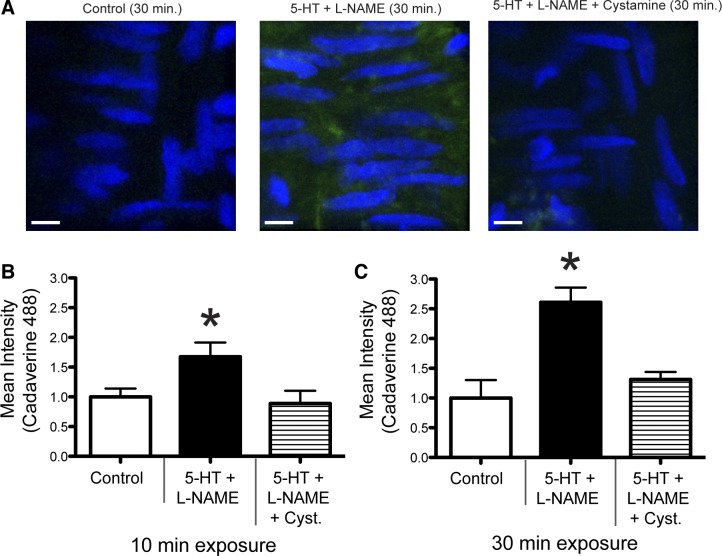
Exposure to 5-HT + l-NAME induces transglutaminase activation in isolated vessels.
To confirm that exposure to 5-HT + l-NAME induced transglutaminase activation, isolated cremasteric 1A arterioles were exposed to these compounds in the presence of fluorescently labeled cadaverine (Fig. 3A). Exposure of vessels to 5-HT + l-NAME for 10 (Fig. 3B) or 30 (Fig. 3C) min caused a significant incorporation of cadaverine compared with vessels not exposed to the vasoconstrictors. To confirm that the incorporation of cadaverine was transglutaminase specific, the transglutaminase inhibitor cystamine was added to the treatment protocol. Vessels exposed to 10−4 M 5-HT + 10−4 M l-NAME + 10−3 M cystamine had significantly reduced levels of cadaverine incorporation compared with vessels treated with 5-HT + l-NAME, similar to control vessels (Fig. 3, B and C).
Fig. 3.
Exposure of isolated arterioles to 5-HT + l-NAME increases transglutaminase activity. A: incorporation of fluorescent cadaverine into isolated arterioles following a 30-min incubation in control conditions, 10−4 M 5-HT + 10−4 M l-NAME, or 10−4 M 5-HT + 10−4 M l-NAME + 10−3 M cystamine. Blue, nuclei [4M,6-diamidino-2-phenylindole (DAPI)]; green, Alexa Fluor 488-cadaverine. Scale bar = 5 μm. B and C: incorporation of fluorescent cadaverine into isolated arterioles expressed as mean fluorescence intensity following a 10- (B) or 30- (C) min incubation in control conditions, 10−4 M 5-HT + 10−4 M l-NAME, or 10−4 M 5-HT + 10−4 M l-NAME + 10−3 M cystamine. Data are means ± SE (n = 4 for all treatments). *P ≤ 0.05 vs. Control.
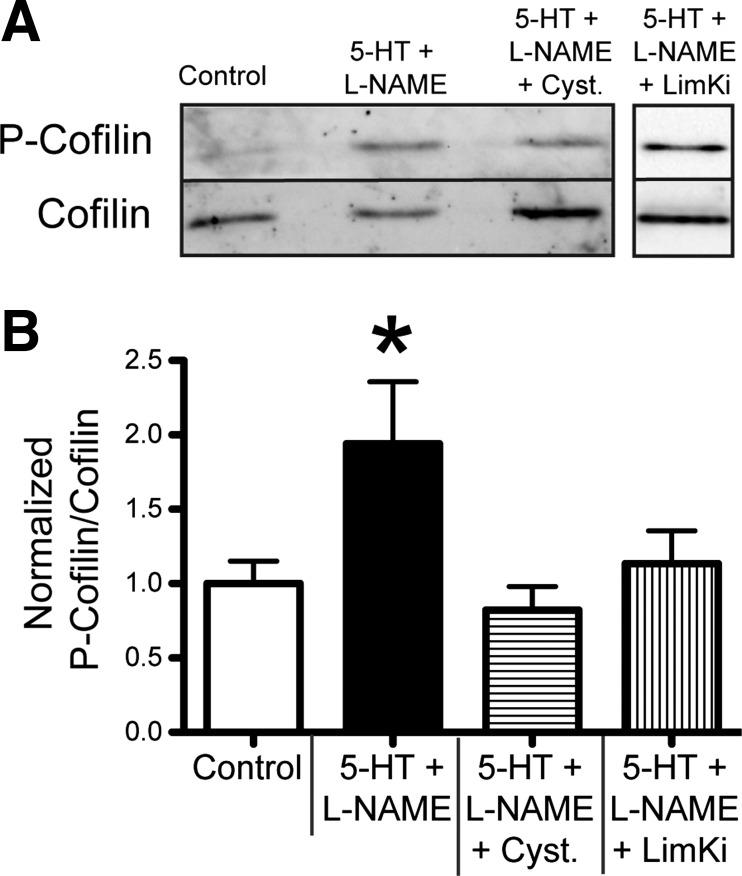
Exposure to 5-HT + l-NAME increases cofilin phosphorylation in isolated vessels.
