Na/Ca exchange (NCX) is normally located predominantly in the T tubules of cardiac ventricular myocytes. However, redistribution of NCX occurs in myocytes from failing hearts, resulting in more uniform distribution between T tubule and surface sarcolemma; this alters access of NCX to Ca released from sarcoplasmic reticulum and thus cellular Ca handling.
Keywords: cardiac myocytes, T tubules, intracellular Ca, heart failure
Abstract
In mammalian cardiac ventricular myocytes, Ca efflux via Na/Ca exchange (NCX) occurs predominantly at T tubules. Heart failure is associated with disrupted t-tubular structure, but its effect on t-tubular function is less clear. We therefore investigated t-tubular NCX activity in ventricular myocytes isolated from rat hearts ∼18 wk after coronary artery ligation (CAL) or corresponding sham operation (Sham). NCX current (INCX) and l-type Ca current (ICa) were recorded using the whole cell, voltage-clamp technique in intact and detubulated (DT) myocytes; intracellular free Ca concentration ([Ca]i) was monitored simultaneously using fluo-4. INCX was activated and measured during application of caffeine to release Ca from sarcoplasmic reticulum (SR). Whole cell INCX was not significantly different in Sham and CAL myocytes and occurred predominantly in the T tubules in Sham myocytes. CAL was associated with redistribution of INCX and ICa away from the T tubules to the cell surface and an increase in t-tubular INCX/ICa density from 0.12 in Sham to 0.30 in CAL myocytes. The decrease in t-tubular INCX in CAL myocytes was accompanied by an increase in the fraction of Ca sequestered by SR. However, SR Ca content was not significantly different in Sham, Sham DT, and CAL myocytes but was significantly increased by DT of CAL myocytes. In Sham myocytes, there was hysteresis between INCX and [Ca]i, which was absent in DT Sham but present in CAL and DT CAL myocytes. These data suggest altered distribution of NCX in CAL myocytes.
NEW & NOTEWORTHY
Na/Ca exchange (NCX) is normally located predominantly in the T tubules of cardiac ventricular myocytes. However, redistribution of NCX occurs in myocytes from failing hearts, resulting in more uniform distribution between T tubule and surface sarcolemma; this alters access of NCX to Ca released from sarcoplasmic reticulum and thus cellular Ca handling.
contraction of cardiac ventricular myocytes is initiated by Ca influx across the cell membrane via the L-type Ca current (ICa), which activates ryanodine receptors (RyRs) in adjacent sarcoplasmic reticulum (SR) membrane, triggering Ca-induced Ca release (CICR) from the SR. Relaxation occurs as a result of Ca removal from the cytoplasm into the SR, via a Ca ATPase (SERCA), and across the cell membrane, predominantly via Na/Ca exchange (NCX) (2).
In mammalian ventricular myocytes, ICa, CICR, and Ca efflux via NCX occur predominantly at invaginations of the surface membrane, called T tubules (5). Presumably because of this colocation, Ca close to the site of CICR appears to be more effective than bulk cytoplasmic Ca at stimulating Ca efflux via NCX, and thus–since NCX carries three Na for each Ca–the associated inward (depolarizing) NCX current (INCX) (35). This is important not only for normal Ca efflux but also because under conditions of Ca overload, spontaneous SR Ca release occurs, activating inward INCX and causing delayed afterdepolarisations (DADs), which can trigger action potentials and arrhythmias (18). Because of the proximity of RyRs and NCX, it seems likely that such activity will arise predominantly at T tubules.
Heart failure (HF) is associated with disruption of the t-tubular network (21, 22) and redistribution of ICa from the T tubules to the surface membrane, although RyR distribution appears unaltered (7). However, the effect of HF on the distribution of INCX is unknown but is important because changes may alter the proximity of NCX to the site of CICR, and thus INCX and Ca efflux. In the present study we therefore investigated the distribution of INCX between the t-tubular and surface membranes, and its functional consequences, in myocytes from normal and HF rats.
MATERIALS AND METHODS
Surgical model of HF.
Ligation of the left anterior descending coronary artery of adult male Wistar rats (CAL) was performed as previously described (7). The corresponding sham operation (Sham) was identical except that no tie was placed around the coronary artery. All procedures were performed in accordance with UK legislation and approved by the University of Bristol Ethics Committee. As reported in another study using cells from these animals, CAL had no significant effect on body weight or tibia length, but significantly increased heart and lung weights relative to body weight and tibia length, decreased ejection fraction, and increased left ventricular diastolic and systolic volumes, indicative of early stage HF (7).
Myocyte isolation and detubulation.
Myocytes were isolated from the left ventricular free wall and septum of Sham and CAL animals 18.6 ± 0.3 and 18.5 ± 0.3 wk after surgery, respectively, as previously described (7) and stored for 2–8 h before use on the day of isolation. Myocyte detubulation (DT), physical and functional uncoupling of the T tubules from the surface membrane, was achieved using formamide-induced osmotic shock, as previously described (4, 6, 7).
