Abstract
Estrogen regulates the expression of many genes and has been correlated with differences in cardiac contraction; however, the underlying mechanisms remain poorly defined. Adrenomedullin (Adm = gene; AM = protein) is a multifunctional peptide with inotropic actions. Previous studies have demonstrated that estrogen enhances the expression of Adm, suggesting a relationship between AM and estrogen in cardiac contraction during physiological and pathological states. In this study, female mice in a mouse model of genetic Adm overexpression, abbreviated as Admhi/hi, were found to express 60 times more Adm in the heart than wild-type littermates, compared with the three-fold elevation of Adm previously reported in Admhi/hi male hearts. Thus, this study sought to further investigate any functional consequences of increased cardiac Adm expression and begin exploring the mechanisms that regulate Adm expression in an estrogen-dependent fashion. This study revealed that heart function is enhanced in Admhi/hi females, which along with Adm expression levels, was reversed following ovariectomization. Since the Admhi/hi line was generated by the displacement of the 3′ untranslated region (UTR), the native 3′UTR was examined for estrogen-induced microRNAs target sites to potentially explain the aberrant overexpression observed in Admhi/hi female hearts. Using a bioinformatic approach, it was determined that the mouse Adm 3′UTR contains many target sites for previously characterized estrogen-induced microRNAs. This study also determined that the novel microRNA, miR-879, is another estrogen-induced microRNA that interacts with the 3′UTR of Adm to destabilize the mRNA. Together, these studies revealed that estrogen-induced microRNAs are important for balancing cardiac Adm expression in females.
Keywords: estrogen, microRNAs, adrenomedullin, contractility
the steroid hormone 17β-estradiol, referred to herein as estrogen, plays an important role in the regulation of gene transcription. Several mechanisms have been described by which estrogen can regulate gene expression. In the classical model of estrogen signaling, estrogen interacts with cytoplasmic estrogen receptor-alpha (ERα) or ER-β (ERβ), resulting in translocation of the estrogen-ER complex to the nucleus (7), where this complex regulates gene transcription. Moreover, phosphorylation of estrogen receptors can result in ligand-independent activation and subsequent gene expression regulation (52). Finally, estrogen can activate estrogen receptors bound to the plasma membrane (41) or G protein-coupled estrogen receptor-1 (18), resulting in activation of downstream signaling and subsequent gene expression changes. Together, the multitude of pathways by which estrogen can influence cellular functions and gene transcription underscores the complexity of estrogen-mediated actions in physiological and pathological states.
MicroRNAs are important regulators of gene expression and, thus, protein levels within cells. These short DNA fragments work primarily to repress gene expression by interacting with the 3′ untranslated region (3′UTR) to either prevent translation of the mRNA into protein or to promote degradation of the mRNA (4). Recently, several groups have demonstrated that estrogen can regulate the expression of microRNAs (5, 12, 14, 37, 39). Since sex-dependent differences are apparent in many disease states, researchers began to ask whether the expression of microRNAs varies between the sexes during disease. Indeed, several studies have demonstrated that some diseases that differ in susceptibility or severity between the sexes also demonstrate a difference in microRNA expression profiles (reviewed in Ref. 45). Although cardiovascular disease susceptibility and outcome differ between the sexes, the expression profile of microRNAs in the heart during disease has not yet been assessed or compared between the sexes. Because of the tendency for families of microRNAs to target multiple genes within a particular network (34), there has been a surge in interest regarding the role of microRNAs during cardiovascular disease, since therapies may be more effective if multiple components of a pathway can be targeted at one time.
For many years, researchers have recognized that differences exist between males and females with regard to cardiac function in both physiological and pathological scenarios (13, 20, 24, 32, 40). For instance, several studies have noted that healthy females have higher ejection fractions compared with age-matched males (13, 40). Interestingly, several studies have demonstrated that women are more likely to have heart failure with preserved ejection fraction than men (20, 24), indicating a role for female sex hormones in systolic heart function. In addition to the effect of female sex hormones on ejection fraction, work by Locati et al. (32) revealed that females are at increased risk for long QT syndrome, indicating that sex hormones might contribute to disease pathology. Animal studies have demonstrated that sex hormones, and, in particular, estrogen, can directly promote an increase in the QT interval (16, 44), confirming the sex-dependent differences observed in humans. Together, these findings, along with studies demonstrating that ERα and ERβ are expressed in endothelial cells (17), smooth muscle cells (43), cardiomyocytes, and fibroblasts (22), underscore a widespread role for female sex hormones in the regulation of cardiac contraction.
Cardiomyocyte contraction is dependent on the generation of an action potential, which is regulated by the flow of ions between the cardiomyocyte and the extracellular space through ion channels. Thus, disruptions in normal ion flow, either by altered levels of the ion channels or mutations in the ion channel components, can impact cardiomyocyte contraction and heart function. In animal models of long QT syndrome, estrogen was found to decrease the levels of several potassium channels in the heart (16, 44), leading to the lengthening of the QT interval and arrhythmias. In humans, many ion channels in the heart are differentially regulated with sex, including the downregulation of several potassium channels in the female heart (19). Together, these studies highlight the sex-dependent differences in cardiac function, mediated, in part, by differences in ion channel expression. Undoubtedly, a further understanding of the effects of estrogen on the cardiovascular system is necessary for developing effective therapeutics for women.