To determine the effects of 5-HT + l-NAME on the activation of cofilin, we measured P-cofilin and total-cofilin in isolated 1A cremaster arterioles after 10 min of exposure to 10−4 M 5-HT + 10−4 M l-NAME. The normalized ratio of P-cofilin to total-cofilin was significantly increased in isolated 1A cremasteric vessels exposed to 10−4 M 5-HT + 10−4 M l-NAME than in control vessels. The increase in P-cofilin relative to total-cofilin in the presence of 10−4 M 5-HT + 10−4 M l-NAME was attenuated by the addition of 10−3 M cystamine. Presence of 10−6 M LimKi, an inhibitor of the kinase (LIMK) that phosphorylates cofilin, also significantly reduced the ratio of P-cofilin to total cofilin in the presence of 10−4 M 5-HT + 10−4 M l-NAME (Fig. 4).
Fig. 4.
Exposure of isolated arterioles to 5-HT + l-NAME induces cofilin phosphorylation. A: representative Western blot image of lysates from cremaster 1A arterioles following a 10-min exposure to control conditions or 10−4 M 5-HT + 10−4 M l-NAME in the presence or absence of 10−3 M cystamine or 10−6 M LIM kinase inhibitor (LimKi). B: ratio of P-cofilin to total-cofilin, expressed as means ± SE, n = 4 for all treatments. *P ≤ 0.05 vs. Control.
Exposure to 5-HT increases the ratio of filamentous F-actin to globular G-actin in cell culture and in live vessels.
To determine the effect of 5-HT on actin polymerization in smooth muscle cells isolated from cremaster 1A arterioles, we measured the fluorescence intensity of DNAse I and Phalloidin staining in control cells and those treated with 10−4 M 5-HT (Fig. 5A). Exposure of cells to 10−4 M 5-HT resulted in a significant increase in the ratio of Phalloidin to DNAse I fluorescence intensity compared with control cells (Fig. 5B). We also assessed changes in actin polymerization dynamics in response to 5-HT + l-NAME in isolated vessels by measuring the fluorescent intensity of the cell permeable F-actin dye, SiR-actin, and DNAse I. Cannulated and pressurized vessels were imaged at 34.5°C and then treated with PSS for 10 min and imaged a second time as a control measurement (Fig. 5C). This was followed by a 10-min treatment with 10−4 M 5-HT + 10−4 M l-NAME, and a third image was obtained (Fig. 5C). The ratio of SiR-actin intensity to DNAse I intensity was significantly higher following the 5-HT + l-NAME treatment than the control treatment (Fig. 5D).
Fig. 5.
Exposure of vascular smooth muscle cells, in culture or in situ, to 5-HT increases the ratio of F-actin to G-actin. A: representative image of cultured arteriolar smooth muscle cells untreated (top; Control) or treated (bottom) with 10−4 M 5-HT for 10 min and stained with fluorescent DNAse I, phalloidin, and DAPI. Scale bar = 20 μm. B: ratio of mean phalloidin intensity to DNAse I intensity, expressed as means ± SE; n = 4 for both treatments. *P ≤ 0.05 vs. Control. C: representative image of a vascular smooth muscle cell within a living isolated cremasteric arteriole after 10-min treatment with vehicle (top; Control) and the same cell following a 10-min treatment with 10−4 M 5-HT + 10−4 M l-NAME (bottom; 5-HT + l-NAME), stained with fluorescent DNAse I and SiR-actin. Scale bar = 15 μm. D: ratio of SiR-actin intensity to DNAse I intensity, expressed as mean percentage normalized to pretreatment ratio, ± SE; n = 4 for both treatments. *P ≤ 0.05 vs. Control.