Solutions.
All reagents were obtained from Sigma-Aldrich (Poole, UK) unless otherwise specified. Cells were superfused with solution containing (in mmol/l) 133 NaCl, 1 MgSO4, 1 CaCl2, 1 Na2HPO4, 10 d-glucose, and 10 HEPES (pH 7.4, NaOH); 5 CsCl was added to inhibit K currents. The pipette solution contained (in mmol/l) 110 CsCl, 20 tetraethylammonium chloride, 0.5 MgCl2, 5 MgATP, 10 HEPES, 0.4 GTP-Tris (pH 7.2, CsOH), and 0.1 pentapotassium salt of the fluorescent Ca indicator fluo-4 (Life Technologies, Paisley, UK).
Measurement of ICa and INCX.
Myocytes were placed in a chamber mounted on a Diaphot inverted microscope (Nikon UK, Kingston-upon-Thames, UK). Membrane currents and cell capacitance were recorded with the whole cell patch-clamp technique using an Axopatch 200B patch clamp amplifier, a Digidata 1440A analog-to-digital converter, and pClamp 10 software (Molecular Devices, Reading, UK), which was used for data acquisition (at 2 kHz) and analysis. Pipette tip resistances were typically 1.5–3.0 MΩ when filled with pipette solution.
Holding potential was −80 mV; a 300-ms ramp to −40 mV was used to inactivate INa, followed by step depolarization to 0 mV for 300 ms to activate ICa, at a frequency of 1 Hz. ICa was measured as the difference between peak inward current and current at the end of the pulse to 0 mV. Once steady-state was achieved, stimulation was stopped, and after 10-s quiescence, caffeine (10 mmol/l) was rapidly applied to the cell to cause spatially and temporally uniform release of SR Ca (3); the resulting inward current due to Ca extrusion via NCX was recorded at −80 mV. Following wash-off of caffeine, stimulation was restarted and continued until a steady-state was reached. The protocol was then repeated, but NiCl2 (10 mmol/l) was applied 10 s before application of caffeine in the continued presence of Ni to inhibit NCX.
Membrane currents were normalized to membrane capacitance (a function of membrane area) to give current density. The distribution of membrane current between the surface and t-tubular membranes was calculated from the currents measured in intact (whole cell) and DT (surface membrane only) myocytes, as previously described (7).
Measurement of intracellular Ca.
Fluo-4 fluorescence was excited at 450–488 nm and emitted fluorescence collected at wavelengths > 560 nm. Normalized fluo-4 fluorescence (F/F0) was converted to intracellular free Ca concentration ([Ca]i) as follows:
| (1) |
where resting Ca ([Ca]rest) was assumed to be 0.1 μmol/l and the dissociation constant (Kd) for fluo-4 in situ was 1.1 μmol/l (8, 20).
The rate of decay of Ca transients was obtained by fitting single exponential functions to the declining phase of the ICa- and caffeine-induced Ca transients. The fitted rate constants (k′) were multiplied by the appropriate (Sham or CAL) buffering power (calculated by plotting [Ca]i against [Ca]total, as previously described) (12, 33) to correct for the effect of buffering power and thus allow comparison of the rate of Ca extrusion in Sham and CAL myocytes. This corrected rate constant (k) during application of caffeine (kCaff) was used as an index of the rate of total sarcolemmal Ca efflux, and that in the presence of caffeine plus Ni (kNi) as the rate of the slow (non-SR, non-NCX) Ca extrusion pathways. The rate of Ca removal via NCX (kNCX) was calculated as kCaff-kNi, and the rate of Ca uptake by SR (kSR) was calculated as the difference between the rate of decline of the ICa-induced Ca transient and kCaff. These rate constants were also used to calculate the percent contribution of these pathways to Ca removal from the cell cytoplasm, as previously described (25, 36).
Statistical analysis.
Data are expressed as means ± SE. The errors of derived variables and the subsequent statistical analysis were calculated using propagation of errors from the constituent measurements. Student's t-tests and 2-way ANOVA with the Bonferroni post hoc test were used as appropriate. Statistical significance was taken as P < 0.05. All statistical tests were performed on the number of cells. Sample sizes (n) are given as c/h, where c is the number of cells used from h hearts.
RESULTS
The effect of CAL on NCX distribution.
Cell capacitance (a function of membrane area) was significantly larger in CAL myocytes [240.2 ± 20.8 pF (Sham) vs. 375.0 ± 63.0 pF (CAL); n = 12/6 and 8/4, respectively; P = 0.004]; this was accompanied by a nonsignificant increase in cell volume [33.5 ± 5.1 pl (Sham) vs. 49.0+6.1 pl (CAL)], resulting in no significant difference in cell surface area-to-volume ratio, as previously reported during cellular hypertrophy (15, 29).