Adrenomedullin (Adm = gene; AM = protein) is a secreted peptide with a multitude of functions (9, 11, 15, 25, 29, 30, 35, 54). Specifically, in the heart, several groups have described an inotropic role for AM (25, 49, 50), although these findings have been controversial (26, 48). These and other studies have indicated that the positive inotropic effect of AM is mediated through calcium channels (6, 50). Specifically, Szokodi et al. (50) found that the positive inotropic effect of AM was dependent on the release of intracellular calcium stores from the sarcoplasmic reticulum, as well as the influx of calcium through L-type calcium channels. During many human disease conditions, including cardiovascular disease, circulating levels of AM in the plasma are significantly elevated above baseline levels (28). Thus, the inotropic role of AM (25, 49, 50), along with other beneficial properties of AM in the heart (2, 10, 46), indicate that the observed elevation of AM in the plasma during cardiovascular disease most likely serves to mitigate the extent of damage. Interestingly, Adm expression is enhanced by estrogen through ERα-dependent mechanisms (55), indicating that AM signaling may represent a novel mechanism by which estrogen improves cardiovascular outcomes in females. In this study, the fortuitous discovery of drastic Adm overexpression in the heart of female Admhi/hi mice was explored. Specifically, since the native Adm 3′UTR was displaced with the generation of the Admhi/hi line, the possibility of estrogen-dependent negative regulation of Adm at the 3′UTR was examined.
MATERIALS AND METHODS
Mice.
The generation of Admhi/hi mice has been documented previously (31, 56). Briefly, gene targeting techniques were used to insert the bovine growth hormone (bGH) 3′UTR upstream of the native Adm 3′UTR, thereby stabilizing the Adm mRNA. Mice used in these studies were either from a mixed genetic background (129S6/SvEv × C57BL6/J) or backcrossed to the C57BL6/J strain for over nine generations. For all experiments, littermate animals were used as controls. To assess the impact of endogenous estrogen on expression levels of Adm wild-type animals from the 129S6/SvEv background, referred to as SvEV, were utilized. Ovariectomized C57BL6/J females and intact controls during estrus and diestrus were used to assess the impact of endogenous estrogen on expression of Eig121l and miR-879.
Ovariectomy was performed to remove the influence of endogenous hormones on gene expression. Mice were 21 to 28 days old at the time of ovariectomy. Mice were anesthetized with avertin (400–500 mg/kg body wt) and placed on a warm heating pad to maintain body temperature throughout the surgical procedure. Hair was removed from the abdomen and a small <1-cm incision was made through the abdominal wall about 1 cm to the left of the spinal cord. The ovary was exteriorized and placed on a sterile drape. Silk sutures were used to ligate the oviduct and anterior connective tissue prior to excising the ovary. The body wall was then sutured with dissolvable sutures, and wound clips were used to close the skin. The procedure was repeated on the animal's right side. Wound clips are removed 4 or 5 days after surgery, and 4 wk later, the ovariectomized mice were used in experiments.
Transthoracic echocardiography was performed on Admhi/hi and littermate wild-type mice under anesthesia (3% isoflurane) with a VisualSonics Vevo770 high-resolution imaging system.
All studies were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Gene expression analysis.
Gene expression was analyzed by quantitative (q)RT-PCR with the Mx3000P real-time PCR System from Stratagene. Adm (RefSeq ID: NM_009627.1) gene expression was assessed using primers and a probe described previously (56). TaqMan gene expression assays for EIG121L (RefSeq ID: NM_172706.3, cat. no. 4351372), miR-879 (RefSeq ID: NR_030537.1, cat. no. 4427975), Gapdh (RefSeq ID: NM_001289726.1, cat. no. 4308313) and snoRNA-202 (cat. no. 4427975), were purchased from Applied Biosystems. Samples were assessed three times in triplicate. Relative levels of gene expression were determined using the ΔΔCt method.
Plasmids, mutagenesis, transfection, and luciferase assay.
Seven hundred base pairs of the 3′UTR region of the mAdm gene (112 bp after Adm stop codon) were cloned into the Xba I site of pGL3-promoter vector (Promega, E1761). The QuikChange II site-directed mutagenesis kit from Stratagene was used to introduce specific point mutations in the miR-25 and miR-879 binding sites. MicroRNA-25 (AM17100-PM10584), microRNA-879 (AM17100-PM12276), and a scrambled negative control microRNA (AM4611) were ordered from Ambion. Human embryonic kidney (HEK) 293T cells were transfected with 10 nM microRNAs, 250 ng/ml plasmids, and 5 ng/ml Renilla luciferase (Rluc) reporter vectors using Lipofectamine 2000 (Invitrogen, 11668-027), in accordance with the manufacturer's protocol. Forty-eight hours after transfection, the cells were lysed and luciferase activities were measured with the Dual-Glo luciferase assay system (Promega, E2920).
Statistics.
Statistical analyses were performed with JMP software (SAS). One-way ANOVA was used to determine significance at P < 0.05. In all figures, error bars represent means ± SE.
RESULTS
Large, estrogen-dependent increase of Adm in female Admhi/hi heart.
Previous studies have characterized the generation of the Adm overexpression model (31), referred to as Admhi/hi mice, as well as the developmental (56) and reproductive phenotypes (31) observed in this line. These mice were generated by the gene-targeted insertion of the stable bovine growth hormone (bGH) 3′UTR upstream of the unstable native Adm 3′UTR (31), thereby resulting in accumulation of Adm mRNA and increased expression. Through this approach, 5′UTR regulatory elements remain unchanged, thereby preventing disruption of the native regulatory elements of Adm. This approach, however, does disrupt the native 3′UTR regulatory elements with the insertion of the bGH 3′UTR. When the expression levels of Adm in female tissues were compared with the levels previously reported in males (56), similar degrees of Adm overexpression in the adrenal gland, reproductive organs, intestine, skin, liver, brain, kidney, and spleen were observed between the two sexes (Fig. 1A). However, upon examination of Adm expression in the heart, a 60-fold increase in Adm was observed in Admhi/hi female hearts relative to wild-type littermates (Fig. 1A). This 60-fold increase in expression was reduced to a four-fold increase of expression with ovariectomization of female animals, which is comparable to the increase observed in male Admhi/hi mice (56). When Adm expression in the spleen and brain, two tissues with similar overexpression of Adm between males and females, was compared between intact and ovariectomized females, no change in Adm overexpression was observed (Fig. 1B). To determine whether endogenous changes in estrogen can regulate Adm expression in vivo, an assessment of Adm expression levels from heart and uterus samples of wild-type 129S6/SvEv, abbreviated as SvEV, female mice in either diestrus or estrus, the low and high phases of the estrus cycle, respectively, was conducted. This approach revealed a modest, but significant, increase of Adm transcript levels in the hearts and uteri of estrus females compared with diestrus females (Fig. 1C), indicating that changes in endogenous estrogen levels can modulate Adm expression in vivo.