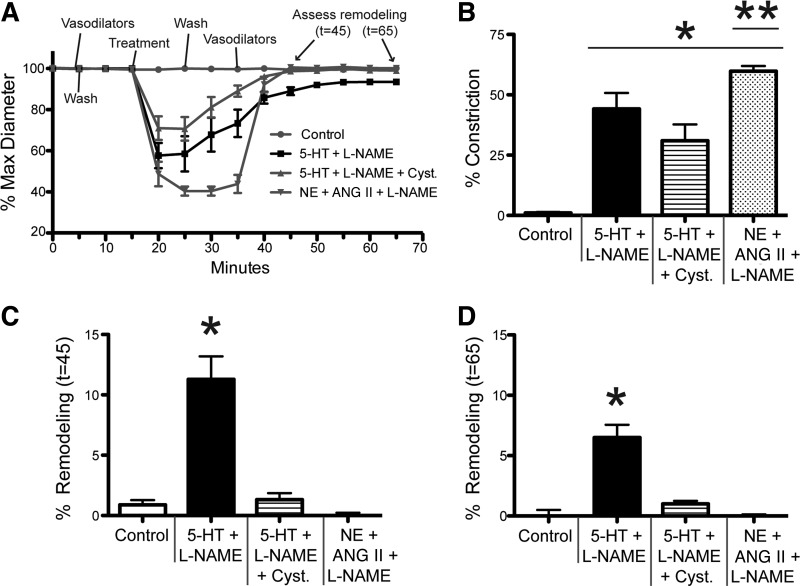
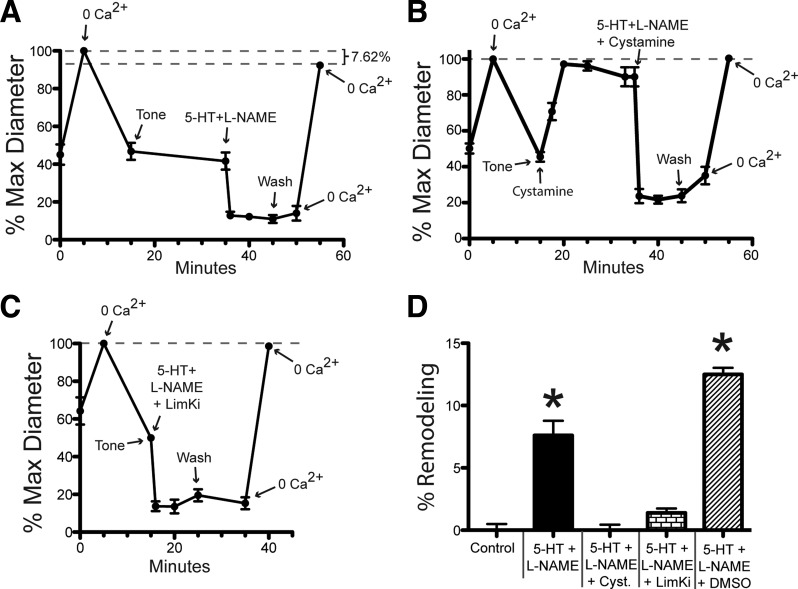
Exposure to 5-HT + l-NAME induces inward remodeling in isolated vessels.
To corroborate that 5-HT + l-NAME also induce inward remodeling in isolated vessels, pressurized 1A cremasteric vessels were exposed for 10 min to 10−4 M 5-HT + 10−4 M l-NAME alone or in the additional presence of 10−3 M cystamine or 10−6 M LimKi. Remodeling was assessed by comparing maximal passive diameters obtained in the presence of Ca2+-free PSS + 2 mM EGTA + 10−4 M adenosine before and after the exposure to the vasoconstrictor agonists (Fig. 6). Only the 5-HT + l-NAME treatment resulted in significant inward remodeling of 7.6 ± 1.1% (Fig. 6D). Presence of either 10−3 M cystamine or 10−6 M LimKi effectively blocked the 5-HT + l-NAME induced remodeling (Fig. 6D). Similar to the intravital data, the inhibitors had a negligible effect on the degree of constriction caused by 5-HT + l-NAME (Fig. 6, A–C).
Fig. 6.
Exposure of isolated arterioles to 5-HT + l-NAME induces inward remodeling. After development of spontaneous myogenic tone, cannulated and pressurized arterioles were exposed to a 5-min incubation in 10−4 M adenosine + 0 Ca2+ + 2 mM EGTA (to establish maximum passive diameter), washed with fresh buffer for 20 min (to re-establish tone), exposed to vasoconstrictor agents for 10 min, washed with fresh buffer for 5 min, and exposed to 10−4 M adenosine + 0 Ca2+ + 2 mM EGTA (to re-assess maximum passive diameter). In A–C, the luminal diameter of arterioles is expressed as percentage of the initial maximal diameter obtained. A: diameter of arterioles exposed to 10−4 M 5-HT + 10−4 M l-NAME as vasoconstrictor agents. B: diameter of arterioles exposed to 10−4 M 5-HT + 10−4 M l-NAME + 10−3 M cystamine, including a 20-min incubation in 10−3 M cystamine before application of vasoconstrictor agents. C: diameter of arterioles exposed 10−4 M 5-HT + 10−4 M l-NAME + 10−6 M LimKi as vasoconstrictor agents. D: percent remodeling expressed as percent change in the maximal passive diameter measured after vs. that measured before exposure to vasoconstrictor agents. Data are means ± SE; n = 3 for 5-HT + l-NAME + DMSO, and for all other treatments n = 6. *P ≤ 0.05 vs. Control.