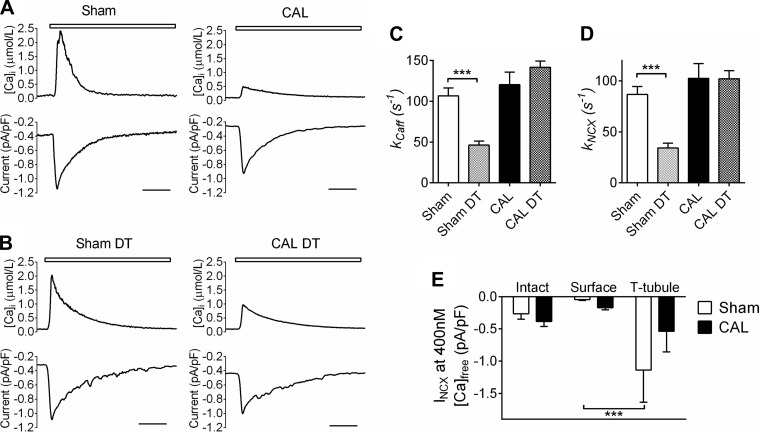
Figure 1A shows representative caffeine-induced Ca transients (top) and accompanying membrane currents (bottom) recorded from Sham and CAL myocytes. Caffeine-induced Ca transient amplitude was significantly smaller in CAL compared with Sham myocytes [2.42 ± 0.63 μM (Sham) vs. 0.78 ± 0.44 μM (CAL); P < 0.05] and buffering power, assessed as described in the materials and methods, was significantly larger in CAL myocytes [134 ± 66 (Sham) vs. 294 ± 46 (CAL); P < 0.01]. Representative caffeine-induced Ca transients and INCX in DT Sham and CAL cells are shown in Fig. 1B: DT did not significantly alter peak [Ca]i in Sham cells (2.06 ± 0.69 μM) but increased peak [Ca]i in CAL cells (to 2.30 ± 1.09 μM).
Fig. 1.
A: representative records of intracellular free Ca concentration ([Ca]i) and the associated inward Na/Ca exchange (NCX) current (INCX) during the application of 10 mM caffeine (white bar) in sham operation (Sham) and coronary artery ligation (CAL) cells. Scale bars represent 2 s. B: representative records of [Ca]i and the associated inward current (INCX) during the application of 10 mM caffeine (white bar) in detubulated (DT) Sham and CAL cells. Scale bars represent 2 s. C: mean rate constants for the decline of the caffeine-induced Ca transient (kCaff); n = 12/6, 11/5, 8/4, 7/4 for Sham, Sham DT, CAL, and CAL DT, respectively. D: mean kNCX; n = 12/6, 11/5, 8/4, 5/3 for Sham, Sham DT, CAL, and CAL DT, respectively. E: INCX density in the whole cell and at the surface and t-tubular membranes, determined at 400 nM [Ca]i during the declining phase of the caffeine transient. ***P < 0.001 with Bonferroni posttest.
In Sham myocytes, the decline of the caffeine-induced Ca transient, corrected for buffering power (see materials and methods), was significantly slowed by DT [kCaff, 106.8 ± 9.5 (Sham) vs. 46.3 ± 5.0 s−1 (Sham DT); P < 0.001; Fig. 1C], consistent with loss of t-tubular Ca efflux pathways. In CAL myocytes, the decline of the caffeine-induced Ca transient was not significantly different from Sham myocytes and not significantly altered by DT [kCaff, 120.4 ± 15.2 (CAL) vs. 141.8 ± 7.7 s−1 (CAL DT); Fig. 1C], consistent with reduced t-tubular sarcolemmal Ca efflux in CAL myocytes.
To investigate the role of NCX in these changes, exposure to caffeine was repeated in the presence of Ni to inhibit NCX. Figure 1D shows the rate of Ca extrusion via NCX (kNCX); DT significantly decreased kNCX in Sham cells [86.8 ± 7.8 (Sham) vs. 34.3 ± 4.8 s−1 (Sham DT); P < 0.0001], compatible with loss of t-tubular NCX. In CAL myocytes, kNCX was not significantly different from Sham myocytes and not significantly altered by DT [102.5 ± 14.5 (CAL) vs. 102.2 ± 8.0 s−1 (CAL DT)], implying that although the rate of Ca extrusion via NCX is similar in Sham and CAL myocytes, there is little Ca extrusion via t-tubular NCX in these cells.
We used INCX in intact and DT myocytes to quantify its distribution between the surface and t-tubular membranes. Since NCX activity depends on Ca adjacent to the exchanger, we measured INCX at a [Ca]i of 400 nmol/l during the descending phase of the caffeine transient, when Ca has been shown to be uniformly distributed throughout the cytoplasm in both intact and DT cells (3). Figure 1E shows that whole cell INCX density determined in this way was not significantly different in Sham and CAL myocytes and that in Sham myocytes, INCX density is significantly greater in the t-tubular membrane than at the cell surface, as previously reported (14), resulting in a T tubule-to-surface sarcolemma INCX ratio of 25:1. However, the distribution of INCX is different in CAL myocytes, decreasing at the T tubules by ∼50% and increasing at the surface membrane by ∼300%, resulting in no significant difference in INCX density between the two membranes in these cells and an INCX T tubule-to-surface sarcolemma ratio of 3:1. This suggests that the slower Ca extrusion via NCX following DT of Sham cells is due to loss of t-tubular NCX and that the lack of effect of DT on the rate of Ca extrusion via NCX in CAL cells is due to its relocation away from T tubules.