Fig. 1.
Adm expression is drastically upregulated in the heart in an estrogen-dependent manner. A: quantitative RT-PCR (qRT-PCR) analysis for Adm from intact Adm+/+ and Admhi/hi females normalized to calibrator. Additionally, Adm expression was analyzed by qRT-PCR in the hearts of ovariectomized (OVX) Adm+/+ and Admhi/hi female mouse hearts and normalized to calibrator. B: qRT-PCR analysis of Adm levels from intact or OVX Adm+/+ and Admhi/hi females in brain and spleen. C: qRT-PCR analysis of Adm levels from intact wild-type SvEV heart and uterus samples isolated during diestrus and estrus. All values represent means ± SE. *P < 0.05, **P < 0.01, ***P < 0.0001.
Enhanced contractility of Admhi/hi females is reversed with ovariectomy.
Cardiac function was compared between intact and ovariectomized wild-type and Admhi/hi females using echocardiography. As the results in Table 1 demonstrate, Admhi/hi females have enhanced heart function, as determined by the ejection fraction and fractional shortening, compared with wild-type littermates, consistent with the published inotropic properties of AM (25, 50). Additionally, ovariectomy of females prior to the onset of puberty reverted the heart function of Admhi/hi females to wild-type levels, indicating that the robust cardiac overexpression of Adm was responsible for the enhanced function. Furthermore, assessment of male cardiac function by echocardiography revealed no significant difference in ejection fraction between wild-type and Admhi/hi males (42.42% ± 5.49 vs. 42.19% ± 3.04), indicating that a three- to four-fold increase of cardiac AM is insufficient for altering cardiac function.
Table 1.
Blood pressure and echocardiogram data
| Adm+/+ ♀ | Admhi/hi♀ | Adm+/+-OVX | Admhi/hi-OVX | |
|---|---|---|---|---|
| n | 6 | 6 | 8 | 9 |
| MAP, mmHg | 124.68 ± 5.68 | 114.25 ± 23.78 | ND | ND |
| Heart rate | 505.67 ± 6.38 | 471.4 ± 15.64 | 505 ± 15.98 | 477.22 ± 14.73 |
| LVED, d, mm | 3.83 ± 0.07 | 4.17 ± 0.15 | 3.94 ± 0.14 | 3.62 ± 0.18† |
| LVED, s, mm | 3.01 ± 0.04 | 2.98 ± 0.12 | 3.03 ± 0.1 | 2.81 ± 0.13 |
| LVPW, d, mm | 0.7 ± 0.03 | 0.73 ± 0.04 | 0.73 ± 0.03 | 0.76 ± 0.04 |
| LVPW, s, mm | 0.92 ± 0.04 | 1.01 ± 0.02 | 0.97 ± 0.04 | 0.92 ± 0.04 |
| LV% FS | 20.32 ± 1.49 | 27.65 ± 1.47* | 21.36 ± 1.02 | 22.1 ± 1.41† |
| LV% EF | 41.97 ± 2.63 | 53.63 ± 2.33* | 43.78 ± 1.74 | 45.15 ± 2.38† |
| LV Vol, d, μl | 66.26 ± 2.67 | 80.82 ± 6.65 | 70.76 ± 5.87 | 61.53 ± 7.14 |
| LV Vol, s, μl | 38.24 ± 1.4 | 37.34 ± 3.66 | 39.58 ± 3.15 | 33.14 ± 3.63 |
| CO, ml/min | 14.24 ± 1.44 | 20.36 ± 1.85* | 15.78 ± 1.82 | 13.37 ± 1.82† |
MAP, mean arterial pressure; LVED, left ventricular end diameter; LVPW, left ventricle posterior wall; LV, left ventricle; %FS, percent fractional shortening; %EF, percent ejection fraction; CO, cardiac output; d, diastolic; s, systolic.
P < 0.05 compared with Adm+/+ females;
P < 0.05 compared with Admhi/hi females.
Estrogen drives the expression of miR-879, which targets the Adm 3′UTR.