DISCUSSION
The primary finding of this study is that a brief topical application of 5-HT + l-NAME initiated arteriolar inward remodeling processes in vivo. The reduction in passive diameter of the resistance vessels occurred in the absence of changes in systemic blood pressure. Ex vivo experiments further indicate that the inward remodeling process induced by 5-HT was dependent on the activity of transglutaminases, LIMK, and the phosphorylation of cofilin.
In accordance with what we and others have previously reported ex vivo in isolated vessels (2, 5, 29, 30), our results demonstrate that in vivo a brief (minutes to hours) exposure to vasoconstrictors is also able to initiate inward remodeling processes in the absence of changes in intravascular pressure. However, one caveat is that the rapid induction of inward remodeling in vivo appears to be specific to certain vasoconstrictor agonists, at least with respect to the cremasteric vasculature. Thus far, our results indicate remodeling occurs in response to 5-HT, and not in response to NE + Ang II. We used NE + Ang II because we have previously shown that a 4-h incubation in this combination of vasoconstrictors consistently induces inward remodeling in isolated arterioles (32, 45). Topical application of this combination of vasoconstrictors in vivo was unable to maintain arteriolar constriction over the duration of the experiment, even when supplemented with l-NAME or indomethacin. This suggests that mechanisms other than NO- and prostacyclin-induced dilations prevent topical NE + Ang II from sustaining vasoconstriction for more than ∼2 h. The only combination of vasoconstrictors tested that maintained a sustained 4-h constriction was 5-HT + l-NAME (Fig. 1A). In addition to maintaining prolonged vasoconstriction, 5-HT + l-NAME also induced inward remodeling following a 4-h incubation (Fig. 1C). We used a 10−4 M concentration of 5-HT to induce near maximal constriction. A literature search revealed that submaximal constriction of cremasteric arterioles to topically applied 5-HT was achieved with a concentration of 10−4 M 5-HT (25).
We also report that just a 10-min superfusion with 5-HT + l-NAME induced a significant inward remodeling of 11.2% when measured after 10 min under passive conditions and 6.50% after 30 min (Fig. 2). These results are comparable with the remodeling we observed following a 4-h incubation with 5-HT + l-NAME at the equivalent time following the removal of the vasoconstrictors, indicating that structural changes to the wall occur relatively early in the remodeling process induced by these agonists. This rapid induction of inward remodeling appeared specific to 5-HT + l-NAME, since a 10-min exposure to NE + Ang II + l-NAME did not induce remodeling (Fig. 2). We have also previously shown that a brief 5- to 10-min or 4-h exposure to a high K+ solution, NE alone (5 μM), or endothelin-1 (10−8 M) does not induce inward remodeling in isolated arterioles (19, 29). Others, however, have found that longer than 4-h incubations with endothelin-1 induce inward remodeling in isolated arterioles (2). This suggests that vasoconstrictor agonists vary in their capacity and rate to initiate inward remodeling processes. Furthermore, it would appear that the degree of constriction is not a primary factor in inducing remodeling, since NE + Ang II + l-NAME significantly constricted vessels to a greater degree than 5-HT + l-NAME, yet those vessels did not remodel (Fig. 2, B–D).
It is our contention that the presence of an incomplete vasodilation capacity following vasoconstriction represents early stages of the inward remodeling process (19, 31). Cumulative data support our concept of a remodeling continuum that allows blood vessels to modify their structural characteristics to accommodate for changes in their neurohumoral and/or mechanical environments (4, 29). The incomplete relaxation response we observed upon exposure to the vasodilator cocktail in vivo, or the calcium-free buffer ex vivo, is in accordance with the definition of inward remodeling introduced by Mulvany et al. (38) and therefore indicates that inward remodeling processes have been initiated in vessels exposed to 5-HT + l-NAME. We have also shown that the inward remodeling induced by a 4-h endogenous activation of transglutaminases is able to withstand a 1-h exposure to calcium-free conditions in the presence of 2 mM EGTA and 10−4 M adenosine. During this time frame, only active disruption of the actin cytoskeleton was able to reverse the inward remodeling (7), which further supports our view that the incomplete vasodilation capacity observed after a 10-min exposure to 5-HT + l-NAME represents the early stages of the inward remodeling process.
A number of reports have implicated transglutaminases in the inward remodeling process. Ex vivo, the activation of TG2 by dithiothreitol induces inward eutrophic remodeling in resistance vessels from mice (1) and rats (7). In addition, previous reports have shown that 5-HT is a potent transglutaminase activator in vascular tissues (40, 49). Therefore, we examined the involvement of transglutaminase activation in the rapid inward remodeling we observed in response to 5-HT + l-NAME exposure. Our evidence suggests that transglutaminases facilitate the remodeling process in vivo, as the addition of 10−3 M cystamine to the superfusate effectively blocked the inward remodeling induced by 5-HT + l-NAME (Fig. 2). Note that although the presence of cystamine dampened the level of constriction induced by 5-HT + l-NAME, this reduction in constriction level was not statistically significant compared with the 5-HT + l-NAME treatment (Fig. 2B). Thus, we cannot ascribe a decrease in constriction as an influencing mechanism that attenuates remodeling.