The effect of CAL on cellular Ca handling.
The preceding data show redistribution of INCX in CAL myocytes. NCX is one of the major Ca efflux pathways that compete for cytoplasmic Ca (1, 25), so that a decrease of NCX activity at the T tubules (the site of CICR and SERCA) (24, 32) might alter the balance of Ca removal via NCX and SR (30), thereby altering SR Ca content and thus Ca release and INCX. We therefore determined the effect of CAL on the contribution of different pathways to Ca removal.
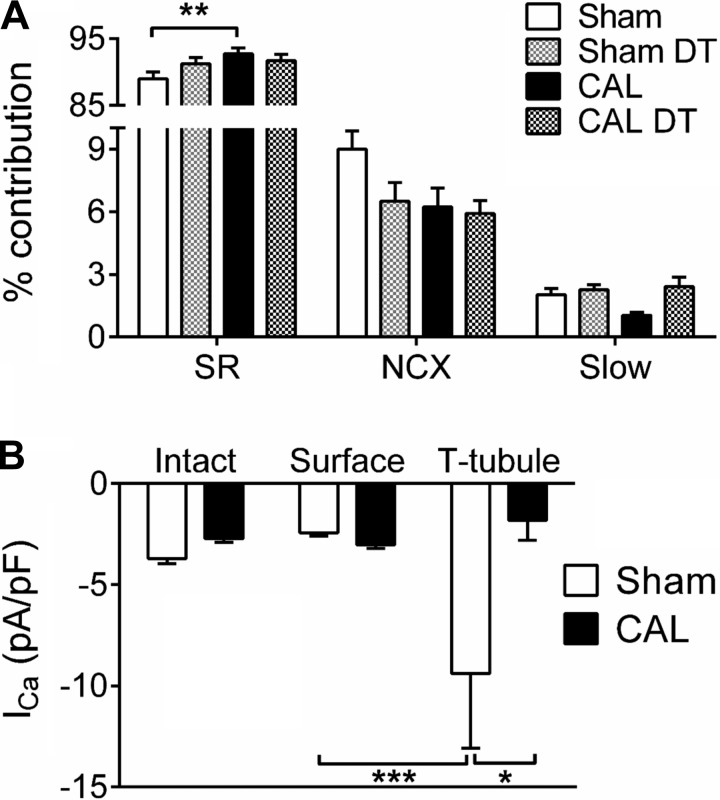
Figure 2A shows the percent contribution of different pathways to Ca removal from the cytoplasm. There was no significant difference in the contribution to Ca removal by the “slow” Ca extrusion pathways (sarcolemmal Ca ATPase and mitochondria) between the four groups. In Sham myocytes, DT caused a small decrease in the fraction of Ca removed via NCX and increase in Ca removal by the SR, consistent with loss of NCX following DT. In CAL myocytes, the fraction of Ca removed via NCX was smaller than in Sham cells, despite no significant change in whole cell INCX density at a given free [Ca]i (Fig. 1E); this was accompanied by a significant increase in the fraction of Ca removed by SR. DT of CAL myocytes had no significant effect on the fraction of Ca removed via NCX or SR, consistent with less t-tubular NCX activity in these cells. These data suggest decreased access of NCX to Ca ions, and thus Ca efflux, in CAL compared with Sham myocytes, presumably as a result of its change in location away from the site of SR Ca release at the T tubules, possibly exacerbated by decreased Ca release at the T tubules as a result of redistribution of ICa and altered t-tubular morphology (7, 21). They also suggest that this redistribution leads to an increase in fractional SR Ca uptake, which would be expected to increase SR Ca release, given its steep dependence on SR Ca content (26, 34).
Fig. 2.
A: mean percent contribution of different pathways to Ca removal from the cytoplasm of Sham, DT Sham, CAL, and DT CAL cells; n = 12/6, 11/5, 8/4, 5/3, respectively. B: distribution of Ca current (ICa) density between the surface and t-tubular membranes; n = 12/6 and 8/4 for Sham and CAL, respectively; only statistical comparisons between t-tubular and surface membranes are shown. *P < 0.05, **P < 0.01, and ***P < 0.001 with Bonferroni posttest.