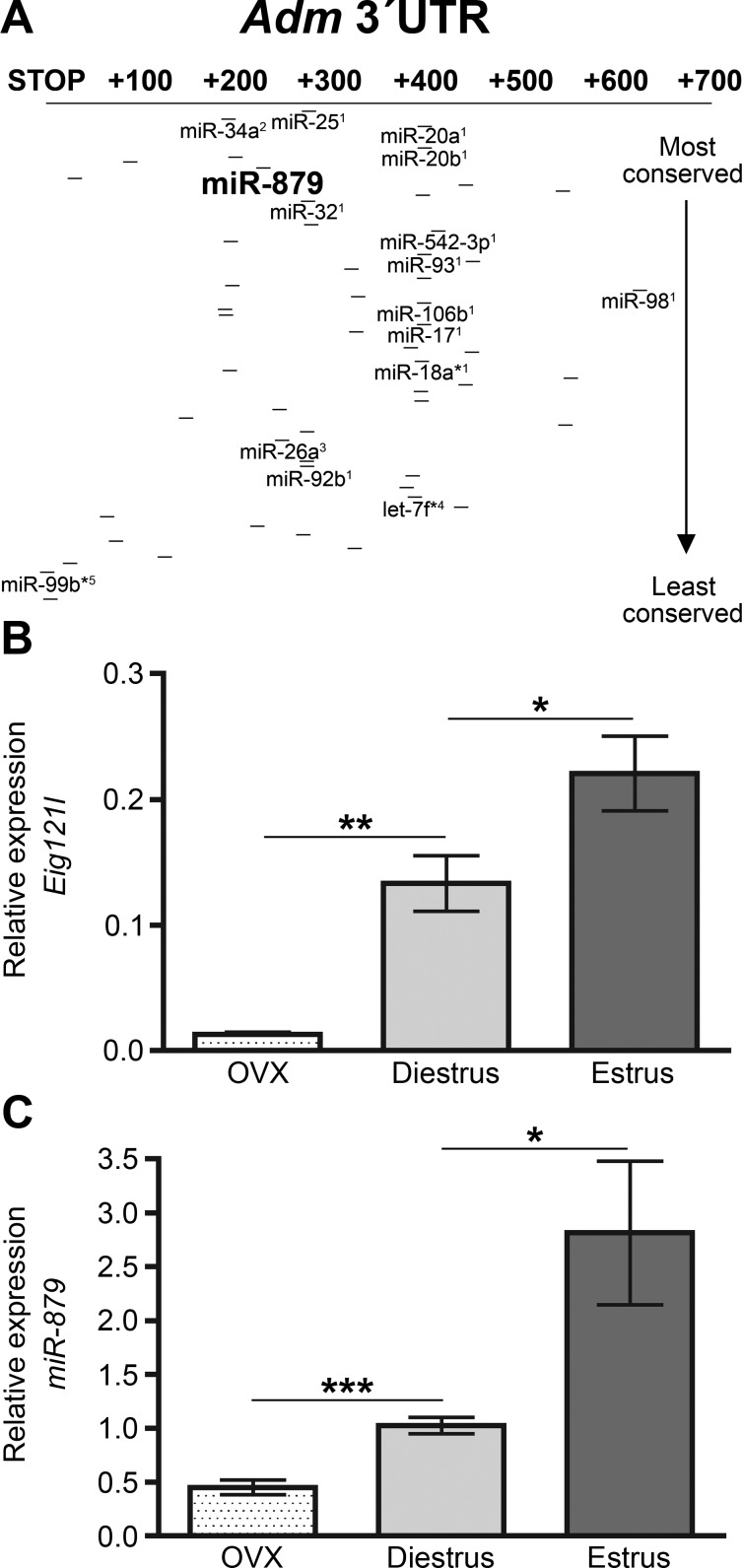
Under basal conditions, Adm expression levels differ in a sex-dependent manner in the heart, highlighting the importance of estrogen regulation of endogenous Adm. Compared with the expression of Adm in the hearts of wild-type males (data previously published in Ref. 56), intact wild-type females express significantly more Adm. This sex-dependent difference in Adm expression in wild-type animals, however, is only about 5-fold, not the 50-fold difference observed between Admhi/hi males (reported in Ref. 56) and intact Admhi/hi females. Consistent with the notion that estrogen mediates Adm expression in an estrogen-dependent manner in the heart, ovariectomization of wild-type females results in Adm expression levels indistinguishable from wild-type males, while ovariectomization of Admhi/hi females results in a four-fold elevation of Adm expression over wild-type animals, which is comparable to the difference observed between male wild-type and Admhi/hi mice (56). Together, these data suggested that estrogen acts on both the 5′UTR and 3′UTR of Adm to provide fine-tuned control of Adm expression in response to hormonal cues. Therefore, the hypothesis was put forth that estrogen-induced microRNAs target Adm to decrease expression levels in the heart in the presence of estrogen and that by inserting the bGH 3′UTR upstream of the native Adm 3′UTR, this important regulatory process had been disrupted. In support of this hypothesis, several groups have identified microRNAs that are, indeed, induced by estrogen (5, 12, 14, 37, 39), and so, the 3′UTR of Adm was examined for any predicted target sites of microRNAs that are upregulated by estrogen. Of the 54 microRNAs that target the mouse Adm 3′UTR, 15 of these predicted target sites were for estrogen-induced microRNAs (Fig. 2A). During this analysis, a target site for the poorly characterized microRNA, miR-879, was noticed in the Adm 3′UTR. This particular microRNA was of interest because it is located within a gene called estrogen-induced gene 121-like, abbreviated as Eig121l. To determine the effect of estrogen on these genes, the relative expression of Eig121l and miR-879 from heart lysates of ovariectomized, diestrus, and estrus females was assessed. This analysis revealed that with increasing levels of endogenous estrogen, the expression of Eig121l (Fig. 2B) and miR-879 (Fig. 2C) increased significantly.
Fig. 2.
Regulation of Adm by estrogen-regulated microRNAs. A: schematic of the microRNA binding sites located in the Mus musculus 3′UTR of Adm as identified by the MicroCosm Targets database (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/). Lines indicate microRNA binding sites, while bold microRNA names indicate estrogen-regulated microRNAs. 1Castellano et al. (12); 2Chung et al. (14); 3Pan et al. (39); 4Bhat-Nakshatri et al. (5); 5Nothnick and Healy (37). B: qRT-PCR analysis for Eig121l from OVX, diestrus, and estrus C57BL6/J female hearts normalized to mouse calibrator. C: qRT-PCR analysis of miR-879 expression from the hearts of OVX, intact diestrus, and intact estrus C57BL6/J females. Expression of miR-879 was normalized to sno-202 and relative expression was calculated using the ΔΔCT method. All values are expressed as means ± SE. *P < 0.05, **P < 0.001, ***P < 0.0001.
MicroRNA-879 destabilizes Adm through its predicted target site.