To confirm that the 5-HT + l-NAME treatment activated transglutaminases, we performed a transglutaminase activity assay on isolated vessels. Both, a 10- and 30-min exposure to 5-HT + l-NAME significantly increased transglutaminase activity in isolated arterioles as determined by the incorporation of the fluorescently labeled transglutaminase substrate, cadaverine, within the vessel wall. Incorporation of the fluorescent cadaverine was attenuated in the presence of cystamine (Fig. 3). These results are in accordance with previous findings implicating 5-HT in the activation of transglutaminases. It has been demonstrated that 5-HT activation of 5-HT2A receptors increases transglutaminase activity in the rat cortical cell line A1A1v (11). In rat aortic smooth muscle cells, a 10-min exposure to 5-HT induces activation of RhoA, via 5-HT1B/1D receptors in a transglutaminase-dependent manner. It was also reported that the transamidation of 5-HT to RhoA was attenuated in the presence of calcium inhibitors (16). It is well established that transglutaminases are activated and secreted in response to increases in intracellular calcium (18, 50) and that 5-HT functions as a vasoconstrictor by increasing intracellular Ca2+. In rat mesenteric arteries, agonist activation of 5-HT2A receptors has been implicated in vasoconstriction. In addition, 5-HT induced vasoconstriction was severely blunted in the presence of l-type Ca2+ channel blockers (48). Therefore, it is likely that the 5-HT–induced activation of transglutaminases is due, in part, to 5-HT receptor-mediated increases in intracellular levels of Ca2+.
In addition to 5-HT, l-NAME also has a positive effect on transglutaminase activity. NO inhibits transglutaminase activity, via s-nitrosylation of cysteine residues, required for transamidation (26). In human aortic smooth muscle cells, inhibition of NO production by incubation in l-NAME increased transglutaminase activity, whereas in vivo a chronic infusion (4 wk) of l-NAME increased transglutaminase activity in mouse aortas (22). In another in vivo study, a 1-wk infusion of l-NAME in mice was shown to induce hypertension and inward remodeling of mesenteric resistance vessels. In a transglutaminase knock-out cohort, l-NAME still induced hypertension, but mesenteric arterioles did not inwardly remodel (41). Thus, in the context of inward remodeling, l-NAME appears to function as a positive modulator of transglutaminase activity. We posit that l-NAME, in our treatment protocol, potentiated the activation of transglutaminases by limiting the bioavailabilty of NO to mediate inhibitory s-nitrosylation.
The role of transglutaminases in vascular remodeling has previously been attributed to modifications of the ECM: during exposure to vasoconstrictors, transglutaminases are activated and secreted from vascular smooth muscle cells and form cross-links that reorganize the ECM around a smaller constricted vessel. Under passive conditions, the newly reorganized ECM constrains the vessel to a smaller diameter. Data presented in this study suggest that transglutaminases also function intracellularly to mediate remodeling. Both in vivo and ex vivo, a 10-min exposure to 5-HT + l-NAME was sufficient to induce remodeling, which was inhibited by cystamine, thus indicating a role for transglutaminases in the process (Figs. 2 and 6). We hypothesize that transglutaminases are acting intracellularly in processes that modify the actin cytoskeleton and constrain the vessel to a smaller passive diameter. We have previously demonstrated that the actin cytoskeleton plays an important role in inward remodeling, following activation of endogenous transglutaminases by dithiothreitol (7). We now demonstrate that in isolated vessels a 10-min exposure to 5-HT + l-NAME increases the ratio of P-cofilin to total-cofilin and that this increase is attenuated by the presence of cystamine (Fig. 4). Cofilin is known to be involved in actin depolymerization, and its phosphorylation by Lim Kinase(s) prevents its association with actin and inhibits its severing activity (37).
To assess if 5-HT affects actin polymerization/depolymerization equilibrium in vascular smooth muscle cells, we costained cells with Phalloidin, to quantitate F-actin, and DNAse I, to quantitate G-actin. We demonstrate that the ratio of Phalloidin to DNAse I fluorescence is increased in vascular smooth muscle cells exposed to 5-HT compared with untreated cells (Fig. 5, A and B). This result is in accordance with previously published data demonstrating that stimulation of vascular smooth muscle cells with a number of distinct vasoconstriction agonists increases the ratio of Phalloidin to DNAse I staining (24). We used a similar approach to examine actin polymerization dynamics in vascular smooth muscle cells in intact live vessels following 5-HT + l-NAME exposure. In lieu of fluorescently labeled phalloidin, which is not cell permeable, we used a silicon rhodamine derivative, SiR-actin, that is reported to increase its fluorescence ≈100-fold when bound to F-actin (28). We were able to repeatedly image vascular smooth muscle cells costained with both SiR-actin and DNAse I within an intact pressurized vessel following a 10-min control treatment and then after a 10-min exposure to 5-HT + l-NAME (Fig. 5C). Similar to the cell culture data, we observed a significant increase in the ratio of the fluorescent probe binding F-actin versus the probe for G-actin following treatment with 5-HT + l-NAME compared with the ratio following control treatment (Fig. 5D). These results support the hypothesis that vasoconstrictors mediate remodeling of the actin cytoskeleton, in part, via mechanism(s) that promote actin polymerization.