To determine whether the changes in NCX distribution were quantitatively different from the redistribution of ICa previously reported (7), which might alter Ca balance across the t-tubular membrane and thus the effect of loss of T tubules during HF on cellular Ca balance, we calculated ICa distribution in Sham and CAL myocytes. We have previously reported changes in ICa distribution in CAL myocytes (7) when Ca was buffered using BAPTA in the patch pipette. It was therefore possible that the redistribution of ICa might differ under the present conditions in which no exogenous Ca buffer apart from fluo-4 was present. However, in agreement with previous work, DT of Sham myocytes decreased ICa density from −3.71 ± 0.25 to −2.70 ± 0.25 pA/pF, whereas DT of CAL myocytes had no significant effect on ICa density [−2.71 ± 0.19 (CAL) vs. −2.93 ± 0.21 pA/pF (CAL DT)]. These data were used to calculate the distribution of ICa between the t-tubular and surface membranes, as previously described (7). Figure 2B shows that consistent with previous work (7), ICa density was significantly higher in the T tubules of Sham myocytes than in their surface membrane and that in CAL myocytes, t-tubular ICa density was significantly smaller than in Sham myocytes [t-tubular ICa, −9.39 ± 3.70 (Sham) vs. −1.80 ± 0.99 pA/pF (CAL); n = 12/6 and 8/4, respectively; P < 0.05] and not significantly different from ICa density in the surface membrane. Since CAL has little effect of the rate of inactivation of ICa at the t-tubular or surface membranes (7), Ca influx via ICa will reflect these changes in current density.
The ratio of t-tubular INCX to ICa density obtained from these data is 0.12 in Sham myocytes and 0.30 in CAL myocytes. Thus t-tubular density of INCX relative to ICa is greater in CAL than in Sham myocytes; this will result in DT causing greater loss of NCX (and thus Ca efflux) relative to ICa (and thus Ca influx) in CAL cells, which would be expected to increase cellular Ca loading. This is consistent with the observation that SR Ca content was not significantly affected by DT in Sham myocytes but was significantly increased (P < 0.05) by DT in CAL myocytes [SR Ca content, 78.8 ± 8.2 μM (Sham); 63.3 ± 9.8 μM (Sham DT); 58.7 ± 5.8 μM (CAL); and 105.4 ± 17.4 μM (CAL DT)].
The effect of CAL on the relationship between [Ca]i and INCX.
The data above suggest that decreased t-tubular NCX in CAL myocytes decreases access of NCX to Ca released from the SR, thereby increasing fractional SR Ca uptake; they also suggest that SR Ca load might be increased following loss of T tubules, as a result of the relative changes in Ca influx and efflux. The consequent increase in SR Ca content will alter NCX activity by altering release. However, INCX activity may also be altered directly as a result of the change in the colocation of NCX and RyRs.
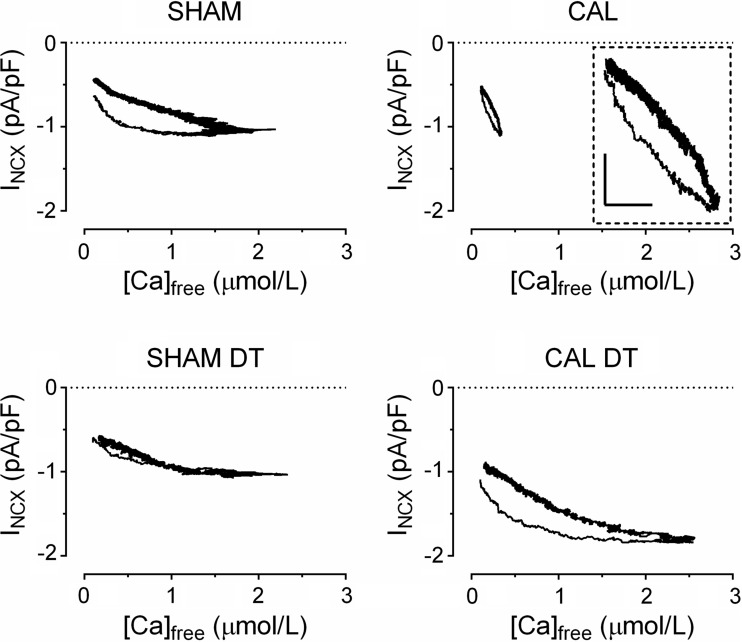
Previous work has shown hysteresis in the relationship between bulk cytoplasmic Ca and INCX during spontaneous and caffeine-induced Ca release, with a larger current for a given Ca when Ca is increasing than when it is decreasing (35). We have proposed that this is because INCX occurs predominantly at T tubules, where NCX will be exposed to a higher Ca than that in the bulk cytoplasm during Ca release (28). If so, DT would be expected to reduce the hysteresis and CAL to change it because of altered NCX distribution.
To test this idea we plotted free [Ca]i against current density during application of caffeine for each of the four groups of cells. Figure 3 shows the average hysteresis loops for each group of cells; each loop consists of the data from all the cells in that group. Figure 3, left, shows the relationship in intact (top) and DT (bottom) Sham myocytes. Intact cells show a hysteresis, as previously described (35), with more current at a given [Ca]i during the rising phase. However, this hysteresis is reduced in DT myocytes, consistent with idea that the hysteresis arises at the T tubules, because of proximity of NCX to SR Ca release via RyRs.
Fig. 3.