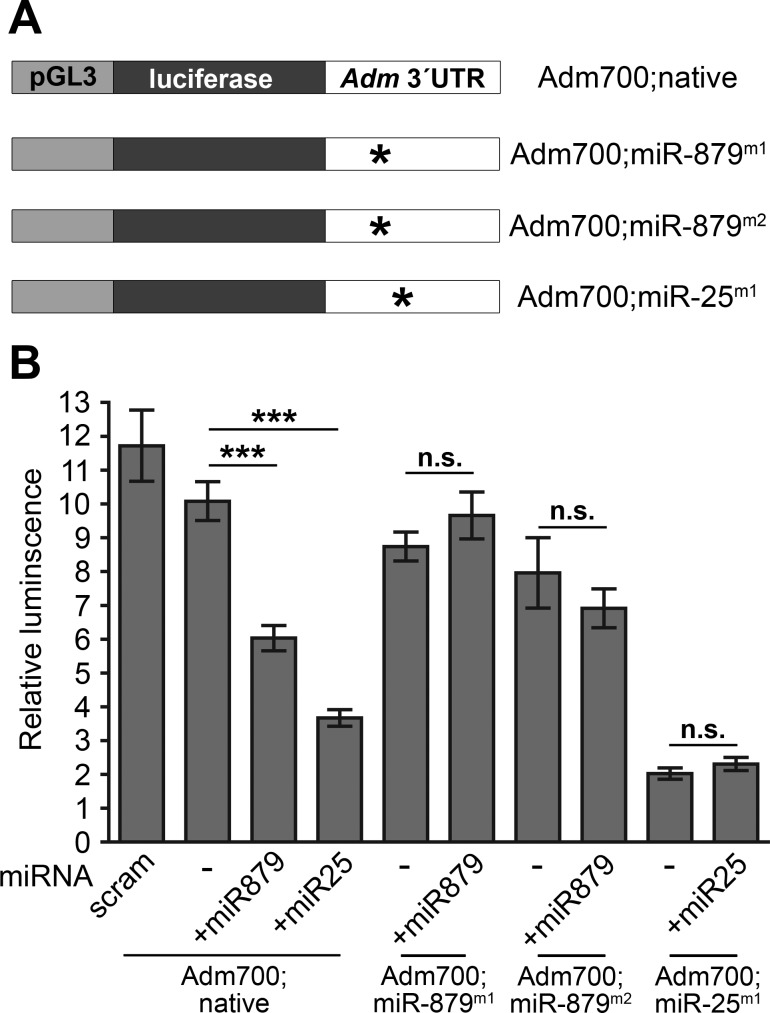
Having confirmed that miR-879 is upregulated by endogenous estrogen in the heart, the next objective was to demonstrate that this microRNA can, indeed, interact with the 3′UTR of Adm to reduce transcript levels. To accomplish this, the luciferase gene within the pGL3-promoter vector was stabilized with 700 base pairs of the mouse Adm 3′UTR, labeled as Adm700;native (Fig. 3A), thereby allowing for the use of the relative amount of luciferase luminescence as a readout of Adm gene stability. To demonstrate that miR-879 works through the predicted target site in the Adm 3′UTR, two vectors were generated in which separate single point mutations were created within the predicted target site for miR-879, designated as Adm700;miR-879m1 and Adm700;miR-879m2 (Fig. 3A). Additionally, a vector was created with a single point mutation in the predicted target site for miR-25, the positive control of Adm destabilization for this experiment, represented as Adm700;miR25m1 (Fig. 3A). The reason for selecting this microRNA as a positive control was twofold; first, miR-25 is the most highly conserved microRNA to target the Adm 3′UTR, and second, miR-25 is also regulated by estrogen (12), further supporting the hypothesis that estrogen-driven microRNAs help balance Adm expression in the female heart. As shown in Fig. 3B, transfection with the Adm700;native vector and either miR-879 or miR-25 resulted in a significant reduction in the amount of luciferase luminescence normalized to the total amount of Renilla luminescence, indicating that both microRNAs can act on the Adm 3′UTR to destabilize expression. This approach also demonstrated that these microRNAs were acting specifically through the predicted target sites, since transfection of the mutated vectors, along with the proper microRNA, did not alter the relative luminescence (Fig. 3B).
Fig. 3.
Estrogen-regulated microRNA-879 negatively regulates Adm expression. A: graphical representation of luciferase vectors used for assessing regulation of Adm by microRNAs. Adm700;native refers to the modified pGL3-promoter vector in which luciferase expression was stabilized by 700-bp of the mouse Adm 3′UTR. Two separate sets of point mutations were made to the predicted miR-879 target sites in the Adm700 construct and are designated as Adm700;miR-879m1 and Adm700;miR-879m2. Additionally, the target site for miR-25 was mutated and is designated as Adm700;miR-25m1. Asterisks indicate location of point mutations. B: relative luminescence was calculated by normalizing luciferase luminescence to Renilla luminescence. Scram refers to the scrambled negative control. All values represent means ± SE. n.s., not significant. ***P < 0.0001.
DISCUSSION
On the basis of the findings in this study, a balanced model of the regulation of Adm by estrogen is proposed (Fig. 4). To summarize, previous studies have demonstrated that Adm expression is upregulated by estrogen through ERα (55). The current study indicates that under normal circumstances, estrogen drives the expression of a cohort of microRNAs, including miR-879, that target the Adm 3′UTR, resulting in balanced Adm expression. In the case of the Admhi/hi mice, the introduction of the bGH 3′UTR most likely displaced the native target sites for these estrogen-induced microRNAs, rendering them ineffective in balancing the positive transcriptional force of estrogen from the 5′UTR of Adm, resulting in 60-fold overexpression of Adm in the female heart. Additionally, results from the luciferase assay revealed that modification of even one base pair within the predicted target site for the estrogen-induced microRNA miR-25 severely destabilized Adm expression, indicating that this microRNA may play a critical role in the regulation of Adm, although this requires further study. This study also found that overexpression of Adm had physiological consequences, including enhanced contractility of cardiomyocytes. Although this study cannot rule out the possibility that the modification of the Adm 3′UTR with the generation of the Admhi/hi line has altered the responsiveness of the promoter due to displacement of repressive cis-acting elements, the large number of estrogen-induced microRNAs that target the Adm 3′UTR indicates an important role for estrogen-induced microRNAs in Adm gene regulation.
Fig. 4.
Model of Adm regulation and physiology. ERα, estrogen receptor-α; Adm, adrenomedullin; Eig121l, estrogen-induced gene 121-like; miR-879, microRNA-879.