Together, our results suggest that modifications to the actin cytoskeleton occur following 5-HT exposure and that these changes are contingent on transglutaminase activity. To further assess the role of the actin cytoskeleton on remodeling, we examined inward remodeling, ex vivo, in vessels exposed to 5-HT + l-NAME + LimKi (an inhibitor of Lim Kinases). Remodeling was effectively blocked when LIM kinases were inhibited compared with vessels treated with 5-HT + l-NAME in the absence of LimKi (Fig. 6). In addition, the ratio of P-cofilin to total-cofilin was also reduced to control levels in the presence of LimKi (Fig. 4). Thus two distinct pharmacological approaches that decrease the phosphorylation state of cofilin also block remodeling. The data support a model in which signaling pathway(s), triggered by 5-HT exposure, lead to transglutaminase activation and the downstream modulation of cofilin phosphorylation by Lim Kinases that favors actin polymerization and/or stress fiber formation.
It is also interesting that cystamine abolished spontaneous myogenic tone but had a negligible impact on the constriction induced by 5-HT + l-NAME. We have previously reported that 10−3 M cystamine does not significantly affect the constriction induced by high K+ membrane depolarization in isolated cremasteric arterioles (7). Others have reported that 10−3 M cystamine blocks the constriction induced by phenylephrine but not that induced by endothelin-1 in mesenteric arteries isolated from spontaneously hypertensive rats (14). We are currently investigating this phenomenon in more detail, but as of the present, we have not identified the mechanism(s) by which cystamine ablates myogenic tone. Our results (this study and Ref. 7) as well as those of Engholm et al. (14) suggest that cystamine affects only specific vasoconstriction pathways that may be more prominently affected by particular stimuli. These pathways may include activation of calcium sensitization mechanisms (31, 47), promotion of actin polymerization (10, 31, 47), and changes in the cellular redox status (27, 46).
The pathophysiological implications of our findings rest on the role that 5-HT at the concentration we used has or may have on reducing the structural diameter of blood vessels during the process of wound healing and/or vasospasm. Although our studies were performed in vessels from skeletal muscle, it has been demonstrated in rat middle cerebral arteries that 5-HT increases the phosphorylation state of cofilin and reduces the concentration of G-actin, indicating that 5-HT–induced remodeling of the actin cytoskeleton also occurs in the cerebral circulation (36), where localized vasoconstriction or vasospasm following subarachnoid hemorrhage or hemorrhagic stroke hinders blood flow re-establishment (8, 12, 39). Moreover, in a rat model of subarachnoid hemorrhage, inward remodeling of middle cerebral arteries, following exposure to hemolyzed blood, is blocked by inhibition of transglutaminases (17). In addition, presence of reversible cerebral vasoconstriction syndrome has been associated with intake of serotonergic drugs (23, 35, 43, 44), which suggest that 5-HT-induced vasoconstriction and acute inward remodeling, via changes to the actin cytoskeleton and upregulation of transglutaminases, may be implicated in the developmental process of this pathological condition.
In conclusion, our results provide novel evidence that suggest exposure of arterioles to 5-HT initiates inward remodeling processes that are linked to transglutaminase activation and affect the actin polymerization/depolymerization equilibrium via alterations of the phosphorylation state of cofilin. Additional experiments are necessary to determine the point(s) at which transglutaminase activities and the actin polymerization signaling pathway(s) intersect. The RhoA-Rho Kinase-Lim Kinase pathway merits particular investigation, since transglutaminases have been demonstrated to activate RhoA in response to 5-HT treatment (16). It is well established that this pathway increases actin polymerization, and we present data that inhibition of Lim Kinases, similar to cystamine addition, attenuates cofilin phosphorylation and blocks remodeling. We postulate that a brief exposure to vasoconstrictors induces the transition from a normal vessel to an inwardly remodeled one via the transglutaminase-dependent formation of persistent actin cytoskeleton structures. Moreover, it is this intracellular actin cytoskeletal alteration, within vascular smooth muscle cells, that stiffens the vascular smooth muscle cell so that it resists expansion under passive conditions, thus contributing to the remodeling process of the vascular wall around a smaller luminal diameter.