Averaged hysteresis loops for INCX density vs. [Ca]i during application of caffeine in Sham, DT Sham, CAL, and DT CAL myocytes; n = 11/5, 9/5, 7/4, 7/4, respectively. Inset: loop on expanded scales: x and y-scale bars represent 0.1 μM and 0.2 pA/pF, respectively.
Figure 3, right, shows similar traces obtained from CAL myocytes. The loop obtained in CAL myocytes was smaller than in Sham cells, presumably because the mean rise of free [Ca]i was smaller in these cells (Fig. 1A), although the hysteresis was maintained (see inset). Interestingly, the hysteresis is still apparent in DT CAL myocytes, suggesting that the hysteresis arises at the surface membrane in contrast to its t-tubular location in Sham myocytes and consistent with redistribution of NCX to the surface membrane in these cells.
DISCUSSION
The present study shows that in CAL myocytes, NCX activity decreases at the T tubules and increases at the surface membrane. This is similar to the redistribution of ICa that we have previously reported (7) and is associated with changes in the fraction of Ca removed by NCX and SR, the balance of Ca influx versus efflux across the t-tubular membrane, and the relationship between [Ca]i and INCX. This is important in understanding the role of the T tubules, and their loss, in Ca handling and the generation of DADs in HF.
Redistribution of NCX in CAL myocytes.
INCX decreases at the T tubules and increases at the cell surface of CAL myocytes, with no change in whole cell current. Although INCX depends on Ca at the cytoplasmic face of the exchanger, this redistribution is unlikely to be due to local differences in Ca, because caffeine was used to release total SR Ca content and INCX distribution calculated at a fixed Ca, late during the descending phase of the caffeine transient, when Ca, and thus stimulation of NCX, is likely to be uniform throughout the cell. The redistribution is also unlikely to be due to changes in T tubule density, which is unaltered in this model of HF (7), nor is it likely that a change in surface area-to-volume ratio, which was not significantly different in Sham and CAL myocytes, contributes to the observed changes.
The mechanism of redistribution is unclear, although reminiscent of the redistribution of β2-adrenoceptors and ICa from their normal t-tubular location to a more uniform distribution in cells from failing hearts (6, 7, 27). It has been suggested that localization of NCX activity at the T tubules is due, in part, to local protein kinase A activity (10); however, protein kinase A activity appears to increase at the T tubules in CAL myocytes (7), making it unlikely that this can explain the decrease in t-tubular INCX observed in these cells. These changes may reflect reversion in HF toward a more neonatal phenotype, in which cell activation is dependent on Ca influx and efflux across the surface, rather than t-tubular, membrane (11, 31) and a general loss of t-tubular protein localization.
Functional consequences of redistribution.
Computer modeling suggests that the relative location of NCX, SERCA, and sarcolemmal Ca ATPase alters their ability to compete for cytoplasmic Ca and thus the amount of Ca removed by each pathway (30). The present work shows that DT of Sham myocytes decreases Ca efflux via NCX, as a result of loss of t-tubular NCX, and increases SR Ca uptake. A similar decrease in Ca efflux via NCX and increased Ca uptake via SERCA occurred in CAL myocytes, compared with Sham cells, even though total NCX density at a given [Ca]i is the same in CAL and Sham myocytes. This can be explained by redistribution of NCX away from the T tubules in CAL cells, so that it no longer has “privileged” access to Ca released from the SR as a result of its proximity to RyRs; this will reduce Ca extrusion via NCX and allow a greater fraction of the cytoplasmic Ca to be removed by SERCA, much of which also appears to be located at T tubules (24) where Ca release occurs. Although a large fractional decrease in Ca extrusion via NCX results in a relatively small fractional increase in SR Ca uptake, this reflects the relatively small fraction of Ca removed by NCX compared with the SR. Altered NCX location in CAL myocytes, which was measured when cytoplasmic Ca was relatively uniformly distributed, is likely to be important during the systolic Ca transient, since NCX activity close to the site of CICR at the T tubules, where the majority of Ca efflux normally occurs (Fig. 1D), will be reduced, although this may be offset by reduced Ca release at the T tubules due to decreased t-tubular ICa.
It is notable that although fractional SR Ca uptake was greater in CAL than in Sham myocytes (Fig. 2A), SR Ca content was not significantly different and the caffeine-induced rise of cytoplasmic Ca was smaller (Fig. 1A). However, calculated peak [Ca]i depends on [Ca]rest, which was taken as 0.1 μmol/l in the present study; increasing [Ca]rest would increase peak [Ca]i, but previous reports of [Ca]rest in CAL have been inconsistent, showing an increase, decrease, or no change (9, 17, 19, 37). Nevertheless, the present observations may be reconciled by increased fractional SR Ca uptake being offset by decreased local ICa, which would tend to decrease SR Ca content and the increased Ca buffering observed in CAL myocytes, which would decrease free Ca for a given release. This may also account for the greater slope of the relationship between Ca and INCX in CAL myocytes (Fig. 3), since a given Ca extrusion would result in a smaller change in free Ca. However, DT sufficiently increased SR Ca content to cause a larger caffeine-induced rise of cytoplasmic Ca with hysteresis evident in INCX between the rising and falling phases of Ca release. An alternative explanation for the increased SR Ca uptake is increased SERCA activity in CAL myocytes, although this seems unlikely since previous work has shown decreased SERCA activity in HF (16) and this alone would not explain the lack of effect of DT on the contribution of different efflux pathways to Ca removal.