Previous work has demonstrated that exogenous administration of estrogen induces Adm expression in the uterus through ERα (55). However, whether endogenous changes in estrogen are sufficient to induce changes in Adm expression remained unclear. The work presented here revealed small, but significant, changes in endogenous Adm within the heart and uterus during different stages of the estrous cycle, although whether these small changes result in physiological consequences remains unanswered. Given that ejection fraction in female Admhi/hi mice was increased only when Adm was overexpressed by 60-fold, it seems unlikely that minor changes in Adm expression levels will greatly impact normal physiological functions. Nonetheless, the possibility exists that estrogen induces a robust increase of cardiac Adm expression during pathological conditions to elicit appreciable effects. Thus, estrogen may regulate cardiac AM in a meaningful manner following injury. However, to firmly establish the role of estrogen in Adm expression, additional studies will be needed. For example, genetic deletion of estrogen receptors and/or select microRNAs in cultured cardiomyocytes or epicardial cells could provide a useful in vitro approach to validate these gene regulatory pathways.
Several clinical studies have demonstrated that circulating levels of AM in the blood plasma are elevated during many human disease conditions, including cardiovascular disease (reviewed in Ref. 28), which is generally thought to improve heart function due to the many beneficial properties of AM in the cardiovascular system (reviewed in Ref. 27). Interestingly, Tambara et al. (51) found that levels of AM in the pericardial fluid are more robustly increased following myocardial infarction compared with the levels in the blood plasma. Although these data were not analyzed with respect to the sex of the study participants, it suggests that cardiac-derived AM is elevated to a greater extent than systemic levels following cardiac injury.
This study revealed that, while Adm overexpression is similar between male and female Admhi/hi animals in the majority of tissues, a robust, estrogen-dependent increase in Adm overexpression is observed exclusively in the hearts of Admhi/hi females. The reasons for the cardiac restriction of this estrogen-dependent increase in Adm remain unclear, although several possibilities exist. One potential explanation is that Adm expression may not be induced by estrogen in other organs of the body under physiological conditions. Perhaps, with various stressors during pathological conditions, Adm expression may be enhanced in the presence of estrogen; however, this remains untested. Additionally, it is possible that the estrogen-induced microRNAs targeting the 3′UTR of Adm may be primarily expressed in the heart, indicating that cardiac Adm may be more sensitive to alterations in estrogen signaling. The lack of change in Adm expression within the brain and spleen following ovariectomy in both wild-type and Admhi/hi females indicates that estrogen may play a lesser role in regulating Adm expression in extra-cardiac tissues under physiological conditions, lending further support to this possibility. Clearly, the work presented here highlights the need for a more detailed analysis regarding the expression of estrogen-induced microRNAs across tissues during physiological and pathological conditions.
The fine-tuned balance of gene expression levels by microRNAs has emerged as a powerful and pervasive mechanism for controlling numerous biological processes from early development to pathological disease progression (1, 47). Indeed, the coordinated expression of microRNAs in response to cellular and physiological stimuli can be exploited to control both positive and negative regulatory factors within a biological pathway or signaling cascade (34). Thus, microRNAs can provide a buffer against large and detrimental variations in gene expression in response to (patho)physiological stimuli, such as estrogen. This study has revealed the importance of this type of balanced gene regulation for a particular estrogen-regulated gene, Adm, in the heart. Considering that it has been notoriously difficult to identify the downstream target genes of estrogen signaling that account for sex differences in cardiovascular disease susceptibility and progression (33), it seemed possible that similar microRNA regulatory mechanisms might be in place for other estrogen-regulated target genes with important roles in cardiovascular disease.
To test this theory, other targets of miR-879 were examined using the microRNA.org database, and it was immediately noted that the Kcnj2 gene, encoding the Kir2.1 potassium channel, was one of the top 10 genes predicted to be downregulated by miR-879. Kcnj2 is highly expressed in the heart (42) and contains species-conserved ERα binding sites within its promoter. Importantly, mutations in this gene are associated with Andersen syndrome (long QT syndrome type-7), of which a symptom is ventricular arrhythmia (53). Therefore, the cardiac function of miR-879 is likely to extend to additional target genes with roles in cardiac conductance and cardiovascular disease, and this will be an exciting area for future study.
It is noteworthy that numerous potassium and cardiac conductance ion channels are significantly downregulated in female hearts compared with males at the basal state (19). However, the regulatory mechanisms contributing to these sex differences in gene expression have yet to be explained. Therefore, the 3′UTRs of the cardiac conductance ion channels identified by Gaborit et al. (19) as being reduced in female hearts compared with males were examined for binding sites of microRNAs that are positively regulated by estrogen (12). This analysis also included KCNA5, a potassium channel differentially expressed with sex (8, 16), and KCND2, a potassium channel associated with long QT syndrome in mice (3). Interestingly, all of the ion channels analyzed contained four or more target sites, and half contained 10 or more target sites, for microRNAs that are upregulated by estrogen. The genes identified with a high proportion of target sites for estrogen-regulated microRNAs were ABCC4, KCNJ11, KCNE1, GJA1, KCNA5, and KCND2. Therefore, future studies to elucidate the molecular networks that underlie the sex-dependent differences in ion channel gene expression in the heart should include an assessment of estrogen-regulated microRNAs.
Finally, considering that the inotropic properties of AM on cardiomyocytes has been controversial (25, 26, 48, 50), the comparison of the contractile function in intact and ovariectomized Admhi/hi female mice herein helps to clarify the effects of AM dosage on cardiac contractility. This study found that the modest, but physiologically relevant, four-fold increase of AM in ovariectomized Admhi/hi females did not alter cardiac function, whereas in intact Admhi/hi females, estrogen drives Adm expression to levels 60 times greater than wild-type counterparts, which, in turn, significantly enhances cardiac function. With regard to the period immediately following the onset of myocardial infarction, it is, therefore, possible that the robust, yet transient, increases in AM, through the action of HIF-1 (21), may occur to help sustain cardiac function. But then, how persistently elevated plasma AM can ultimately be correlated to poor patient survival remains curious. It also remains understudied whether the clinically diagnostic plasma levels of AM in cardiovascular disease differ between men and women. The results from this study highlight the importance of carefully considering hormonal status when evaluating AM during cardiovascular disease. Results from this work underscore the importance of carefully evaluating estrogen-regulated genes and pathways, including microRNAs, as underlying factors contributing to sex differences in cardiovascular disease susceptibility and progression.