GRANTS
This research was supported by grants from the National Heart, Lung, and Blood Institute (R01-HL-088105 to L. A. Martinez-Lemus and 1PO1-HL-095486 to G. A. Meininger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.A.F., P.S.C., M.A.H., G.A.M., and L.A.M.-L. conception and design of research; C.A.F., J.A.C.-G., and M.C.S. performed experiments; C.A.F., J.A.C.-G., M.C.S., and L.A.M.-L. analyzed data; C.A.F., J.A.C.-G., M.C.S., and L.A.M.-L. interpreted results of experiments; C.A.F. and J.A.C.-G. prepared figures; C.A.F. and L.A.M.-L. drafted manuscript; C.A.F., M.A.H., G.A.M., and L.A.M.-L. edited and revised manuscript; C.A.F., J.A.C.-G., P.S.C., M.A.H., G.A.M., and L.A.M.-L. approved final version of manuscript.
REFERENCES
- 1.Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res 96: 119–126, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bakker EN, Buus CL, VanBavel E, Mulvany MJ. Activation of resistance arteries with endothelin-1: from vasoconstriction to functional adaptation and remodeling. J Vasc Res 41: 174–182, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bakker EN, Matlung HL, Bonta P, de Vries CJ, van Rooijen N, Vanbavel E. Blood flow-dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovasc Res 78: 341–348, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Bakker EN, Pistea A, Spaan JA, Rolf T, de Vries CJ, van Rooijen N, Candi E, VanBavel E. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res 99: 86–92, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bakker EN, van der Meulen ET, van den Berg BM, Everts V, Spaan JA, VanBavel E. Inward remodeling follows chronic vasoconstriction in isolated resistance arteries. J Vasc Res 39: 12–20, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Buus NH, Mathiassen ON, Fenger-Gron M, Praestholm MN, Sihm I, Thybo NK, Schroeder AP, Thygesen K, Aalkjaer C, Pedersen OL, Mulvany MJ, Christensen KL. Small artery structure during antihypertensive therapy is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 31: 791–797, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Castorena-Gonzalez JA, Staiculescu MC, Foote CA, Polo-Parada L, Martinez-Lemus LA. The obligatory role of the actin cytoskeleton on inward remodeling induced by dithiothreitol activation of endogenous transglutaminase in isolated arterioles. Am J Physiol Heart Circ Physiol 306: H485–H495, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalouhi N, Tjoumakaris S, Thakkar V, Theofanis T, Hammer C, Hasan D, Starke RM, Wu C, Gonzalez LF, Rosenwasser R, Jabbour P. Endovascular management of cerebral vasospasm following aneurysm rupture: outcomes and predictors in 116 patients. Clin Neurol Neurosurg 118: 26–31, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Christensen KL, Mulvany MJ. Vasodilatation, not hypotension, improves resistance vessel design during treatment of essential hypertension: a literature survey. J Hypertens 19: 1001–1006, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J 16: 72–76, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Dudek NL, Patel TB, Muma NA. Transglutaminase-catalyzed transamidation: a novel mechanism for Rac1 activation by 5-hydroxytryptamine2A receptor stimulation. J Pharmacol Exp Ther 326: 153–162, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 11: 906–917, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Eftekhari A, Rahman A, Schaebel LH, Chen H, Rasmussen CV, Aalkjaer C, Buus CL, Mulvany MJ. Chronic cystamine treatment inhibits small artery remodelling in rats. J Vasc Res 44: 471–482, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Engholm M, Eftekhari A, Chwatko G, Bald E, Mulvany MJ. Effect of cystamine on blood pressure and vascular characteristics in spontaneously hypertensive rats. J Vasc Res 48: 476–484, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res 78: 274–285, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Guilluy C, Rolli-Derkinderen M, Tharaux PL, Melino G, Pacaud P, Loirand G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J Biol Chem 282: 2918–2928, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Guvenc Tuna B, Lachkar N, de Vos J, Bakker EN, VanBavel E. Cerebral artery remodeling in rodent models of subarachnoid hemorrhage. J Vasc Res 52: 103–115, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Hand D, Bungay PJ, Elliott BM, Griffin M. Activation of transglutaminase at calcium levels consistent with a role for this enzyme as a calcium receptor protein. Biosci Rep 5: 1079–1086, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Hill MA, Potocnik SJ, Martinez-Lemus LA, Meininger GA. Delayed arteriolar relaxation after prolonged agonist exposure: functional remodeling involving tyrosine phosphorylation. Am J Physiol Heart Circ Physiol 285: H849–H856, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Hill MA, Simpson BE, Meininger GA. Altered cremaster muscle hemodynamics due to disruption of the deferential feed vessels. Microvasc Res 39: 349–363, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Hungerford JE, Sessa WC, Segal SS. Vasomotor control in arterioles of the mouse cremaster muscle. FASEB J 14: 197–207, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Jandu SK, Webb AK, Pak A, Sevinc B, Nyhan D, Belkin AM, Flavahan NA, Berkowitz DE, Santhanam L. Nitric oxide regulates tissue transglutaminase localization and function in the vasculature. Amino acids 44: 261–269, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.John S, Donnelly M, Uchino K. Catastrophic reversible cerebral vasoconstriction syndrome associated with serotonin syndrome. Headache 53: 1482–1487, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol 295: C768–C778, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch LG, Alsip NL, Feige BD, Wead WB, Harris PD. In vivo effect of naftidrofuryl on 5-hydroxytryptamine-mediated constriction in rat peripheral microcirculation. Eur J Pharmacol 254: 249–255, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry 40: 4904–4910, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem 278: 3825–3830, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Lukinavicius G, Reymond L, D′Este E, Masharina A, Gottfert F, Ta H, Guther A, Fournier M, Rizzo S, Waldmann H, Blaukopf C, Sommer C, Gerlich DW, Arndt HD, Hell SW, Johnsson K. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat Methods 11: 731–733, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Lemus LA. Persistent agonist-induced vasoconstriction is not required for angiotensin II to mediate inward remodeling of isolated arterioles with myogenic tone. J Vasc Res 45: 211–221, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Lemus LA, Hill MA, Bolz SS, Pohl U, Meininger GA. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. FASEB J 18: 708–710, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24: 45–57, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Lemus LA, Zhao G, Galinanes EL, Boone M. Inward remodeling of resistance arteries requires reactive oxygen species-dependent activation of matrix metalloproteinases. Am J Physiol Heart Circ Physiol 300: H2005–H2015, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathiassen ON, Buus NH, Larsen ML, Mulvany MJ, Christensen KL. Small artery structure adapts to vasodilatation rather than to blood pressure during antihypertensive treatment. J Hypertens 25: 1027–1034, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Mathiassen ON, Buus NH, Sihm I, Thybo NK, Morn B, Schroeder AP, Thygesen K, Aalkjaer C, Lederballe O, Mulvany MJ, Christensen KL. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 25: 1021–1026, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Mawet J, Boukobza M, Franc J, Sarov M, Arnold M, Bousser MG, Ducros A. Reversible cerebral vasoconstriction syndrome and cervical artery dissection in 20 patients. Neurology 81: 821–824, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Moreno-Dominguez A, El-Yazbi AF, Zhu HL, Colinas O, Zhong XZ, Walsh EJ, Cole DM, Kargacin GJ, Walsh MP, Cole WC. Cytoskeletal reorganization evoked by Rho-associated kinase- and protein kinase C-catalyzed phosphorylation of cofilin and heat shock protein 27, respectively, contributes to myogenic constriction of rat cerebral arteries. J Biol Chem 289: 20939–20952, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriyama K, Iida K, Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells 1: 73–86, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension 28: 505–506, 1996. [PubMed] [Google Scholar]
- 39.Otite F, Mink S, Tan CO, Puri A, Zamani AA, Mehregan A, Chou S, Orzell S, Purkayastha S, Du R, Sorond FA. Impaired cerebral autoregulation is associated with vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. Stroke 45: 677–682, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penumatsa K, Abualkhair S, Wei L, Warburton R, Preston I, Hill NS, Watts SW, Fanburg BL, Toksoz D. Tissue transglutaminase promotes serotonin-induced AKT signaling and mitogenesis in pulmonary vascular smooth muscle cells. Cell Signal 26: 2818–2825, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pistea A, Bakker EN, Spaan JA, Hardeman MR, van Rooijen N, VanBavel E. Small artery remodeling and erythrocyte deformability in l-NAME-induced hypertension: role of transglutaminases. J Vasc Res 45: 10–18, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation 108: 2230–2235, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Shaik S, Chhetri SK, Roberts G, Wuppalapati S, Emsley HC. Reversible cerebral vasoconstriction syndrome with involvement of external carotid artery branches. Neurohospitalist 4: 141–143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singhal AB, Caviness VS, Begleiter AF, Mark EJ, Rordorf G, Koroshetz WJ. Cerebral vasoconstriction and stroke after use of serotonergic drugs. Neurology 58: 130–133, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Staiculescu MC, Galinanes EL, Zhao G, Ulloa U, Jin M, Beig MI, Meininger GA, Martinez-Lemus LA. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res 98: 428–436, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Velmurugan GV, Sundaresan NR, Gupta MP, White C. Defective Nrf2-dependent redox signalling contributes to microvascular dysfunction in type 2 diabetes. Cardiovasc Res 100: 143–150, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh MP, Cole WC. The role of actin filament dynamics in the myogenic response of cerebral resistance arteries. J Cereb Blood Flow Metab 33: 1–12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watts SW. Serotonin-induced contraction in mesenteric resistance arteries: signaling and changes in deoxycorticosterone acetate-salt hypertension. Hypertension 39: 825–829, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS ONE 4: e5682, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zemskov EA, Mikhailenko I, Hsia RC, Zaritskaya L, Belkin AM. Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One 6: e19414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]