It is also notable that despite the decreased percent contribution of NCX to Ca removal in CAL myocytes, kNCX was not significantly different from that in Sham cells. Thus it appears that NCX can rapidly remove Ca from the cytoplasm in the absence of a functional SR (kNCX), but its fractional contribution is decreased, presumably because its ability to compete with SERCA is decreased as a result of its relocation.
This redistribution of INCX is also likely to be important because HF is associated with disorganization and loss of T tubules (13, 21–23) and redistribution of ICa (and thus Ca release) from the T tubules to the surface membrane. The present work shows that t-tubular INCX/ICa density is higher in CAL myocytes than in Sham, suggesting that loss of T tubules will lead to greater loss of Ca efflux, compared with influx, in CAL myocytes, and thus greater Ca accumulation consistent with the observed effect of DT on SR Ca content in these cells. Thus loss of T tubules in HF may result in increased SR Ca content, which will increase both systolic Ca release and the probability of spontaneous SR Ca release and thus of DADs.
The proximity of the majority of NCX adjacent to RyRs at the T tubules may also be important in the genesis of arrhythmias due to activation of NCX by spontaneous SR Ca release in conditions of Ca overload (28). The hysteresis between bulk cytoplasmic Ca and INCX observed during application of caffeine or during spontaneous Ca release (35; Fig. 3) is consistent with Ca released from SR having privileged access to NCX. The observation that DT of Sham myocytes decreased this hysteresis suggests that it arises at the T tubules as a result of the proximity of the majority of NCX to the site of Ca release in the T tubules. However, this hysteresis was evident in CAL and DT CAL myocytes, so that it appears to be occurring at the surface of these cells. The hysteresis in CAL cells cannot be explained by a change in [Ca]rest altering the calibration of [Ca]i, which would alter the x-axis gain of the hysteresis loops, but the hysteresis would remain. It seems likely, therefore, that the hysteresis in CAL myocytes is due to the redistribution of INCX to the surface membrane, resulting in enhanced INCX in response to Ca at the cell surface, which itself may be increased by the observed redistribution of ICa, even in the apparent absence of changes in RyR distribution (7). Thus it appears that privileged access occurs at the cell surface in CAL myocytes, so that loss of T tubules in HF may not protect against DADs, which may be generated at the cell surface and exacerbated by the increase in SR Ca content that accompanies loss of T tubules in these cells.
Conclusions.
These data suggest that the cellular distribution of NCX is altered in CAL myocytes and that this will alter NCX activity both directly, by altering the proximity of NCX to the site of SR Ca release, and indirectly, by increasing SR Ca uptake, both in intact myocytes, by decreasing the ability of NCX to compete with SERCA, and following loss of T tubules, which will result in greater loss of NCX than ICa. These changes will alter INCX and thus action potential configuration, Ca balance, and the probability, magnitude and site of DAD generation in HF.
GRANTS
This work was funded by British Heart Foundation Grants PG/10/91/28644, PG/14/65/31055, and RG/12/10/29802.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.C.G. and S.M.B. performed experiments; H.C.G. analyzed data; H.C.G. prepared figures; H.C.G., S.M.B., A.F.J., and C.H.O. edited and revised manuscript; S.M.B., A.F.J., and C.H.O. interpreted results of experiments; A.F.J. and C.H.O. conception and design of research; A.F.J. and C.H.O. approved final version of manuscript; C.H.O. drafted manuscript.