Perspectives and Significance
Hormonal signals, including estrogen, mediate gene expression changes that contribute to sex-dependent differences in physiological and pathological states. In this study, displacement of the native Adm 3′UTR in the generation of the Admhi/hi mouse line led to the unexpected appreciation of the important role played by estrogen-induced microRNAs in the regulation of Adm. Additionally, this study alludes to the presence of tissue-specific regulatory networks of microRNAs, as the 60-fold elevation of Adm observed in Admhi/hi females was restricted to the heart. Given the elevation of plasma AM levels during human disease (28) and the multitude of beneficial effects it imparts on the heart (reduced fibrosis, inotropic factor, anti-inflammatory) (10, 23, 36, 38, 46), future studies should examine the implications of this elevation of AM on cardiovascular pathology following an insult. Additionally, further work investigating the relative levels of estrogen-induced microRNAs across tissues should be conducted to enhance the understanding of the complex networks regulating Adm expression. Finally, further exploration into the role of estrogen-induced microRNAs in the regulation of cardiac ion channels should be investigated as a potential mechanism underlying sex-dependent differences in diseases involving cardiac contraction.
GRANTS
This article is supported by an American Heart Association Established Investigator Award and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) R01 DK-099156, and NIH/National Institute of Child Health and Human Development HD-060860 to K. M. Caron, as well as American Heart Association PRE11710002 to S. E. Wetzel-Strong.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.E.W.-S., M.L., and S.T.E. performed experiments; S.E.W.-S., M.L., S.T.E., and K.M.C. analyzed data; S.E.W.-S., M.L., S.T.E., and K.M.C. interpreted results of experiments; S.E.W.-S. prepared figures; S.E.W.-S. drafted manuscript; S.E.W.-S. and K.M.C. edited and revised manuscript; S.E.W.-S., M.L., S.T.E., and K.M.C. approved final version of manuscript; M.L. and K.M.C. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Claire Trincot and all other current and past members of the Caron Lab, as well as members of the Rodent Advanced Surgical Models Core for technical assistance and helpful discussions.
REFERENCES
- 1.Abdellatif M. Differential expression of microRNAs in different disease states. Circ Res 110: 638–650, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrick CJ, Lenhart PM, Dackor RT, Nagle E, Caron KM. Loss of receptor activity-modifying protein 3 exacerbates cardiac hypertrophy and transition to heart failure in a sex-dependent manner. J Mol Cell Cardiol 52: 165–174, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit. Circ Res 83: 560–567, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, Brown M, Hammond S, Srour EF, Liu Y, Nakshatri H. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res 37: 4850–4861, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisping E, Tenderich G, Barckhausen P, Stumme B, Bruns S, von Lewinski D, Pieske B. Atrial myocardium is the predominant inotropic target of adrenomedullin in the human heart. Am J Physiol Heart Circ Physiol 293: H3001–H3007, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19: 833–842, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Brouillette J, Rivard K, Lizotte E, Fiset C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res 65: 148–157, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Cao YN, Kuwasako K, Kato J, Yanagita T, Tsuruda T, Kawano J, Nagoshi Y, Chen AF, Wada A, Suganuma T, Eto T, Kitamura K. Beyond vasodilation: the antioxidant effect of adrenomedullin in Dahl salt-sensitive rat aorta. Biochem Biophys Res Commun 332: 866–872, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Caron K, Hagaman J, Nishikimi T, Kim HS, Smithies O. Adrenomedullin gene expression differences in mice do not affect blood pressure but modulate hypertension-induced pathology in males. Proc Natl Acad Sci USA 104: 3420–3425, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional adrenomedullin gene. Proc Natl Acad Sci USA 98: 615–619, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellano L, Giamas G, Jacob J, Coombes RC, Lucchesi W, Thiruchelvam P, Barton G, Jiao LR, Wait R, Waxman J, Hannon GJ, Stebbing J. The estrogen receptor-α-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci USA 106: 15,732–15,737, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, Canham RM, Levine BD, Drazner MH. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation 113: 1597–1604, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Chung TK, Cheung TH, Huen NY, Wong KW, Lo KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW, Yu MY, To KF, Mok SC, Wang VW, Li C, Cheung AY, Doran G, Birrer MJ, Smith DI, Wong YF. Dysregulated microRNAs and their predicted targets associated with endometrioid endometrial adenocarcinoma in Hong Kong women. Int J Cancer 124: 1358–1365, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clementi G, Caruso A, Cutuli VM, Prato A, Mangano NG, Amico-Roxas M. Antiinflammatory activity of adrenomedullin in the acetic acid peritonitis in rats. Life Sci 65: PL203–PL208, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation 94: 1471–1474, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Favre J, Gao J, Henry JP, Remy-Jouet I, Fourquaux I, Billon-Gales A, Thuillez C, Arnal JF, Lenfant F, Richard V. Endothelial estrogen receptor α plays an essential role in the coronary and myocardial protective effects of estradiol in ischemia/reperfusion. Arterioscler Thromb Vasc Biol 30: 2562–2567, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14: 1649–1660, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Gaborit N, Varro A, Le Bouter S, Szuts V, Escande D, Nattel S, Demolombe S. Gender-related differences in ion-channel and transporter subunit expression in non-diseased human hearts. J Mol Cell Cardiol 49: 639–646, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Galvao M, ADHERE. Scientific Advisory Committee (SAC) Investigators, Coordinators, and Study Group . Reshaping our perception of the typical hospitalized heart failure patient: a gender analysis of data from the ADHERE Heart Failure Registry. J Cardiovasc Nurs 20: 442–450, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Garayoa M, Martinez A, Lee S, Pio R, An WG, Neckers L, Trepel J, Montuenga LM, Ryan H, Johnson R, Gassmann M, Cuttitta F. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol Endocrinol 14: 848–862, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Grohe C, Kahlert S, Lobbert K, Stimpel M, Karas RH, Vetter H, Neyses L. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett 416: 107–112, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Hamid SA, Totzeck M, Drexhage C, Thompson I, Fowkes RC, Rassaf T, Baxter GF. Nitric oxide/cGMP signalling mediates the cardioprotective action of adrenomedullin in reperfused myocardium. Basic Res Cardiol 105: 257–266, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Hellermann JP, Jacobsen SJ, Reeder GS, Lopez-Jimenez F, Weston SA, Roger VL. Heart failure after myocardial infarction: prevalence of preserved left ventricular systolic function in the community. Am Heart J 145: 742–748, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Ihara T, Ikeda U, Tate Y, Ishibashi S, Shimada K. Positive inotropic effects of adrenomedullin on rat papillary muscle. Eur J Pharmacol 390: 167–172, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Ikenouchi H, Kangawa K, Matsuo H, Hirata Y. Negative inotropic effect of adrenomedullin in isolated adult rabbit cardiac ventricular myocytes. Circulation 95: 2318–2324, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Ishimitsu T, Ono H, Minami J, Matsuoka H. Pathophysiologic and therapeutic implications of adrenomedullin in cardiovascular disorders. Pharmacol Ther 111: 909–927, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Karpinich NO, Hoopes SL, Kechele DO, Lenhart PM, Caron KM. Adrenomedullin function in vascular endothelial cells: insights from genetic mouse models. Curr Hypertens Rev 7: 228–239, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim W, Moon SO, Sung MJ, Kim SH, Lee S, So JN, Park SK. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J 17: 1937–1939, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 192: 553–560, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Schwerbrock NM, Lenhart PM, Fritz-Six KL, Kadmiel M, Christine KS, Kraus DM, Espenschied ST, Willcockson HH, Mack CP, Caron KM. Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. J Clin Invest 123: 2408–2420, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, Towbin JA, Priori SG, Napolitano C, Robinson JL, Andrews M, Timothy K, Hall WJ. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation 97: 2237–2244, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol 71: 1–18, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 148: 1172–1187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikimi T, Tadokoro K, Akimoto K, Mori Y, Ishikawa Y, Ishimura K, Horio T, Kangawa K, Matsuoka H. Response of adrenomedullin system to cytokine in cardiac fibroblasts-role of adrenomedullin as an antifibrotic factor. Cardiovasc Res 66: 104–113, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Nishikimi T, Yoshihara F, Horinaka S, Kobayashi N, Mori Y, Tadokoro K, Akimoto K, Minamino N, Kangawa K, Matsuoka H. Chronic administration of adrenomedullin attenuates transition from left ventricular hypertrophy to heart failure in rats. Hypertension 42: 1034–1041, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Nothnick WB, Healy C. Estrogen induces distinct patterns of microRNA expression within the mouse uterus. Reprod Sci 17: 987–994, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okumura H, Nagaya N, Itoh T, Okano I, Hino J, Mori K, Tsukamoto Y, Ishibashi-Ueda H, Miwa S, Tambara K, Toyokuni S, Yutani C, Kangawa K. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation 109: 242–248, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod 13: 797–806, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Peace RA, Adams PC, Lloyd JJ. Effect of sex, age, and weight on ejection fraction and end-systolic volume reference limits in gated myocardial perfusion SPECT. J Nucl Cardiol 15: 86–93, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Pedram A, Razandi M, O'Mahony F, Harvey H, Harvey BJ, Levin ER. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci Signal 6: ra36, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Raab-Graham KF, Radeke CM, Vandenberg CA. Molecular cloning and expression of a human heart inward rectifier potassium channel. Neuroreport 5: 2501–2505, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Register TC, Adams MR. Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J Steroid Biochem Mol Biol 64: 187–191, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Saito T, Ciobotaru A, Bopassa JC, Toro L, Stefani E, Eghbali M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circ Res 105: 343–352, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma S, Eghbali M. Influence of sex differences on microRNA gene regulation in disease. Biol Sex Diff 5: 3, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimosawa T, Shibagaki Y, Ishibashi K, Kitamura K, Kangawa K, Kato S, Ando K, Fujita T. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation 105: 106–111, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stangl V, Dschietzig T, Bramlage P, Boye P, Kinkel HT, Staudt A, Baumann G, Felix SB, Stangl K. Adrenomedullin and myocardial contractility in the rat. Eur J Pharmacol 408: 83–89, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Szokodi I, Kinnunen P, Ruskoaho H. Inotropic effect of adrenomedullin in the isolated perfused rat heart. Acta Physiol Scand 156: 151–152, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Szokodi I, Kinnunen P, Tavi P, Weckstrom M, Toth M, Ruskoaho H. Evidence for cAMP-independent mechanisms mediating the effects of adrenomedullin, a new inotropic peptide. Circulation 97: 1062–1070, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Tambara K, Fujita M, Nagaya N, Miyamoto S, Iwakura A, Doi K, Sakaguchi G, Nishimura K, Kangawa K, Komeda M. Increased pericardial fluid concentrations of the mature form of adrenomedullin in patients with cardiac remodelling. Heart 87: 242–246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol Cell 3: 513–519, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J Clin Invest 110: 381–388, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuruda T, Kato J, Kitamura K, Kuwasako K, Imamura T, Koiwaya Y, Tsuji T, Kangawa K, Eto T. Adrenomedullin: a possible autocrine or paracrine inhibitor of hypertrophy of cardiomyocytes. Hypertension 31: 505–510, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe H, Takahashi E, Kobayashi M, Goto M, Krust A, Chambon P, Iguchi T. The estrogen-responsive adrenomedullin and receptor-modifying protein 3 gene identified by DNA microarray analysis are directly regulated by estrogen receptor. J Mol Endocrinol 36: 81–89, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Wetzel-Strong SE, Li M, Klein KR, Nishikimi T, Caron KM. Epicardial-derived adrenomedullin drives cardiac hyperplasia during embryogenesis. Dev Dyn 243: 243–256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]