REFERENCES
- 1.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol 476: 279–293, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Brette F, Despa S, Bers DM, Orchard CH. Spatiotemporal characteristics of SR Ca2+ uptake and release in detubulated rat ventricular myocytes. J Mol Cell Cardiol 39: 804–812, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Brette F, Komukai K, Orchard CH. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol Heart Circ Physiol 283: H1720–H1728, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology 22: 167–173, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bryant S, Kimura TE, Kong CH, Watson JJ, Chase A, Suleiman MS, James AF, Orchard CH. Stimulation of ICa by basal PKA activity is facilitated by caveolin-3 in cardiac ventricular myocytes. J Mol Cell Cardiol 68: 47–55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant SM, Kong CH, Watson J, Cannell MB, James AF, Orchard CH. Altered distribution of ICa impairs Ca release at the t-tubules of ventricular myocytes from failing hearts. J Mol Cell Cardiol 86: 23–31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca]i during excitation-contraction coupling in cardiac myocytes. Biophys J 67: 1942–1956, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capasso JM, Li P, Anversa P. Cytosolic calcium transients in myocytes isolated from rats with ischemic heart failure. Am J Physiol Heart Circ Physiol 265: H1953–H1964, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Chase A, Orchard CH. Ca efflux via the sarcolemmal Ca ATPase occurs only in the t-tubules of rat ventricular myocytes. J Mol Cell Cardiol 50: 187–193, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Guo A, Zhang C, Chen R, Zhu Y, Hong J, Kutschke W, Zimmerman K, Weiss RM, Zingman L, Anderson ME, Wehrens XH, Song LS. Critical roles of junctophilin-2 in T-tubule and excitation-contraction coupling maturation during postnatal development. Cardiovasc Res 100: 54–62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke JD, Caldwell JL, Horn MA, Bode EF, Richards MA, Hall MCS, Graham HK, Briston SJ, Greensmith DJ, Eisner DA, Dibb KM, Trafford AW. Perturbed atrial calcium handling in an ovine model of heart failure: Potential roles for reductions in the L-type calcium current. J Mol Cell Cardiol 79: 169–179, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crossman DJ, Ruygrok PN, Ruygrok PR, Soeller C, Cannell MB. Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS One 6: e17901, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Despa S, Brette F, Orchard CH, Bers DM. Na/Ca exchange and Na/K-ATPase function are equally concentrated in transverse tubules of rat ventricular myocytes. Biophys J 85: 3388–3396, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisk M, Koivumäki JT, Norseng PA, Maleckar MM, Sejersted OM, Louch WE. Variable t-tubule organization and Ca2+ homeostasis across the atria. Am J Physiol Heart Circ Physiol 307: H609–H620, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res 37: 279–289, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Holt E, Tønnessen T, Lunde PK, Semb SO, Wasserstrom JA, Sejersted OM, Christensen G. Mechanisms of cardiomyocyte dysfunction in heart failure following myocardial infarction in rats. J Mol Cell Cardiol 30: 1581–1593, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Kass RS, Lederer WJ, Tsien RW, Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol 281: 187–208, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Stagg MA, Fukushima S, Soppa GK, Siedlecka U, Youssef SJ, Suzuki K, Yacoub MH, Terracciano CM. Adult progenitor cell transplantation influences contractile performance and calcium handling of recipient cardiomyocytes. Am J Physiol Heart Circ Physiol 296: H927–H936, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljubojević S, Walther S, Asgarzoei M, Sedej S, Pieske B, Kockskämper J. In situ calibration of nucleoplasmic versus cytoplasmic Ca concentration in adult cardiomyocytes. Biophys J 100: 2356–2366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca release with loss of T-tubules-a comparison to Ca release in human failing cardiomyocytes. Cardiovasc Res 62: 63–73, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Louch WE, Mørk HK, Sexton J, Strømme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca release in murine cardiomyocytes following myocardial infarction. J Physiol 574: 519–533, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci USA 106: 6854–6859, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musa H, Lei M, Honjo H, Jones SA, Dobrzynski H, Lancaster MK, Takagishi Y, Henderson Z, Kodama I, Boyett MR. Heterogeneous expression of Ca handling proteins in rabbit sinoatrial node. J Histochem Cytochem 50: 311–324, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Negretti N, O'Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc Res 27: 1826–1830, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Negretti N, Varro A, Eisner DA. Estimate of net calcium fluxes and sarcoplasmic reticulum calcium content during systole in rat ventricular myocytes. J Physiol 486: 581–591, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327: 1653–1657, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Orchard CH, Bryant SM, James AF. Do t-tubules play a role in arrhythmogenesis in cardiac ventricular myocytes? J Physiol 591: 4141–4147, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page E, McCallister LP. Quantitative electron microscopic description of heart muscle cells. Application to normal, hypertrophied and thyroxin-stimulated hearts. Am J Cardiol 31: 172–181, 1973. [DOI] [PubMed] [Google Scholar]
- 30.Pásek M, Simurda J, Orchard CH. Effect of Ca2+ efflux pathway distribution and exogenous Ca2+ buffers on intracellular Ca2+ dynamics in the rat ventricular myocyte: a simulation study. Biomed Res Int 2014: 920208, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev 12: 331–343, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Shacklock PS, Wier WG, Balke CW. Local Ca2+ transients (Ca2+ sparks) originate at transverse tubules in rat heart cells. J Physiol 487: 601–608, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trafford AW, Díaz ME, Eisner DA. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflügers Arch 437: 501–503, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Trafford AW, Diaz ME, Negretti N, Eisner DA. Enhanced Ca2+ current and decreased Ca2+ efflux restore sarcoplasmic reticulum Ca2+ content after depletion. Circ Res 81: 477–484, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J Physiol 488: 577–586, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol 46: 463–473, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Zhang XQ, Moore RL, Tenhave T, Cheung JY. [Ca2+]i transients in hypertensive and postinfarction myocytes. Am J Physiol Cell Physiol 269: C632–C640, 1995. [DOI] [PubMed] [Google Scholar]